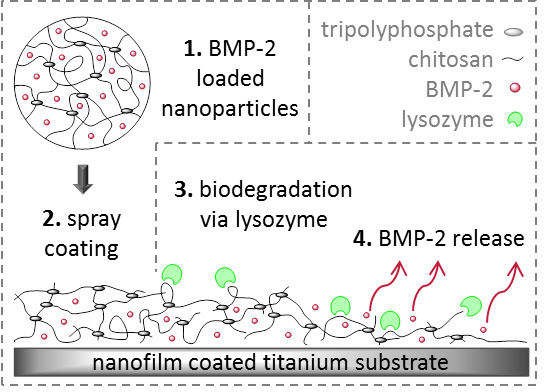
Biodegradable Chitosan Nanoparticle Coatings on Titanium for the Delivery of BMP-2
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chitosan Purification and Acetylation
2.2. Chitosan Degradation: Viscometry

2.3. Nanoparticle Synthesis
2.4. Nanoparticle Degradation
2.5. Coated Titanium Substrates




2.6. BMP-2 in CS-TPP Nanoparticles
2.7. Cell Response to BMP-2-Loaded Surfaces


2.8. In Vivo Study: Implantation of Nanoparticles in Mice
3. Experimental Section
3.1. Materials
3.2. Chitosan Purification
3.3. Chitosan Acetylation
3.4. NMR Characterization
3.5. Viscometry
3.6. Nanoparticle Synthesis
3.7. Particle Size and Zeta Potential
3.8. Nanoparticle Degradation
3.9. Surface Modification
3.10. Ellipsometry
3.11. Scanning Electron Microscopy
3.12. X-ray Photoelectron Spectroscopy
3.13. Cell Response to BMP-2-Loaded Surfaces
3.14. In Vivo Study: Implantation of Nanoparticles in Mice
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Otto, M.; Kriegsmann, J.; Gehrke, T.; Bertz, S. Abriebpartikel. Der Pathol. 2006, 27, 447–460. [Google Scholar] [CrossRef]
- Zysk, S.; Gebhard, H.; Plitz, W.; Meßmer, K.; Veihelmann, A.; Pellengahr, C.; Refior, H.J. Inflammatorische Reaktion auf Abriebpartikel von Endoprothesen in vivo. Orthopade 2003, 32, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Statistisches Bundesamt (Destatis) Fallpauschalenbezogene Krankenhausstatistik (DRG-Statistik) Operationen und Prozeduren der vollstationären Patientinnen und Patienten in Krankenhäusern Ausführliche Darstellung 2012. Available online: https://www.destatis.de/DE/Publikationen/Thematisch/Gesundheit/Krankenhaeuser/OperationenProzeduren5231401127014%3F__blob%3DpublicationFile (accessed on 4 January 2015).
- Gristina, A.G. Biomaterial-centered infection: Microbial adhesion versus tissue integration. Science 1987, 237, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.M.; Hwang, K.; Winn, S.R.; Hollinger, J.O. Bone morphogenetic proteins: An update on basic biology and clinical relevance. J. Orthop. Res. 1999, 17, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Hollinger, J.O.; Schmitt, J.M.; Buck, D.C.; Shannon, R.; Joh, S.-P.; Zegzula, H.D.; Wozney, J. Recombinant human bone morphogenetic protein-2 and collagen for bone regeneration. J. Biomed. Mater. Res. 1998, 43, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Wozney, J.M. Bone morphogenetic proteins. Prog. Growth Factor Res. 1989, 1, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Kim, J. Bone morphogenetic proteins in spine surgery. Spine 2008, 3, 18–20. [Google Scholar]
- Li, R.H.; Wozney, J.M. Delivering on the promise of bone morphogenetic proteins. Trends Biotechnol. 2001, 19, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Chatzinikolaidou, M.; Lichtinger, T.K.; Müller, R.T.; Jennissen, H.P. Peri-implant reactivity and osteoinductive potential of immobilized rhBMP-2 on titanium carriers. Acta Biomater. 2010, 6, 4405–4421. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, C.; Hasegawa, U.; Saita, Y.; Hemmi, H.; Hayata, T.; Nakashima, K.; Ezura, Y.; Amagasa, T.; Akiyoshi, K.; Noda, M. Osteoblastic bone formation is induced by using nanogel-crosslinking hydrogel as novel scaffold for bone growth factor. J. Cell. Physiol. 2009, 220, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Palomares, C.; Ferrand, A.; Facca, S.; Fioretti, F.; Ladam, G.; Kuchler-Bopp, S.; Regnier, T.; Mainard, D.; Benkirane-Jessel, N. Smart Hybrid Materials Equipped by Nanoreservoirs of Therapeutics. ACS Nano 2012, 6, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-W.; Yun, Y.-P.; Park, K.; Kim, S.E. Gentamicin and bone morphogenic protein-2 (BMP-2)-delivering heparinized-titanium implant with enhanced antibacterial activity and osteointegration. Bone 2012, 50, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Neoh, K.G.; Kang, E.T.; Poh, C.K.; Wang, W. Surface functionalization of titanium with carboxymethyl chitosan and immobilized bone morphogenetic protein-2 for enhanced osseointegration. Biomacromolecules 2009, 10, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-I.; Ahn, K.-M.; Jeon, S.-H.; Lee, S.-Y.; Lee, J.-H.; Tae, G. Enhanced bone regeneration with BMP-2 loaded functional nanoparticle-hydrogel complex. J. Controll. Release 2007, 121, 91–99. [Google Scholar] [CrossRef]
- Abarrategi, A.; García-Cantalejo, J.; Moreno-Vicente, C.; Civantos, A.; Ramos, V.; Casado, J.V.S.; Pérez-Rial, S.; Martnez-Corriá, R.; López-Lacomba, J.L. Gene expression profile on chitosan/rhBMP-2 films: A novel osteoinductive coating for implantable materials. Acta Biomater. 2009, 5, 2633–2646. [Google Scholar] [CrossRef] [PubMed]
- Facca, S.; Cortez, C.; Mendoza-Palomares, C.; Messadeq, N.; Dierich, A.; Johnston, A.P.R.; Mainard, D.; Voegel, J.-C.; Caruso, F.; Benkirane-Jessel, N. Active multilayered capsules for in vivo bone formation. Proc. Natl. Acad. Sci. USA 2010, 107, 3406–3411. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.J.; Hong, J.; Hyder, M.D.N.; Hammond, P.T. Osteophilic multilayer coatings for accelerated bone tissue growth. Adv. Mater. 2012, 24, 1445–1450. [Google Scholar] [CrossRef] [PubMed]
- Jennissen, H.P.; Zumbrink, T.; Chatzinikolaidou, M.; Steppuhn, J. Biocoating of implants with mediator molecules: Surface enhancement of metals by treatment with chromosulfuric acid. Mater. Werkst. 1999, 30, 838–845. [Google Scholar] [CrossRef]
- Crouzier, T.; Szarpak, A.; Boudou, T.; Auzély-Velty, R.; Picart, C. Polysaccharide-blend multilayers containing hyaluronan and heparin as a delivery system for rhBMP-2. Small 2010, 6, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Puleo, D.A.; Kissling, R.A.; Sheu, M.S. A technique to immobilize bioactive proteins, including bone morphogenetic protein-4 (BMP-4), on titanium alloy. Biomaterials 2002, 23, 2079–2087. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, C.; Hoffmann, A.; Gross, G.; Windhagen, H.; Dellinger, P.; Möhwald, K.; Dempwolf, W.; Menzel, H. Coating of titanium implant materials with thin polymeric films for binding the signaling protein BMP2. Macromol. Biosci. 2011, 11, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Adden, N.; Gamble, L.J.; Castner, D.G.; Hoffmann, A.; Gross, G.; Menzel, H. Phosphonic acid monolayers for binding of bioactive molecules to titanium surfaces. Langmuir 2006, 22, 8197–8204. [Google Scholar] [CrossRef] [PubMed]
- Crouzier, T.; Ren, K.; Nicolas, C.; Roy, C.; Picart, C. Layer-by-layer films as a biomimetic reservoir for rhBMP-2 delivery: Controlled differentiation of myoblasts to osteoblasts. Small 2009, 5, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Karl, M.; Pendrys, D.; Shafer, D.; Freilich, M.; Kuhn, L. An evaluation of BMP-2 delivery from scaffolds with miniaturized dental implants in a novel rat mandible model. J. Biomed. Mater. Res. Part B 2011, 97, 315–326. [Google Scholar] [CrossRef]
- Bae, S.E.; Choi, J.; Joung, Y.K.; Park, K.; Han, D.K. Controlled release of bone morphogenetic protein (BMP)-2 from nanocomplex incorporated on hydroxyapatite-formed titanium surface. J. Controll. Release 2012, 160, 676–684. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, S.-M.; Lee, I.-S. Immobilizing bioactive molecules onto titanium implants to improve osseointegration. Surf. Coat. Technol. 2013, 22, 312–317. [Google Scholar]
- Gan, Q.; Wang, T. Chitosan nanoparticle as protein delivery carrier—Systematic examination of fabrication conditions for efficient loading and release. Colloids Surf. B 2007, 59, 24–34. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; Peng, Z.; She, F.; Kong, L. Development of chitosan nanoparticles as drug delivery systems for 5-fluorouracil and leucovorin blends. Carbohydr. Polym. 2011, 85, 698–704. [Google Scholar] [CrossRef]
- Ji, J.; Hao, S.; Wu, D.; Huang, R.; Xu, Y. Preparation, characterization and in vitro release of chitosan nanoparticles loaded with gentamicin and salicylic acid. Carbohydr. Polym. 2011, 85, 803–808. [Google Scholar] [CrossRef]
- Alishahi, A.; Mirvaghefi, A.; Tehrani, M.R.; Farahmand, H.; Shojaosadati, S.A.; Dorkoosh, F.A.; Elsabee, M.Z. Shelf life and delivery enhancement of vitamin C using chitosan nanoparticles. Food Chem. 2011, 126, 935–940. [Google Scholar] [CrossRef]
- Leedy, M.; Martin, H.; Norowski, P.A.; Jennings, J.A.; Haggard, W.; Bumgardner, J. Use of Chitosan as a Bioactive Implant Coating for Bone-Implant Applications. In Chitosan for Biomaterials II; Jayakumar, R., Prabaharan, M., Muzzarelli Riccardo, A.A., Eds.; Springer: Berlin, Heidelberg, Germany, 2011; pp. 129–165. [Google Scholar]
- Ohara, N.; Hayashi, Y.; Yamada, S.; Kim, S.-K.; Matsunaga, T.; Yanagiguchi, K.; Ikeda, T. Early gene expression analyzed by cDNA microarray and RT-PCR in osteoblasts cultured with water-soluble and low molecular chitooligosaccharide. Biomaterials 2004, 25, 1749–1754. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, T.; Yanagiguchi, K.; Yamada, S.; Ohara, N.; Ikeda, T.; Hayashi, Y. Chitosan monomer promotes tissue regeneration on dental pulp wounds. J. Biomed. Mater. Res. Part A 2006, 76A, 711–720. [Google Scholar] [CrossRef]
- Golub, E.E.; Boesze-Battaglia, K. The role of alkaline phosphatase in mineralization. Curr. Opin. Orthop. 2007, 18, 444–448. [Google Scholar] [CrossRef]
- Heppel, L.A.; Harkness, D.R.; Hilmoe, R.J. A study of the substrate specificity and other properties a study of the substrate specificity and other properties of the alkaline phosphatase of Escherichia coli. J. Biol. Chem. 1962, 237, 841–846. [Google Scholar] [PubMed]
- Gan, Q.; Wang, T.; Cochrane, C.; McCarron, P. Modulation of surface charge, particle size and morphological properties of chitosan-TPP nanoparticles intended for gene delivery. Colloids Surf. B 2005, 44, 65–73. [Google Scholar] [CrossRef]
- Freier, T.; Koh, H.S.; Kazazian, K.; Shoichet, M.S. Controlling cell adhesion and degradation of chitosan films by N-acetylation. Biomaterials 2005, 26, 5872–5878. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Ha, W.S.; Park, W.H. Blood compatibility and biodegradability of partially N-acylated chitosan derivatives. Biomaterials 1995, 16, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Franca, E.F.; Freitas, L.C.G.; Lins, R.D. Chitosan molecular structure as a function of N-acetylation. Biopolymers 2011, 95, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, J.; van Leeuwen-Herberts, T.; van de Ruit, M.O. Determination of lysozyme in serum, urine, cerebrospinal fluid and feces by enzyme immunoassay. Clin. Chim. Acta 1984, 142, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Hankiewicz, J.; Swierczek, E. Lysozyme in human body fluids. Clin. Chim. Acta 1974, 57, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Venge, P.; Foucard, T.; Henriksen, J.; Håkansson, L.; Kreuger, A. Serum-levels of lactoferrin, lysozyme and myeloperoxidase in normal, infection-prone and leukemic children. Clin. Chim. Acta 1984, 136, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Vårum, K.M.; Myhr, M.M.; Hjerde, R.J.N.; Smidsrød, O. In vitro degradation rates of partially N-acetylated chitosans in human serum. Carbohydr. Res. 1997, 299, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Du, W.-L.; Niu, S.-S.; Xu, Y.-L.; Xu, Z.-R.; Fan, C.-L. Antibacterial activity of chitosan tripolyphosphate nanoparticles loaded with various metal ions. Carbohydr. Polym. 2009, 75, 385–389. [Google Scholar] [CrossRef]
- Kaloti, M.; Bohidar, H.B. Kinetics of coacervation transition versus nanoparticle formation in chitosan-sodium tripolyphosphate solutions. Colloids Surf. B 2010, 81, 165–173. [Google Scholar] [CrossRef]
- Pourshahab, P.S.; Gilani, K.; Moazeni, E.; Eslahi, H.; Fazeli, M.R.; Jamalifar, H. Preparation and characterization of spray dried inhalable powders containing chitosan nanoparticles for pulmonary delivery of isoniazid. J. Microencapsul. 2011, 28, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Ucar, S.; Yilgor, P.; Hasirci, V.; Hasirci, N. Chitosan-based wet-spun scaffolds for bioactive agent delivery. J. Appl. Polym. Sci. 2013, 130, 3759–3769. [Google Scholar] [CrossRef]
- Ren, D.; Yi, H.; Wang, W.; Ma, X. The enzymatic degradation and swelling properties of chitosan matrices with different degrees of N-acetylation. Carbohydr. Res. 2005, 340, 2403–2410. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.-Y.; Zhou, C.-R.; Luo, B.-H. Preparation and characterization of chitosan nanoparticles containing lysozyme. Pharm. Biol. 2006, 44, 336–342. [Google Scholar] [CrossRef]
- Piras, A.M.; Maisetta, G.; Sandreschi, S.; Esin, S.; Gazzarri, M.; Batoni, G.; Chiellini, F. Preparation, physical–chemical and biological characterization of chitosan nanoparticles loaded with lysozyme. Int. J. Biol. Macromol. 2014, 67, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Meier-Westhues, U. Polyurethane: Lacke, Kleb- und Dichtstoffe; Vincentz Network: Hannover, Germany, 2007. [Google Scholar]
- Macdonald, M.L.; Samuel, R.E.; Shah, N.J.; Padera, R.F.; Beben, Y.M.; Hammond, P.T. Tissue integration of growth factor-eluting layer-by-layer polyelectrolyte multilayer coated implants. Biomaterials 2011, 32, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, L.F.; Brokelmann, M.; Marten, S.; Trappe, S.; Cabrera-Crespo, J.; Hoffmann, A.; Gross, G.; Weich, H.A.; Rinas, U. Renaturation and purification of bone morphogenetic protein-2 produced as inclusion bodies in high-cell-density cultures of recombinant Escherichia coli. J. Biotechnol. 2002, 94, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Ehlert, N.; Hoffmann, A.; Luessenhop, T.; Gross, G.; Mueller, P.P.; Stieve, M.; Lenarz, T.; Behrens, P. Amino-modified silica surfaces efficiently immobilize bone morphogenetic protein 2 (BMP2) for medical purposes. Acta Biomater. 2011, 7, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poth, N.; Seiffart, V.; Gross, G.; Menzel, H.; Dempwolf, W. Biodegradable Chitosan Nanoparticle Coatings on Titanium for the Delivery of BMP-2. Biomolecules 2015, 5, 3-19. https://doi.org/10.3390/biom5010003
Poth N, Seiffart V, Gross G, Menzel H, Dempwolf W. Biodegradable Chitosan Nanoparticle Coatings on Titanium for the Delivery of BMP-2. Biomolecules. 2015; 5(1):3-19. https://doi.org/10.3390/biom5010003
Chicago/Turabian StylePoth, Nils, Virginia Seiffart, Gerhard Gross, Henning Menzel, and Wibke Dempwolf. 2015. "Biodegradable Chitosan Nanoparticle Coatings on Titanium for the Delivery of BMP-2" Biomolecules 5, no. 1: 3-19. https://doi.org/10.3390/biom5010003