Evaluation of Stability of Amylose Inclusion Complexes Depending on Guest Polymers and Their Application to Supramolecular Polymeric Materials
Abstract
:1. Introduction
2. Results and Discussion
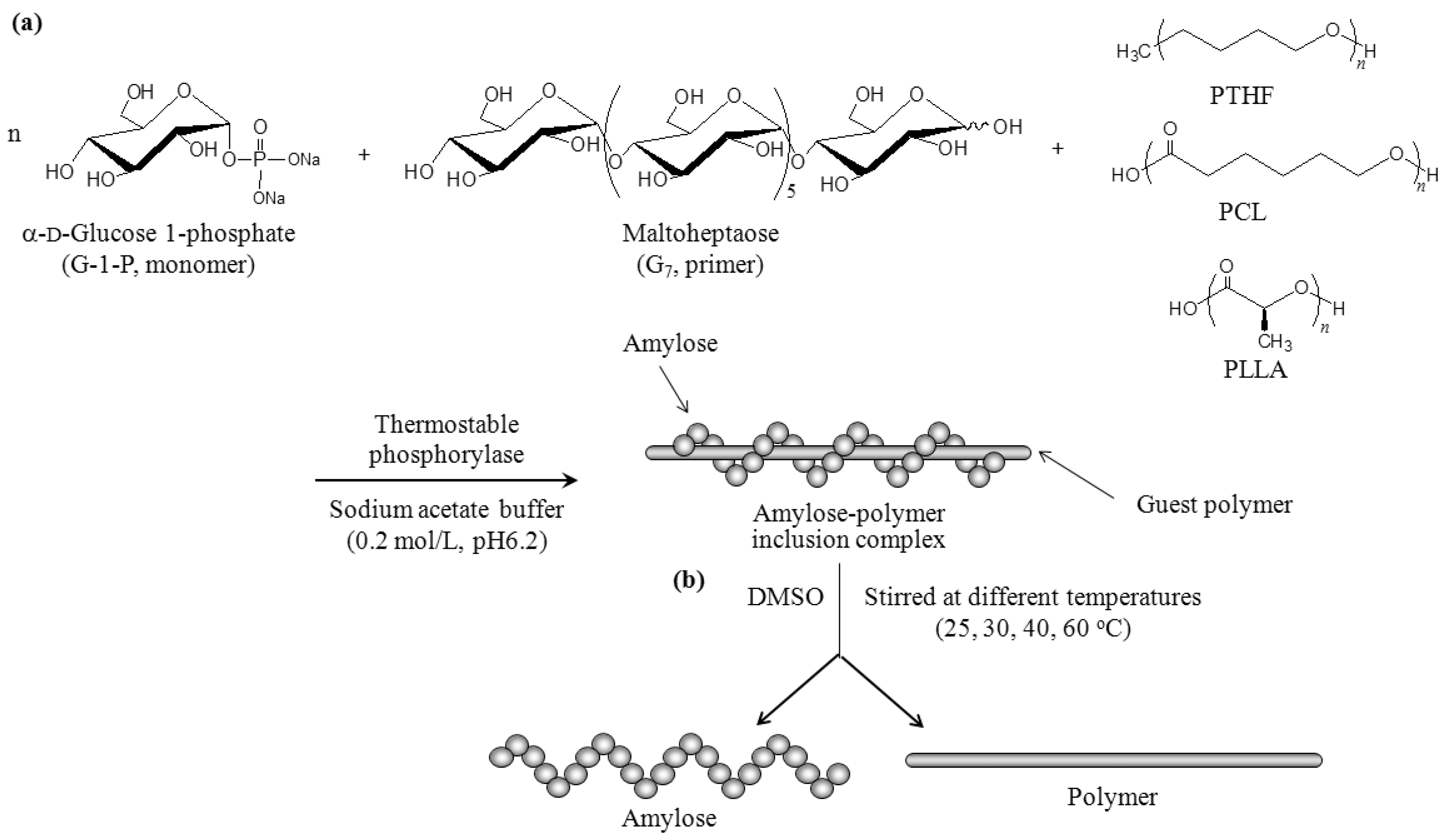
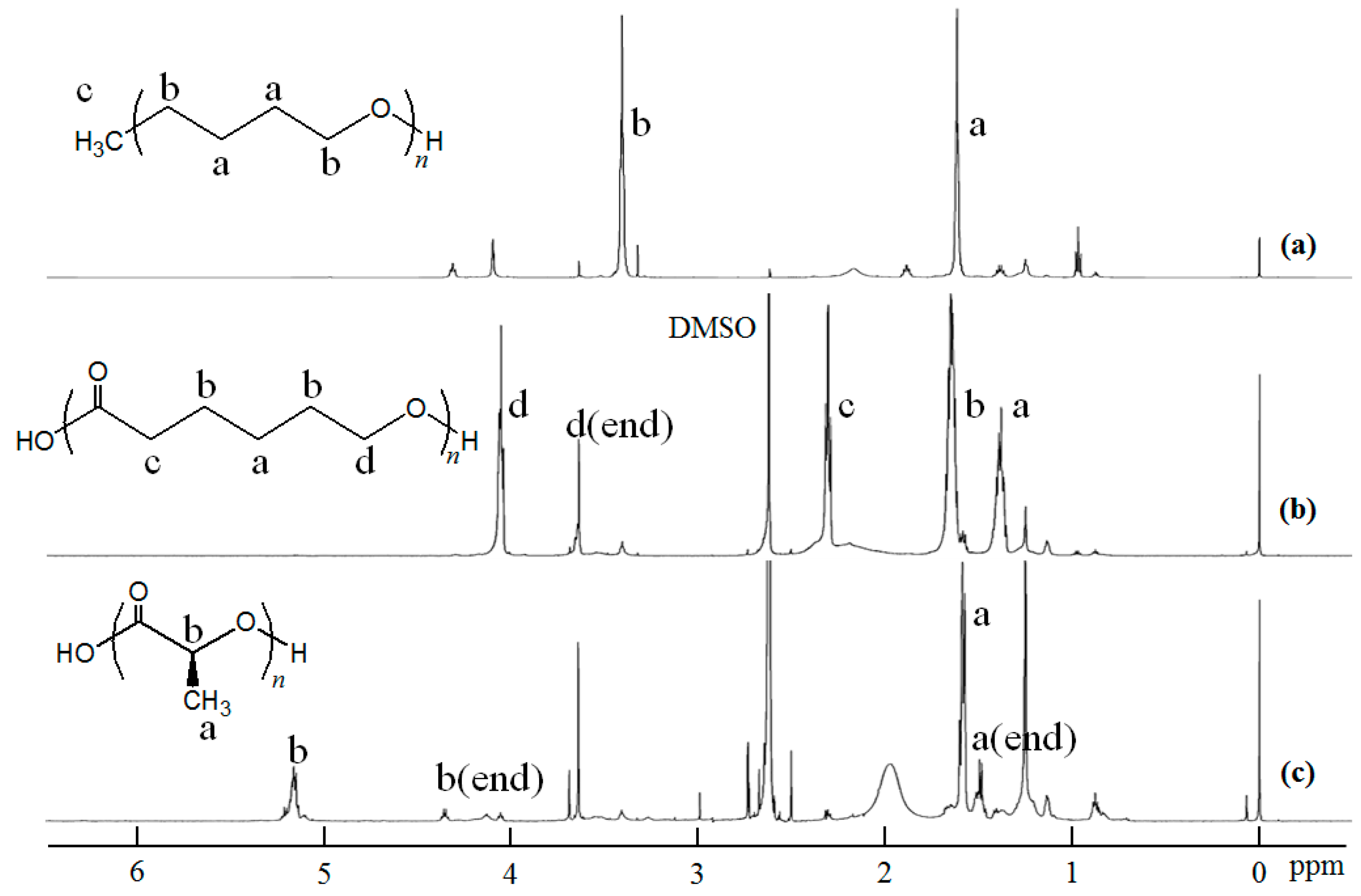
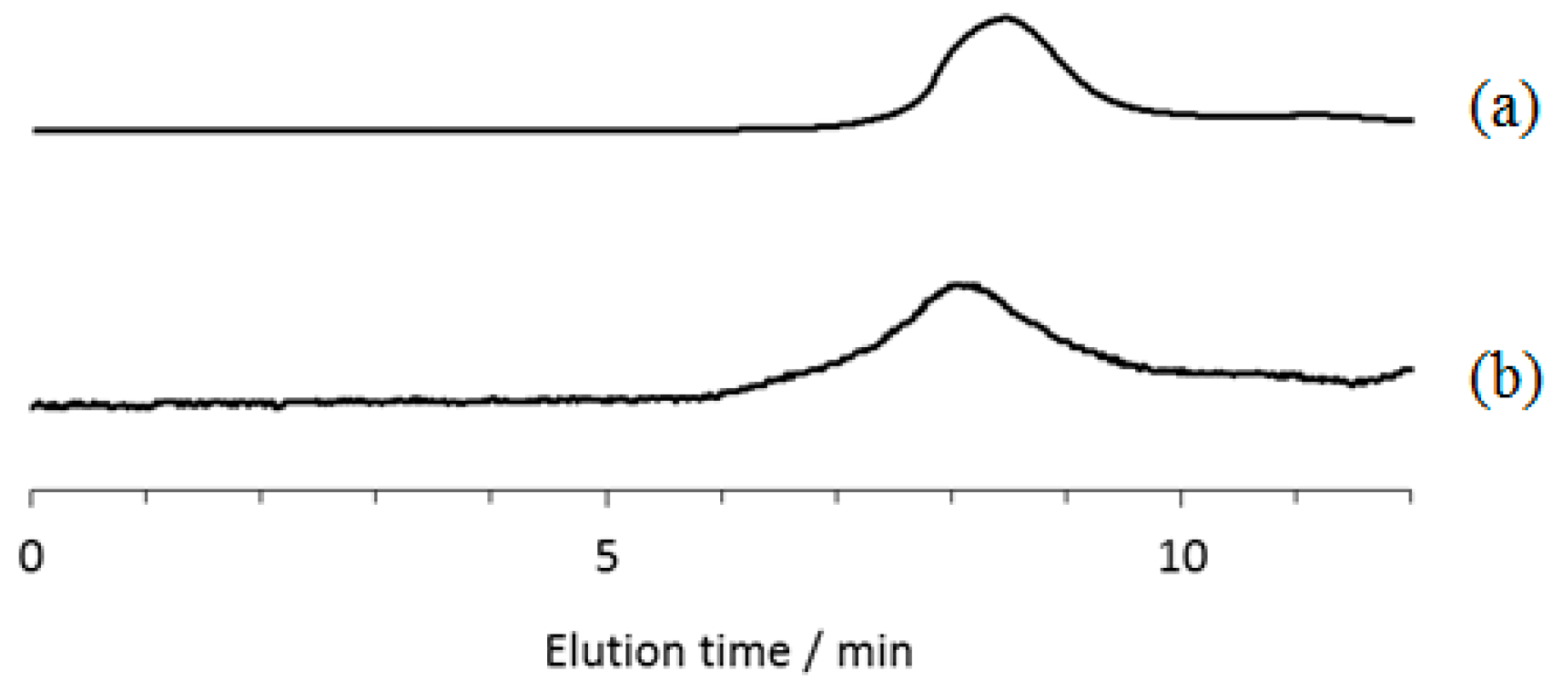
2.1. Evaluation of the Stability of Inclusion Complexes Under Solution State
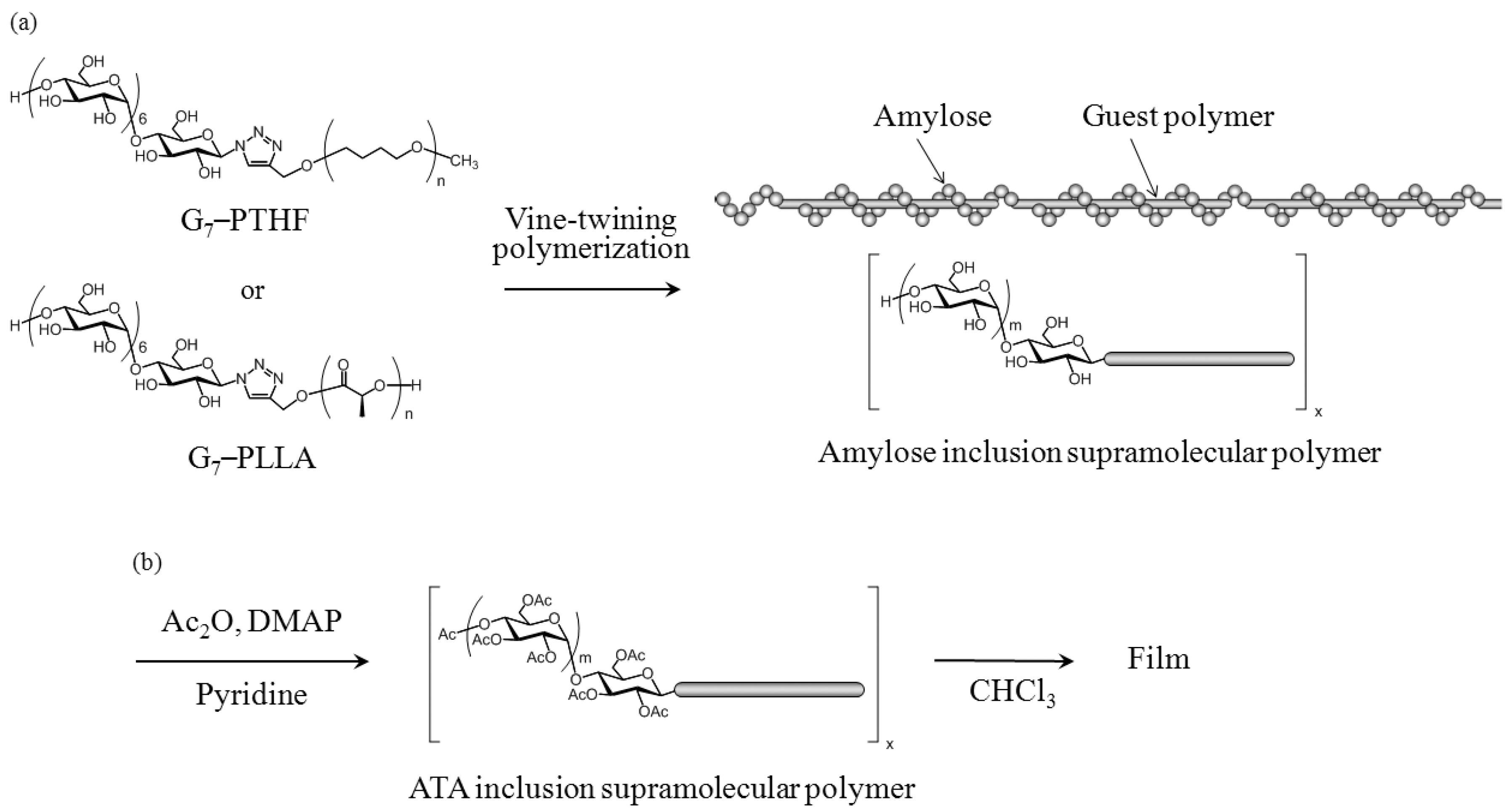
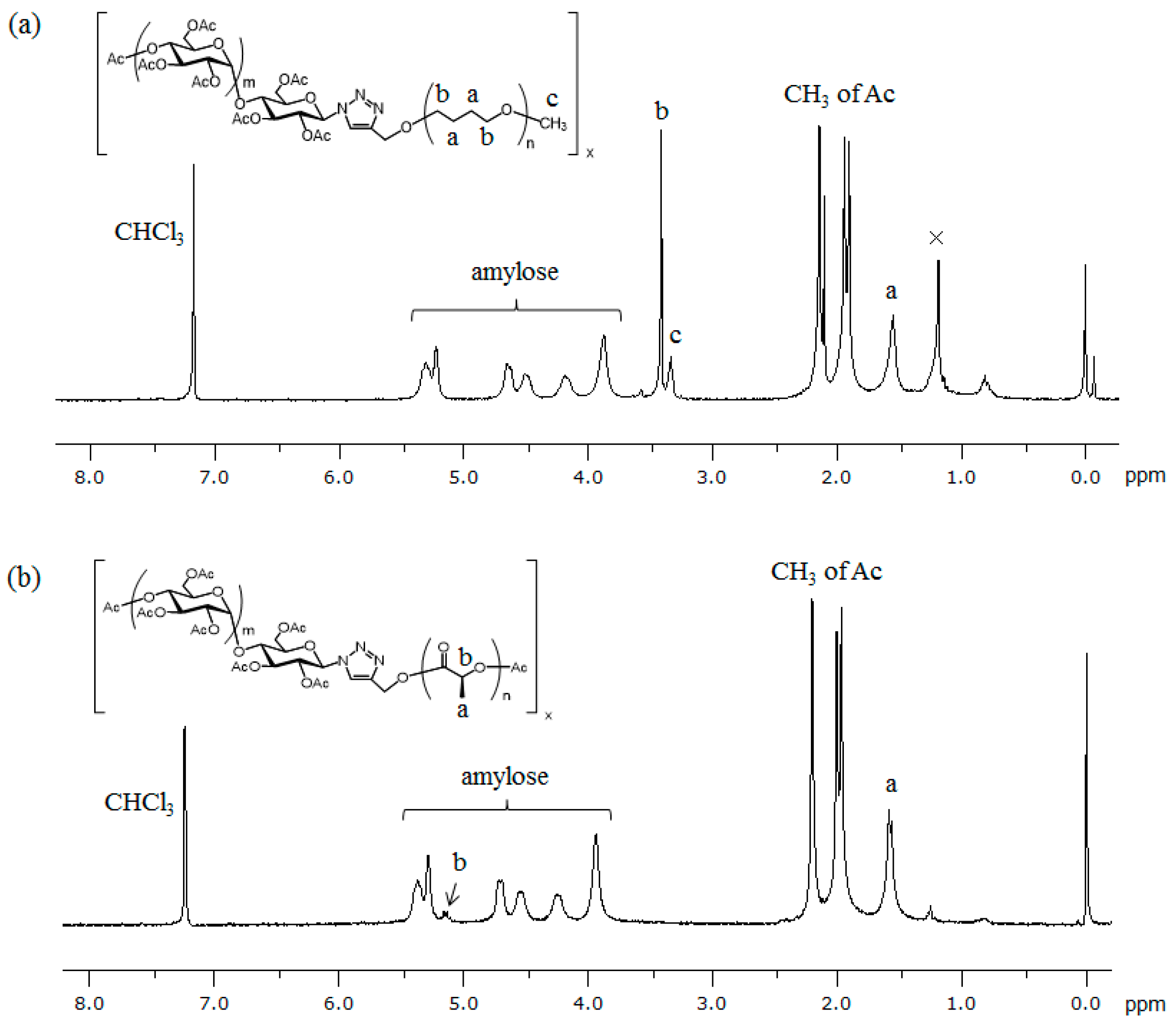
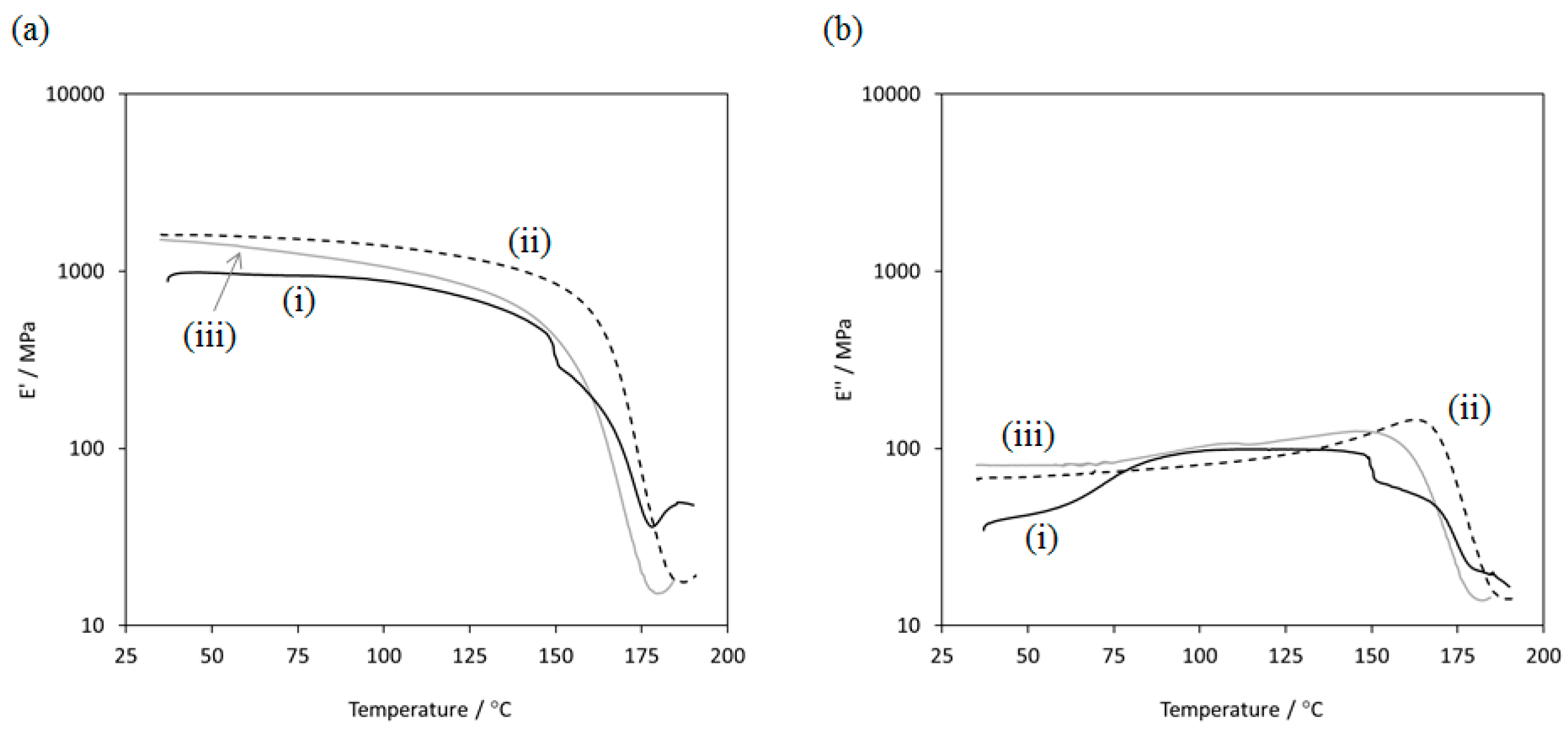
2.2. Preparation and Mechanical Properties of ATA Supramolecular Polymeric Films
3. Materials and Methods
3.1. Materials
3.2. Preparation of Amylose–Polymer Inclusion Complexes
3.3. Evaluation of the Stability of Inclusion Complexes
3.4. Preparation of ATA Inclusion Supramolecular Polymeric Films
3.5. Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schuerch, C. Polysaccharides. In Encyclopedia of Polymer Science and Engineering, 2nd ed.; Mark, H.F., Bilkales, N., Overberger, C.G., Eds.; John Wiley & Sons: New York, NY, USA, 1986; Volume 13, pp. 87–162. [Google Scholar]
- Putseys, J.A.; Lamberts, L.; Delcour, J.A. Amylose-inclusion complexes: Formation, identity and physico-chemical properties. J. Cereal Sci. 2010, 51, 238–247. [Google Scholar] [CrossRef]
- Shogren, R.L.; Greene, R.V.; Wu, Y.V. Complexes of starch polysaccharides and poly(ethylene-co-acrylic acid): Structure and stability in solution. J. Appl. Polym. Sci. 1991, 42, 1701–1709. [Google Scholar] [CrossRef]
- Shogren, R.L. Complexes of starch with telechelic poly(e-caprolactone) phosphate. Carbohydr. Polym. 1993, 22, 93–98. [Google Scholar] [CrossRef]
- Star, A.; Steuerman, D.W.; Heath, J.R.; Stoddart, J.F. Starched carbon nanotubes. Angew. Chem. Int. Ed. 2002, 41, 2508–2512. [Google Scholar] [CrossRef]
- Ikeda, M.; Furusho, Y.; Okoshi, K.; Tanahara, S.; Maeda, K.; Nishino, S.; Mori, T.; Yashima, E. A luminescent poly(phenylenevinylene)-amylose composite with supramolecular liquid crystallinity. Angew. Chem. Int. Ed. 2006, 45, 6491–6495. [Google Scholar] [CrossRef] [PubMed]
- Kida, T.; Minabe, T.; Okabe, S.; Akashi, M. Partially-methylated amyloses as effective hosts for inclusion complex formation with polymeric guests. Chem. Commun. 2007, 1559–1561. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Kyutoku, T.; Shimomura, N.; Kadokawa, J. Formation of amylose-poly(tetrahydrofuran) inclusion complexes in ionic liquid media. Chem. Lett. 2011, 40, 31–33. [Google Scholar] [CrossRef]
- Rachmawati, R.; Woortman, A.J.J.; Loos, K. Facile preparation method for inclusion complexes between amylose and polytetrahydrofurans. Biomacromolecules 2013, 14, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Woortman, A.J.J.; Loos, K. Synthesis of amylose-polystyrene inclusion complexes by a facile preparation route. Biomacromolecules 2013, 14, 1955–1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rachmawati, R.; Woortman, A.J.J.; Loos, K. Tunable properties of inclusion complexes between amylose and polytetrahydrofuran. Macromol. Biosci. 2013, 13, 767–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rachmawati, R.; Woortman, A.J.J.; Loos, K. Solvent-responsive behavior of inclusion complexes between amylose and polytetrahydrofuran. Macromol. Biosci. 2014, 14, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Kadokawa, J. Vine-twining polymerization: A new preparation method for well-defined supramolecules composed of amylose and synthetic polymers. Chem. Rec. 2005, 5, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Kadokawa, J. Synthesis of nanostructured bio-related materials by hybridization of synthetic polymers with polysaccharides or saccharide residues. J. Biomater. Sci. Polym. Ed. 2006, 17, 1269–1284. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Kadokawa, J. Preparation of Polymers with Well-Defined Nanostructure in the Polymerization Field. In Modern Trends in Macromolecular Chemistry; Lee, J.N., Ed.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2009; pp. 199–217. [Google Scholar]
- Kadokawa, J. Precision polysaccharide synthesis catalyzed by enzymes. Chem. Rev. 2011, 111, 4308–4345. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J. Preparation and applications of amylose supramolecules by means of phosphorylase-catalyzed enzymatic polymerization. Polymers 2012, 4, 116–133. [Google Scholar] [CrossRef]
- Kadokawa, J. Architecture of amylose supramolecules in form of inclusion complexes by phosphorylase-catalyzed enzymatic polymerization. Biomolecules 2013, 3, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J. Chemoenzymatic synthesis of functional amylosic materials. Pure Appl. Chem. 2014, 86, 701–709. [Google Scholar] [CrossRef]
- Shoda, S.; Uyama, H.; Kadokawa, J.; Kimura, S.; Kobayashi, S. Enzymes as green catalysts for precision macromolecular synthesis. Chem. Rev. 2016, 116, 2307–2413. [Google Scholar] [CrossRef] [PubMed]
- Ziegast, G.; Pfannemüller, B. Linear and star-shaped hybrid polymers. Phosphorolytic syntheses with di-functional, oligo-functional and multifunctional primers. Carbohydr. Res. 1987, 160, 185–204. [Google Scholar] [CrossRef]
- Kitaoka, M.; Hayashi, K. Carbohydrate-processing phosphorolytic enzymes. Trends Glycosci. Glycotechnol. 2002, 14, 35–50. [Google Scholar] [CrossRef]
- Yanase, M.; Takaha, T.; Kuriki, T. α-Glucan phosphorylase and its use in carbohydrate engineering. J. Sci. Food Agric. 2006, 86, 1631–1635. [Google Scholar] [CrossRef]
- Ohdan, K.; Fujii, K.; Yanase, M.; Takaha, T.; Kuriki, T. Enzymatic synthesis of amylose. Biocatal. Biotransform. 2006, 24, 77–81. [Google Scholar] [CrossRef]
- Kadokawa, J. Precision synthesis of functional polysaccharide materials by phosphorylase-catalyzed enzymatic reactions. Polymers 2016, 8, 138. [Google Scholar] [CrossRef]
- Kadokawa, J.; Kaneko, Y.; Tagaya, H.; Chiba, K. Synthesis of an amylose-polymer inclusion complex by enzymatic polymerization of glucose 1-phosphate catalyzed by phosphorylase enzyme in the presence of polyTHF: A new method for synthesis of polymer–polymer inclusion complexes. Chem. Commun. 2001, 449–450. [Google Scholar] [CrossRef]
- Kadokawa, J.; Kaneko, Y.; Nagase, S.; Takahashi, T.; Tagaya, H. Vine-twining polymerization: Amylose twines around polyethers to form amylose-Polyether inclusion complexes. Chem. Eur. J. 2002, 8, 3321–3326. [Google Scholar] [CrossRef]
- Kadokawa, J.; Kaneko, Y.; Nakaya, A.; Tagaya, H. Formation of an amylose-polyester inclusion complex by means of phosphorylase-catalyzed enzymatic polymerization of α-d-glucose 1-phosphate monomer in the presence of poly(e-caprolactone). Macromolecules 2001, 34, 6536–6538. [Google Scholar] [CrossRef]
- Kadokawa, J.; Nakaya, A.; Kaneko, Y.; Tagaya, H. Preparation of inclusion complexes between amylose and ester-containing polymers by means of vine-twining polymerization. Macromol. Chem. Phys. 2003, 204, 1451–1457. [Google Scholar] [CrossRef]
- Kaneko, Y.; Ueno, K.; Yui, T.; Nakahara, K.; Kadokawa, J. Amylose’s recognition of chirality in polylactides on formation of inclusion complexes in vine-twining polymerization. Macromol. Biosci. 2011, 11, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Sasayama, S.; Nomura, S.; Yamamoto, K.; Kimura, Y.; Kadokawa, J. An amylose-poly(l-lactide) inclusion supramolecular polymer: Enzymatic synthesis by means of vine-twining polymerization using a primer-guest conjugate. Macromol. Chem. Phys. 2013, 214, 2829–2834. [Google Scholar] [CrossRef]
- Tanaka, T.; Sasayama, S.; Yamamoto, K.; Kimura, Y.; Kadokawa, J. Evaluating relative chain orientation of amylose and poly(l-lactide) in inclusion complexes formed by vine-twining polymerization using primer-guest conjugates. Macromol. Chem. Phys. 2015, 216, 794–800. [Google Scholar] [CrossRef]
- Tanaka, T.; Tsutsui, A.; Gotanda, R.; Sasayama, S.; Yamamoto, K.; Kadokawa, J. Synthesis of amylose-polyether inclusion supramolecular polymers by vine-twining polymerization using maltoheptaose-functionalized poly(tetrahydrofuran) as a primer-guest conjugate. J. Appl. Glycosci. 2015, 62, 135–141. [Google Scholar] [CrossRef]
- Tanaka, T.; Gotanda, R.; Tsutsui, A.; Sasayama, S.; Yamamoto, K.; Kimura, Y.; Kadokawa, J. Synthesis and gel formation of hyperbranched supramolecular polymer by vine-twining polymerization using branched primer-guest conjugate. Polymer 2015, 73, 9–16. [Google Scholar] [CrossRef]
- Kadokawa, J.; Nomura, S.; Hatanaka, D.; Yamamoto, K. Preparation of polysaccharide supramolecular films by vine-twining polymerization approach. Carbohydr. Polym. 2013, 98, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Von Braunmühl, V.; Jonas, G.; Stadler, R. Enzymatic grafting of amylose from poly(dimethylsiloxanes). Macromolecules 1995, 28, 17–24. [Google Scholar] [CrossRef]
- Smith, S.; Hubin, A.J. The preparation and chemistry of dicationically active polymers of tetrahydrofuran. J. Macromol. Sci. A Chem. 1973, 7, 1399–1413. [Google Scholar] [CrossRef]
- Nomura, N.; Taira, A.; Tomioka, T.; Okada, M. A catalytic approach for cationic living polymerization: Sc(OTf)3-catalyzed ring-opening polymerization of lactones. Macromolecules 2000, 33, 1497–1499. [Google Scholar] [CrossRef]
- Fan, Y.J.; Chen, G.P.; Tanaka, J.; Tateishi, T. l-Phe end-capped poly(l-lactide) as macroinitiator for the synthesis of poly(l-lactide)-b-poly(l-lysine) block copolymer. Biomacromolecules 2005, 6, 3051–3056. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, S.H.; Rus’d, A.A.; Kitaoka, M.; Hayashi, K. Characterization of a hyperthermostable glycogen phosphorylase from Aquifex aeolicus expressed in Escherichia coli. J. Mol. Catal. B Enzym. 2003, 22, 173–180. [Google Scholar] [CrossRef]
- Yanase, M.; Takata, H.; Fujii, K.; Takaha, T.; Kuriki, T. Cumulative effect of amino acid replacements results in enhanced thermostability of potato type L α-glucan phosphorylase. Appl. Environ. Microbiol. 2005, 71, 5433–5439. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, T.; Tsutsui, A.; Tanaka, K.; Yamamoto, K.; Kadokawa, J.-i. Evaluation of Stability of Amylose Inclusion Complexes Depending on Guest Polymers and Their Application to Supramolecular Polymeric Materials. Biomolecules 2017, 7, 28. https://doi.org/10.3390/biom7010028
Tanaka T, Tsutsui A, Tanaka K, Yamamoto K, Kadokawa J-i. Evaluation of Stability of Amylose Inclusion Complexes Depending on Guest Polymers and Their Application to Supramolecular Polymeric Materials. Biomolecules. 2017; 7(1):28. https://doi.org/10.3390/biom7010028
Chicago/Turabian StyleTanaka, Tomonari, Atsushi Tsutsui, Kazuya Tanaka, Kazuya Yamamoto, and Jun-ichi Kadokawa. 2017. "Evaluation of Stability of Amylose Inclusion Complexes Depending on Guest Polymers and Their Application to Supramolecular Polymeric Materials" Biomolecules 7, no. 1: 28. https://doi.org/10.3390/biom7010028





