An Epigenomic Approach to Improving Response to Neoadjuvant Cisplatin Chemotherapy in Bladder Cancer
Abstract
:1. Introduction
2. Results
2.1. Bladder Cancer Cell Lines Have a Distinct Resistance Pattern to Chemotherapeutic Drugs
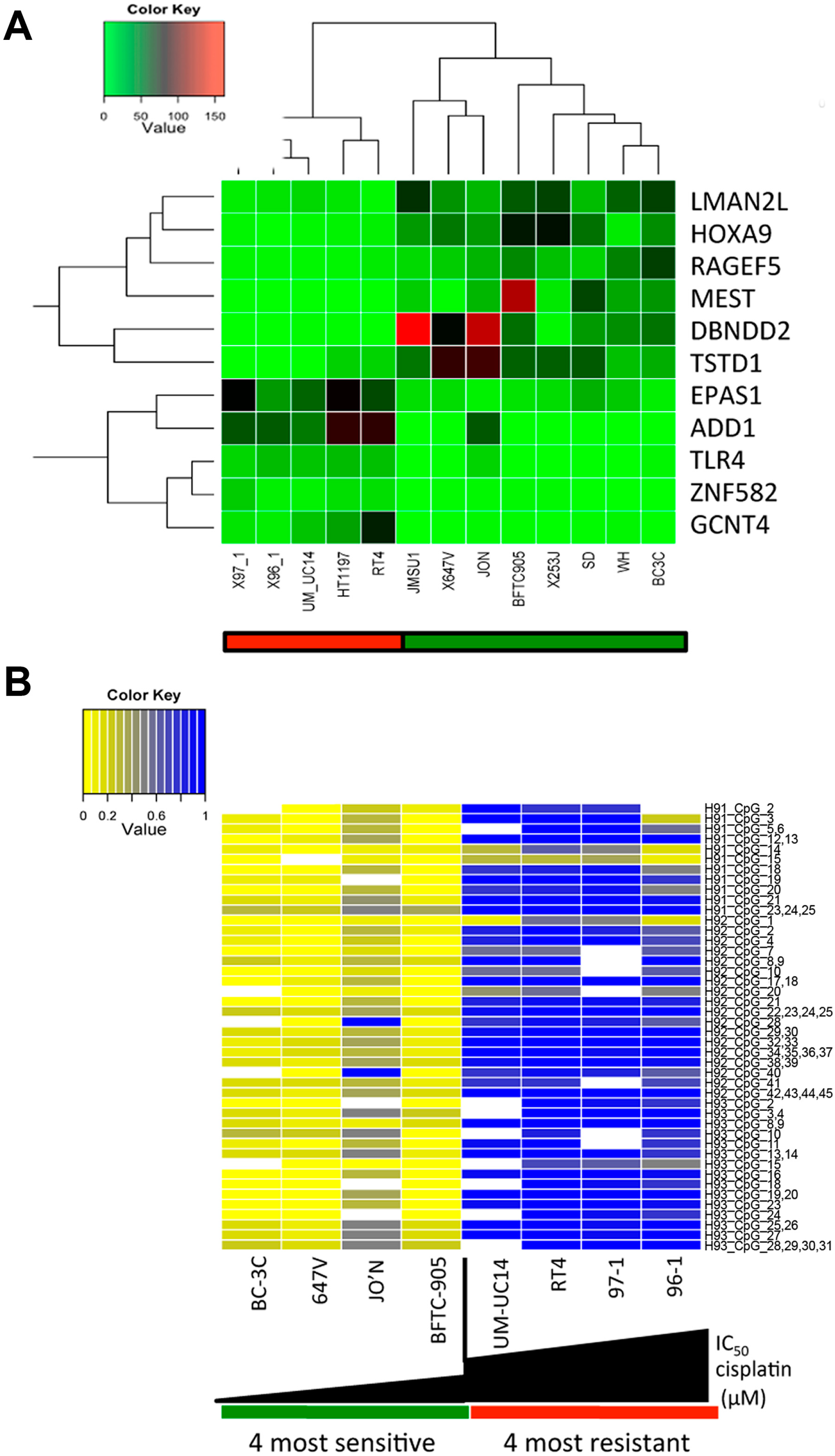
2.2. A Specific Gene Expression Signature is Associated with Cisplatin Resistance
2.3. HOXA9 Promoter Methylation Levels Are Associated with Cisplatin Resistance in Bladder Cancer Cell Lines
2.4. HOXA9 Promoter Methylation Levels Are Associated with Response to Neoadjuvant Cisplatin-Based Combination Chemotherapy in Patients with MIBC
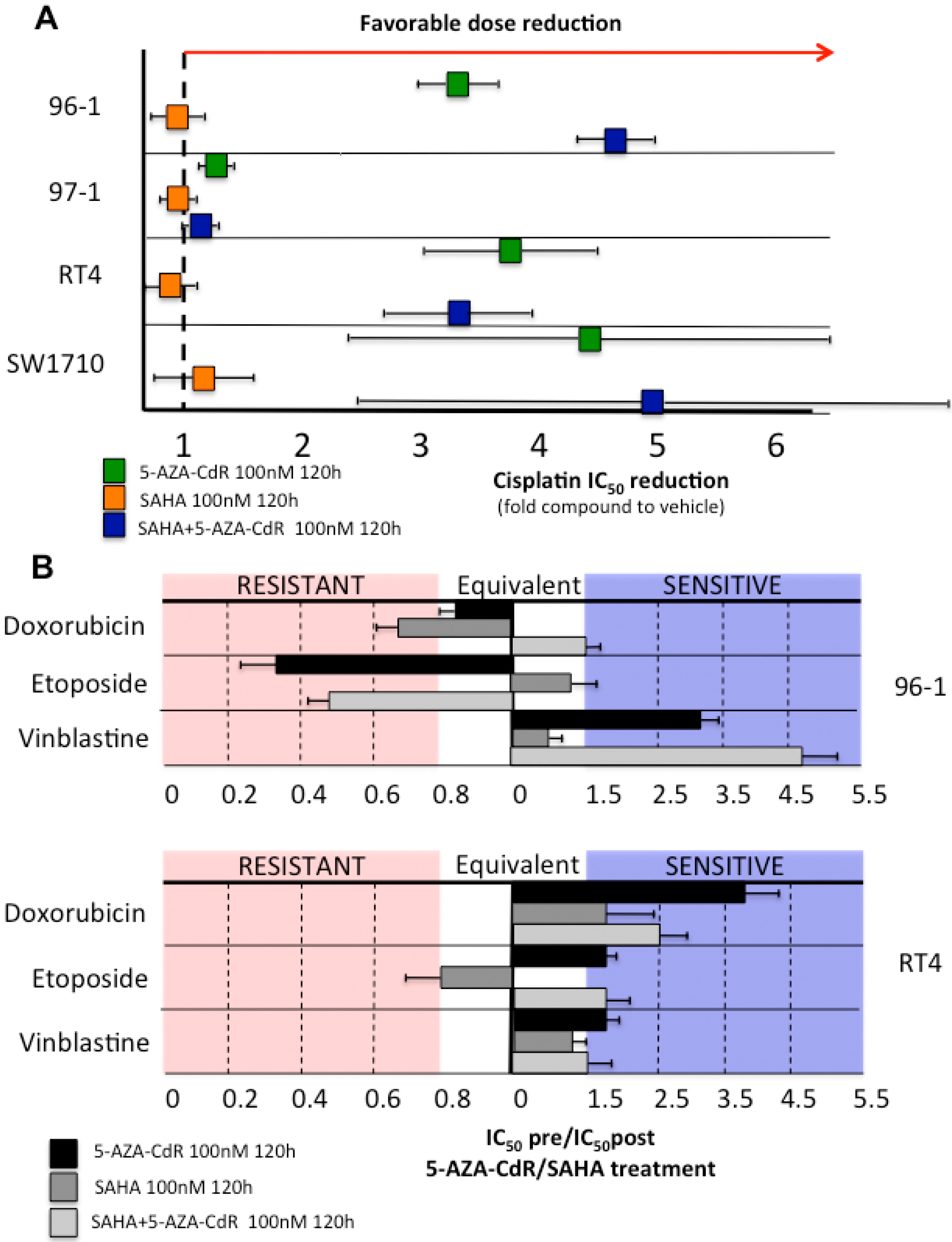
2.5. Decitabine Administration Reduces Cisplatin Dose Application and Induces in Vitro Sensitization of Initially Resistant Bladder Cancer Cell Lines
3. Discussion
4. Materials and Methods
4.1. Cell Line Collection
4.2. Cell Lines and Drugs
4.3. IC50 Determination
4.4. Cisplatin Sensitivity
4.5. RNA Extraction and Sequencing
4.6. Sensitization of Cell Lines by Epigenetic Drugs
4.7. DNA Methylation Assays
4.8. Patient Selection and Pathological Analysis
4.9. DNA Extraction from Tumor Tissue of Patients with Muscle-Invasive Bladder Cancer
4.10. Statistics and Bioinformatics
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Stenzl, A.; Cowan, N.C.; De Santis, M.; Kuczyk, M.A.; Merseburger, A.S.; Ribal, M.J.; Sherif, A.; Witjes, J.A.; European Association of Urology (EAU). Treatment of muscle-invasive and metastatic bladder cancer: Update of the EAU guidelines. Eur. Urol. 2011, 59, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Vale, C.L. Neoadjuvant Chemotherapy in Invasive Bladder Cancer: Update of a Systematic Review and Meta-Analysis of Individual Patient Data: Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Eur. Urol. 2005, 48, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Raj, G.V.; Karavadia, S.; Schlomer, B.; Arriaga, Y.; Lotan, Y.; Sagalowsky, A.; Frenkel, E. Contemporary use of perioperative cisplatin-based chemotherapy in patients with muscle-invasive bladder cancer. Cancer 2011, 117, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Vale, C.L. Adjuvant Chemotherapy in Invasive Bladder Cancer: A Systematic Review and Meta-Analysis of Individual Patient Data: Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Eur. Urol. 2005, 48, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Oronsky, N.; Knox, S.; Fanger, G.; Scicinski, J. Episensitization: Therapeutic tumor resensitization by epigenetic agents: A review and reassessment. Anticancer Agents Med. Chem. 2014, 14, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Drayton, R.M.; Catto, J.W. Molecular mechanisms of cisplatin resistance in bladder cancer. Expert Rev. Anticancer Ther. 2012, 12, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Van Allen, E.M.; Mouw, K.W.; Kim, P.; Iyer, G.; Wagle, N.; Al-Ahmadie, H.; Zhu, C.; Ostrovnaya, I.; Kryukov, G.V.; O’Connor, K.W.; et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov. 2014, 4, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Juergens, R.A.; Wrangle, J.; Vendetti, F.P.; Murphy, S.C.; Zhao, M.; Coleman, B.; Sebree, R.; Rodgers, K.; Hooker, C.M.; Franco, N. Combination epigenetic therapy has efficacy in patients with refractory advanced non-small cell lung cancer. Cancer Discov. 2011, 1, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Clozel, T.; Yang, S.N.; Elstrom, L.R.; Tam, W.; Martin, P.; Kormaksson, M.; Banerjee, S.; Vasanthakumar, A.; Culjkovic, B.; Scott, D.W.; et al. Mechanism-based epigenetic chemosensitization therapy of diffuse large B-cell lymphoma. Cancer Discov. 2013, 3, 1002–1019. [Google Scholar] [CrossRef] [PubMed]
- Matei, D.; Yang, S.; Elstrom, R.L.; Tam, W.; Martin, P.; Kormaksson, M.; Banerjee, S.; Vasanthakumar, A.; Culjkovic, B.; Scott, D.W. Epigenetic resensitization to platinum in ovarian cancer. Cancer Res. 2012, 72, 2197–2205. [Google Scholar] [CrossRef] [PubMed]
- Reinert, T.; Borre, M.; Christiansen, A.; Hermann, G.G.; Ørntoft, T.F.; Dyrskjøt, L. Diagnosis of Bladder Cancer Recurrence Based on Urinary Levels of EOMES, HOXA9, POU4F2, TWIST1, VIM, and ZNF154 Hypermethylation. PLoS ONE 2012, 7, e46297. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Yoon, H.Y.; Kim, J.S.; Kang, H.W.; Min, B.D.; Kim, S.K.; Ha, Y.S.; Kim, I.Y.; Ryu, K.H.; Lee, S.C.; et al. HOXA9, ISL1 and ALDH1A3 methylation patterns as prognostic markers for nonmuscle invasive bladder cancer: Array-based DNA methylation and expression profiling. Int. J. Cancer 2013, 133, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, M.O.; Bryan, R.T.; Haworth, K.E.; Emes, R.D.; Luscombe, C.; Gommersall, L.; Cheng, K.K.; Zeegers, M.P.; James, N.D.; Devall, A.J. Methylation of HOXA9 and ISL1 Predicts Patient Outcome in High-Grade Non-Invasive Bladder Cancer. PLoS ONE 2015, 10, e0137003. [Google Scholar] [CrossRef] [PubMed]
- Mikeska, T.; Bock, C.; Do, H.; Dobrovic, A. DNA methylation biomarkers in cancer: Progress towards clinical implementation. Expert Rev. Mol. Diagn. 2012, 12, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Reinert, T.; Modin, C.; Castano, F.M.; Lamy, P.; Wojdacz, T.K.; Hansen, L.L.; Wiuf, C.; Borre, M.; Dyrskjøt, L.; Orntoft, T.F. Comprehensive genome methylation analysis in bladder cancer: Identification and validation of novel methylated genes and application of these as urinary tumor markers. Clin. Cancer Res. 2011, 17, 5582–5592. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Veeramachaneni, R.; Huey, B.; Bhattacharya, A.; Schmidt, B.L.; Albertson, D.G. Investigation of HOXA9 promoter methylation as a biomarker to distinguish oral cancer patients at low risk of neck metastasis. BMC Cancer 2014, 14, 353. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Lothe, R.A.; Ahlquist, T.; Silins, I.; Tropé, C.G.; Micci, F.; Nesland, J.M.; Suo, Z.; Lind, G.E. DNA methylation profiling of ovarian carcinomas and their in vitro models identifies HOXA9, HOXB5, SCGB3A1, and CRABP1 as novel targets. Mol. Cancer 2007, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Rauch, T.; Wang, Z.; Zhang, X.; Zhong, X.; Wu, X.; Lau, S.K.; Kernstine, K.H.; Riggs, A.D.; Pfeifer, G.P. Homeobox gene methylation in lung cancer studied by genome-wide analysis with a microarray-based methylated CpG island recovery assay. Proc. Natl. Acad. Sci. USA 2007, 104, 5527–5532. [Google Scholar] [CrossRef] [PubMed]
- Pilato, B.; Pinto, R.; De Summa, S.; Lambo, R.; Paradiso, A.; Tommasi, S. HOX gene methylation status analysis in patients with hereditary breast cancer. J. Hum. Genet. 2013, 58, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Kawagoe, H.; Humphries, R.K.; Blair, A.; Sutherland, H.J.; Hogge, D.E. Expression of HOX genes, HOX cofactors, and MLL in phenotypically and functionally defined subpopulations of leukemic and normal human hematopoietic cells. Leukemia 1999, 13, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.M.; Mouw, J.K.; Unger, M.A.; Lakins, N.J.; Gbegnon, M.K.; Clemmer, V.B.; Benezra, M.; Licht, J.D.; Boudreau, N.J.; Tsai, K.K.C.; et al. HOXA9 regulates BRCA1 expression to modulate human breast tumor phenotype. J. Clin. Investig. 2010, 120, 1535–1550. [Google Scholar] [CrossRef] [PubMed]
- Widschwendter, M.; Fiegl, H.; Egle, D.; Mueller-Holzner, E.; Spizzo, G.; Marth, C.; Weisenberger, D.J.; Campan, M.; Young, J.; Jacobs, I. Epigenetic stem cell signature in cancer. Nat. Genet. 2007, 39, 157–158. [Google Scholar] [CrossRef] [PubMed]
- Gal-Yam, E.N.; Egger, G.; Iniguez, L.; Holster, H.; Einarsson, S.; Zhang, X.; Lin, J.C.; Liang, G.; Jones, P.A.; Tanay, A. Frequent switching of Polycomb repressive marks and DNA hypermethylation in the PC3 prostate cancer cell line. Proc. Natl. Acad. Sci. USA 2008, 105, 12979–12984. [Google Scholar] [CrossRef] [PubMed]
- Shang, D.; Liu, Y.; Matsui, Y.; Ito, N.; Nishiyama, H.; Kamoto, T.; Ogawa, O. Demethylating agent 5-aza-2′-deoxycytidine enhances susceptibility of bladder transitional cell carcinoma to Cisplatin. Urology 2008, 71, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Almstedt, M.; Blagitko-Dorfs, N.; Duque-Afonso, J.; Karbach, J.; Pfeifer, D.; Jäger, E.; Lübbert, M. The DNA demethylating agent 5-aza-2′-deoxycytidine induces expression of NY-ESO-1 and other cancer/testis antigens in myeloid leukemia cells. Leuk. Res. 2010, 34, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Claus, R.; Almstedt, M.; Lubbert, M. Epigenetic treatment of hematopoietic malignancies: In vivo targets of demethylating agents. Semin. Oncol. 2005, 32, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Atanackovic, D.; Luetkens, T.; Kloth, B.; Fuchs, G.; Cao, Y.; Hildebrandt, Y.; Meyer, S.; Bartels, K.; Reinhard, H.; Lajmi, N. Cancer-testis antigen expression and its epigenetic modulation in acute myeloid leukemia. Am. J. Hematol. 2011, 86, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.M.; Issa, J.P. Decitabine dosing schedules. Semin. Hematol. 2005, 42 (Suppl. 2), S17–S22. [Google Scholar] [CrossRef] [PubMed]
- Glasspool, R.M.; Brown, R.; Gore, M.E.; Rustin, G.J.; McNeish, I.A.; Wilson, R.H.; Pledge, S.; Paul, J.; Mackean, M.; Hall, G.D. A randomised, phase II trial of the DNA-hypomethylating agent 5-aza-2′-deoxycytidine (decitabine) in combination with carboplatin vs. carboplatin alone in patients with recurrent, partially platinum-sensitive ovarian cancer. Br. J. Cancer 2014, 110, 1923–1929. [Google Scholar] [CrossRef] [PubMed]
- Bensalah, K.; Montorsi, F.; Shariat, S.F. Challenges of cancer biomarker profiling. Eur. Urol. 2007, 52, 1601–1609. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xylinas, E.; Hassler, M.R.; Zhuang, D.; Krzywinski, M.; Erdem, Z.; Robinson, B.D.; Elemento, O.; Clozel, T.; Shariat, S.F. An Epigenomic Approach to Improving Response to Neoadjuvant Cisplatin Chemotherapy in Bladder Cancer. Biomolecules 2016, 6, 37. https://doi.org/10.3390/biom6030037
Xylinas E, Hassler MR, Zhuang D, Krzywinski M, Erdem Z, Robinson BD, Elemento O, Clozel T, Shariat SF. An Epigenomic Approach to Improving Response to Neoadjuvant Cisplatin Chemotherapy in Bladder Cancer. Biomolecules. 2016; 6(3):37. https://doi.org/10.3390/biom6030037
Chicago/Turabian StyleXylinas, Evanguelos, Melanie R. Hassler, Dazhong Zhuang, Martin Krzywinski, Zeynep Erdem, Brian D. Robinson, Olivier Elemento, Thomas Clozel, and Shahrokh F. Shariat. 2016. "An Epigenomic Approach to Improving Response to Neoadjuvant Cisplatin Chemotherapy in Bladder Cancer" Biomolecules 6, no. 3: 37. https://doi.org/10.3390/biom6030037




