Squalene Found in Alpine Grassland Soils under a Harsh Environment in the Tibetan Plateau, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Soil Sampling
2.3. Soil Analyses
2.4. Data Analysis and Statistics
3. Results
3.1. Squalene Relative Abundance
3.2. Soil Chemical/Microbial Characteristics
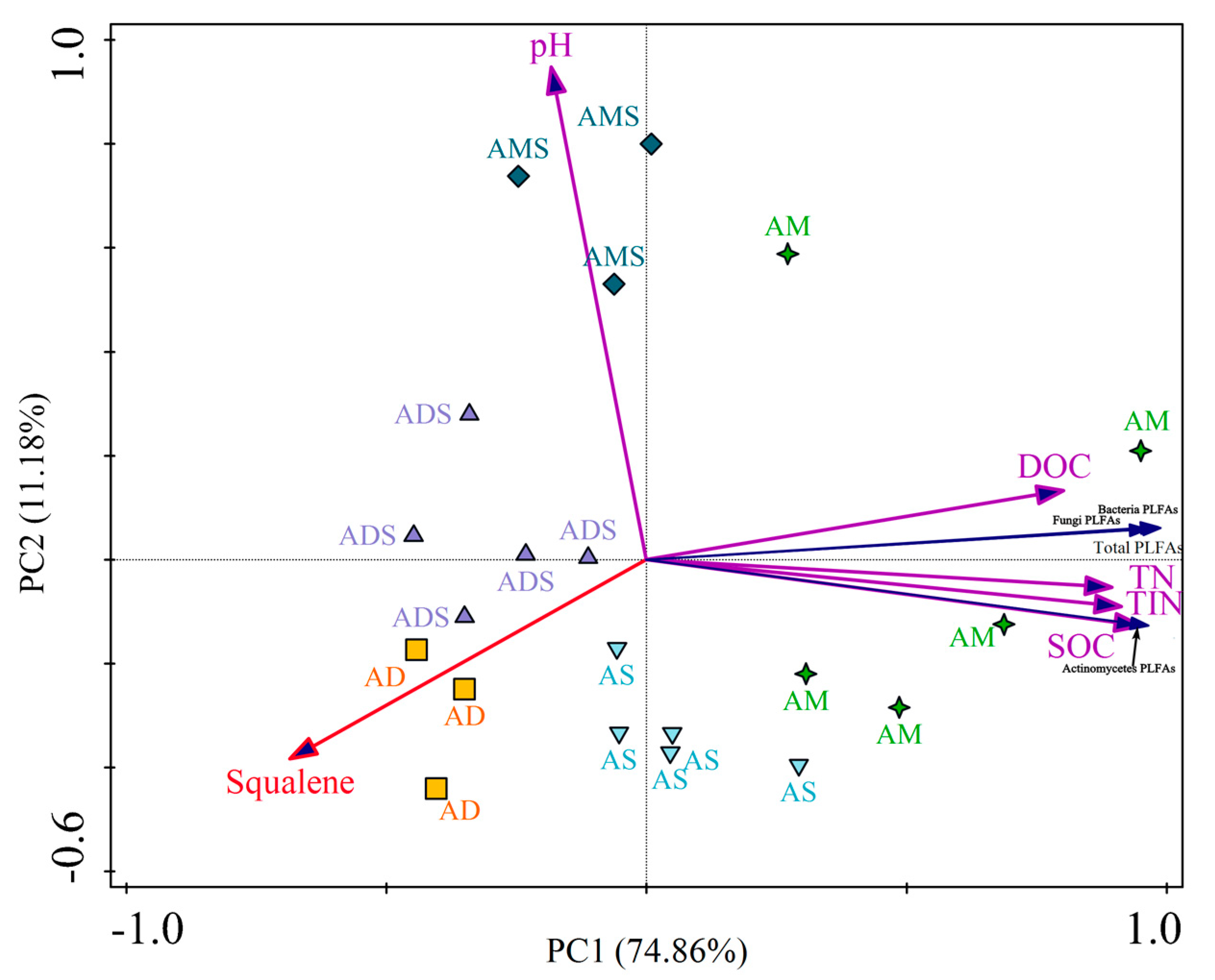
3.3. Relationships between Squalene Relative Abundance and Soil Chemical/Microbial Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Amarowicz, P. Squalene: A natural antioxidant? Eur. J. Lipid Sci. Technol. 2009, 111, 411–412. [Google Scholar] [CrossRef] [Green Version]
- Popa, O.; Bsbeanu, N.E.; Popa, I.; Nitã, S.; Dinu-Pârvu, C.E. Methods for obtaining and determination of squalene from natural sources. BioMed Res. Int. 2015, 2015, 367202. [Google Scholar] [CrossRef] [PubMed]
- Spanova, M.; Daum, G. Squalene—Biochemistry, molecular biology, process biotechnology, and applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1299–1320. [Google Scholar] [CrossRef]
- Katabami, A.; Li, L.; Iwasaki, M.; Furubayashi, M.; Saito, K.; Umeno, D. Production of squalene by squalene synthases and their truncated mutants in Escherichia coli. J. Biosci. Bioeng. 2015, 119, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Lou-Bonafonte, J.M.; Martínez-Beamonte, R.; Sanclemente, T.; Surra, J.C.; Herrera-Marcos, L.V.; Sanchez-Marco, J.; Arnal, C.; Osada, J. Current insights into the biological action of squalene. Mol. Nutr. Food Res. 2018, 62, e1800136. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.W.; Marshall, S.N.; Gordon, K.C.; Killee, D.P. Rapid quantitative determination of squalene in shark liver oils by Raman and IR spectroscopy. Lipids 2016, 51, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Vadalà, M.; Laurino, C.; Palmieri, L.; Palmieri, B. Shark derivatives (Alkylglycerols, Squalene, Cartilage) as putative nutraceuticals in oncology. Eur. J. Oncol. 2017, 22, 5–20. [Google Scholar]
- Yuan, C.; Xie, Y.; Jin, R.; Ren, L.; Zhou, L.; Zhu, M.; Ju, Y. Simultaneous analysis of tocopherols, phytosterols, and squalene in vegetable oils by high-performance liquid chromatography. Food Anal. Methods 2017, 10, 3716–3722. [Google Scholar] [CrossRef]
- Fooshee, D.R.; Aiona, P.K.; Laskin, A.; Laskin, J.; Nizkorodov, S.A.; Baldi, P.F. Atmospheric oxidation of squalene: Molecular study using COBRA modeling and high-resolution mass spectrometry. Environ. Sci. Technol. 2015, 49, 13304–13313. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, G.P.; Thuan, N.H.; Koirala, N.; Sohng, J.K. Advances in biochemistry and microbial production of squalene and its derivatives. J. Microbiol. Biotechnol. 2016, 26, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Ma, X.; Wang, Y. Production of squalene by microbes: An update. World J. Microbiol. Biotechnol. 2016, 32, 195. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, M.B.; Vendruscolo, R.G.; Maroneze, M.M.; Barin, J.S.; de Menezes, C.R.; Zepka, L.Q.; Jacob-Lopes, E.; Wagner, R. Towards a sustainable route for the production of squalene using cyanobacteria. Waste Biomass Valorization 2018, 3, 1–8. [Google Scholar] [CrossRef]
- Qiu, J. China: The third pole. Nature 2008, 454, 393–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, T.; Thompson, L.G.; Mosbrugger, V.; Zhang, F.; Ma, Y.; Luo, T.; Xu, B.; Yang, X.; Joswiak, D.R.; Wang, W.; et al. Third Pole Environment (TPE). Environ. Dev. 2012, 3, 52–64. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, Y.; Wang, S.; Xu, D.; Yu, H.; Wu, L.; Lin, Q.; Hu, Y.; Li, X.; He, Z.; et al. The microbial gene diversity along an elevation gradient of the Tibetan grassland. ISME J. 2014, 8, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Kong, W.; Liu, J.; Zhao, J.; Du, H.; Zhang, X.; Xia, P. Diversity and distribution of autotrophic microbial community along environmental gradients in grassland soils on the Tibetan Plateau. Appl. Microbiol. Biotechnol. 2015, 99, 8765–8776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pugnaire, F.I.; Zhang, L.; Li, R.; Luo, T. No evidence of facilitation collapse in the Tibetan plateau. J. Veg. Sci. 2015, 26, 233–242. [Google Scholar] [CrossRef]
- Pan, S.; Zhang, T.; Rong, Z.; Hu, L.; Gu, Z.; Wu, Q.; Dong, S.; Liu, Q.; Lin, Z.; Deutschova, L.; et al. Population transcriptomes reveal synergistic responses of DNA polymorphism and RNA expression to extreme environments on the Qinghai-Tibetan Plateau in a predatory bird. Mol. Ecol. 2017, 26, 2993–3010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Nan, P.; Wang, L.; Wang, Q.; Tsering, T.; Zhong, Y. Chemical variation in lipophilic composition of Lamiophlomis rotata from the Qinghai-Tibetan Plateau. Chem. Nat. Compd. 2006, 42, 525–528. [Google Scholar] [CrossRef]
- Yuan, L.; Zhong, G.; Quan, H.; Tian, F.; Zhong, Z.; Lan, X. GC-MS study on chemical components of volatile oil from roots of Rhodiola crenulata growing in Tibet. Chin. J. Exp. Tradit. Med. Formulae 2012, 18, 67–70, (In Chinese with English Abstract). [Google Scholar]
- Liu, C.; Jin, G.; Luo, Z.; Li, S.; Sun, S.; Li, Y.; Ma, M. Chinese yak and yellow cattle exhibit considerable differences in tissue content of squalene, tocopherol, and fatty acids. Eur. J. Lipid Sci. Technol. 2015, 117, 899–902. [Google Scholar] [CrossRef]
- Chen, Y.L.; Ding, J.Z.; Peng, Y.F.; Li, F.; Yang, G.B.; Liu, L.; Qin, S.Q.; Fang, K.; Yang, Y.H. Patterns and drivers of soil microbial communities in Tibetan alpine and global terrestrial ecosystems. J. Biogeogr. 2016, 43, 2027–2039. [Google Scholar] [CrossRef]
- Qi, Q.; Zhao, M.; Wang, S.; Ma, X.; Wang, Y.; Gao, Y.; Lin, Q.; Li, X.; Gu, B.; Li, G.; et al. The biogeographic pattern of microbial functional genes along an altitudinal gradient of the Tibetan pasture. Front. Microbiol. 2017, 8, 976. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Adams, J.M.; Shi, Y.; He, J.S.; Jing, X.; Chen, L.; Tedersoo, L.; Chu, H. Soil fungal diversity in natural grasslands of the Tibetan Plateau: Associations with plant diversity and productivity. New Phytol. 2017, 215, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Immerzeel, W.W.; Beek, L.P.H.; Bierkens, M.F.P. Climate change will affect the Asian water towers. Science 2010, 328, 1382–1385. [Google Scholar] [CrossRef] [PubMed]
- Bibi, S.; Wang, L.; Li, X.; Zhou, J.; Chen, D.; Yao, T. Climatic and associated cryospheric, biospheric, and hydrological changes on the Tibetan Plateau: A review. Int. J. Climatol. 2018, 38, e1–e17. [Google Scholar] [CrossRef]
- Lu, X.; Yan, Y.; Sun, J.; Zhang, X.; Chen, Y.; Wang, X.; Cheng, G. Carbon, nitrogen, and phosphorus storage in alpine grassland ecosystems of Tibet: Effects of grazing exclusion. Ecol. Evol. 2015, 5, 4492–4504. [Google Scholar] [CrossRef] [PubMed]
- Gai, J.P.; Christie, P.; Cai, X.B.; Fan, J.Q.; Zhang, J.L.; Feng, G.; Li, X.L. Occurrence and distribution of arbuscular mycorrhizal fungal species in three types of grassland community of the Tibetan Plateau. Ecol. Res. 2009, 24, 1345–1350. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Yan, Y.; Sun, J.; Zhang, X.; Chen, Y.; Wang, X.; Cheng, G. Short-term grazing exclusion has no impact on soil properties and nutrients of degraded alpine grassland in Tibet, China. Solid Earth 2015, 6, 1195–1205. [Google Scholar] [CrossRef] [Green Version]
- Prayogo, C.; Jones, J.E.; Baeyens, J.; Bending, G.D. Impact of biochar on mineralisation of C and N from soil and willow litter and its relationship with microbial community biomass and structure. Biol. Fertil. Soils 2014, 50, 695–702. [Google Scholar] [CrossRef]
- Hannam, K.D.; Quideau, S.A.; Kishchuk, B.E. Forest floor microbial communities in relation to stand composition and timber harvesting in northern Alberta. Soil Biol. Biochem. 2006, 38, 2565–2575. [Google Scholar] [CrossRef]
- Pankhurst, C.E.; Yu, S.; Hawke, B.G.; Harch, B.D. Capacity of fatty acid profiles and substrate utilization patterns to describe differences in soil microbial communities associated with increased salinity or alkalinity at three locations in South Australia. Biol. Fertil. Soils 2001, 33, 204–217. [Google Scholar] [CrossRef]
- Arthur, M.A.; Bray, S.R.; Kuchle, C.R.; McEwan, R.W. The influence of the invasive shrub, Lonicera maackii, on leaf decomposition and microbial community dynamics. Plant Ecol. 2012, 213, 1571–1582. [Google Scholar] [CrossRef]
- McMahon, S.; Schimel, J.P. Shifting patterns of microbial N-metabolism across seasons in upland Alaskan tundra soils. Soil Biol. Biochem. 2017, 105, 96–107. [Google Scholar] [CrossRef]
- Sun, S.Q.; Liu, T.; Wu, Y.H.; Wang, G.X.; Zhu, B.; DeLuca, T.H.; Wang, Y.Q.; Luo, J. Ground bryophytes regulate net soil carbon efflux: Evidence from two subalpine ecosystems on the east edge of the Tibet Plateau. Plant Soil 2017, 417, 363–375. [Google Scholar] [CrossRef]
- Jílková, V.; Cajthaml, T.; Frouz, J. Relative importance of honeydew and resin for the microbial activity in wood ant nest and forest floor substrate—A laboratory study. Soil Biol. Biochem. 2018, 117, 1–4. [Google Scholar] [CrossRef]
- Yang, B.; Pang, X.Y.; Hu, B.; Bao, W.K.; Tian, G.L. Does thinning-induced gap size result in altered soil microbial community in pine plantaton in eastern Tibetan Plateau? Ecol. Evol. 2017, 7, 2986–2993. [Google Scholar] [CrossRef] [PubMed]
- Jiao, F.; Shi, X.R.; Han, F.P.; Yuan, Z.Y. Increasing aridity, temperature and soil pH induce soil C-N-P imbalance in grasslands. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Q.; Sun, Y.M.; Shen, C.D.; Peng, S.L.; Yi, W.X.; Li, Z.A.; Jiang, M.T. Organic matter turnover rates and CO2 flux from organic matter decomposition of mountain soil profiles in the subtropical area, south China. Catena 2002, 49, 217–229. [Google Scholar] [CrossRef]
- Liang, B.; Yang, X.Y.; He, X.H.; Zhou, J.B. Effects of 17-year fertilization on soil microbial biomass C and N and soluble organic C and N in loessial soil during maize growth. Biol. Fertil. Soils 2011, 47, 121–128. [Google Scholar] [CrossRef]
- Kalbitz, K.; Geyer, S. Different effects of peat degradation on dissolved organic carbon and nitrogen. Org. Geochem. 2002, 33, 319–326. [Google Scholar] [CrossRef]
- Downing, D.T.; Stewart, M.E. Skin surface lipids of the mole Scalopus aquaticus. Comp. Biochem. Physiol. Part B Comp. Biochem. 1987, 86, 667–670. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, T.; Liu, M.; Chen, Y.; Shang, H.; Zhu, L.; Shedayi, A.A.; Yu, H.; Cheng, G.; Liu, G.; et al. Linkages of the dynamics of glaciers and lakes with the climate elements over the Tibetan Plateau. Earth-Sci. Rev. 2018, 185, 308–324. [Google Scholar] [CrossRef]
- Dai, X.Y.; Ping, C.L.; Michaelson, G. J. Characterizing soil organic matter in Arctic tundra soil by different analytical approaches. Org. Geochem. 2002, 33, 407–419. [Google Scholar] [CrossRef]
- Grandy, A.; Sinsabaugh, R.; Neff, J.; Stursova, M.; Zak, D. Nitrogen deposition effects on soil organic matter chemistry are linked to variation in enzymes, ecosystems and size fractions. Biogeochemistry 2008, 91, 37–49. [Google Scholar] [CrossRef]
- Ma, S.Q.; Chen, Y.C.; Lu, X.Y.; Wang, X.D. Soil Organic Matter Chemistry: Based on Pyrolysis-Gas Chromatography- Mass Spectrometry (Py-GC/MS). Mini-Rev. Org. Matter 2018, 15, 389–403. [Google Scholar] [CrossRef]
- Yassir, I.; Buurman, P. Soil organic matter chemistry changes upon secondary succession in Imperata Grasslands, Indonesia: A pyrolysis–GC/MS study. Geoderma 2012, 173–174, 94–103. [Google Scholar] [CrossRef]
- Vancampenhout, K.; De Vos, B.; Wouters, K.; Swennen, R.; Buurman, P.; Deckers, J. Organic matter of subsoil horizons under broadleaved forest: Highly processed or labile and plant-derived? Soil Biol. Biochem. 2012, 50, 40–46. [Google Scholar] [CrossRef]
- Campo, J.; Nierop, K.G.J.; Cammeraat, E.; Andreu, V.; Rubio, J.L. Application of pyrolysis-gas chromatography/mass spectrometry to study changes in the organic matter of macro- and microaggregates of a Mediterranean soil upon heating. J. Chromatogr. A 2011, 1218, 4817–4827. [Google Scholar] [CrossRef] [PubMed]
- Jeannottea, R.; Hamela, C.; Jabaji, S.; Whalena, J.K. Pyrolysis-mass spectrometry and gas chromatography-flame ionization detection as complementary tools for soil lipid characterization. J. Anal. Appl. Pyrolysis 2011, 90, 232–237. [Google Scholar] [CrossRef]
- Dai, X.Y.; White, D.; Ping, C.L. Comparing bioavailability in five Arctic soils by pyrolysis-gas chromatography/mass spectrometry. J. Anal. Appl. Pyrolysis 2002, 62, 249–258. [Google Scholar] [CrossRef]
- Buurman, P.; Peterse, F.; Martin, G.A. Soil organic matter chemistry in allophanic soils: A pyrolysis-GC/MS study of a Costa Rican Andosol catena. Eur. J. Soil Sci. 2007, 58, 1330–1347. [Google Scholar] [CrossRef]
- Grandy, A.S.; Strickland, M.S.; Lauber, C.L.; Bradford, M.A.; Fierer, N. The influence of microbial communities, management, and soil texture on soil organic matter chemistry. Geoderma 2009, 150, 278–286. [Google Scholar] [CrossRef]
- Carr, A.S.; Boom, A.; Chase, B.M.; Meadows, M.E.; Roberts, Z.E.; Britton, M.N.; Cumming, A.M.J. Biome-scale characterisation and differentiation of semi-arid and arid zone soil organic matter compositions using pyrolysis–GC/MS analysis. Geoderma 2013, 200–201, 189–201. [Google Scholar] [CrossRef]
- Land Management Bureau of Tibet. Grassland Resources in Tibet Autonomous Region; Sciences Press: Beijing, China, 1994. (In Chinese) [Google Scholar]
- Yu, G.; Tang, L.; Yang, X.; Ke, X.; Harrison, S.P. Modern pollen samples from alpine vegetation on the Tibetan Plateau. Glob. Ecol. Biogeogr. 2001, 10, 503–520. [Google Scholar] [CrossRef]
- Yan, Y.; Lu, X. Is grazing exclusion effective in restoring vegetation in degraded alpine grasslands in Tibet, China? PeerJ 2015, 3, e1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.; Li, X.; Cai, X.; Gai, J.; Li, X.; Christie, P.; Zhang, J. Soil microbial community structure and activity along a montane elevational gradient on the Tibetan Plateau. Eur. J. Soil Biol. 2014, 64, 6–14. [Google Scholar] [CrossRef]
- Liu, X.; Cong, J.; Lu, H.; Xue, Y.; Wang, X.; Li, D.; Zhang, Y. Community structure and elevational distribution pattern of soil Actinobacteria in alpine grasslands. Acta Ecol. Sin. 2017, 37, 213–218. [Google Scholar] [CrossRef]
- Takishita, K.; Chikaraishi, Y.; Leger, M.M.; Kim, E.; Yabuki, A.; Ohkouchi, N.; Roger, A.J. Lateral transfer of tetrahymanol-synthesizing genes has allowed multiple diverse eukaryote lineages to independently adapt to environments without oxygen. Biol. Direct 2012, 7, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilmore, S.F.; Yao, A.I.; Tietel, Z.; Kind, T.; Facciotti, M.T.; Parikh, A.N. Role of squalene in the organization of monolayers derived from lipid extracts of Halobacterium salinarum. Langmuir 2013, 29, 7922–7930. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, N.; Xue, M.; Lu, S.T.; Tao, S. Effects of soil organic matter on the development of the microbial polycyclic aromatic hydrocarbons (PAHs) degradation potentials. Environ. Pollut. 2011, 159, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Kuzyakov, Y.; Xu, X. Competition between roots and microorganisms for nitrogen: Mechanisms and ecological relevance. New Phytol. 2013, 198, 656–669. [Google Scholar] [CrossRef] [PubMed]


| Soil Chemical Indexes | pH | SOC (g kg−1) | DOC (mg kg−1) | TN (g kg−1) | TIN (mg kg−1) |
|---|---|---|---|---|---|
| AM | 8.00 ± 0.15 c | 34.97 ± 2.89 a | 98.39 ± 27.30 a | 1.19 ± 0.54 a | 39.65 ± 6.68 a |
| AS | 7.57 ± 0.03 d | 17.26 ± 2.48 b | 66.21 ± 9.03 ab | 0.75 ± 0.32 ab | 14.52 ± 2.39 b |
| AMS | 9.52 ± 0.21 a | 9.75 ± 5.58 bc | 41.91 ± 12.84 b | 0.42 ± 0.20 bc | 13.58 ± 1.16 b |
| ADS | 8.46 ± 0.29 b | 8.74 ± 1.99 c | 36.38 ± 6.28 b | 0.34 ± 0.12 bc | 5.72 ± 2.71 b |
| AD | 8.16 ± 0.11 bc | 4.36 ± 0.58 c | 21.24 ± 2.73 b | 0.15 ± 0.10 c | 2.72 ± 1.48 b |
| PLFAs (nmol g−1) | Bacteria | Fungi | Actinomycetes | Total |
|---|---|---|---|---|
| AM | 22.84 ± 2.95 a | 3.70 ± 0.54 a | 1.96 ± 0.27 a | 23.58 ± 2.76 a |
| AS | 11.32 ± 1.43 b | 2.03 ± 0.31 b | 1.22 ± 0.15 b | 11.81 ± 1.41 b |
| AMS | 9.23 ± 1.22 bc | 1.86 ± 0.30 b | 0.84 ± 0.18 bc | 9.96 ± 1.27 bc |
| ADS | 3.72 ± 0.93 cd | 0.96 ± 0.15 b | 0.36 ± 0.15 c | 4.45 ± 0.98 cd |
| AD | 2.57 ± 0.69 d | 1.05 ± 0.25 b | 0.55 ± 0.11 c | 3.66 ± 0.93 d |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Ma, S.; Chen, Y.; Yangzom, D.; Jiang, H. Squalene Found in Alpine Grassland Soils under a Harsh Environment in the Tibetan Plateau, China. Biomolecules 2018, 8, 154. https://doi.org/10.3390/biom8040154
Lu X, Ma S, Chen Y, Yangzom D, Jiang H. Squalene Found in Alpine Grassland Soils under a Harsh Environment in the Tibetan Plateau, China. Biomolecules. 2018; 8(4):154. https://doi.org/10.3390/biom8040154
Chicago/Turabian StyleLu, Xuyang, Shuqin Ma, Youchao Chen, Degyi Yangzom, and Hongmao Jiang. 2018. "Squalene Found in Alpine Grassland Soils under a Harsh Environment in the Tibetan Plateau, China" Biomolecules 8, no. 4: 154. https://doi.org/10.3390/biom8040154




