Enzyme-Catalyzed Synthesis of Unsaturated Aliphatic Polyesters Based on Green Monomers from Renewable Resources
Abstract
:1. Introduction
2. Results and Discussion
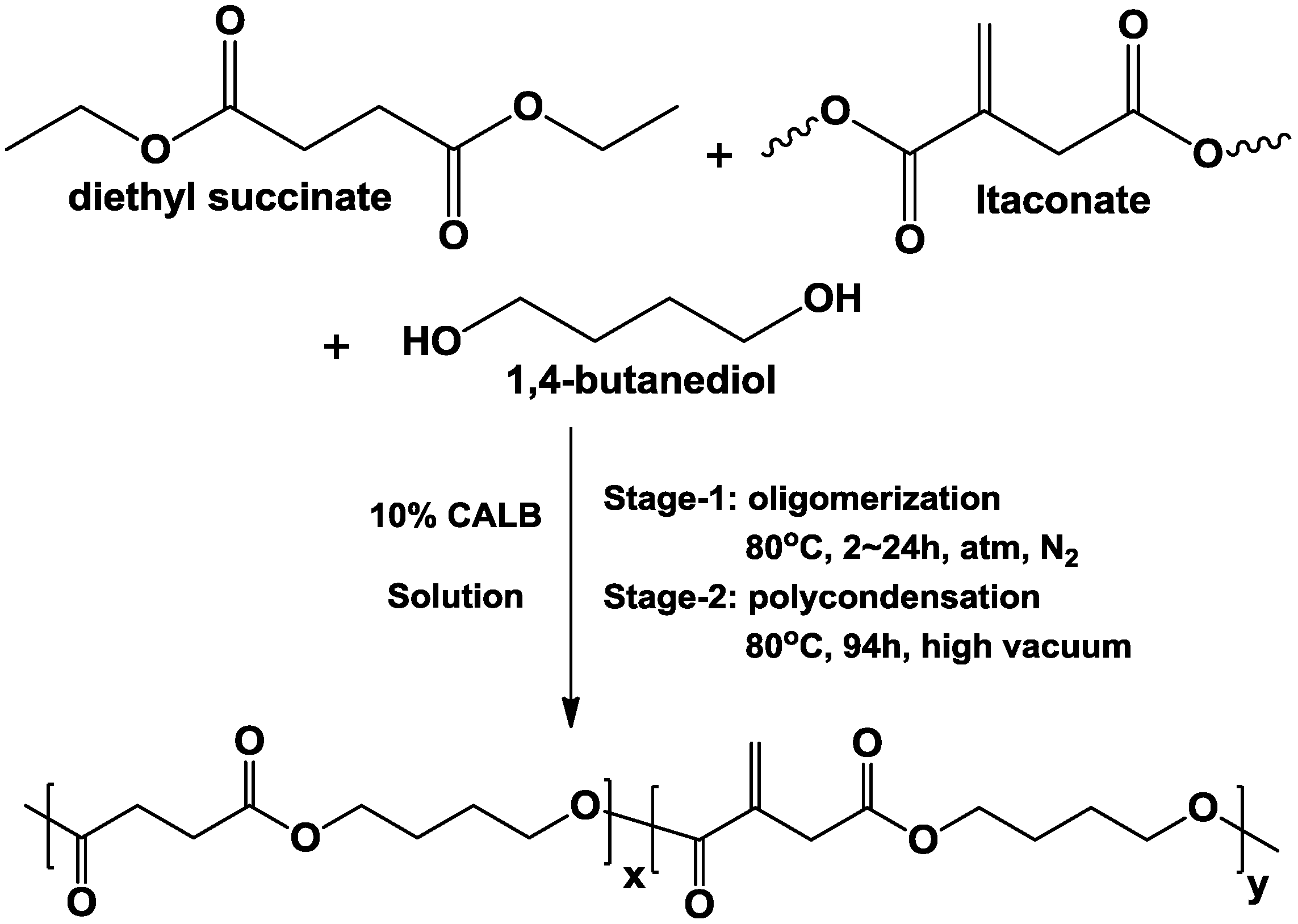
2.1. Effects of Polymerization Conditions on CALB-Catalyzed Co-Polymerization of Diethyl Succinate, Dimethyl Itaconate and 1,4-Butanediol
2.1.1. Effect of Solvent on CALB-Catalyzed Co-Polymerization of Diethyl Succinate, Dimethyl Itaconate and 1,4-Butanediol
| Molar composition | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Feed (%) | PBSI a (%) | Molecular weight | |||||||
| Solvent | log P | FS | FI | FB | XS | XI | XB | Mn (g/mol) b | Yield (%) |
| Diphenyl ether | 4.1 | 35 | 15 | 50 | 40.5 | 10.5 | 49.0 | 1,304 | 55.5 |
| Dodecane | 6.8 | 35 | 15 | 50 | 46.5 | 3.5 | 50.0 | 963 | 54.3 |
| Diglyme | −1.3 | 35 | 15 | 50 | 49.2 | 0.3 | 50.5 | 854 | 68.8 |
| Diphenyl ether | 4.1 | 25 | 25 | 50 | 35.3 | 15.9 | 48.8 | 1,403 | 22.6 |
| Dodecane | 6.8 | 25 | 25 | 50 | 46.5 | 3.7 | 49.8 | 730 | 18.3 |
| Diglyme | −1.3 | 25 | 25 | 50 | 43.2 | 5.9 | 50.9 | 795 | 30.2 |
2.1.2. Effect of Solvent Dosage on CALB-Catalyzed Co-Polymerization of Diethyl Succinate, Dimethyl Itaconate and 1,4-Butanediol
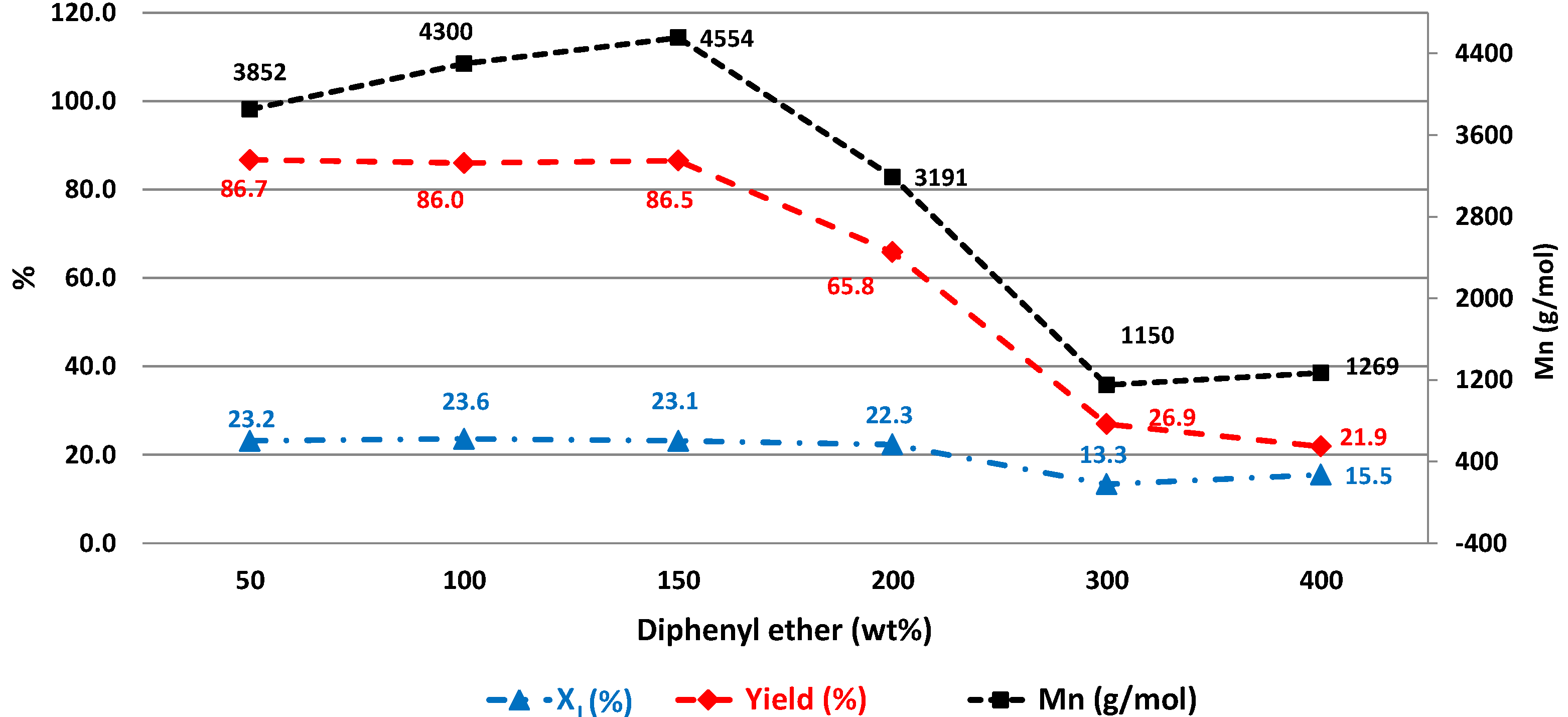
2.1.3. Effect of Oligomerization Time during the First Stage on CALB-Catalyzed Co-Polymerization of Diethyl Succinate, Dimethyl Itaconate and 1,4-Butanediol
| Molar composition | ||||||||
|---|---|---|---|---|---|---|---|---|
| Feed (%) | PBSI a (%) | Molecular weight | ||||||
| Oligomerization time | FS | FI | FB | XS | XI | XB | Mn (g/mol) b | Yield (%) |
| 2 h | 35 | 15 | 50 | 35.7 | 14.1 | 50.2 | 3,936 | 87.7 |
| 6 h | 35 | 15 | 50 | 35.2 | 14.9 | 49.9 | 3,212 | 87.6 |
| 12 h | 35 | 15 | 50 | 36.3 | 14.4 | 49.3 | 2,960 | 84.1 |
| 2 h | 25 | 25 | 50 | 27.0 | 23.1 | 49.9 | 2,935 | 73.1 |
| 6 h | 25 | 25 | 50 | 28.9 | 21.5 | 49.6 | 2,459 | 68.5 |
| 12 h | 25 | 25 | 50 | 27.5 | 22.8 | 49.7 | 2,609 | 71.6 |

2.1.4. Effect of Vacuum during the Second Stage on CALB-Catalyzed Co-Polymerization of Diethyl Succinate, Dimethyl Itaconate and 1,4-Butanediol
| Molar composition | ||||||||
|---|---|---|---|---|---|---|---|---|
| Feed (%) | PBSI a (%) | Molecular weight | ||||||
| Vacuum | FS | FI | FB | XS | XI | XB | Mn (g/mol) b | Yield (%) |
| 2 mmHg | 35 | 15 | 50 | 35.7 | 14.1 | 50.2 | 3,936 | 87.7 |
| 10–20 mmHg | 35 | 15 | 50 | 37.1 | 13.1 | 49.8 | 2,552 | 56.2 |
| 40 mmHg | 35 | 15 | 50 | 40.5 | 10.5 | 49.0 | 1,304 | 55.5 |
| 2 mmHg | 25 | 25 | 50 | 27.0 | 23.1 | 49.9 | 2,935 | 73.1 |
| 10–20 mmHg | 25 | 25 | 50 | 31.7 | 19.1 | 49.2 | 2,328 | 19.5 |
| 40 mmHg | 25 | 25 | 50 | 35.2 | 15.9 | 48.9 | 1,203 | 22.6 |
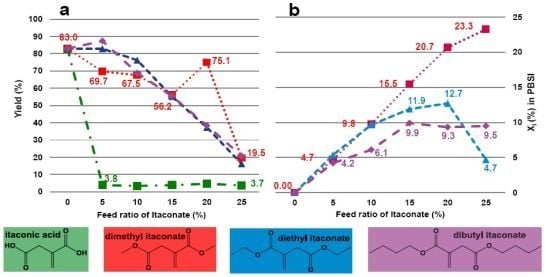
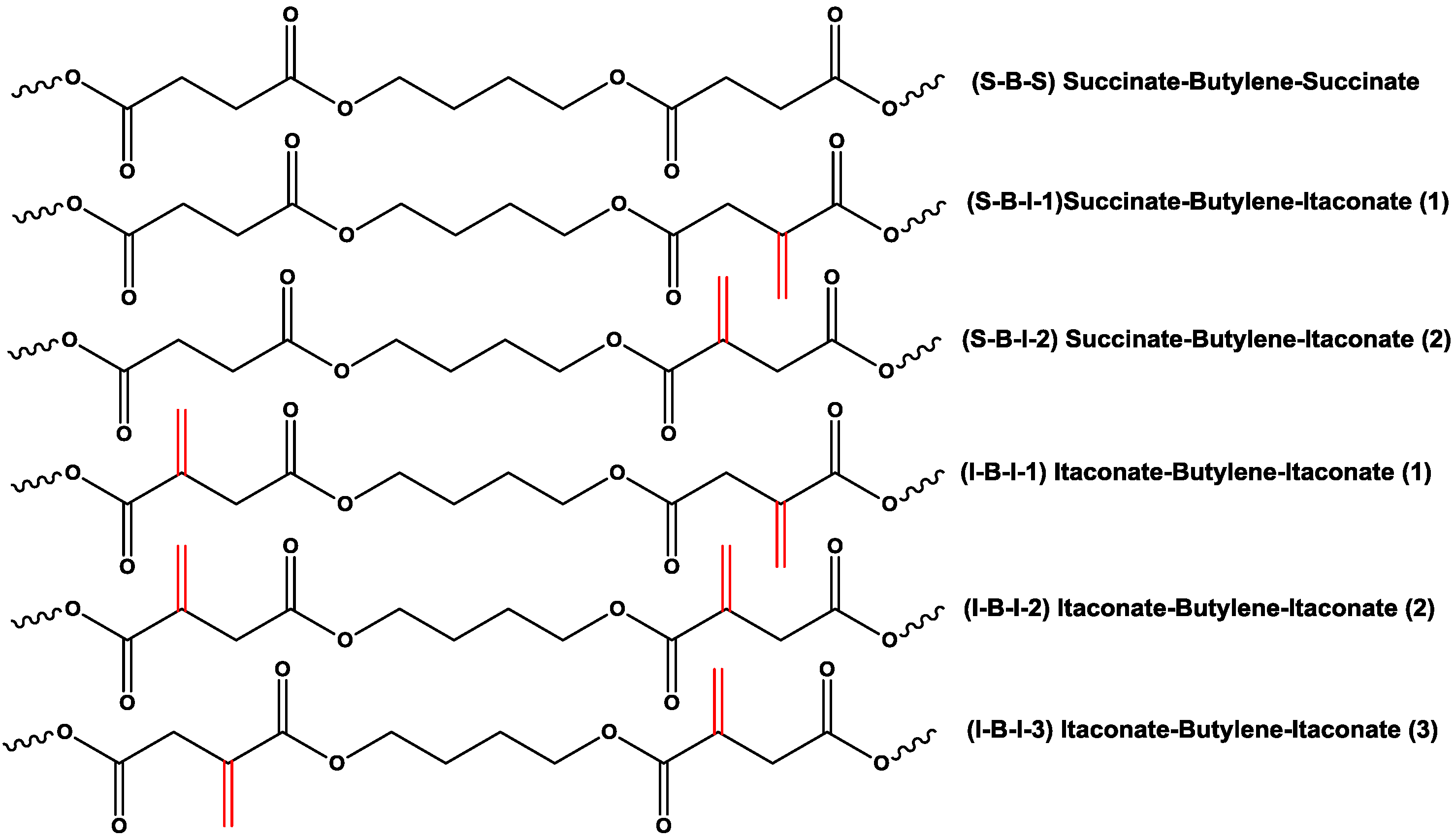
2.2. Effect of Itaconate Structure on CALB-Catalyzed Synthesis of PBSI in Diphenyl Ether
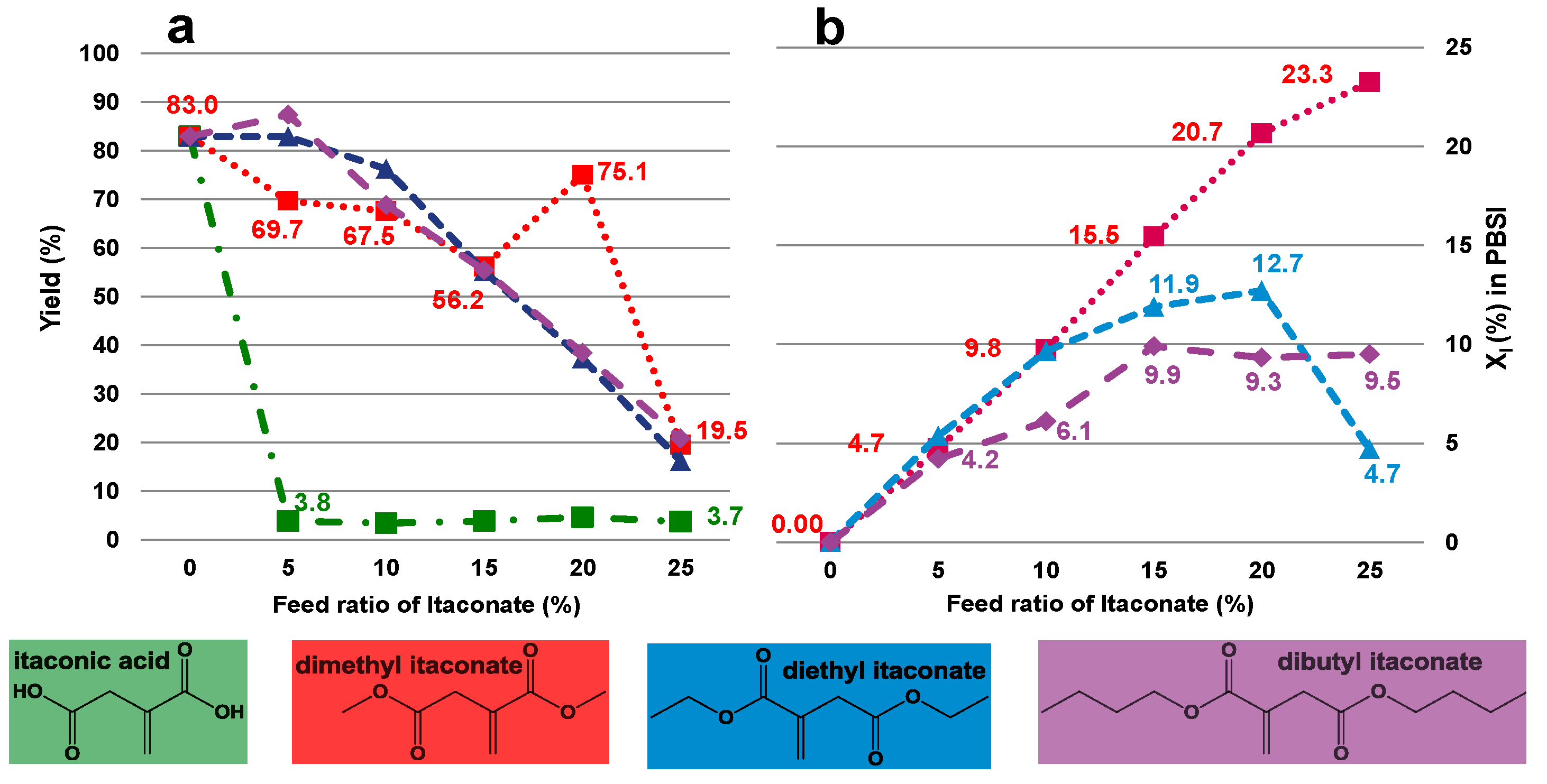
- (1)
- using diphenyl ether as solvent;
- (2)
- the dosage of diphenyl ether is 150 wt% (in relation to the total amount of monomers);
- (3)
- oligomerization for 2 h during the first stage;
- (4)
- regulating vacuum to 2 mmHg during the second stage;
- (5)
- applying dimethyl itaconate as the unsaturated monomer.
2.3. CALB-Catalyzed Synthesis of PBSI Using Optimal Polymerization Conditions
| Molar composition | Molecular Weight | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Feed (%) | PBSI a (%) | NMR | GPC | |||||||||||
| Co-polyester | FS | FI | FB | XS | XI | XB | Yield (%) | Tg b (°C) | Tm c (°C) | Td d (°C) | Mn e | Mn | Mw | Mw/Mn |
| PBS | 50 | 0 | 50 | 50.5 | 0.0 | 49.5 | 85.7 | −35.9 | 112.9 | 406.1 | 4,463 | 6,017 | 11,520 | 1.91 |
| PB50S40I10 | 40 | 10 | 50 | 39.7 | 10.4 | 49.9 | 84.6 | −38.8 | 94.6 | 402.0 | 4,696 | 10,128 | 19,236 | 1.90 |
| PB50S35I15 | 35 | 15 | 50 | 35.0 | 15.7 | 49.3 | 90.0 | −36.7 | 80.3 | 410.9 | 6,494 | 13,288 | 22,642 | 1.70 |
| PB50S30I20 | 30 | 20 | 50 | 30.9 | 20.1 | 49.0 | 75.1 | −37.5 | 71.2 | 404.7 | 5,670 | 8,394 | 11,892 | 1.42 |
| PB50S25I25 | 25 | 25 | 50 | 27.2 | 23.5 | 49.3 | 86.5 | −38.4 | 57.4 | 407.9 | 4,554 | 11,096 | 16,587 | 1.49 |
| PBSI | 20 | 30 | 50 | 36.4 | 15.6 | 48.0 | 20.9 | −49.6 | 69.8 | 396.9 | 1,004 | 1,948 | 2,203 | 1.13 |
| NA f | 15 | 35 | 50 | - | - | - | - | - | - | - | - | - | - | - |
| NA f | 0 | 50 | 50 | - | - | - | - | - | - | - | - | - | - | - |
| Control-1 g | 50 | 0 | 50 | - | - | - | - | - | - | - | - | - | - | - |
| Control-2 g | 25 | 25 | 50 | - | - | - | - | - | - | - | - | - | - | - |
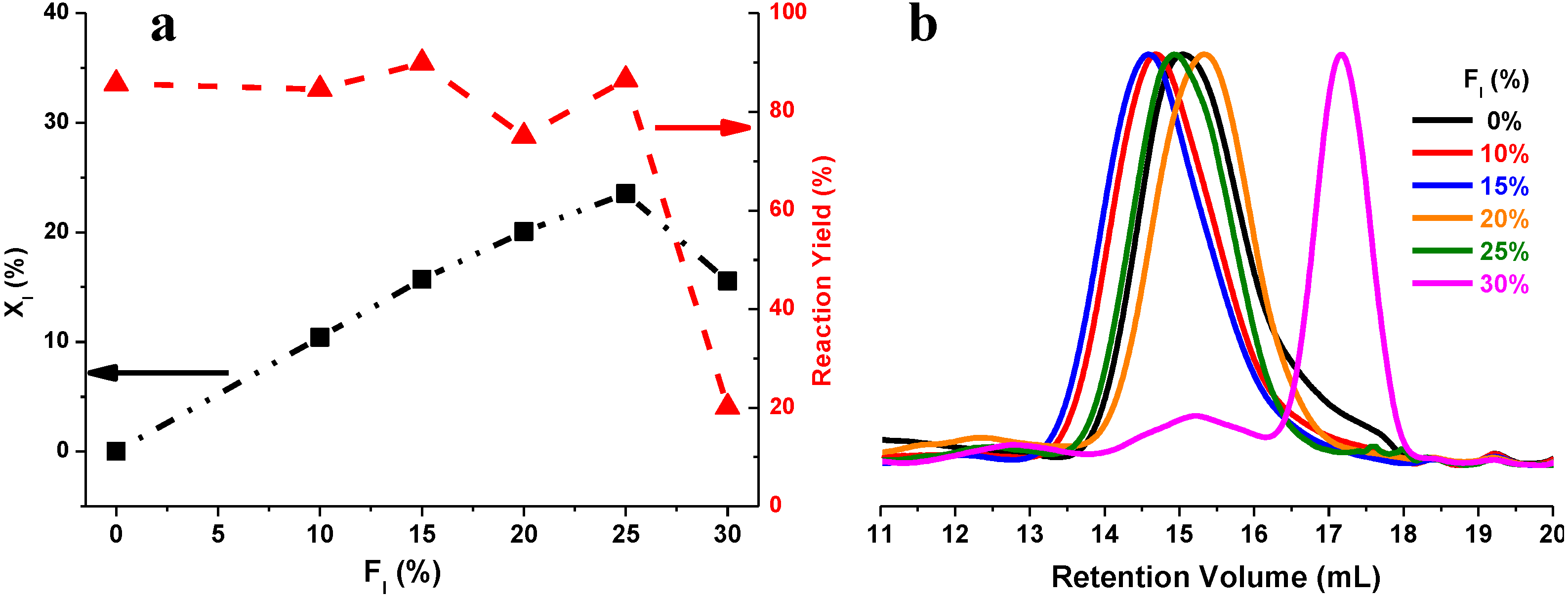

2.4. Thermal Properties of PBS and PBSI

3. Experimental
3.1. Materials
3.2. General Procedure for CALB-Catalyzed Co-Polymerization of Succinate, Itaconate and 1,4-Butanediol in Solution
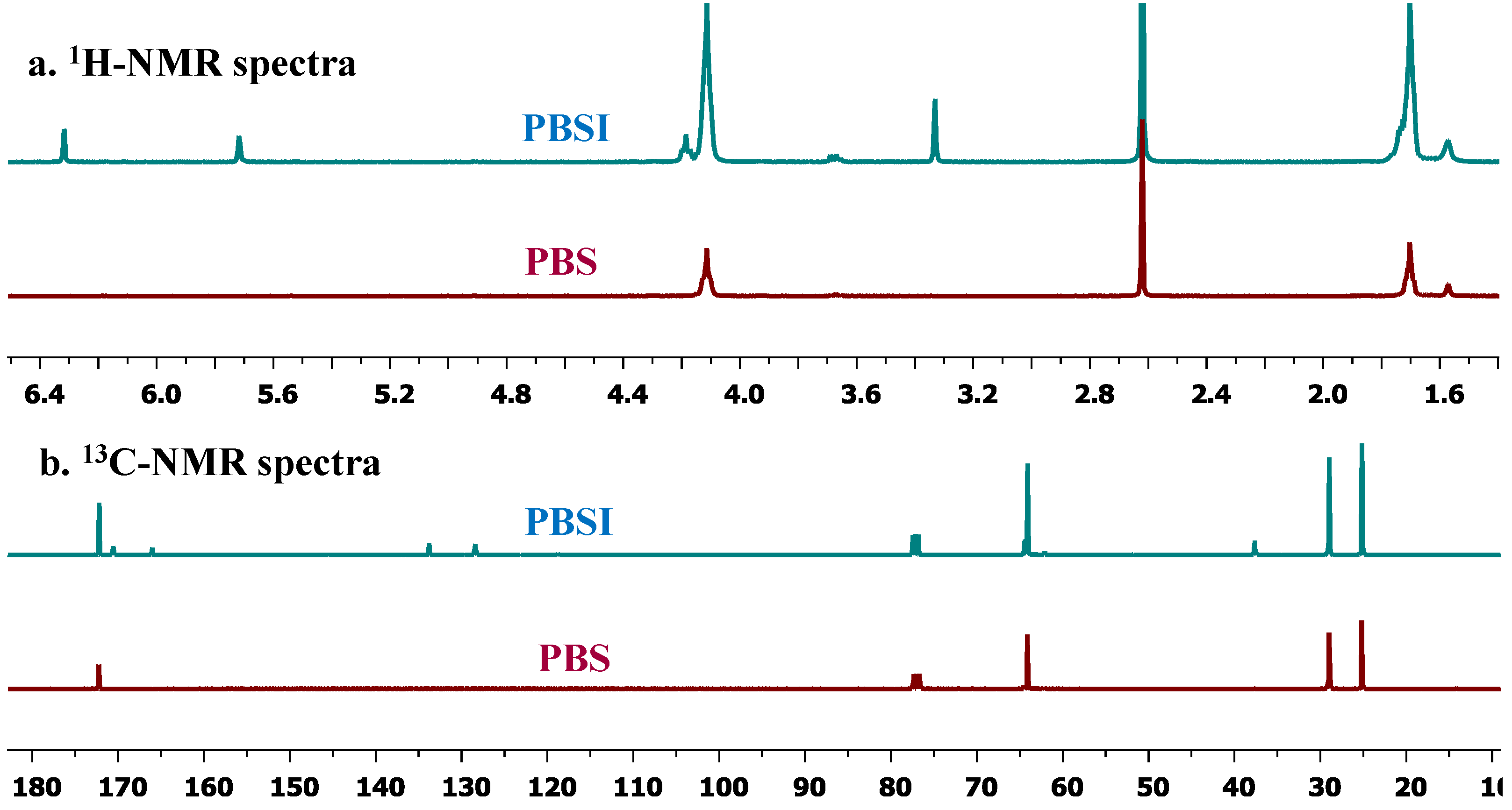
3.3. Control Reactions for Enzymatic Polymerization
3.4. In situ 1H-NMR Investigation of the Oligomerization Process of Diethyl Succinate, Dimethyl Itaconate and 1,4-Butanediol in Diphenyl Ether
3.5. Instrumental Methods
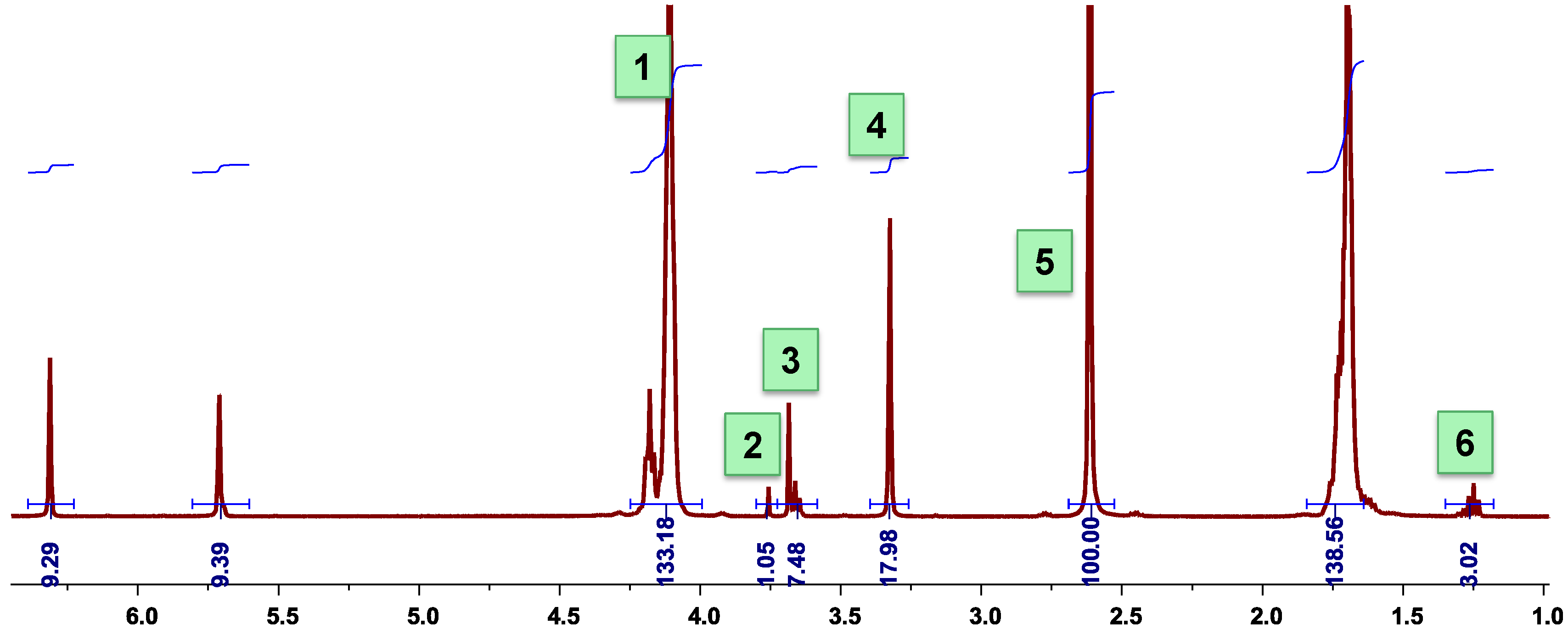
3.6. Calculation of the Molar Composition of PBSI from 1H-NMR Spectra
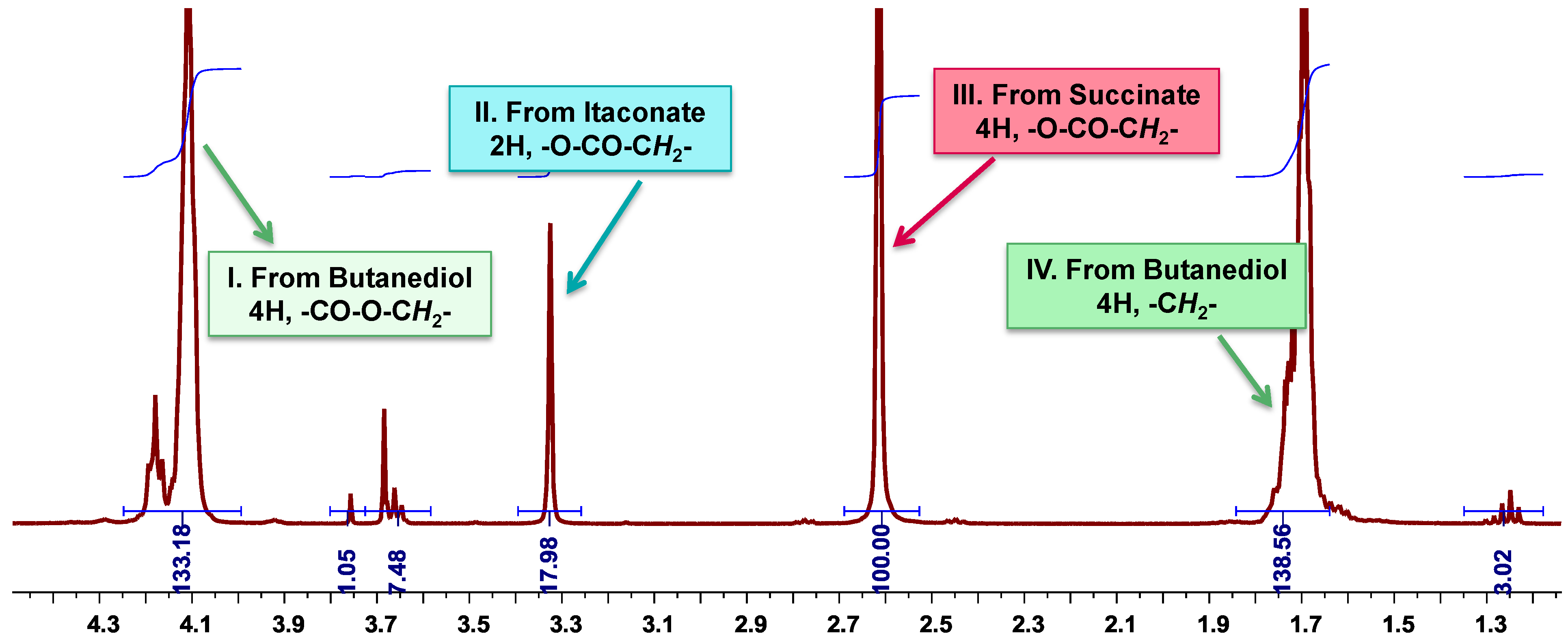

3.7. Calculation of the Number Average Molecular Weight of PBSI from 1H-NMR Spectra

4. Conclusions
Acknowledgments
Conflict of Interest
References
- Gandini, A. Monomers and macromonomers from renewable resources. In Biocatalysis in Polymer Chemistry; Loos, K., Ed.; Wiley-VCH: Weinheim, Germany, 2010; pp. 1–34. [Google Scholar]
- Dove, A. Polymer science tries to make it easy to be green. Science 2012, 335, 1382–1384. [Google Scholar] [CrossRef]
- Mathers, R.T. How well can renewable resources mimic commodity monomers and polymers? J. Polym. Sci. Pol. Chem. 2012, 50, 1–15. [Google Scholar] [CrossRef]
- Mülhaupt, R. Green polymer chemistry and bio-based plastics: Dreams and reality. Macromol. Chem. Phys. 2013, 214, 159–174. [Google Scholar] [CrossRef]
- Robert, C.; de Montigny, F.; Thomas, C.M. Tandem synthesis of alternating polyesters from renewable resources. Nat. Commun. 2011, 2, e586. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G.R. Top Value Added Chemicals from Biomass Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; US Department of Energy: Oak Ridge, TN, USA, 2004; pp. 1–67. [Google Scholar]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Teramoto, N.; Ozeki, M.; Fujiwara, I.; Shibata, M. Crosslinking and biodegradation of poly(butylene succinate) prepolymers containing itaconic or maleic acid units in the main chain. J. Appl. Polym. Sci. 2005, 95, 1473–1480. [Google Scholar] [CrossRef]
- Barrett, D.G.; Merkel, T.J.; Luft, J.C.; Yousaf, M.N. One-step syntheses of photocurable polyesters based on a renewable resource. Macromolecules 2010, 43, 9660–9667. [Google Scholar] [CrossRef]
- Jasinska, L.; Koning, C.E. Unsaturated, biobased polyesters and their cross-linking via radical copolymerization. J. Polym. Sci. Pol. Chem. 2010, 48, 2885–2895. [Google Scholar] [CrossRef]
- Zhang, Y.-R.; Spinella, S.; Xie, W.; Cai, J.; Yang, Y.; Wang, Y.-Z.; Gross, R.A. Polymeric triglyceride analogs prepared by enzyme-catalyzed condensation polymerization. Eur. Polym. J. 2013, 49, 793–803. [Google Scholar] [CrossRef]
- Gross, R.A.; Ganesh, M.; Lu, W. Enzyme-catalysis breathes new life into polyester condensation polymerizations. Trends Biotechnol. 2010, 28, 435–443. [Google Scholar] [CrossRef]
- Mahapatro, A.; Kalra, B.; Kumar, A.; Gross, R.A. Lipase-catalyzed polycondensations: Effect of substrates and solvent on chain formation, dispersity, and end-group structure. Biomacromolecules 2003, 4, 544–551. [Google Scholar] [CrossRef]
- Albertsson, A.C.; Srivastava, R.K. Recent developments in enzyme-catalyzed ring-opening polymerization. Adv. Drug Deliv. Rev. 2008, 60, 1077–1093. [Google Scholar] [CrossRef]
- Kobayashi, S. Recent developments in lipase-catalyzed synthesis of polyesters. Macromol. Rapid Commun. 2009, 30, 237–266. [Google Scholar] [CrossRef]
- Uyama, H.; Kobayashi, S. Enzymatic synthesis of polyesters via polycondensation. In Enzyme-Catalyzed Synthesis of Polymers; Kobayashi, S., Ritter, H., Kaplan, D., Eds.; Springer-Verlag Berlin: Berlin, Germany, 2006; Volume 194, pp. 133–158. [Google Scholar]
- Miletic, N.; Loos, K.; Gross, R.A. Enzymatic polymerization of polyester. In Biocatalysis in Polymer Chemistry; Loos, K., Ed.; Wiley-VCH: Weinheim, Germany, 2010; pp. 83–130. [Google Scholar]
- Gross, R.A.; Kumar, A.; Kalra, B. Polymer synthesis by in vitro enzyme catalysis. Chem. Rev. 2001, 101, 2097–2124. [Google Scholar] [CrossRef]
- Miletic, N.; Abetz, V.; Ebert, K.; Loos, K. Immobilization of Candida antarctica lipase B on polystyrene nanoparticles. Macromol. Rapid Commun. 2010, 31, 71–74. [Google Scholar] [CrossRef]
- Matsumura, S. Enzymatic synthesis of polyesters via ring-opening polymerization. In Enzyme-Catalyzed Synthesis of Polymers; Kobayashi, S., Ritter, H., Kaplan, D., Eds.; Springer-Verlag Berlin: Berlin, Germany, 2006; Volume 194, pp. 95–132. [Google Scholar]
- Stavila, E.; Alberda van Ekenstein, G.O.R.; Loos, K. Enzyme-catalyzed synthesis of aliphatic-aromatic oligoamides. Biomacromolecules 2013, 14, 1600–1606. [Google Scholar] [CrossRef]
- Miletic, N.; Fahriansyah; Nguyen, L.T.T.; Loos, K. Formation, topography and reactivity of Candida antarctica lipase B immobilized on silicon surface. Biocatal. Biotransform. 2010, 28, 357–369. [Google Scholar] [CrossRef]
- Miletic, N.; Nastasovic, A.; Loos, K. Immobilization of biocatalysts for enzymatic polymerizations: Possibilities, advantages, applications. Bioresour. Technol. 2012, 115, 126–135. [Google Scholar] [CrossRef]
- Stavila, E.; Arsyi, R.Z.; Petrovic, D.M.; Loos, K. Fusarium solani pisi cutinase-catalyzed synthesis of polyamides. Eur. Polym. J. 2013, 49, 834–842. [Google Scholar] [CrossRef]
- Stavila, E.; Loos, K. Synthesis of lactams using enzyme-catalyzed aminolysis. Tetrahedron Lett. 2013, 54, 370–372. [Google Scholar] [CrossRef]
- Binns, F.; Harffey, P.; Roberts, S.M.; Taylor, A. Studies of lipase-catalyzed polyesterification of an unactivated diacid/diol system. J. Polym. Sci. Pol. Chem. 1998, 36, 2069–2079. [Google Scholar]
- Binns, F.; Harffey, P.; Roberts, S.M.; Taylor, A. Studies leading to the large scale synthesis of polyesters using enzymes. J. Chem. Soc. Perkin Trans. 1 1999, 2671–2676. [Google Scholar]
- Azim, H.; Dekhterman, A.; Jiang, Z.; Gross, R.A. Candida antarctica lipase B-catalyzed synthesis of poly(butylene succinate): Shorter chain building blocks also work. Biomacromolecules 2006, 7, 3093–3097. [Google Scholar] [CrossRef]
- Habeych, D.I.; Juhl, P.B.; Pleiss, J.; Vanegas, D.; Eggink, G.; Boeriu, C.G. Biocatalytic synthesis of polyesters from sugar-based building blocks using immobilized Candida antarctica lipase B. J. Mol. Catal. B-Enzym. 2011, 71, 1–9. [Google Scholar] [CrossRef]
- Juais, D.; Naves, A.F.; Li, C.; Gross, R.A.; Catalani, L.H. Isosorbide polyesters from enzymatic catalysis. Macromolecules 2010, 43, 10315–10319. [Google Scholar] [CrossRef]
- Jiang, Z. Lipase-catalyzed synthesis of aliphatic polyesters via copolymerization of lactone, dialkyl diester, and diol. Biomacromolecules 2008, 9, 3246–3251. [Google Scholar] [CrossRef]
- Ebata, H.; Toshima, K.; Matsumura, S. Lipase-catalyzed synthesis and properties of poly[(12-hydroxydodecanoate)-co-(12-hydroxystearate)] directed towards novel green and sustainable elastomers. Macromol. Biosci. 2008, 8, 38–45. [Google Scholar] [CrossRef]
- Kobayashi, T.; Matsumura, S. Enzymatic synthesis and properties of novel biodegradable and biobased thermoplastic elastomers. Polym. Degrad. Stab. 2011, 96, 2071–2079. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Uyama, H.; Kobayashi, S. Enzymatic synthesis of cross-linkable polyesters from renewable resources. Biomacromolecules 2001, 2, 29–31. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Uyama, H.; Kobayashi, S. Enzymatic synthesis and curing of biodegradable crosslinkable polyesters. Macromol. Biosci. 2002, 2, 329–335. [Google Scholar] [CrossRef]
- Uyama, H.; Kuwabara, M.; Tsujimoto, T.; Kobayashi, S. Enzymatic synthesis and curing of biodegradable epoxide-containing polyesters from renewable resources. Biomacromolecules 2003, 4, 211–215. [Google Scholar] [CrossRef]
- Jiang, Z.; Azim, H.; Gross, R.A.; Focarete, M.L.; Scandola, M. Lipase-catalyzed copolymerization of ω-pentadecalactone with p-dioxanone and characterization of copolymer thermal and crystalline properties. Biomacromolecules 2007, 8, 2262–2269. [Google Scholar] [CrossRef]
- Mazzocchetti, L.; Scandola, M.; Jiang, Z. Enzymatic synthesis and structural and thermal properties of poly(ω-pentadecalactone-co-butylene-co-succinate). Macromolecules 2009, 42, 7811–7819. [Google Scholar] [CrossRef]
- Liu, W.; Wang, F.; Tan, T.; Chen, B. Lipase-catalyzed synthesis and characterization of polymers by cyclodextrin as support architecture. Carbohydr. Polym. 2013, 92, 633–640. [Google Scholar] [CrossRef]
- Jiang, Z. Lipase-catalyzed copolymerization of dialkyl carbonate with 1,4-butanediol and omega-pentadecalactone: Synthesis of poly-(omega-pentadecalactone-co-butylene-co-carbonate). Biomacromolecules 2011, 12, 1912–1919. [Google Scholar] [CrossRef]
- MuralidharaRao, D.; Hussain, S.M.D.J.; Rangadu, V.P.; Subramanyam, K.; Krishna, G.S.; Swamy, A.V.N. Fermentatative production of itaconic acid by aspergillus terreus using Jatropha seed cake. Afr. J. Biotechnol. 2007, 6, 2140–2142. [Google Scholar]
- Guo, B.; Chen, Y.; Lei, Y.; Zhang, L.; Zhou, W.Y.; Rabie, A.B.M.; Zhao, J. Biobased poly(propylene sebacate) as shape memory polymer with tunable switching temperature for potential biomedical applications. Biomacromolecules 2011, 12, 1312–1321. [Google Scholar] [CrossRef]
- Wei, T.; Lei, L.; Kang, H.; Qiao, B.; Wang, Z.; Zhang, L.; Coates, P.; Hua, K.-C.; Kulig, J. Tough bio-based elastomer nanocomposites with high performance for engineering applications. Adv. Eng. Mater. 2012, 14, 112–118. [Google Scholar] [CrossRef]
- Retuert, J.; Yazdanipedram, M.; Martinez, F.; Jeria, M. Soluble itaconic acid ethylene-glycol polyesters. Bull. Chem. Soc. Jpn. 1993, 66, 1707–1708. [Google Scholar] [CrossRef]
- Ramo, V.; Anghelescu-Hakala, A.; Nurmi, L.; Mehtio, T.; Salomaki, E.; Harkonen, M.; Harlin, A. Preparation of aqueous crosslinked dispersions of functionalized poly(d,l-lactic acid) with a thermomechanical method. Eur. Polym. J. 2012, 48, 1495–1503. [Google Scholar] [CrossRef]
- Sakuma, T.; Kumagai, A.; Teramoto, N.; Shibata, M. Thermal and dynamic mechanical properties of organic-inorganic hybrid composites of itaconate-containing poly(butylene succinate) and methacrylate-substituted polysilsesquioxane. J. Appl. Polym. Sci. 2008, 107, 2159–2164. [Google Scholar] [CrossRef]
- Brugel, W.; Demmler, K. Synthesis and properties of itaconic acid-containing polyester resins. J. Polym. Sci. Pol. Sym. 1969, 22, 1117–1137. [Google Scholar] [CrossRef]
- Sepulchre, M.O.; Sepulchre, M. Synthesis of unsaturated polyesters from dipotassium salts of cis-aconitic, itaconic and mesaconic acids and 1,4-dibromobutane. Macromol. Symp. 1997, 122, 291–296. [Google Scholar] [CrossRef]
- Rajkhowa, R.; Varma, I.K.; Albertsson, A.C.; Edlund, W. Enzyme-catalyzed copolymerization of oxiranes with dicarboxylic acid anhydrides. J. Appl. Polym. Sci. 2005, 97, 697–704. [Google Scholar] [CrossRef]
- Linko, Y.Y.; Wang, Z.L.; Seppala, J. Lipase-catalyzed linear aliphatic polyester synthesis in organic-solvent. Enzyme Microb. Technol. 1995, 17, 506–511. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jiang, Y.; Woortman, A.J.J.; Van Ekenstein, G.O.R.A.; Loos, K. Enzyme-Catalyzed Synthesis of Unsaturated Aliphatic Polyesters Based on Green Monomers from Renewable Resources. Biomolecules 2013, 3, 461-480. https://doi.org/10.3390/biom3030461
Jiang Y, Woortman AJJ, Van Ekenstein GORA, Loos K. Enzyme-Catalyzed Synthesis of Unsaturated Aliphatic Polyesters Based on Green Monomers from Renewable Resources. Biomolecules. 2013; 3(3):461-480. https://doi.org/10.3390/biom3030461
Chicago/Turabian StyleJiang, Yi, Albert J.J. Woortman, Gert O.R. Alberda Van Ekenstein, and Katja Loos. 2013. "Enzyme-Catalyzed Synthesis of Unsaturated Aliphatic Polyesters Based on Green Monomers from Renewable Resources" Biomolecules 3, no. 3: 461-480. https://doi.org/10.3390/biom3030461
APA StyleJiang, Y., Woortman, A. J. J., Van Ekenstein, G. O. R. A., & Loos, K. (2013). Enzyme-Catalyzed Synthesis of Unsaturated Aliphatic Polyesters Based on Green Monomers from Renewable Resources. Biomolecules, 3(3), 461-480. https://doi.org/10.3390/biom3030461