QM/MM Molecular Dynamics Studies of Metal Binding Proteins
Abstract
:1. Introduction
2. QM/MM Calculations: An Introduction



3. QM/MM Applications to the Study of Enzymatic Reactivity and Metal Binding to Biomolecules
3.1. High Redox Intermediates in Enzymatic Cycles: Heme Hydroperoxidase Catalysis
3.1.1. Mechanism of Cpd I Formation in Peroxidases

3.1.2. Characterization of Cpd I in Catalase-Peroxidases
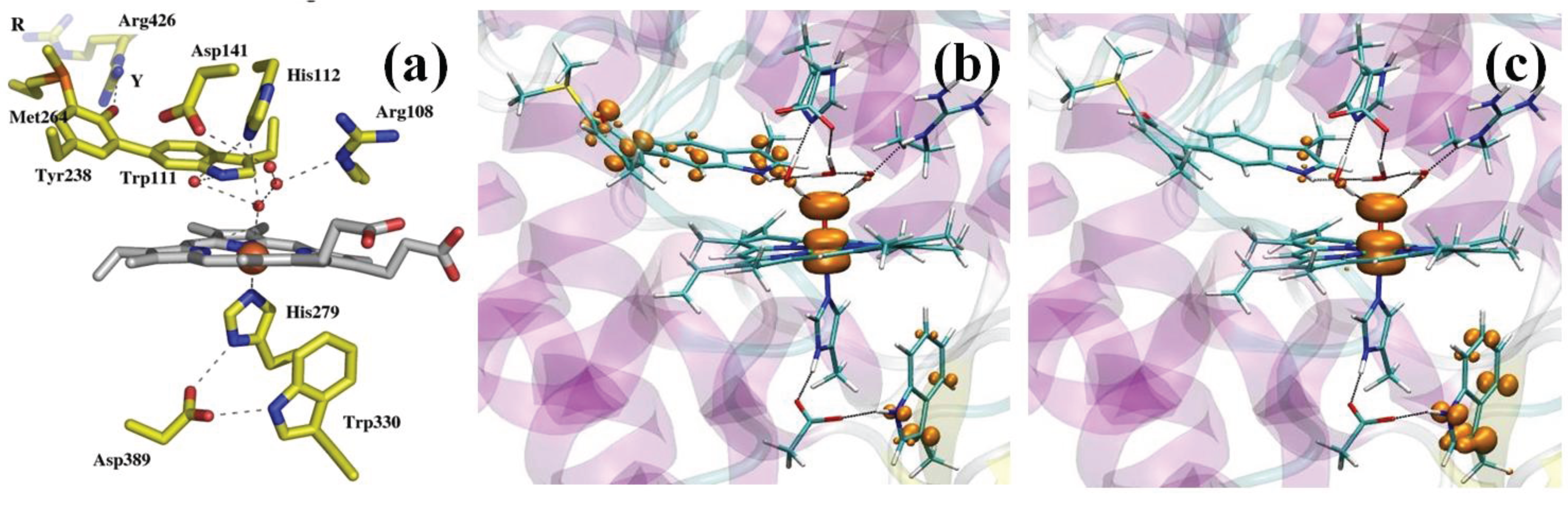
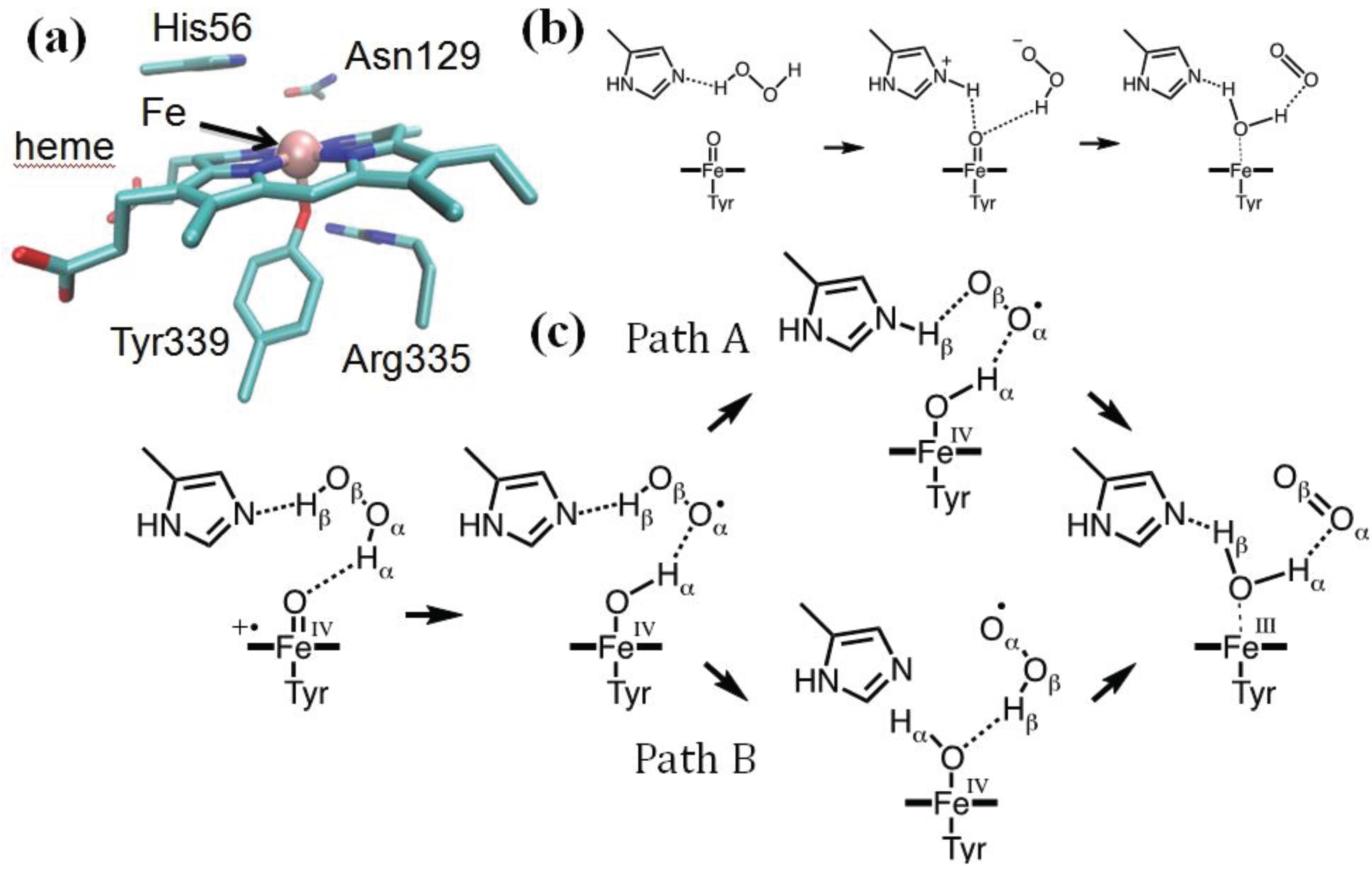
3.1.3. Mechanism of Cpd I Reduction in Catalases
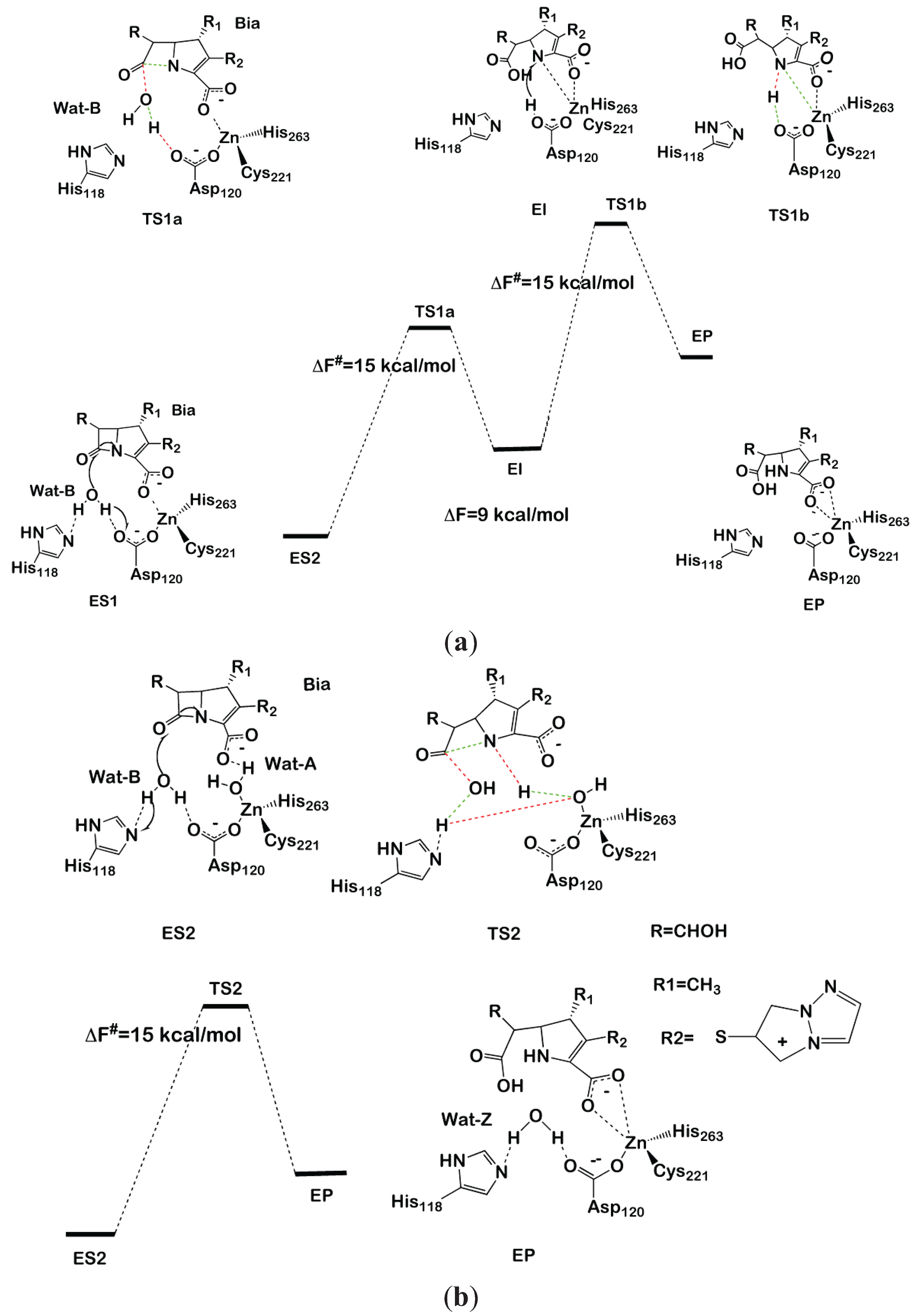
3.2. Zn Enzymatic Drug Metabolism: Antibiotic Hydrolysis by Metallo-β-Lactamases Enzymes
3.3. Cu-Mediated Amyloid Formation: Cu(II)-A-synuclein Adducts in Parkinson’s Disease
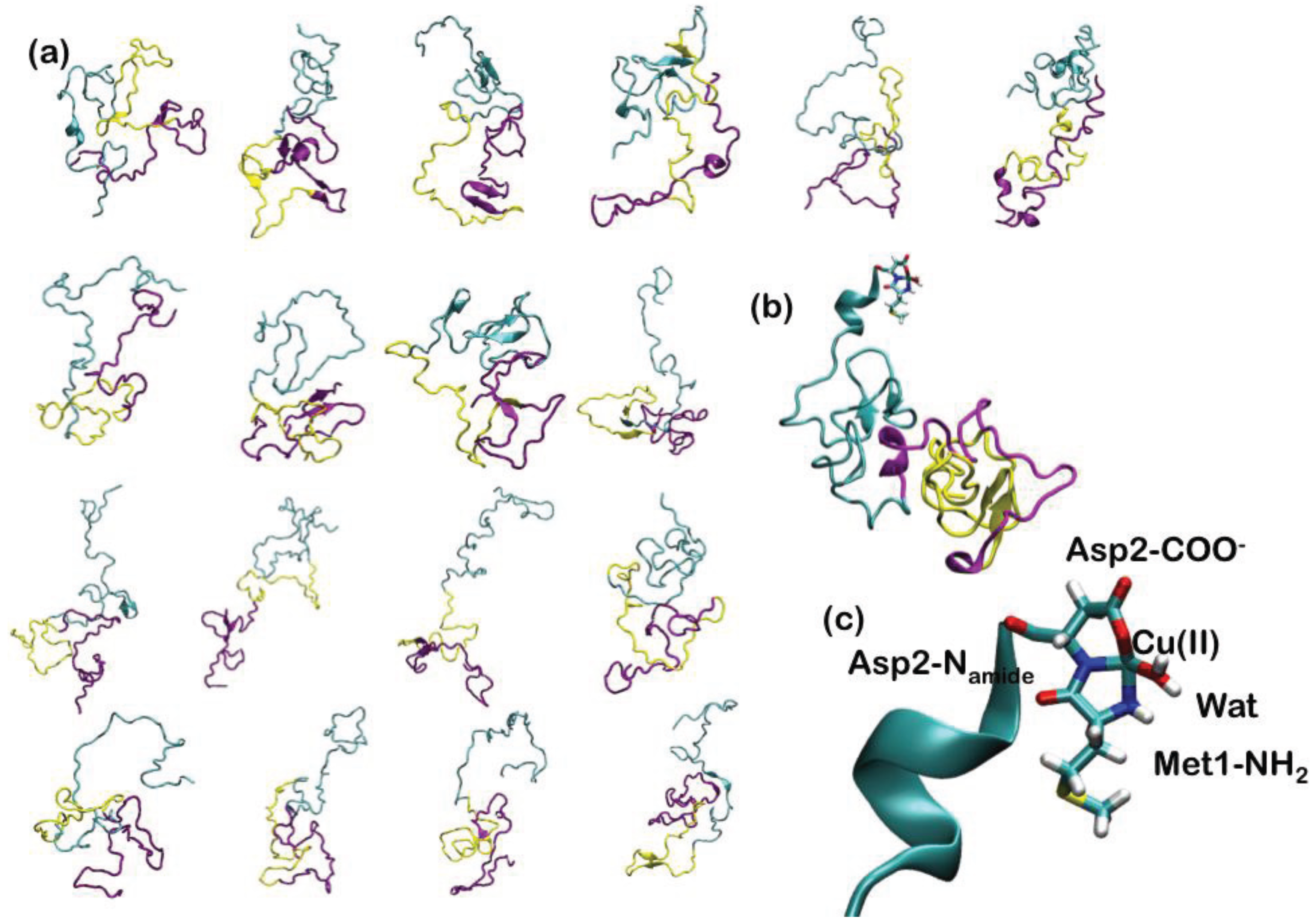
| Cu(II)-ligand bond lengths and angles for the AS-Cu(II) | X-ray | |
|---|---|---|
| Cu(II)-NH2@Met1 | 2.06 (0.02) | 2.00 |
| Cu(II)-N−amide@Asp2 | 1.92 (0.02) | 1.92 |
| Cu(II)-O−@Asp2 | 2.01 (0.02) | 1.98 |
| Cu(II)-O@Wat | 2.09 (0.02) | 1.97 |
| Asp2@O-Cu(II)-NH2@Met1 | 164 (2) | 167 |
| Wat@O-Cu(II)-N−amide@Asp2 | 168 (2) | 166 |
| N-amide-Cu(II)-NH2@Met1 | 84 (1) | 84 |
| Wat@O-Cu(II)-O−@Asp2 | 88 (1) | 84 |
3.4. Mg(II) Ions in Nucleic Acids Synthesis: The Case of the RNA Ligase Ribozymes
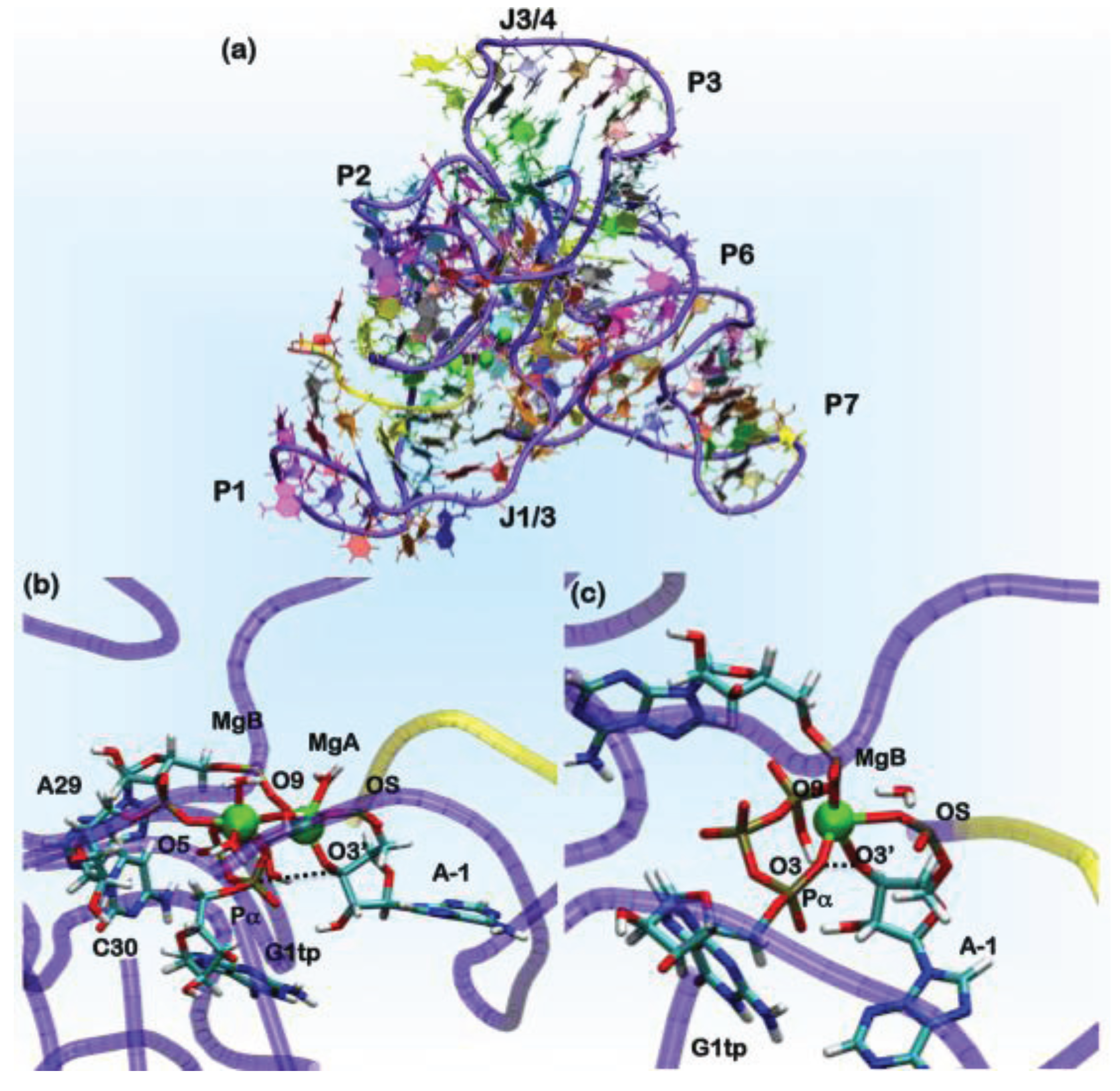
| AdMgAB | AdMgB | |||||||
|---|---|---|---|---|---|---|---|---|
| Atom-1 | Atom-2 | Distance (Å) | Atom-1 | Atom-2 | Distance (Å) | Atom-1 | Atom-2 | Distance (Å) |
| O(R)@C30 | MgA | 2.50 (0.06) | O3@G1tp | MgB | 2.26 (0.11) | O3@ G1tp | MgB | 2.12 (0.06) |
| O(S)@A29 | MgA | 2.06 (0.06) | O(S)@A29 | MgB | 2.26 (0.06) | O(S)@A29 | MgB | 1.86 (0.12) |
| O5@G1tp | MgA | 2.06 (0.06) | O9@G1tp | MgB | 2.98 (0.10) | O9@ G1tp | MgB | 2.01 (0.06) |
| O@Wat2 | MgA | 2.35 (0.11) | O3’@A-1 | MgB | 2.00 (0.05) | O3'@A-1 | MgB | 2.46 (0.13) |
| O@Wat3 | MgA | 2.47 (0.07) | O(S)@A-1 | MgB | 2.17 (0.07) | O4@G1tp | MgB | 2.26 (0.06) |
| O@Wat4 | MgA | 2.18 (0.10) | O@Wat1 | MgB | 2.28 (0.09) | O(S)@A-1 | MgB | 2.36 (0.05) |
| MgA | MgB | 4.05 (0.0001) | O4@G1tp | MgB | 2.67 (0.12) | |||
| O3’@A-1 | Pa@G1tp | 4.08 (0.10) | O3’@A-1 | Pa@G1tp | 3.96 (0.20) | |||
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shi, W.; Chance, M.R. Metalloproteomics: Forward and reverse approaches in metalloprotein structural and functional characterization. Curr. Opin. Chem. Biol. 2011, 15, 144–148. [Google Scholar] [CrossRef]
- Warshel, A.; Levitt, M. Theoretical studies on enzymic reactions—Dielectric, electrostatic and steric stabilization of carbonium-ion in reaction of lysozyme. J. Mol. Biol. 1976, 103, 227–249. [Google Scholar] [CrossRef]
- Field, M.J.; Bash, P.A.; Karplus, M. A combined quantum-mechanical and molecular-mechanical potential for molecular-dynamics simulations. J. Comput. Chem. 1990, 11, 700–733. [Google Scholar] [CrossRef]
- Singh, U.C.; Kollman, P.A. A combined ab initio quatum-mechanical and molecular mechanical method for carrying out simulations on complex molecular-systems—Applications to the CH3Cl + Cl− exchange reaction and gas-phase protonation of polyethers. J. Comput. Chem. 1986, 7, 718–730. [Google Scholar] [CrossRef]
- 2013 Nobel Prize in Chemistry. Available online: http://www.nobelprize.org/nobel_prizes/chemistry/ (accessed on 17 June 2014).
- Ditzler, M.A.; Otyepka, M.; Sponer, J.; Walter, N.G. Molecular dynamics and quantum mechanics of RNA: Conformational and chemical change we can believe in. Acc. Chem. Res. 2010, 43, 40–47. [Google Scholar] [CrossRef]
- Yang, L.; Arora, K.; Beard, W.A.; Wilson, S.H.; Schlick, T. Critical role of magnesium ions in DNA polymerase beta’s closing and active site assembly. J. Am. Chem. Soc. 2004, 126, 8441–8453. [Google Scholar] [CrossRef]
- Orcellet, M.L.; Fernández, C.O. Structures behind the amyloid aggregation of α-synuclein: An NMR based approach. Curr. Protein Pept. Sci. 2011, 12, 188–204. [Google Scholar] [CrossRef]
- Crowder, M.W.; Spencer, J.; Vila, A.J. Metallo-beta-lactamases: Novel weaponry for antibiotic resistance in bacteria. Acc. Chem. Res. 2006, 39, 721–728. [Google Scholar] [CrossRef]
- Meini, M.R.; González, L.J.; Vila, A.J. Antibiotic resistance in Zn(II)-deficient environments: Metallo-β-lactamase activation in the periplasm. Future Microbiol. 2013, 8, 947–979. [Google Scholar] [CrossRef]
- Magistrato, A.; Ruggerone, P.; Spiegel, K.; Carloni, P.; Reedijk, J. Binding of novel azole-bridged dinuclear platinum(II) anticancer drugs to DNA: Insights from hybrid QM/MM molecular dynamics simulations. J. Phys. Chem. B 2006, 110, 3604–3613. [Google Scholar] [CrossRef]
- Spiegel, K.; Rothlisberger, U.; Carloni, P. Cisplatin binding to DNA oligomers from hybrid Car–Parrinello/molecular dynamics simulations. J. Phys. Chem. B 2004, 108, 2699–2707. [Google Scholar] [CrossRef]
- Gossens, C.; Tavernelli, I.; Rothlisberger, U. DNA structural distortions induced by ruthenium-arene anticancer compounds. J. Am. Chem. Soc. 2008, 130, 10921–10928. [Google Scholar] [CrossRef]
- Vargiu, A.V.; Magistrato, A. Detecting DNA mismatches with metallo-insertors: A molecular simulation study. Inorg. Chem. 2012, 51, 2046–2057. [Google Scholar] [CrossRef]
- Vargiu, A.V.; Robertazzi, A.; Magistrato, A.; Ruggerone, P.; Carloni, P. The hydrolysis mechanism of the anticancer ruthenium drugs NAMI-A and ICR investigated by DFT-PCM calculations. J. Phys. Chem. B 2008, 112, 4401–4409. [Google Scholar] [CrossRef]
- Robertazzi, A.; Vargiu, A.V.; Magistrato, A.; Ruggerone, P.; Carloni, P.; de Hoog, P.; Reedijk, J. Copper-1,10-phenanthroline complexes binding to DNA: Structural predictions from molecular simulations. J. Phys. Chem. B 2009, 113, 10881–10890. [Google Scholar]
- Zastrow, M.L.; Pecoraro, V.L. Designing functional metalloproteins: From structural to catalytic metal sites. Coord. Chem. Rev. 2013, 257, 2565–2588. [Google Scholar] [CrossRef]
- Bell, C.B.; Calhoun, J.R.; Bobyr, E.; Wei, P.P.; Hedman, B.; Hodgson, K.O.; Degrado, W.F.; Solomon, E.I. Spectroscopic definition of the biferrous and biferric sites in de novo designed four-helix bundle DFsc peptides: Implications for O2 reactivity of binuclear non-heme iron enzymes. Biochemistry 2009, 48, 59–73. [Google Scholar] [CrossRef]
- Bovi, D.; Narzi, D.; Guidoni, L. The S-2 state of the oxygen-evolving complex of Photosystem II explored by QM/MM dynamics: Spin surfaces and metastable states suggest a reaction path towards the S-3 state. Angew. Chem. Int. Ed. 2013, 52, 11744–11749. [Google Scholar] [CrossRef]
- Magistrato, A.; DeGrado, W.F.; Laio, A.; Rothlisberger, U.; VandeVondele, J.; Klein, M.L. Characterization of the dizinc analogue of the synthetic diiron protein DF1 using ab initio and hybrid quantum/classical molecular dynamics simulations. J. Phys. Chem. B 2003, 107, 4182–4188. [Google Scholar] [CrossRef]
- Burton, S.G. Oxidizing enzymes as biocatalysts. Trends Biotechnol. 2003, 21, 543–549. [Google Scholar] [CrossRef]
- Reedy, C.J.; Gibney, B.R. Heme protein assemblies. Chem. Rev. 2004, 104, 617–649. [Google Scholar] [CrossRef]
- Watanabe, Y. Construction of heme enzymes: Four approaches. Curr. Opin. Chem. Biol. 2002, 6, 208–216. [Google Scholar]
- Alfonso-Prieto, M.; Klein, M.L. Density functional theory-based treatments of metal binding sites in metalloenzymes: Challenges and opportunities. In Metalloproteins: Structure, Function and Interactions; Cho, A.E., Goddard III, W.A., Eds.; CRC Press: Boca Raton, FL, USA, 2014; in press. [Google Scholar]
- Barducci, A.; Bonomi, M.; Parrinello, M. Metadynamics. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2011, 1, 826–843. [Google Scholar] [CrossRef]
- Dal Peraro, M.; Ruggerone, P.; Raugei, S.; Gervasio, F.L.; Carloni, P. Investigating biological systems using first principles Car–Parrinello molecular dynamics simulations. Curr. Opin. Struct. Biol. 2007, 17, 149–156. [Google Scholar] [CrossRef]
- Rovira, C. The description of electronic processes inside proteins from Car–Parrinello molecular dynamics: Chemical transformations. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2013, 3, 393–407. [Google Scholar] [CrossRef]
- Senn, H.M.; Thiel, W. QM/MM methods for biomolecular systems. Angew. Chem. Int. Ed. 2009, 48, 1198–1229. [Google Scholar] [CrossRef]
- Cornell, W.D.; Cieplak, P.; Bayly, C.I.; Gould, I.R.; Merz, K.M.; Ferguson, D.M.; Spellmeyer, D.C.; Fox, T.; Caldwell, J.W.; Kollman, P.A. A 2nd generation force-field for the simulation of proteins, nucleic acids and organic molecules. J. Am. Chem. Soc. 1995, 117, 5179–5197. [Google Scholar] [CrossRef]
- Comba, P.; Remenyi, R. Inorganic and bioinorganic molecular mechanics modeling—The problem of the force field parameterization. Coord. Chem. Rev. 2003, 238, 9–20. [Google Scholar] [CrossRef]
- Deeth, R.J. Computational bioinorganic chemistry. In Principles and Applications of Density in Inorganic Chemistry II; Kaltsoyannis, N., McGrady, J.E., Eds.; Springer: Berlin, Germany, 2004; pp. 37–69. [Google Scholar]
- Deeth, R.J.; Anastasi, A.; Diedrich, C.; Randell, K. Molecular modelling for transition metal complexes: Dealing with d-electron effects. Coord. Chem. Rev. 2009, 253, 795–816. [Google Scholar] [CrossRef]
- Hambley, T.W.; Jones, A.R. Molecular mechanics modelling of Pt/nucleotide and Pt/DNA interactions. Coord. Chem. Rev. 2001, 212, 35–59. [Google Scholar] [CrossRef]
- Zimmer, M. Are classical molecular mechanics calculations still useful in bioinorganic simulations? Coord. Chem. Rev. 2009, 253, 817–826. [Google Scholar] [CrossRef]
- Burger, S.K.; Lacasse, M.; Verstraelen, T.; Drewry, J.; Gunning, P.; Ayers, P.W. Automated parametrization of AMBER force field terms from vibrational analysis with a focus on functionalizing dinuclear Zinc(II) scaffolds. J. Chem. Theory Comput. 2012, 8, 554–562. [Google Scholar] [CrossRef]
- Hu, L.; Ryde, U. Comparison of methods to obtain force-field parameters for metal sites. J. Chem. Theory Comput. 2011, 7, 2452–2463. [Google Scholar] [CrossRef]
- Tafipolsky, M.; Schmid, R. Systematic first principles parameterization of force fields for metal-organic frameworks using a genetic algorithm approach. J. Phys. Chem. B 2009, 113, 1341–1352. [Google Scholar] [CrossRef]
- Balcells, D.; Clot, E.; Eisenstein, O. C–H bond activation in transition metal species from a computational perspective. Chem. Rev. 2010, 110, 749–823. [Google Scholar] [CrossRef]
- Davidson, E.R. Computational transition metal chemistry. Chem. Rev. 2000, 100, 351–352. [Google Scholar] [CrossRef]
- Garcia-Melchor, M.; Braga, A.A.C.; Lledos, A.; Ujaque, G.; Maseras, F. Computational perspective on Pd-catalyzed C–C cross-coupling reaction mechanisms. Acc. Chem. Res. 2013, 46, 2626–2634. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. B 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, 1133–1138. [Google Scholar] [CrossRef]
- Cramer, C.J.; Truhlar, D.G. Density functional theory for transition metals and transition metal chemistry. Phys. Chem. Chem. Phys. 2009, 11, 10757–10816. [Google Scholar] [CrossRef]
- Neese, F. Prediction of molecular properties and molecular spectroscopy with density functional theory: From fundamental theory to exchange-coupling. Coord. Chem. Rev. 2009, 253, 526–563. [Google Scholar] [CrossRef]
- Becke, A.D. Perspective: Fifty years of density functional theory in chemical physics. J. Chem. Phys. 2014. [Google Scholar] [CrossRef]
- Cohen, A.J.; Mori-Sanchez, P.; Yang, W. Challenges for density functional theory. Chem. Rev. 2012, 112, 289–320. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010. [Google Scholar] [CrossRef]
- Kulik, H.J.; Cococcioni, M.; Scherlis, D.A.; Marzari, N. Density functional theory in transition-metal chemistry: A self-consistent Hubbard U approach. Phys. Rev. Lett. 2006. [Google Scholar] [CrossRef]
- VandeVondele, J.; Sprik, M. A molecular dynamics study of the hydroxyl radical in solution applying self-interaction-corrected density functional methods. Phys. Chem. Chem. Phys. 2005, 7, 1363–1367. [Google Scholar] [CrossRef]
- Marques, M.A.L.; Gross, E.K.U. Time-dependent density functional theory. Annu. Rev. Phys. Chem. 2004, 55, 427–455. [Google Scholar] [CrossRef]
- Neese, F. A critical evaluation of DFT, including time-dependent DFT, applied to bioinorganic chemistry. J. Biol. Inorg. Chem. 2006, 11, 702–711. [Google Scholar] [CrossRef]
- Goedecker, S. Linear scaling electronic structure methods. Rev. Mod. Phys. 1999, 71, 1085–1123. [Google Scholar] [CrossRef]
- Sulpizi, M.; Raugei, S.; VandeVondele, J.; Carloni, P.; Sprik, M. Calculation of redox properties: Understanding short- and long-range effects in rubredoxin. J. Phys. Chem. B 2007, 111, 3969–3976. [Google Scholar] [CrossRef]
- Laio, A.; VandeVondele, J.; Rothlisberger, U. D-RESP: Dynamically generated electrostatic potential derived charges from quantum mechanics/molecular mechanics simulations. J. Phys. Chem. B 2002, 106, 7300–7307. [Google Scholar] [CrossRef]
- Laio, A.; VandeVondele, J.; Rothlisberger, U. A Hamiltonian electrostatic coupling scheme for hybrid Car–Parrinello molecular dynamics simulations. J. Chem. Phys. 2002, 116, 6941–6947. [Google Scholar] [CrossRef]
- Laino, T.; Mohamed, F.; Laio, A.; Parrinello, M. An efficient real space multigrid QM/MM electrostatic coupling. J. Chem. Theory Comput. 2005, 1, 1176–1184. [Google Scholar] [CrossRef]
- Laino, T.; Mohamed, F.; Laio, A.; Parrinello, M. An efficient linear-scaling electrostatic coupling for treating periodic boundary conditions in QM/MM simulations. J. Chem. Theory Comput. 2006, 2, 1370–1378. [Google Scholar] [CrossRef]
- Laio, A.; Gervasio, F.L.; VandeVondele, J.; Sulpizi, M.; Rothlisberger, U. A variational definition of electrostatic potential derived charges. J. Phys. Chem. B 2004, 108, 7963–7968. [Google Scholar] [CrossRef]
- Reuter, N.; Dejaegere, A.; Maigret, B.; Karplus, M. Frontier bonds in QM/MM methods: A comparison of different approaches. J. Phys. Chem. A 2000, 104, 1720–1735. [Google Scholar] [CrossRef]
- Vreven, T.; Frisch, M.J.; Kudin, K.N.; Schlegel, H.B.; Morokuma, K. Geometry optimization with QM/MM methods II: Explicit quadratic coupling. Mol. Phys. 2006, 104, 701–714. [Google Scholar] [CrossRef]
- Vreven, T.; Morokuma, K.; Farkas, O.; Schlegel, H.B.; Frisch, M.J. Geometry optimization with QM/MM, ONIOM, and other combined methods. I. Microiterations and constraints. J. Comput. Chem. 2003, 24, 760–769. [Google Scholar] [CrossRef]
- Carloni, P.; Rothlisberger, U.; Parrinello, M. The role and perspective of a initio molecular dynamics in the study of biological systems. Acc. Chem. Res. 2002, 35, 455–464. [Google Scholar]
- Verlet, L. Computer experiments on classical fluids I. Thermodynamical properties of Lennard-Jones molecules. Phys. Rev. 1967, 159, 98–103. [Google Scholar] [CrossRef]
- Marx, D.; Hutter, J. Ab Initio Molecular Dynamics: Basic Theory and Advanced Methods; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Car, R.; Parrinello, M. Unified approach for molecular-dynamics and density-functional theory. Phys. Rev. Lett. 1985, 55, 2471–2474. [Google Scholar] [CrossRef]
- Iannuzzi, M.; Laio, A.; Parrinello, M. Efficient exploration of reactive potential energy surfaces using Car–Parrinello molecular dynamics. Phys. Rev. Lett. 2003. [Google Scholar] [CrossRef]
- Laio, A.; Parrinello, M. Escaping free-energy minima. Proc. Natl. Acad. Sci. USA 2002, 99, 12562–12566. [Google Scholar] [CrossRef]
- Carter, E.A.; Ciccotti, G.; Hynes, J.T.; Kapral, R. Constrained reaction coordinate dynamics for the simulation of rare events. Chem. Phys. Lett. 1989, 156, 472–477. [Google Scholar] [CrossRef]
- Sprik, M.; Ciccotti, G. Free energy from constrained molecular dynamics. J. Chem. Phys. 1998, 109, 7737–7744. [Google Scholar] [CrossRef]
- Souaille, M.; Roux, B. Extension to the weighted histogram analysis method: Combining umbrella sampling with free energy calculations. Comput. Phys. Commun. 2001, 135, 40–57. [Google Scholar] [CrossRef]
- Torrie, G.M.; Valleau, J.P. Non-physical sampling distributions in monte-carlo free-energy estimation—Umbrella sampling. J. Comput. Phys. 1977, 23, 187–199. [Google Scholar]
- The CPMD Consortium Page. Available online: http://www.cpmd.org/ (accessed on 17 June 2014).
- CP2K Open Source Molecular Dynamics. Available online: http://www.cp2k.org/ (accessed on 17 June 2014).
- List of Quantum Chemistry and Solid-State Physics Software. Available online: http://en.wikipedia.org/wiki/List_of_quantum_chemistry_and_solid-state_physics_software/ (accessed on 17 June 2014).
- List of Software for Molecular Mechanics Modeling. Available online: http://en.wikipedia.org/wiki/List_of_software_for_molecular_mechanics_modeling (accessed on 17 June 2014).
- Dlouhy, A.C.O.; Caryn, E. The iron metallome in eukaryotic organisms. In Metallomics and the Cell; Banci, L., Ed.; Springer: Berlin, Germany, 2013; pp. 241–278. [Google Scholar]
- Aisen, P.; Listowsky, I. Iron transport and storage proteins. Annu. Rev. Biochem. 1980, 49, 357–393. [Google Scholar] [CrossRef]
- Beinert, H.; Holm, R.H.; Munck, E. Iron-sulfur clusters: Nature’s modular, multipurpose structures. Science 1997, 277, 653–659. [Google Scholar] [CrossRef]
- Poulos, T.L. The Janus nature of heme. Nat. Prod. Rep. 2007, 24, 504–510. [Google Scholar] [CrossRef]
- Ryle, M.J.; Hausinger, R.P. Non-heme iron oxygenases. Curr. Opin. Chem. Biol. 2002, 6, 193–201. [Google Scholar] [CrossRef]
- Poulos, T.L. Thirty years of heme peroxidase structural biology. Arch. Biochem. Biophys. 2010, 500, 3–12. [Google Scholar] [CrossRef]
- Dunford, B.H. Heme Peroxidases; Wiley-VCH: New York, NY, USA, 1999. [Google Scholar]
- Alfonso-Prieto, M.; Biarnes, X.; Vidossich, P.; Rovira, C. The molecular mechanism of the catalase reaction. J. Am. Chem. Soc. 2009, 131, 11751–11761. [Google Scholar] [CrossRef]
- Scherlis, D.A.; Cococcioni, M.; Sit, P.; Marzari, N. Simulation of heme using DFT + U: A step toward accurate spin-state energetics. J. Phys. Chem. B 2007, 111, 7384–7391. [Google Scholar] [CrossRef]
- Sit, P.H.L.; Migliore, A.; Ho, M.-H.; Klein, M.L. Quantum mechanical and quantum mechanical/molecular mechanical studies of the iron-dioxygen intermediates and proton transfer in superoxide reductase. J. Chem. Theory Comput. 2010, 6, 2896–2909. [Google Scholar] [CrossRef]
- Porstmann, T.; Kiessig, S. Enzyme immunoassay techniques: An overview. J. Immunol. Methods 1992, 150, 5–21. [Google Scholar] [CrossRef]
- Erman, J.E.; Vitello, L.B.; Miller, M.A.; Shaw, A.; Brown, K.A.; Kraut, J. Histidine-52 is a critical residue for rapid formation of cytochrome-C peroxidase compound-I. Biochemistry 1993, 32, 9798–9806. [Google Scholar] [CrossRef]
- Vitello, L.B.; Erman, J.E.; Miller, M.A.; Wang, J.; Kraut, J. Effect of arginine-48 replacement on the reaction between cytochrome-C peroxidase and hydrogen-peroxide. Biochemistry 1993, 32, 9807–9818. [Google Scholar] [CrossRef]
- Poulos, T.L.; Kraut, J. The stereochemistry of peroxidase catalysis. J. Biol. Chem. 1980, 255, 8199–8205. [Google Scholar]
- Baek, H.K.; Vanwart, H.E. Elementary steps in the formation of horseradish-peroxidase compound-I—Direct observation of compound 0, a new intermediate with a hyperporphyrin spectrum. Biochemistry 1989, 28, 5714–5719. [Google Scholar] [CrossRef]
- Rodriguez-Lopez, J.N.; Gilabert, M.A.; Tudela, J.; Thorneley, R.N.F.; Garcia-Canovas, F. Reactivity of horseradish peroxidase compound II toward substrates: Kinetic evidence for a two-step mechanism. Biochemistry 2000, 39, 13201–13209. [Google Scholar] [CrossRef]
- Jones, P.; Dunford, H.B. Mechanism of compound-I formation from peroxidases and catalases. J. Theor. Biol. 1977, 69, 457–470. [Google Scholar] [CrossRef]
- Derat, E.; Shaik, S.; Rovira, C.; Vidossich, P.; Alfonso-Prito, M. The effect of a water molecule on the mechanism of formation of compound 0 in horseradish peroxidase. J. Am. Chem. Soc. 2007, 129, 6346–6347. [Google Scholar]
- Vidossich, P.; Florin, G.; Alfonso-Prieto, M.; Derat, E.; Shaik, S.; Rovira, C. On the role of water in peroxidase catalysis: A theoretical investigation of HRP compound I formation. J. Phys. Chem. B 2010, 114, 5161–5169. [Google Scholar]
- Becke, A.D. Density functional calculations of molecular bond energies. J. Chem. Phys. 1986, 84, 4524–4529. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation-energy of the inhomogeneous electron-gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry 3 The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Vidossich, P.; Alfonso-Prieto, M.; Carpena, X.; Fita, I.; Loewen, P.C.; Rovira, C. The dynamic role of distal side residues in heme hydroperoxidase catalysis. Interplay between X-ray crystallography and ab initio MD simulations. Arch. Biochem. Biophys. 2010, 500, 37–44. [Google Scholar]
- Smulevich, G.; Jakopitsch, C.; Droghetti, E.; Obinger, C. Probing the structure and bifunctionality of catalase-peroxidase (KatG). J. Inorg. Biochem. 2006, 100, 568–585. [Google Scholar]
- Vlasits, J.; Jakopitsch, C.; Bernroitner, M.; Zamocky, M.; Furtmueller, P.G.; Obinger, C. Mechanisms of catalase activity of heme peroxidases. Arch. Biochem. Biophys. 2010, 500, 74–81. [Google Scholar]
- Carpena, X.; Loprasert, S.; Mongkolsuk, S.; Switala, J.; Loewen, P.C.; Fita, I. Catalase-peroxidase KatG of Burkholderia pseudomallei at 1.7 Å resolution. J. Mol. Biol. 2003, 327, 475–489. [Google Scholar] [CrossRef]
- Carpena, X.; Wiseman, B.; Deemagarn, T.; Singh, R.; Switala, J.; Ivancich, A.; Fita, I.; Loewen, P.C. A molecular switch and electronic circuit modulate catalase activity in catalase-peroxidases. EMBO Rep. 2005, 6, 1156–1162. [Google Scholar]
- Vidossich, P.; Alfonso-Prieto, M.; Carpena, X.; Loewen, P.C.; Fita, I.; Rovira, C. Versatility of the electronic structure of compound I in catalase-peroxidases. J. Am. Chem. Soc. 2007, 129, 13436–13446. [Google Scholar]
- Singh, R.; Switala, J.; Loewen, P.C.; Ivancich, A. Two Fe(IV) = O Trp(center dot) intermediates in M-tuberculosis catalase-peroxidase discriminated by multifrequency (9–285 GHz) EPR spectroscopy: Reactivity toward isoniazid. J. Am. Chem. Soc. 2007, 129, 15954–15963. [Google Scholar]
- Sivaraja, M.; Goodin, D.B.; Smith, M.; Hoffman, B.M. Identification by endor of Trp191 as the free-radical site in cytochrome-C peroxidase compound ES. Science 1989, 245, 738–740. [Google Scholar]
- Zhao, X.; Khajo, A.; Jarrett, S.; Suarez, J.; Levitsky, Y.; Burger, R.M.; Jarzecki, A.A.; Magliozzo, R.S. Specific function of the Met-Tyr-Trp adduct radical and residues Arg-418 and Asp-137 in the atypical catalase reaction of catalase-peroxidase KatG. J. Biol. Chem. 2012, 287, 37057–37065. [Google Scholar] [CrossRef]
- Vidossich, P.; Carpena, X.; Loewen, P.C.; Fita, I.; Rovira, C. Oxygen binding to catalase-peroxidase. J. Phys. Chem. Lett. 2011, 2, 196–200. [Google Scholar] [CrossRef]
- Jones, D.P.; Eklow, L.; Thor, H.; Orrenius, S. Metabolism of hydrogen-proxide in isolated hepatocytes-relative contributions of catalase and glutathione-peroxidase in decomposition of endogenously generated H2O2. Arch. Biochem. Biophys. 1981, 210, 505–516. [Google Scholar] [CrossRef]
- Chelikani, P.; Fita, I.; Loewen, P.C. Diversity of structures and properties among catalases. Cell Mol. Life Sci. 2004, 61, 192–208. [Google Scholar] [CrossRef]
- Alfonso-Prieto, M.; Borovik, A.; Carpena, X.; Murshudov, G.; Melik-Adamyan, W.; Fita, I.; Rovira, C.; Loewen, P.C. The structures and electronic configuration of compound I intermediates of Helicobacter pylori and Penicillium vitale catalases determined by X-ray crystallography and QM/MM density functional theory calculations. J. Am. Chem. Soc. 2007, 129, 4193–4205. [Google Scholar] [CrossRef]
- Fita, I.; Rossmann, M.G. The active-center of catalase. J. Mol. Biol. 1985, 185, 21–37. [Google Scholar] [CrossRef]
- Vainshtein, B.K.; Melikadamyan, W.R.; Barynin, V.V.; Vagin, A.A.; Grebenko, A.I.; Borisov, V.V.; Bartels, K.S.; Fita, I.; Rossmann, M.G. 3-Dimensional structure of catalase from Penicillium vitale at 2.0 Å resolution. J. Mol. Biol. 1986, 188, 49–61. [Google Scholar] [CrossRef]
- Vidossich, P.; Alfonso-Prieto, M.; Rovira, C. Catalases versus peroxidases: DFT investigation of H2O2 oxidation in models systems and implications for heme protein engineering. J. Inorg. Biochem. 2012, 117, 292–297. [Google Scholar]
- Andreini, C.; Bertini, I.; Rosato, A. Metalloproteomes: A bioinformatic approach. Acc. Chem. Res. 2009, 42, 1471–1479. [Google Scholar] [CrossRef]
- Frere, J.M. Beta-lactamases and bacterial-resistance to antibiotics. Mol. Microbiol. 1995, 16, 385–395. [Google Scholar]
- Sgrignani, J.; Magistrato, A.; dal Peraro, M.; Vila, A.J.; Carloni, P.; Pierattelli, R. On the active site of mononuclear B1 metallo beta-lactamases: A computational study. J. Comput. Aided Mol. Des. 2012, 26, 425–435. [Google Scholar]
- Ackerman, S.H.; Gatti, D.L. Biapenem inactivation by B2 metallo beta-lactamases: Energy landscape of the hydrolysis reaction. PLoS One 2013, 8, e55136. [Google Scholar] [CrossRef]
- Dal Peraro, M.; Llarrull, L.I.; Rothlisberger, U.; Vila, A.J.; Carloni, P. Water-assisted reaction mechanism of monozinc beta-lactamases. J. Am. Chem. Soc. 2004, 126, 12661–12668. [Google Scholar]
- Dal Peraro, M.; Vila, A.J.; Carloni, P.; Klein, M.L. Role of Zinc content on the catalytic efficiency of B1 metallo beta-lactamases. J. Am. Chem. Soc. 2007, 129, 2808–2816. [Google Scholar] [CrossRef]
- Diaz, N.; Suarez, D.; Merz, K.M. Molecular dynamics simulations of the mononuclear Zinc-beta-lactamase from Bacillus cereus complexed with benzylpenicillin and a quantum chemical study of the reaction mechanism. J. Am. Chem. Soc. 2001, 123, 9867–9879. [Google Scholar] [CrossRef]
- Diaz, N.; Suarez, D.; Merz, K.M.M. Zinc metallo-beta-lactamase from Bacteroides fragilis: A quantum chemical study on model systems of the active site. J. Am. Chem. Soc. 2000, 122, 4197–4208. [Google Scholar]
- Simona, F.; Magistrato, A.; Vera, D.M.A.; Garau, G.; Vila, A.J.; Carloni, P. Protonation state and substrate binding to B2 metallo-beta-lactamase CphA from Aeromonas hydrofila. Proteins 2007, 69, 595–605. [Google Scholar]
- Suarez, D.; Diaz, N.; Merz, K.M. Molecular dynamics simulations of the dinuclear Zinc-beta-lactamase from bacteroides fragilis complexed with imipenem. J. Comput. Chem. 2002, 23, 1587–1600. [Google Scholar]
- Wu, S.; Xu, D.; Guo, H. QM/MM studies of monozinc beta-lactamase CphA suggest that the crystal structure of an enzyme-intermediate complex represents a minor pathway. J. Am. Chem. Soc. 2010, 132, 17986–17988. [Google Scholar]
- Zhu, K.; Lu, J.; Liang, Z.; Kong, X.; Ye, F.; Jin, L.; Geng, H.; Chen, Y.; Zheng, M.; Jiang, H.; et al. A quantum mechanics/molecular mechanics study on the hydrolysis mechanism of New Delhi metallo-beta-lactamase-1. J. Comput. Aided Mol. Des. 2013, 27, 247–256. [Google Scholar] [CrossRef]
- Simona, F.; Magistrato, A.; dal Peraro, M.; Cavalli, A.; Vila, A.J.; Carloni, P. Common mechanistic features among metallo-beta-lactamases. A computational study of Aeromonas hydrophila CphA enzyme. J. Biol. Chem. 2009, 284, 28164–28171. [Google Scholar]
- Garau, G.; Bebrone, C.; Anne, C.; Galleni, M.; Frere, J.M.; Dideberg, O. A metallo-beta-lactamase enzyme in action: Crystal structures of the monozinc carbapenemase CphA and its complex with biapenem. J. Mol. Biol. 2005, 345, 785–795. [Google Scholar]
- Gatti, D.L. Biapenem inactivation by B2 metallo β-lactamases: Energy landscape of the post-hydrolysis reactions. PLoS One 2012, 7, e30079. [Google Scholar]
- De Vivo, M. Bridging quantum mechanics and structure-based drug design. Front. Biosci. 2011, 16, 1619–1633. [Google Scholar] [CrossRef]
- Jomova, K.; Vondrakova, D.; Lawson, M.; Valko, M. Metals, oxidative stress and neurodegenerative disorders. Mol. Cell Biochem. 2010, 345, 91–104. [Google Scholar]
- Viles, J.H. Metal ions and amyloid fiber formation in neurodegenerative diseases. Copper, Zinc and iron in Alzheimer’s, Parkinson’s and prion diseases. Coord. Chem. Rev. 2012, 256, 2271–2284. [Google Scholar] [CrossRef]
- Faller, P.; Hureau, C. A bioinorganic view of Alzheimer’s disease: When misplaced metal ions (re)direct the electrons to the wrong target. Chemistry 2012, 18, 15910–15920. [Google Scholar] [CrossRef]
- Goedert, M. Alpha-synuclein and neurodegenerative diseases. Nat. Rev. Neurosci. 2001, 2, 492–501. [Google Scholar] [CrossRef]
- Sayre, L.M.; Perry, G.; Smith, M.A. Redox metals and neurodegenerative disease. Curr. Opin. Chem. Biol. 1999, 3, 220–225. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.Y.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar]
- Binolfi, A.; Quintanar, L.; Bertoncini, C.W.; Griesinger, C.; Fernandez, C.O. Bioinorganic chemistry of copper coordination to alpha-synuclein: Relevance to Parkinson’s disease. Coord. Chem. Rev. 2012, 256, 2188–2201. [Google Scholar]
- Bertoncini, C.W.; Jung, Y.S.; Fernandez, C.O.; Hoyer, W.; Griesinger, C.; Jovin, T.M.; Zweckstetter, M. Release of long-range tertiary interactions potentiates aggregation of natively unstructured alpha-synuclein. Proc. Natl. Acad. Sci. USA 2005, 102, 1430–1435. [Google Scholar] [CrossRef]
- Dedmon, M.M.; Lindorff-Larsen, K.; Christodoulou, J.; Vendruscolo, M.; Dobson, C.M. Mapping long-range interactions in alpha-synuclein using spin-label NMR and ensemble molecular dynamics simulations. J. Am. Chem. Soc. 2005, 127, 476–477. [Google Scholar]
- Binolfi, A.; Rodriguez, E.E.; Valensin, D.; D’Amelio, N.; Ippoliti, E.; Obal, G.; Duran, R.; Magistrato, A.; Pritsch, O.; Zweckstetter, M.; et al. Bioinorganic chemistry of Parkinson’s disease: Structural determinants for the Copper-mediated amyloid formation of alpha-synuclein. Inorg. Chem. 2010, 49, 10668–10679. [Google Scholar] [CrossRef]
- Binolfi, A.; Valiente-Gabioud, A.A.; Duran, R.; Zweckstetter, M.; Griesinger, C.; Fernandez, C.O. Exploring the structural details of Cu(I) binding to α-synuclein by NMR spectroscopy. J. Am. Chem. Soc. 2011, 133, 194–196. [Google Scholar]
- Rasia, R.M.; Bertoncini, C.W.; Marsh, D.; Hoyer, W.; Cherny, D.; Zweckstetter, M.; Griesinger, C.; Jovin, T.M.; Fernandez, C.O. Structural characterization of Copper(II) binding to alpha-synuclein: Insights into the bioinorganic chemistry of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2005, 102, 4294–4299. [Google Scholar] [CrossRef]
- Perdew, J.B.K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Bowman, J.C.; Lenz, T.K.; Hud, N.V.; Williams, L.D. Cations in charge: Magnesium ions in RNA folding and catalysis. Curr. Opin. Struct. Biol. 2012, 22, 262–272. [Google Scholar] [CrossRef]
- Rosta, E.; Yang, W.; Hummer, G. Calcium inhibition of ribonuclease H1 two-metal ion catalysis. J. Am. Chem. Soc. 2014. [Google Scholar] [CrossRef]
- Palermo, G.; Stenta, M.; Cavalli, A.; dal Peraro, M.; de Vivo, M. Molecular simulations highlight the role of metals in catalysis and inhibition of type II topoisomerase. J. Chem. Theory Comput. 2013, 9, 857–862. [Google Scholar] [CrossRef]
- Robertson, M.P.; Joyce, G.F. The origins of the RNA world. Cold Spring Harb. Perspect. Biol. 2012. [Google Scholar] [CrossRef]
- Shechner, D.M.; Grant, R.A.; Bagby, S.C.; Koldobskaya, Y.; Piccirilli, J.A.; Bartel, D.P. Crystal structure of the catalytic core of an RNA-polymerase ribozyme. Science 2009, 326, 1271–1275. [Google Scholar]
- Attwater, J.; Wochner, A.; Holliger, P. In-ice evolution of RNA polymerase ribozyme activity. Nat. Chem. 2013, 5, 1011–1018. [Google Scholar] [CrossRef]
- Wochner, A.; Attwater, J.; Coulson, A.; Holliger, P. Ribozyme-catalyzed transcription of an active ribozyme. Science 2011, 332, 209–212. [Google Scholar] [CrossRef]
- Shechner, D.M.; Bartel, D.P. The structural basis of RNA-catalyzed RNA polymerization. Nat. Struct. Mol. Biol. 2011, 18, 1036–1042. [Google Scholar] [CrossRef]
- Sgrignani, J.; Magistrato, A. The structural role of Mg2+ ions in a class I RNA polymerase ribozyme: A molecular simulation study. J. Phys. Chem. B 2012, 116, 2259–2268. [Google Scholar] [CrossRef]
- Aqvist, J. Ion water interaction potentials derived from free-energy perturbation simulations. J. Phys. Chem. 1990, 94, 8021–8024. [Google Scholar] [CrossRef]
- Oelschlaeger, P.; Klahn, M.; Beard, W.A.; Wilson, S.H.; Warshel, A. Magnesium-cationic dummy atom molecules enhance representation of DNA polymerase beta in molecular dynamics simulations: improved accuracy in studies of structural features and mutational effects. J. Mol. Biol. 2007, 366, 687–701. [Google Scholar] [CrossRef]
- Pérez, A.; Marchán, I.; Svozil, D.; Sponer, J.; Cheatham Iii, T.E.; Laughton, C.A.; Orozco, M. Refinement of the AMBER force field for nucleic acids: Improving the description of α/γ conformers. Biophys. J. 2007, 92, 3817–3829. [Google Scholar] [CrossRef]
- De Vivo, M.; dal Peraro, M.; Klein, M.L. Phosphodiester cleavage in ribonuclease H occurs via an associative two-metal-aided catalytic mechanism. J. Am. Chem. Soc. 2008, 130, 10955–10962. [Google Scholar] [CrossRef]
- Boero, M.; Tateno, M.; Terakura, K.; Oshiyama, A. Double-metal-ion/single-metal-ion mechanisms of the cleavage reaction of ribozymes: First-principles molecular dynamics simulations of a fully hydrated model system. J. Chem. Theory Comput. 2005, 1, 925–934. [Google Scholar]
- Stefan, L.R.; Zhang, R.; Levitan, A.G.; Hendrix, D.K.; Brenner, S.E.; Holbrook, S.R. MeRNA: A database of metal ion binding sites in RNA structures. Nucleic Acids Res. 2006, 34, D131–D134. [Google Scholar] [CrossRef]
- Steitz, T.A. Structural biology—A mechanism for all polymerases. Nature 1998, 391, 231–232. [Google Scholar] [CrossRef]
- Dror, R.O.; Dirks, R.M.; Grossman, J.P.; Xu, H.F.; Shaw, D.E. Biomolecular simulation: A computational microscope for molecular biology. Annu. Rev. Biophys. 2012, 41, 429–452. [Google Scholar] [CrossRef]
- Hamelberg, D.; Mongan, J.; McCammon, J.A. Accelerated molecular dynamics: A promising and efficient simulation method for biomolecules. J. Chem. Phys. 2004, 120, 11919–11929. [Google Scholar] [CrossRef]
- Liu, P.; Kim, B.; Friesner, R.A.; Berne, B.J. Replica exchange with solute tempering: A method for sampling biological systems in explicit water. Proc. Natl. Acad. Sci. USA 2005, 102, 13749–13754. [Google Scholar]
- Tribello, G.A.; Ceriotti, M.; Parrinello, M. A self-learning algorithm for biased molecular dynamics. Proc. Natl. Acad. Sci. USA 2010, 107, 17509–17514. [Google Scholar] [Green Version]
- Maurer, P.; Laio, A.; Hugosson, H.W.; Colombo, M.C.; Rothlisberger, U. Automated parametrization of biomolecular force fields from quantum mechanics/molecular mechanics (QM/MM) simulations through force matching. J. Chem. Theory Comput. 2007, 3, 628–639. [Google Scholar]
- Spiegel, K.; Magistrato, A.; Maurer, P.; Ruggerone, P.; Rothlisberger, U.; Carloni, P.; Reedijk, J.; Klein, M.L. Parameterization of azole-bridged dinuclear platinum anticancer drugs via a QM/MM force matching procedure. J. Comput. Chem. 2008, 29, 38–49. [Google Scholar] [CrossRef]
- Spiegel, K.; Magistrato, A.; Carloni, P.; Reedijk, J.; Klein, M.L. Azole-bridged diplatinum anticancer compounds. Modulating DNA flexibility to escape repair mechanism and avoid cross resistance. J. Phys. Chem. B 2007, 111, 11873–11876. [Google Scholar]
- Nguyen, T.H.; Arnesano, F.; Scintilla, S.; Rossetti, G.; Ippoliti, E.; Carloni, P.; Natile, G. Structural determinants of cisplatin and transplatin binding to the Met-rich motif of Ctr1: A computational spectroscopy approach. J. Chem. Theory Comput. 2012, 8, 2912–2920. [Google Scholar] [CrossRef]
- Cascella, M.; Cuendet, M.A.; Tavernelli, I.; Rothlisberger, U. Optical spectra of Cu(II)-azurin by hybrid TDDFT-molecular dynamics simulations. J. Phys. Chem. B 2007, 111, 10248–10252. [Google Scholar] [CrossRef]
- Miani, A.; Raugei, S.; Carloni, P.; Helfand, M.S. Structure and Raman spectrum of clavulanic acid in aqueous solution. J. Phys. Chem. B 2007, 111, 2621–2630. [Google Scholar] [CrossRef]
- Cascella, M.; Magistrato, A.; Tavernelli, I.; Carloni, P.; Rothlisberger, U. Role of protein frame and solvent for the redox properties of azurin from Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2006, 103, 19641–19646. [Google Scholar] [CrossRef]
- Breuer, M.; Rosso, K.M.; Blumberger, J. Electron flow in multiheme bacterial cytochromes is a balancing act between heme electronic interaction and redox potentials. Proc. Natl. Acad. Sci. USA 2014, 111, 611–616. [Google Scholar] [CrossRef]
- Blumberger, J. Free energies for biological electron transfer from QM/MM calculation: Method, application and critical assessment. Phys. Chem. Chem. Phys. 2008, 10, 5651–5667. [Google Scholar] [CrossRef]
- Cho, A.E.; Rinaldo, D. Extension of QM/MM docking and its applications to metalloproteins. J. Comput. Chem. 2009, 30, 2609–2616. [Google Scholar] [CrossRef]
- Sgrignani, J.; Magistrato, A. First-principles modeling of biological systems and structure-based drug-design. Curr. Comput. Aided Drug Des. 2013, 9, 15–34. [Google Scholar]
- Khandelwal, A.; Lukacova, V.; Comez, D.; Kroll, D.M.; Raha, S.; Balaz, S. A combination of docking, QM/MM methods, and MD simulation for binding affinity estimation of metalloprotein ligands. J. Med. Chem. 2005, 48, 5437–5447. [Google Scholar] [CrossRef]
- Hayik, S.A.; Dunbrack, R., Jr.; Merz, K.M., Jr. A mixed QM/MM scoring function to predict protein-ligand binding affinity. J. Chem. Theory Comput. 2010, 6, 3079–3091. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Vidossich, P.; Magistrato, A. QM/MM Molecular Dynamics Studies of Metal Binding Proteins. Biomolecules 2014, 4, 616-645. https://doi.org/10.3390/biom4030616
Vidossich P, Magistrato A. QM/MM Molecular Dynamics Studies of Metal Binding Proteins. Biomolecules. 2014; 4(3):616-645. https://doi.org/10.3390/biom4030616
Chicago/Turabian StyleVidossich, Pietro, and Alessandra Magistrato. 2014. "QM/MM Molecular Dynamics Studies of Metal Binding Proteins" Biomolecules 4, no. 3: 616-645. https://doi.org/10.3390/biom4030616




