Mucin-Type O-Glycosylation in Gastric Carcinogenesis
Abstract
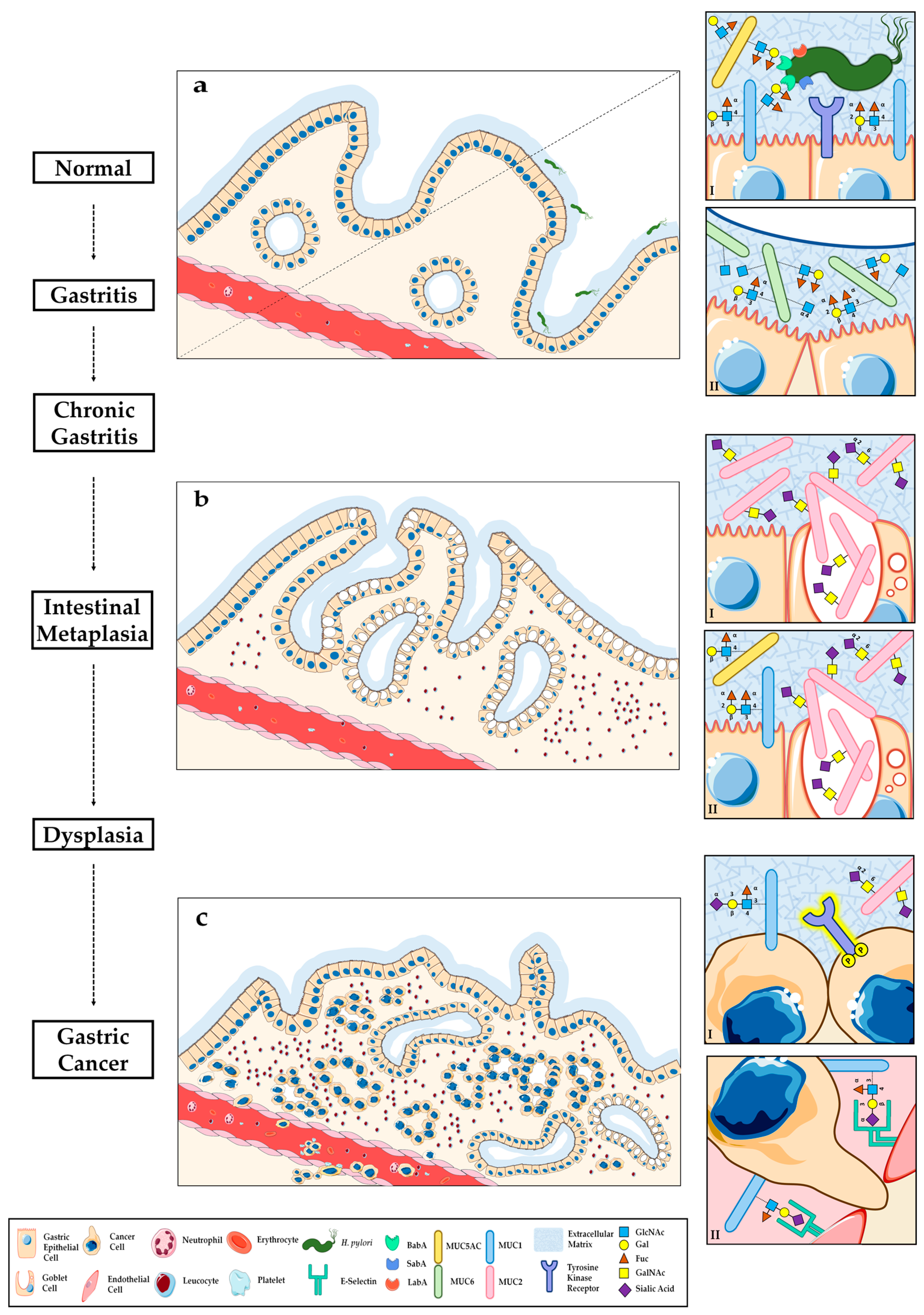
:1. Introduction
2. Mucin and O-Glycosylation in Healthy Gastric Mucosa
3. O-Glycosylation Alterations during Gastric Carcinogenesis
3.1. Helicobacter Pylori Host Glycan Receptors and Infection
3.1.1. Modulation of Gastric Glycosylation Induced by Helicobacter pylori Infection and Inflammation
3.1.2. Novel Treatment Strategies Targeting Helicobacter pylori Glycan-Mediated Adhesion
3.2. Glycosylation Alterations in Gastric Premalignant Conditions
4. O-Glycosylation Alterations in Gastric Carcinoma
4.1. Aberrant Glycophenotype of Gastric Carcinoma Cells
4.2. Crosstalk between Altered Glycosylation and Tyrosine Kinase Receptor Activation in Gastric Cancer
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MUC | Mucin |
| ppGalNAc-T | UDP-GalNAc:polypeptide N-Acetylgalactosaminyltransferase |
| SLea | Sialyl-Lewisa |
| SLex | Sialyl-Lewisx |
| FUT | Fucosyltransferase |
| H. pylori | Helicobacter pylori |
| GC | Gastric Cancer |
| OMP | Outer Membrane Proteins |
| BabA | Blood Group Antigen Binding Adhesin |
| LabA | LacdiNAc-Binding Adhesin |
| SabA | Sialic Acid Binding Adhesin |
| PPI | Proton-Pump Inhibitor |
| IM | Intestinal Metaplasia |
| STn | Sialyl-Tn |
| ST | Sialyl-T |
| TKR | Tyrosine Kinase Receptor |
References
- Correa, P. Human gastric carcinogenesis: A multistep and multifactorial process—First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992, 52, 6735–6740. [Google Scholar] [PubMed]
- IARC. Schistosomes, liver flukes and Helicobacter pylori. IARC working group on the evaluation of carcinogenic risks to humans. Lyon, 7–14 June 1994. IARC Monogr. Eval. Carcinog. Risks Hum. 1994, 61, 1–241. [Google Scholar]
- Polk, D.B.; Peek, R.M., Jr. Helicobacter pylori: Gastric cancer and beyond. Nat. Rev. Cancer 2010, 10, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Correa, P.; Houghton, J. Carcinogenesis of Helicobacter pylori. Gastroenterology 2007, 133, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.S.; Carvalho, S.; Marcos-Pinto, R.; Magalhães, A.; Oliveira, C.; Gu, J.; Dinis-Ribeiro, M.; Carneiro, F.; Seruca, R.; Reis, C.A. Gastric cancer: Adding glycosylation to the equation. Trends Mol. Med. 2013, 19, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Robbe, C.; Capon, C.; Coddeville, B.; Michalski, J.C. Structural diversity and specific distribution of O-glycans in normal human mucins along the intestinal tract. Biochem. J. 2004, 384, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Kufe, D.W. Mucins in cancer: Function, prognosis and therapy. Nat. Rev. Cancer 2009, 9, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, M.A.; Swanson, B.J. Mucins in cancer: Protection and control of the cell surface. Nat. Rev. Cancer 2004, 4, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Kumar, S.; Momi, N.; Sasson, A.R.; Batra, S.K. Mucins in pancreatic cancer and its microenvironment. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.B.; Shekels, L.L.; Toribara, N.W.; Kim, Y.S.; Lyftogt, C.; Cherwitz, D.L.; Niehans, G.A. Mucin gene expression in normal, preneoplastic, and neoplastic human gastric epithelium. Cancer Res. 1995, 55, 2681–2690. [Google Scholar] [PubMed]
- De Bolos, C.; Garrido, M.; Real, F.X. MUC6 apomucin shows a distinct normal tissue distribution that correlates with Lewis antigen expression in the human stomach. Gastroenterology 1995, 109, 723–734. [Google Scholar] [CrossRef]
- Reis, C.A.; David, L.; Carvalho, F.; Mandel, U.; de Bolos, C.; Mirgorodskaya, E.; Clausen, H.; Sobrinho-Simoes, M. Immunohistochemical study of the expression of MUC6 mucin and co-expression of other secreted mucins (MUC5AC and MUC2) in human gastric carcinomas. J. Histochem. Cytochem. 2000, 48, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.A.; David, L.; Nielsen, P.A.; Clausen, H.; Mirgorodskaya, K.; Roepstorff, P.; Sobrinho-Simoes, M. Immunohistochemical study of MUC5AC expression in human gastric carcinomas using a novel monoclonal antibody. Int. J. Cancer 1997, 74, 112–121. [Google Scholar] [CrossRef]
- Reis, C.A.; David, L.; Seixas, M.; Burchell, J.; Sobrinho-Simoes, M. Expression of fully and under-glycosylated forms of MUC1 mucin in gastric carcinoma. Int. J. Cancer 1998, 79, 402–410. [Google Scholar] [CrossRef]
- Timpte, C.S.; Eckhardt, A.E.; Abernethy, J.L.; Hill, R.L. Porcine submaxillary gland apomucin contains tandemly repeated, identical sequences of 81 residues. J. Biol. Chem. 1988, 263, 1081–1088. [Google Scholar] [PubMed]
- Gupta, R.; Jentoft, N. Subunit structure of porcine submaxillary mucin. Biochemistry 1989, 28, 6114–6121. [Google Scholar] [CrossRef] [PubMed]
- Carlstedt, I.; Sheehan, J.K.; Corfield, A.P.; Gallagher, J.T. Mucous glycoproteins: A gel of a problem. Essays Biochem. 1985, 20, 40–76. [Google Scholar] [PubMed]
- Perez-Vilar, J.; Hill, R.L. The structure and assembly of secreted mucins. J. Biol. Chem. 1999, 274, 31751–31754. [Google Scholar] [CrossRef] [PubMed]
- Bennett, E.P.; Hassan, H.; Mandel, U.; Mirgorodskaya, E.; Roepstorff, P.; Burchell, J.; Taylor-Papadimitriou, J.; Hollingsworth, M.A.; Merkx, G.; van Kessel, A.G.; et al. Cloning of a human UDP-N-acetyl-alpha-d-Galactosamine:polypeptide N-acetylgalactosaminyltransferase that complements other GalNAc-transferases in complete O-glycosylation of the MUC1 tandem repeat. J. Biol. Chem. 1998, 273, 30472–30481. [Google Scholar] [CrossRef] [PubMed]
- Clausen, H.; Bennett, E.P. A family of UDP-GalNAc: Polypeptide N-acetylgalactosaminyl-transferases control the initiation of mucin-type O-linked glycosylation. Glycobiology 1996, 6, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Bennett, E.P.; Mandel, U.; Clausen, H.; Gerken, T.A.; Fritz, T.A.; Tabak, L.A. Control of mucin-type O-glycosylation: A classification of the polypeptide GalNAc-transferase gene family. Glycobiology 2012, 22, 736–756. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.A.; Osorio, H.; Silva, L.; Gomes, C.; David, L. Alterations in glycosylation as biomarkers for cancer detection. J. Clin. Pathol. 2010, 63, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Kitada, S.; Yamada, S.; Kuma, A.; Ouchi, S.; Tasaki, T.; Nabeshima, A.; Noguchi, H.; Wang, K.Y.; Shimajiri, S.; Nakano, R.; et al. Polypeptide N-acetylgalactosaminyl transferase 3 independently predicts high-grade tumours and poor prognosis in patients with renal cell carcinomas. Br. J. Cancer 2013, 109, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Hennebicq, S.; Tetaert, D.; Soudan, B.; Boersma, A.; Briand, G.; Richet, C.; Gagnon, J.; Degand, P. Influence of the amino acid sequence on the MUC5AC motif peptide O-glycosylation by human gastric UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase(s). Glycoconj. J. 1998, 15, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Gerken, T.A.; Raman, J.; Fritz, T.A.; Jamison, O. Identification of common and unique peptide substrate preferences for the UDP-GalNAc: Polypeptide alpha-N-acetylgalactosaminyltransferases T1 and T2 derived from oriented random peptide substrates. J. Biol. Chem. 2006, 281, 32403–32416. [Google Scholar] [CrossRef] [PubMed]
- Wandall, H.H.; Hassan, H.; Mirgorodskaya, E.; Kristensen, A.K.; Roepstorff, P.; Bennett, E.P.; Nielsen, P.A.; Hollingsworth, M.A.; Burchell, J.; Taylor-Papadimitriou, J.; et al. Substrate specificities of three members of the human UDP-N-acetyl-alpha-d-galactosamine:Polypeptide N-acetylgalactosaminyltransferase family, GalNAc-T1, -T2, and -T3. J. Biol. Chem. 1997, 272, 23503–23514. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Shen, Z.; Zhang, H.; Wang, X.; Tang, Z.; Xu, J.; Sun, Y. Clinical significance of polypeptide N-acetylgalactosaminyl transferase-5 (GalNAc-T5) expression in patients with gastric cancer. Br. J. Cancer 2014, 110, 2021–2029. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Marcos, N.T.; Berois, N.; Osinaga, E.; Magalhaes, A.; Pinto-de-Sousa, J.; Almeida, R.; Gartner, F.; Reis, C.A. Expression of UDP-N-acetyl-d-galactosamine: Polypeptide N-acetylgalactosaminyltransferase-6 in gastric mucosa, intestinal metaplasia, and gastric carcinoma. J. Histochem. Cytochem. 2009, 57, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, Z.; Feng, J.; Sun, Q.; Zhang, B.; Zheng, W.; Ma, W. Expression pattern of polypeptide N-acetylgalactosaminyltransferase-10 in gastric carcinoma. Oncol. Lett. 2013, 5, 113–116. [Google Scholar] [PubMed]
- Cheng, L.; Tachibana, K.; Zhang, Y.; Guo, J.M.; Kahori Tachibana, K.; Kameyama, A.; Wang, H.; Hiruma, T.; Iwasaki, H.; Togayachi, A.; et al. Characterization of a novel human UDP-GalNAc transferase, pp-GalNAc-T10. FEBS Lett. 2002, 531, 115–121. [Google Scholar] [CrossRef]
- Teixeira, A.; David, L.; Reis, C.A.; Costa, J.; Sobrinho-Simoes, M. Expression of mucins (MUC1, MUC2, MUC5AC, and MUC6) and type 1 Lewis antigens in cases with and without Helicobacter pylori colonization in metaplastic glands of the human stomach. J. Pathol. 2002, 197, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Watanabe, T.; Tokumaru, T.; Takagi, H.; Nakazato, H.; Lloyd, K.O. Expression of Lewisa, Lewisb, Lewisx, Lewisy, siayl-Lewisa, and sialyl-Lewisx blood group antigens in human gastric carcinoma and in normal gastric tissue. Cancer Res. 1989, 49, 745–752. [Google Scholar] [PubMed]
- Lopez-Ferrer, A.; de Bolos, C.; Barranco, C.; Garrido, M.; Isern, J.; Carlstedt, I.; Reis, C.A.; Torrado, J.; Real, F.X. Role of fucosyltransferases in the association between apomucin and Lewis antigen expression in normal and malignant gastric epithelium. Gut 2000, 47, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Kurihara, M.; Goso, Y.; Urata, T.; Ota, H.; Katsuyama, T.; Hotta, K. Peripheral alpha-linked N-acetylglucosamine on the carbohydrate moiety of mucin derived from mammalian gastric gland mucous cells: Epitope recognized by a newly characterized monoclonal antibody. Biochem. J. 1996, 318, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Kawakubo, M.; Ito, Y.; Okimura, Y.; Kobayashi, M.; Sakura, K.; Kasama, S.; Fukuda, M.N.; Fukuda, M.; Katsuyama, T.; Nakayama, J.; et al. Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science 2004, 305, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, B.; Marcos, N.T.; David, L.; Nakayama, J.; Reis, C.A. Terminal alpha1,4-linked N-acetylglucosamine in Helicobacter pylori-associated intestinal metaplasia of the human stomach and gastric carcinoma cell lines. J. Histochem. Cytochem. 2006, 54, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, A.; Ismail, M.N.; Reis, C.A. Sweet receptors mediate the adhesion of the gastric pathogen Helicobacter pylori: Glycoproteomic strategies. Expert Rev. Proteom. 2010, 7, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.E.; Boren, T.; Solnick, J.V. Life at the margins: Modulation of attachment proteins in Helicobacter pylori. Gut Microbes 2011, 2, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Amieva, M.; Peek, R.M., Jr. Pathobiology of Helicobacter pylori-induced gastric cancer. Gastroenterology 2016, 150, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Selgrad, M. Helicobacter pylori. Curr. Opin. Gastroenterol. 2014, 30, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Chiang, T.H.; Chou, C.K.; Tu, Y.K.; Liao, W.C.; Wu, M.S.; Graham, D.Y. Association between Helicobacter pylori eradication and gastric cancer incidence: A systematic review and meta-analysis. Gastroenterology 2016, 150, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Boren, T.; Falk, P.; Roth, K.A.; Larson, G.; Normark, S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science 1993, 262, 1892–1895. [Google Scholar] [CrossRef] [PubMed]
- Ilver, D.; Arnqvist, A.; Ogren, J.; Frick, I.M.; Kersulyte, D.; Incecik, E.T.; Berg, D.E.; Covacci, A.; Engstrand, L.; Borén, T. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science 1998, 279, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Aspholm-Hurtig, M.; Dailide, G.; Lahmann, M.; Kalia, A.; Ilver, D.; Roche, N.; Vikström, S.; Sjöström, R.; Lindén, S.; Bäckström, A.; et al. Functional adaptation of BabA, the H. pylori ABO blood group antigen binding adhesin. Science 2004, 305, 519–522. [Google Scholar] [PubMed]
- Moonens, K.; Gideonsson, P.; Subedi, S.; Bugaytsova, J.; Romaõ, E.; Mendez, M.; Nordén, J.; Fallah, M.; Rakhimova, L.; Shevtsova, A.; et al. Structural insights into polymorphic ABO glycan binding by Helicobacter pylori. Cell Host Microbe 2016, 19, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.; Eriksson, S.; Mendes, N.; Serpa, J.; Figueiredo, C.; Resende, L.P.; Ruvoën-Clouet, N.; Haas, R.; Borén, T.; Le Pendu, J.; et al. Infection by Helicobacter pylori expressing the BabA adhesin is influenced by the secretor phenotype. J. Pathol. 2008, 215, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Linden, S.; Mahdavi, J.; Semino-Mora, C.; Olsen, C.; Carlstedt, I.; Boren, T.; Dubois, A. Role of ABO secretor status in mucosal innate immunity and H. pylori infection. PLoS Pathog. 2008, 4, e2. [Google Scholar]
- Ikehara, Y.; Nishihara, S.; Yasutomi, H.; Kitamura, T.; Matsuo, K.; Shimizu, N.; Inada, K.; Kodera, Y.; Yamamura, Y.; Narimatsu, H.; et al. Polymorphisms of two fucosyltransferase genes (Lewis and Secretor genes) involving type I Lewis antigens are associated with the presence of anti-Helicobacter pylori IgG antibody. Cancer Epidemiol. Biomark. Prev. 2001, 10, 971–977. [Google Scholar]
- Magalhaes, A.; Gomes, J.; Ismail, M.N.; Haslam, S.M.; Mendes, N.; Osório, H.; David, L.; Le Pendu, J.; Haas, R.; Dell, A.; et al. Fut2-null mice display an altered glycosylation profile and impaired BabA-mediated Helicobacter pylori adhesion to gastric mucosa. Glycobiology 2009, 19, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, M.; Lehn, N.; Neumayer, N.; Boren, T.; Rad, R.; Schepp, W.; Miehlke, S.; Classen, M.; Prinz, C. Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proc. Natl. Acad. Sci. USA 1999, 96, 12778–12783. [Google Scholar] [CrossRef] [PubMed]
- Alm, R.A.; Bina, J.; Andrews, B.M.; Doig, P.; Hancock, R.E.; Trust, T.J. Comparative genomics of Helicobacter pylori: Analysis of the outer membrane protein families. Infect. Immun. 2000, 68, 4155–4168. [Google Scholar] [CrossRef] [PubMed]
- Rossez, Y.; Gosset, P.; Boneca, I.G.; Magalhães, A.; Ecobichon, C.; Reis, C.A.; Cieniewski-Bernard, C.; Joncquel Chevalier Curt, M.; Léonard, R.; Maes, E.; et al. The lacdiNAc-specific adhesin LabA mediates adhesion of Helicobacter pylori to human gastric mucosa. J. Infect. Dis. 2014, 210, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, J.; Sondén, B.; Hurtig, M.; Olfat, F.O.; Forsberg, L.; Roche, N.; Angstrom, J.; Larsson, T.; Teneberg, S.; Karlsson, K.A.; et al. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science 2002, 297, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Ota, H.; Nakayama, J.; Momose, M.; Hayama, M.; Akamatsu, T.; Katsuyama, T.; Graham, D.Y.; Genta, R.M. Helicobacter pylori infection produces reversible glycosylation changes to gastric mucins. Virchows Arch. 1998, 433, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, A.; Marcos-Pinto, R.; Nairn, A.V.; Dela Rosa, M.; Ferreira, R.M.; Junqueira-Neto, S.; Freitas, D.; Gomes, J.; Oliveira, P.; Santos, M.R.; et al. Helicobacter pylori chronic infection and mucosal inflammation switches the human gastric glycosylation pathways. Biochim. Biophys. Acta 2015, 1852, 1928–1939. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, A.; Marcos, N.T.; Carvalho, A.S.; David, L.; Figueiredo, C.; Bastos, J.; David, G.; Reis, C.A. Helicobacter pylori cag pathogenicity island-positive strains induce syndecan-4 expression in gastric epithelial cells. FEMS Immunol. Med. Microbiol. 2009, 56, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Marcos, N.T.; Magalhães, A.; Ferreira, B.; Oliveira, M.J.; Carvalho, A.S.; Mendes, N.; Gilmartin, T.; Head, S.R.; Figueiredo, C.; David, L.; Santos-Silva, F.; et al. Helicobacter pylori induces beta3GnT5 in human Gastric Cell Lines, Modulating Expression of the SabA ligand sialyl-Lewis x. J. Clin. Investig. 2008, 118, 2325–2336. [Google Scholar] [PubMed]
- Aspholm, M.; Olfat, F.O.; Nordén, J.; Sondén, B.; Lundberg, C.; Sjöström, R.; Altraja, S.; Odenbreit, S.; Haas, R.; Wadström, T.; et al. SabA is the H. pylori hemagglutinin and is polymorphic in binding to sialylated glycans. PLoS Pathog. 2006, 2, e110. [Google Scholar]
- Pang, S.S.; Nguyen, S.T.; Perry, A.J.; Day, C.J.; Panjikar, S.; Tiralongo, J.; Whisstock, J.C.; Kwok, T. The three-dimensional structure of the extracellular adhesion domain of the sialic acid-binding adhesin SabA from Helicobacter pylori. J. Biol. Chem. 2014, 289, 6332–6340. [Google Scholar] [CrossRef] [PubMed]
- Talarico, S.; Whitefield, S.E.; Fero, J.; Haas, R.; Salama, N.R. Regulation of Helicobacter pylori adherence by gene conversion. Mol. Microbiol. 2012, 84, 1050–1061. [Google Scholar] [CrossRef] [PubMed]
- Gatta, L.; Vakil, N.; Vaira, D.; Scarpignato, C. Global eradication rates for Helicobacter pylori infection: Systematic review and meta-analysis of sequential therapy. BMJ 2013. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, A.C.; Weinberger, D.M.; Ford, C.B.; Nelson, J.C.; Snider, J.D.; Hall, J.D.; Paules, C.I.; Peek, R.M., Jr.; Forsyth, M.H. Expression of the Helicobacter pylori adhesin SabA is controlled via phase variation and the ArsRS signal transduction system. Microbiology 2008, 154, 2231–2240. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, Y.; Ojo, O.; Fujimoto, S.; Odenbreit, S.; Haas, R.; Gutierrez, O.; El-Zimaity, H.M.; Reddy, R.; Arnqvist, A.; Graham, D.Y. Helicobacter pylori outer membrane proteins and gastroduodenal disease. Gut 2006, 55, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Atherton, J.; Axon, A.T.; Bazzoli, F.; Gensini, G.F.; Gisbert, J.P.; Graham, D.Y.; Rokkas, T.; et al. Management of Helicobacter pylori infection—the Maastricht IV/Florence consensus report. Gut 2012, 61, 646–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goncalves, I.C.; Magalhaes, A.; Costa, A.M.; Oliveira, J.R.; Henriques, P.C.; Gomes, P.; Reis, C.A.; Martins, M.C. Bacteria-targeted biomaterials: Glycan-coated microspheres to bind Helicobacter pylori. Acta Biomater. 2016, 33, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, I.C.; Magalhaes, A.; Fernandes, M.; Rodrigues, I.V.; Reis, C.A.; Martins, M.C. Bacterial-binding chitosan microspheres for gastric infection treatment and prevention. Acta Biomater. 2013, 9, 9370–9378. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Wang, P.; Hoshino, H.; Ito, Y.; Kobayashi, M.; Nakayama, J.; Seeberger, P.H.; Fukuda, M. Alpha1,4GlcNAc-capped mucin-type O-glycan inhibits cholesterol alpha-glucosyltransferase from Helicobacter pylori and suppresses H. pylori growth. Glycobiology 2008, 18, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, F.G.; Bonar, D.; Schloerer, N.; Schroten, H. Human trefoil factor 2 is a lectin that binds alpha-GlcNAc-capped mucin glycans with antibiotic activity against Helicobacter pylori. J. Biol. Chem. 2014, 289, 27363–27375. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Naughton, J.; Clyne, M.; Murphy, P.V. Synthesis of bivalent glycoclusters containing GlcNAc as hexasaccharide mimetics. Bactericidal activity against Helicobacter pylori. Carbohydr. Res. 2012, 360, 1–7. [Google Scholar] [PubMed]
- Kobayashi, M.; Fukuda, M.; Nakayama, J. Role of sulfated O-glycans expressed by high endothelial venule-like vessels in pathogenesis of chronic inflammatory gastrointestinal diseases. Biol. Pharm. Bull. 2009, 32, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Correa, P. A human model of gastric carcinogenesis. Cancer Res. 1988, 48, 3554–3560. [Google Scholar] [PubMed]
- Heilmann, K.L.; Hopker, W.W. Loss of differentiation in intestinal metaplasia in cancerous stomachs. A comparative morphologic study. Pathol. Res. Pract. 1979, 164, 249–258. [Google Scholar] [CrossRef]
- Sipponen, P.; Seppala, K.; Varis, K.; Hjelt, L.; Ihamaki, T.; Kekki, M.; Siurala, M. Intestinal metaplasia with colonic-type sulphomucins in the gastric mucosa; its association with gastric carcinoma. Acta Pathol. Microbiol. Scand. A 1980, 88, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.A.; David, L.; Correa, P.; Carneiro, F.; de Bolos, C.; Garcia, E.; Mandel, U.; Clausen, H.; Sobrinho-Simoes, M. Intestinal metaplasia of human stomach displays distinct patterns of mucin (MUC1, MUC2, MUC5AC, and MUC6) expression. Cancer Res. 1999, 59, 1003–1007. [Google Scholar] [PubMed]
- Filipe, M.I.; Jass, J.R. Intestinal metaplasia subtypes and cancer risk. In Gastric Carcinoma; Filipe, M.I., Jass, J.R., Eds.; Churchill Livingstone: London, UK, 1986; pp. 87–115. [Google Scholar]
- Filipe, M.I. Borderline lesions of the gastric epithelium: New indicators of gastric risk and clinical implications. In Progress in Surgical Pathology; Springer Berlin Heidelberg: Berlin, Heidelberg, Germany, 1992; pp. 269–290. [Google Scholar]
- Dinis-Ribeiro, M.; Areia, M.; de Vries, A.C.; Marcos-Pinto, R.; Monteiro-Soares, M.; O’Connor, A.; Pereira, C.; Pimentel-Nunes, P.; Correia, R.; Ensari, A.; et al. Management of precancerous conditions and lesions in the stomach (MAPS): Guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Virchows Arch. 2012, 460, 19–46. [Google Scholar] [PubMed]
- Torrado, J.; Correa, P.; Ruiz, B.; Bernardi, P.; Zavala, D.; Bara, J. Lewis antigen alterations in gastric cancer precursors. Gastroenterology 1992, 102, 424–430. [Google Scholar] [CrossRef]
- Murata, K.; Egami, H.; Shibata, Y.; Sakamoto, K.; Misumi, A.; Ogawa, M. Expression of blood group-related antigens, ABH, Lewis(a), Lewis(b), Lewis(x), Lewis(y), CA19-9, and CSLEX1 in early cancer, intestinal metaplasia, and uninvolved mucosa of the stomach. Am. J. Clin. Pathol. 1992, 98, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Torrado, J.; Blasco, E.; Gutierrez-Hoyos, A.; Cosme, A.; Lojendio, M.; Arenas, J.I. Lewis system alterations in gastric carcinogenesis. Cancer 1990, 66, 1769–1774. [Google Scholar] [CrossRef]
- Carneiro, F.; Santos, L.; David, L.; Dabelsteen, E.; Clausen, H.; Sobrinho-Simoes, M. T (Thomsen-Friedenreich) antigen and other simple mucin-type carbohydrate antigens in precursor lesions of gastric carcinoma. Histopathology 1994, 24, 105–113. [Google Scholar] [CrossRef] [PubMed]
- David, L.; Nesland, J.M.; Clausen, H.; Carneiro, F.; Sobrinho-Simoes, M. Simple mucin-type carbohydrate antigens (Tn, sialosyl-Tn and T) in Gastric Mucosa, Carcinomas and metastases. APMIS Suppl. 1992, 27, 162–172. [Google Scholar] [PubMed]
- Marcos, N.T.; Pinho, S.; Grandela, C.; Cruz, A.; Samyn-Petit, B.; Harduin-Lepers, A.; Almeida, R.; Silva, F.; Morais, V.; Costa, J.; Kihlberg, J.; et al. Role of the human ST6GalNAc-I and ST6GalNAc-II in the synthesis of the cancer-associated sialyl-Tn antigen. Cancer Res. 2004, 64, 7050–7057. [Google Scholar] [CrossRef] [PubMed]
- Marcos, N.T.; Bennett, E.P.; Gomes, J.; Magalhaes, A.; Gomes, C.; David, L.; Dar, I.; Jeanneau, C.; DeFrees, S.; Krustrup, D.; et al. ST6GalNAc-I controls expression of sialyl-Tn antigen in gastrointestinal tissues. Front. Biosci. 2011, 3, 1443–1455. [Google Scholar] [CrossRef]
- Pinto, R.; Barros, R.; Pereira-Castro, I.; Mesquita, P.; da Costa, L.T.; Bennett, E.P.; Almeida, R.; David, L. CDX2 homeoprotein is involved in the regulation of ST6GalNAc-I gene in intestinal metaplasia. Lab. Investig. 2015, 95, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Conze, T.; Carvalho, A.S.; Landegren, U.; Almeida, R.; Reis, C.A.; David, L.; Soderberg, O. MUC2 mucin is a major carrier of the cancer-associated sialyl-Tn antigen in intestinal metaplasia and gastric carcinomas. Glycobiology 2010, 20, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.; Almeida, A.; Ferreira, J.A.; Silva, L.; Santos-Sousa, H.; Pinto-de-Sousa, J.; Santos, L.L.; Amado, F.; Schwientek, T.; Levery, S.B.; et al. Glycoproteomic analysis of serum from patients with gastric precancerous lesions. J. Proteome Res. 2013, 12, 1454–1466. [Google Scholar] [CrossRef] [PubMed]
- Rugge, M.; Capelle, L.G.; Cappellesso, R.; Nitti, D.; Kuipers, E.J. Precancerous lesions in the stomach: From biology to clinical patient management. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Pinto-de-Sousa, J.; David, L.; Reis, C.A.; Gomes, R.; Silva, L.; Pimenta, A. Mucins MUC1, MUC2, MUC5AC and MUC6 expression in the evaluation of differentiation and clinico-biological behaviour of gastric carcinoma. Virchows Arch. 2002, 440, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Jass, J.R.; Filipe, M.I. The mucin profiles of normal gastric mucosa, intestinal metaplasia and its variants and gastric carcinoma. Histochem. J. 1981, 13, 931–939. [Google Scholar] [CrossRef] [PubMed]
- De Bolos, C.; Real, F.X.; Lopez-Ferrer, A. Regulation of mucin and glycoconjugate expression: From normal epithelium to gastric tumors. Front. Biosci. 2001, 6, D1256–D1263. [Google Scholar] [CrossRef] [PubMed]
- Perrais, M.; Pigny, P.; Buisine, M.P.; Porchet, N.; Aubert, J.P.; van Seuningen-Lempire, I. Aberrant expression of human mucin gene MUC5B in gastric carcinoma and cancer cells. Identification and regulation of a distal promoter. J. Biol. Chem. 2001, 276, 15386–15396. [Google Scholar] [PubMed]
- Senapati, S.; Chaturvedi, P.; Sharma, P.; Venkatraman, G.; Meza, J.L.; El-Rifai, W.; Roy, H.K.; Batra, S.K. Deregulation of MUC4 in gastric adenocarcinoma: Potential pathobiological implication in poorly differentiated non-signet ring cell type gastric cancer. Br. J. Cancer 2008, 99, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, T.; Yonezawa, S.; Sakamoto, H.; Kitamura, H.; Hokita, S.; Aiko, T.; Tanaka, S.; Irimura, T.; Kim, Y.S.; Sato, E. Expression of MUC1 and MUC2 mucins in gastric carcinomas: Its relationship with the prognosis of the patients. Clin. Cancer Res. 1998, 4, 2605–2614. [Google Scholar] [PubMed]
- Kufe, D.W. MUC1-C oncoprotein as a target in breast cancer: Activation of signaling pathways and therapeutic approaches. Oncogene 2013, 32, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Papadimitriou, J.; Burchell, J.; Miles, D.W.; Dalziel, M. MUC1 and cancer. Biochim. Biophys. Acta 1999, 1455, 301–313. [Google Scholar] [CrossRef]
- Costa, N.R.; Mendes, N.; Marcos, N.T.; Reis, C.A.; Caffrey, T.; Hollingsworth, M.A.; Santos-Silva, F. Relevance of MUC1 mucin variable number of tandem repeats polymorphism in H pylori adhesion to gastric epithelial cells. World J. Gastroenterol. 2008, 14, 1411–1414. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.; Gao, F.; Zhou, C.J.; Jia, Y.B. Polymorphisms in mucin genes in the development of gastric cancer. World J. Gastrointest. Oncol. 2015, 7, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Santos-Silva, F.; Fonseca, A.; Caffrey, T.; Carvalho, F.; Mesquita, P.; Reis, C.; Almeida, R.; David, L.; Hollingsworth, M.A. Thomsen-Friedenreich antigen expression in gastric carcinomas is associated with MUC1 mucin VNTR polymorphism. Glycobiology 2005, 15, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.A. Ana magalhaes, glycosyltransferases and gastric cancer. In Glycosignals in Cancer: Mechanisms of Malignant Phenotypes; Springer: New York, NY, USA, 2016; pp. 17–32. [Google Scholar]
- Ishikawa, M.; Kitayama, J.; Nariko, H.; Kohno, K.; Nagawa, H. The expression pattern of UDP-N-acetyl-alpha-d-galactosamine: Polypeptide N-acetylgalactosaminyl transferase-3 in early gastric carcinoma. J. Surg. Oncol. 2004, 86, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Marcos, N.T.; Cruz, A.; Silva, F.; Almeida, R.; David, L.; Mandel, U.; Clausen, H.; von Mensdorff-Pouilly, S.; Reis, C.A. Polypeptide GalNAc-transferases, ST6GalNAc-transferase I, and ST3Gal-transferase I expression in gastric carcinoma cell lines. J. Histochem. Cytochem. 2003, 51, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Shen, L.; Xu, L.; Jiang, Z.; Zhou, Y.; Yue, A.; Zou, S.; Cheng, Z.; Wu, S. Polypeptide N-acetylgalactosaminyltransferase 2 regulates cellular metastasis-associated behavior in gastric cancer. Int. J. Mol. Med. 2012, 30, 1267–1274. [Google Scholar] [PubMed]
- Liu, S.Y.; Shun, C.T.; Hung, K.Y.; Juan, H.F.; Hsu, C.L.; Huang, M.C.; Lai, I.R. Mucin glycosylating enzyme GALNT2 suppresses malignancy in gastric adenocarcinoma by reducing MET phosphorylation. Oncotarget 2016, 7, 11251–11262. [Google Scholar] [PubMed]
- Springer, G.F. T and Tn, general carcinoma autoantigens. Science 1984, 224, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.S.; Yamashita, Y.; Kato, Y.; Nakata, B.; Sawada, T.; Sowa, M. Prognostic significance of T antigen expression in patients with gastric carcinoma. Cancer 1996, 77, 1768–1773. [Google Scholar] [CrossRef]
- Pinho, S.; Marcos, N.T.; Ferreira, B.; Carvalho, A.S.; Oliveira, M.J.; Santos-Silva, F.; Harduin-Lepers, A.; Reis, C.A. Biological significance of cancer-associated sialyl-Tn antigen: Modulation of malignant phenotype in gastric carcinoma cells. Cancer Lett. 2007, 249, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Ikehara, Y.; Nishihara, S.; Kudo, T.; Hiraga, T.; Morozumi, K.; Hattori, T.; Narimatsu, H. The aberrant expression of Lewis a antigen in intestinal metaplastic cells of gastric mucosa is caused by augmentation of Lewis enzyme expression. Glycoconj. J. 1998, 15, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Serpa, J.; Mesquita, P.; Mendes, N.; Oliveira, C.; Almeida, R.; Santos-Silva, F.; Reis, C.A.; LePendu, J.; David, L. Expression of Lea in gastric cancer cell lines depends on FUT3 expression regulated by promoter methylation. Cancer Lett. 2006, 242, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Sakamoto, J.; Kito, T.; Yamamura, Y.; Koshikawa, T.; Fujita, M.; Watanabe, T.; Nakazato, H. Lewis blood group-related antigen expression in normal gastric epithelium, intestinal metaplasia, gastric adenoma, and gastric carcinoma. Am. J. Gastroenterol. 1993, 88, 919–924. [Google Scholar] [PubMed]
- Dohi, T.; Hashiguchi, M.; Yamamoto, S.; Morita, H.; Oshima, M. Fucosyltransferase-producing sialyl Le(a) and sialyl Le(x) carbohydrate antigen in benign and malignant gastrointestinal mucosa. Cancer 1994, 73, 1552–1561. [Google Scholar] [CrossRef]
- Ashizawa, T.; Aoki, T.; Yamazaki, T.; Katayanagi, S.; Shimizu, H.; Koyanagi, Y. The clinical significance of sialyl Lewis antigen expression in the spread of gastric cancer. Flow cytometric DNA analysis. J. Exp. Clin. Cancer Res. 2003, 22, 91–98. [Google Scholar] [PubMed]
- Sumikura, S.; Ishigami, S.; Natsugoe, S.; Miyazono, F.; Tokuda, K.; Nakajo, A.; Okumura, H.; Matsumoto, M.; Hokita, S.; Aikou, T. Disseminated cancer cells in the blood and expression of sialylated antigen in gastric cancer. Cancer Lett. 2003, 200, 77–83. [Google Scholar] [CrossRef]
- Jun, L.; Yuanshu, W.; Yanying, X.; Zhongfa, X.; Jian, Y.; Fengling, W.; Xianjun, Q.; Kokudo, N.; Wei, T.; Weixia, Z.; et al. Altered mRNA expressions of sialyltransferases in human gastric cancer tissues. Med. Oncol. 2012, 29, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Paschos, K.A.; Canovas, D.; Bird, N.C. The engagement of selectins and their ligands in colorectal cancer liver metastases. J. Cell. Mol. Med. 2010, 14, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Laubli, H.; Borsig, L. Selectins promote tumor metastasis. Semin. Cancer Biol. 2010, 20, 169–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amado, M.; Carneiro, F.; Seixas, M.; Clausen, H.; Sobrinho-Simoes, M. Dimeric sialyl-Le(x) expression in gastric carcinoma correlates with venous invasion and poor outcome. Gastroenterology 1998, 114, 462–470. [Google Scholar] [CrossRef]
- Ura, H.; Denno, R.; Hirata, K.; Yamaguchi, K.; Yasoshima, T.; Shishido, T. Close correlation between increased sialyl-Lewisx expression and metastasis in human gastric carcinoma. World J. Surg. 1997, 21, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Futamura, N.; Nakamura, S.; Tatematsu, M.; Yamamura, Y.; Kannagi, R.; Hirose, H. Clinicopathologic significance of sialyl Le(x) expression in advanced gastric carcinoma. Br. J. Cancer 2000, 83, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Baldus, S.E.; Zirbes, T.K.; Mönig, S.P.; Engel, S.; Monaca, E.; Rafiqpoor, K.; Hanisch, F.G.; Hanski, C.; Thiele, J.; Pichlmaier, H.; et al. Histopathological subtypes and prognosis of gastric cancer are correlated with the expression of mucin-associated sialylated antigens: Sialosyl-Lewis(a), Sialosyl-Lewis(x) and sialosyl-Tn. Tumour Biol. 1998, 19, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Gretschel, S.; Haensch, W.; Schlag, P.M.; Kemmner, W. Clinical relevance of sialyltransferases ST6GAL-I and ST3GAL-III in gastric cancer. Oncology 2003, 65, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.S.; Harduin-Lepers, A.; Magalhães, A.; Machado, E.; Mendes, N.; Costa, L.T.; Matthiesen, R.; Almeida, R.; Costa, J.; Reis, C.A. Differential expression of alpha-2,3-sialyltransferases and alpha-1,3/4-fucosyltransferases regulates the levels of sialyl Lewis a and sialyl Lewis x in gastrointestinal carcinoma cells. Int. J. Biochem. Cell. Biol. 2010, 42, 80–89. [Google Scholar] [CrossRef] [PubMed]
- David, L.; Nesland, J.M.; Funderud, S.; Sobrinho-Simoes, M. CDw75 antigen expression in human gastric carcinoma and adjacent mucosa. Cancer 1993, 72, 1522–1527. [Google Scholar] [CrossRef]
- Campos, D.; Freitas, D.; Gomes, J.; Magalhães, A.; Steentoft, C.; Gomes, C.; Vester-Christensen, M.B.; Ferreira, J.A.; Afonso, L.P.; Santos, L.L.; et al. Probing the O-glycoproteome of gastric cancer cell lines for biomarker discovery. Mol. Cell. Proteom. 2015, 14, 1616–1629. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.; Freitas, D.; Gomes, J.; Reis, C.A. Glycoengineered cell models for the characterization of cancer O-glycoproteome: An innovative strategy for biomarker discovery. Expert Rev. Proteom. 2015, 12, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.P.; Zhou, Y.Q.; Zhang, R.; Wang, M.H. MSP-RON signalling in cancer: Pathogenesis and therapeutic potential. Nat. Rev. Cancer 2013, 13, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, J.; Cai, J.; Song, X.; Deng, J.; Huang, X.; Chen, D.; Yang, M.; Wery, J.P.; Li, S.; et al. A subset of gastric cancers with EGFR amplification and overexpression respond to cetuximab therapy. Sci. Rep. 2013, 3, 2992. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, H.; Okamoto, I.; Arao, T.; Okamoto, W.; Matsumoto, K.; Taniguchi, H.; Kuwata, K.; Yamaguchi, H.; Nishio, K.; Nakagawa, K.; et al. MET amplification as a potential therapeutic target in gastric cancer. Oncotarget 2013, 4, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Zhu, Y.; Wang, Q.; Gao, J.; Li, Y.; Li, Y.; Ge, S.; Shen, L. Prognostic significance of MET amplification and expression in gastric cancer: A systematic review with meta-analysis. PLoS ONE 2014, 9, e84502. [Google Scholar] [CrossRef] [PubMed]
- Gravalos, C.; Jimeno, A. HER2 in gastric cancer: A new prognostic factor and a novel therapeutic target. Ann. Oncol. 2008, 19, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Mereiter, S.; Magalhães, A.; Adamczyk, B.; Jin, C.; Almeida, A.; Drici, L.; Ibáñez-Vea, M.; Gomes, C.; Ferreira, J.A.; Afonso, L.P.; et al. Glycomic analysis of gastric carcinoma cells discloses glycans as modulators of RON receptor tyrosine kinase activation in cancer. Biochim. Biophys. Acta 2015. [Google Scholar] [CrossRef] [PubMed]
- Morishita, A.; Gong, J.; Masaki, T. Targeting receptor tyrosine kinases in gastric cancer. World J. Gastroenterol. 2014, 20, 4536–4545. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Kao, H.W.; Robinson, D.; Kung, H.J.; Wu, C.W.; Chen, H.C. Tyrosine kinases and gastric cancer. Oncogene 2000, 19, 5680–5689. [Google Scholar] [CrossRef] [PubMed]
- Stowell, S.R.; Ju, T.; D, R. Cummings, Protein glycosylation in cancer. Ann. Rev. Pathol. 2015, 10, 473–510. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.; Osorio, H.; Pinto, M.T.; Campos, D.; Oliveira, M.J.; Reis, C.A. Expression of ST3GAL4 leads to SLe(x) expression and induces c-Met activation and an invasive phenotype in gastric carcinoma cells. PLoS ONE 2013, 8, e66737. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.E.; Kim, M.A.; Lee, H.S.; Jung, E.J.; Yang, H.K.; Lee, B.L.; Bang, Y.J.; Kim, W.H. MET in gastric carcinomas: Comparison between protein expression and gene copy number and impact on clinical outcome. Br. J. Cancer 2012, 107, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Inokuchi, M.; Otsuki, S.; Fujimori, Y.; Sato, Y.; Nakagawa, M.; Kojima, K. Clinical significance of MET in gastric cancer. World J. Gastrointest. Oncol. 2015, 7, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Graziano, F.; Galluccio, N.; Lorenzini, P.; Ruzzo, A.; Canestrari, E.; D’Emidio, S.; Catalano, V.; Sisti, V.; Ligorio, C.; Andreoni, F.; et al. Genetic activation of the MET pathway and prognosis of patients with high-risk, radically resected gastric cancer. J. Clin. Oncol. 2011, 29, 4789–4795. [Google Scholar] [CrossRef] [PubMed]
- Churin, Y.; Al-Ghoul, L.; Kepp, O.; Meyer, T.F.; Birchmeier, W.; Naumann, M. Helicobacter pylori CagA protein targets the c-Met receptor and enhances the motogenic response. J. Cell Biol. 2003, 161, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.J.; Costa, A.M.; Costa, A.C.; Ferreira, R.M.; Sampaio, P.; Machado, J.C.; Seruca, R.; Mareel, M.; Figueiredo, C. CagA associates with c-Met, E-cadherin, and p120-catenin in a multiproteic complex that suppresses Helicobacter pylori-induced cell-invasive phenotype. J. Infect. Dis. 2009, 200, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.J.; Costa, A.C.; Costa, A.M.; Henriques, L.; Suriano, G.; Atherton, J.C.; Machado, J.C.; Carneiro, F.; Seruca, R.; Mareel, M.; et al. Helicobacter pylori induces gastric epithelial cell invasion in a c-Met and type IV secretion system-dependent manner. J. Biol. Chem. 2006, 281, 34888–34896. [Google Scholar] [CrossRef] [PubMed]
- Odenbreit, S.; Puls, J.; Sedlmaier, B.; Gerland, E.; Fischer, W.; Haas, R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 2000, 287, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Mimuro, H.; Suzuki, T.; Park, M.; Yamamoto, T.; Sasakawa, C. Interaction of CagA with Crk plays an important role in Helicobacter pylori-induced loss of gastric epithelial cell adhesion. J. Exp. Med. 2005, 202, 1235–1247. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, S.; Okumura, T.; Tu, S.; Wang, S.S.; Shibata, W.; Vigneshwaran, R.; Gordon, S.A.; Shimada, Y.; Wang, T.C. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells 2009, 27, 1006–1020. [Google Scholar] [CrossRef] [PubMed]
- Orian-Rousseau, V.; Chen, L.; Sleeman, J.P.; Herrlich, P.; Ponta, H. CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev. 2002, 16, 3074–3086. [Google Scholar] [CrossRef] [PubMed]
- Bertaux-Skeirik, N.; Feng, R.; Schumacher, M.A.; Li, J.; Mahe, M.M.; Engevik, A.C.; Javier, J.E.; Peek, R.M., Jr.; Ottemann, K.; Orian-Rousseau, V.; et al. CD44 plays a functional role in Helicobacter pylori-induced epithelial cell proliferation. PLoS Pathog. 2015, 11, e1004663. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Long, A.; Goggins, M.; Fan, X.; Keeling, P.W.; Kelleher, D. Expression of CD44 and its variants on gastric epithelial cells of patients with Helicobacter pylori colonisation. Gut 1996, 38, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, C.B.; Oliveira, C.; Wen, X.; Gomes, B.; Sousa, S.; Suriano, G.; Grellier, M.; Huntsman, D.G.; Carneiro, F.; Granja, P.L.; et al. De novo expression of CD44 variants in sporadic and hereditary gastric cancer. Lab. Investig. 2010, 90, 1604–1614. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Hascall, V.C.; Markwald, R.R.; Ghatak, S. Interactions between hyaluronan and its receptors (CD44, RHAMM) regulate the activities of inflammation and cancer. Front. Immunol. 2015, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Naor, D.; Nedvetzki, S.; Golan, I.; Melnik, L.; Faitelson, Y. CD44 in cancer. Crit. Rev. Clin. Lab. Sci. 2002, 39, 527–579. [Google Scholar] [CrossRef] [PubMed]
- Naor, D.; Sionov, R.V.; Ish-Shalom, D. CD44: Structure, function, and association with the malignant process. Adv. Cancer Res. 1997, 71, 241–319. [Google Scholar] [PubMed]
- Hsieh, H.F.; Yu, J.C.; Ho, L.I.; Chiu, S.C.; Harn, H.J. Molecular studies into the role of CD44 variants in metastasis in gastric cancer. Mol. Pathol. 1999, 52, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Aruffo, A.; Stamenkovic, I.; Melnick, M.; Underhill, C.B.; Seed, B. CD44 is the principal cell surface receptor for hyaluronate. Cell 1990, 61, 1303–1313. [Google Scholar] [CrossRef]
- Turley, E.A.; Noble, P.W.; Bourguignon, L.Y. Signaling properties of hyaluronan receptors. J. Biol. Chem. 2002, 277, 4589–4592. [Google Scholar] [CrossRef] [PubMed]
- Mereiter, S.; Magalhães, A.; Adamczyk, B.; Jin, C.; Almeida, A.; Drici, L.; Ibáñez-Vea, M.; Larsen, M.R.; Kolarich, D.; Karlsson, N.G.; et al. Glycomic and sialoproteomic data of gastric carcinoma cells overexpressing ST3GAL4. Data Brief 2016, 7, 814–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magalhaes, A.; Mereiter, S.; Reis, C. Reciprocal modulation of terminal sialylation and bisecting N-glycans: A new axis of cancer-cell glycome regulation? J. Biol. Chem. 2016, 291, 8308. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte, H.O.; Freitas, D.; Gomes, C.; Gomes, J.; Magalhães, A.; Reis, C.A. Mucin-Type O-Glycosylation in Gastric Carcinogenesis. Biomolecules 2016, 6, 33. https://doi.org/10.3390/biom6030033
Duarte HO, Freitas D, Gomes C, Gomes J, Magalhães A, Reis CA. Mucin-Type O-Glycosylation in Gastric Carcinogenesis. Biomolecules. 2016; 6(3):33. https://doi.org/10.3390/biom6030033
Chicago/Turabian StyleDuarte, Henrique O., Daniela Freitas, Catarina Gomes, Joana Gomes, Ana Magalhães, and Celso A. Reis. 2016. "Mucin-Type O-Glycosylation in Gastric Carcinogenesis" Biomolecules 6, no. 3: 33. https://doi.org/10.3390/biom6030033







