Colonic Bacteria-Transformed Catechin Metabolite Response to Cytokine Production by Human Peripheral Blood Mononuclear Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Fecal Slurry
2.3. Fecal Batch-Culture Fermentation
2.4. Separation and Purification of Biotransformed Metabolites
2.5. GC–MS Analysis
2.6. HPLC-MS Analysis
2.7. Immunomodulatory Activity of Biotransformed Metabolites on PBMCs
2.8. Cytotoxicity (MTT) Assay
2.9. Cytokines Analysis
2.10. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
2.11. Statistical Analysis
3. Results and Discussion
3.1. Profiling of Biotransformed Metabolites
3.2. Effect of Biotransformed Catechin Metabolites on the Cell Population of Human PBMCs
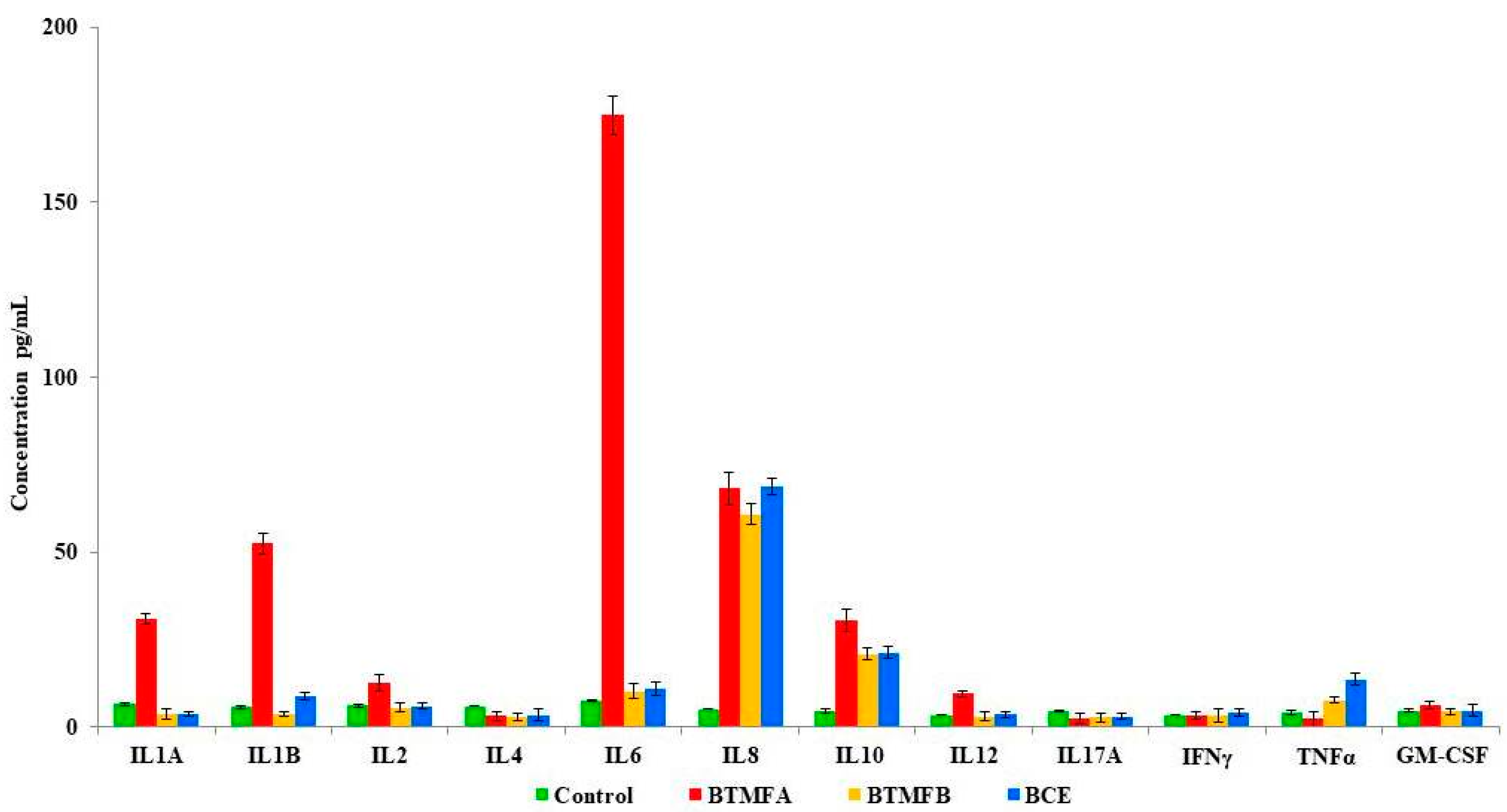
3.3. Effect of Biotransformed Metabolites on Cytokine Secretion in PBMCs
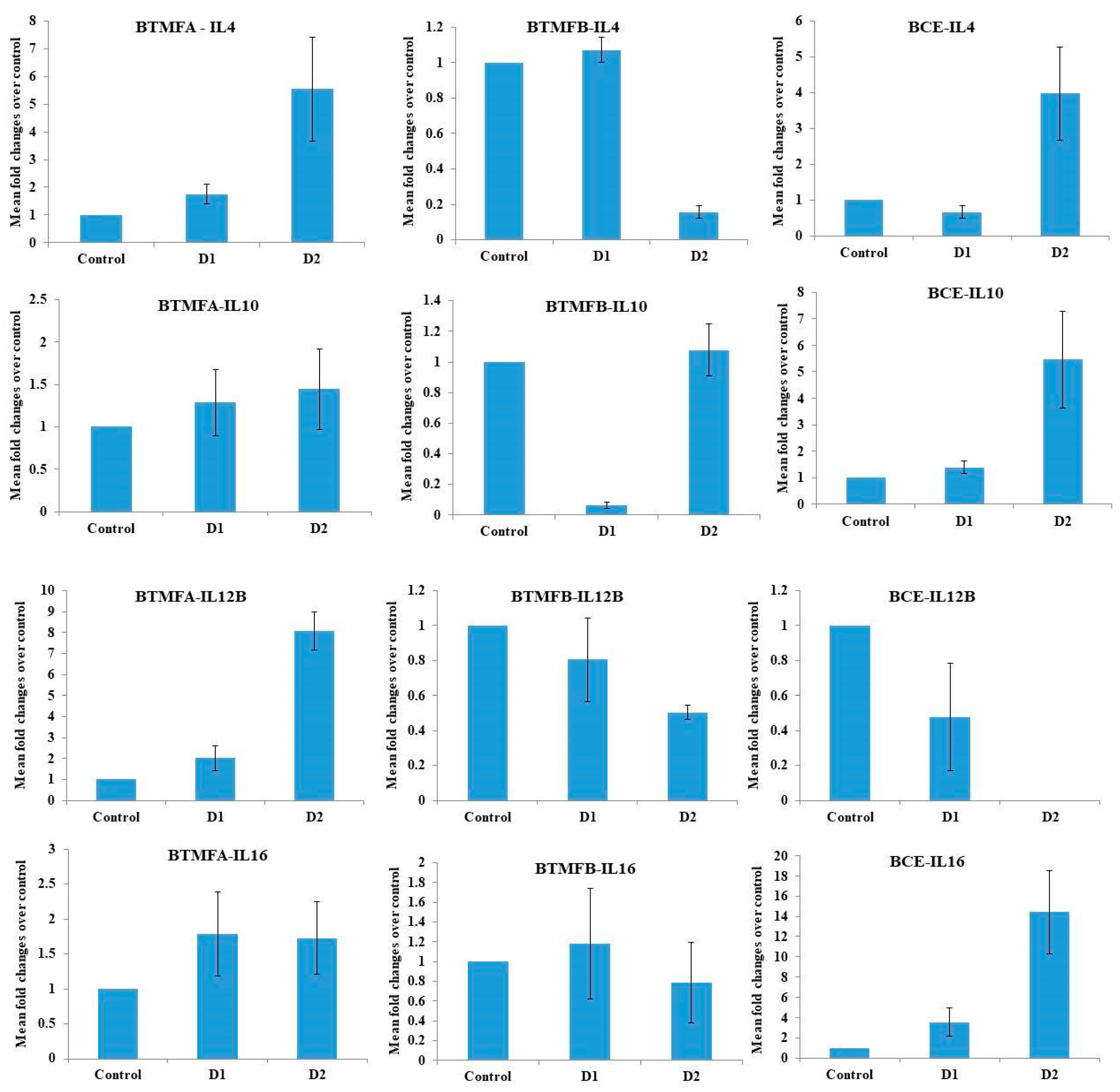
3.4. Effect of Biotransformed Metabolites on the Expression of IL mRNA in PBMCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nii-Trebi, N.I. Emerging and Neglected Infectious Diseases: Insights, Advances, and Challenges. BioMed Res. Int. 2017, 4, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Baba, S.; Osakabe, N.; Natsume, M.; Muto, Y.; Takizawa, T.; Terao, J. In Vivo Comparison of the Bioavailability of (+)-Catechin, (−)-Epicatechin and Their Mixture in Orally Administered Rats. J. Nutr. 2011, 131, 2885–2891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frei, B.; Higdon, J.V. Antioxidant activity of tea polyphenols in vivo: Evidence from animal studies. J. Nutr. 2003, 133, 3275S–3284S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thilakarathna, S.; Rupasinghe, H. Flavonoid Bioavailability and Attempts for Bioavailability Enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N. Diet-Derived Phenols in Plasma and Tissues and their Implications for Health. Planta Med. 2004, 70, 1103–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takagaki, A.; Nanjo, F. Biotransformation of (−)-epicatechin, (+)-epicatechin, (−)catechin, and (+)-catechin by intestinal bacteria involved in isoflavone metabolism. Biosci. Biotechnol. Biochem. 2016, 80, 199–202. [Google Scholar] [CrossRef] [Green Version]
- Rechner, A.R.; Kroner, C. Anthocyanins and colonic metabolites of dietary polyphenols inhibit platelet function. Thromb. Res. 2005, 116, 327–334. [Google Scholar] [CrossRef]
- Kim, Y.H.; Won, Y.; Yang, X.; Kumazoe, M.; Yamashita, S.; Hara, A.; Takagaki, A.; Goto, K.; Nanjo, F.; Tachibana, H. Green Tea Catechin Metabolites Exert Immunoregulatory Effects on CD4+ T Cell and Natural Killer Cell Activities. J. Agric. Food Chem. 2016, 64, 3591–3597. [Google Scholar] [CrossRef]
- Gao, K.; Xu, A.; Krul, C.; Venema, K.; Liu, Y.; Niu, Y.; Lu, J.; Bensoussan, L.; Seeram, N.P.; Heber, D.; et al. Of the Major Phenolic Acids Formed during Human Microbial Fermentation of Tea, Citrus, and Soy Flavonoid Supplements, Only 3,4-Dihydroxyphenylacetic Acid Has Antiproliferative Activity. J. Nutr. 2006, 136, 52–57. [Google Scholar] [CrossRef]
- Tedgui, A.; Mallat, Z. Cytokines in atherosclerosis: Pathogenic and regulatory pathways. Physiol. Rev. 2006, 86, 515–581. [Google Scholar] [CrossRef] [Green Version]
- Contreras, T.C.; Ricciardi, E.; Cremonini, E.; Oteiza, P.I. (-)-Epicatechin in the prevention of tumor necrosis alpha-induced loss of Caco-2 cell barrier integrity. Arch. Biochem. Biophys. 2015, 573, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Kook, S.H.; Choi, K.C.; Cho, S.W.; Cho, H.K.; Lee, K.D.; Lee, J.C. Catechin-7-O-β-D-glucopyranoside isolated from the seed of Phaseolus calcaratus Roxburgh ameliorates experimental colitis in rats. Int. Immunopharmacol. 2015, 29, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Porath, D.; Riegger, C.; Drewe, J.; Schwager, J. Epigallocatechin-3-gallate impairs chemokine production in human colon epithelial cell lines. J. Pharmacol. Exp. Ther. 2005, 315, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Leyva-López, N.; Gutierrez-Grijalva, E.P.; Ambriz-Perez, D.L.; Heredia, J.B. Flavonoids as Cytokine Modulators: A Possible Therapy for Inflammation-Related Diseases. Int. J. Mol. Sci. 2016, 17, 921. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, L.; Wolfson, S.; Kelly, L. The human gut chemical landscape predicts microbe-mediated biotransformation of foods and drugs. eLife 2019, 8, e42866. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.M.; Rea, M.C.; O’Sullivan, Ã.; Flynn, C.; Jones, B.; McQuaid, A.; Shanahan, F.; Ross, R.P. Preparation of a standardised faecal slurry for ex-vivo microbiota studies which reduces inter-individual donor bias. J. Microbiol. Methods 2016, 129, 109–116. [Google Scholar] [CrossRef]
- Tzounis, X.; Vulevic, J.; Kuhnle, G.G.; George, T.; Leonczak, J.; Gibson, G.R.; Spencer, J.P. Flavanol monomer-induced changes to the human faecal microflora. Br. J. Nutr. 2007, 99, 782–792. [Google Scholar] [CrossRef] [Green Version]
- Takagaki, A.; Nanjo, F. Catabolism of (+)-Catechin and (−)-Epicatechin by Rat Intestinal Microbiota. J. Agric. Food Chem. 2013, 61, 4927–4935. [Google Scholar] [CrossRef]
- Roowi, S.; Stalmach, A.; Mullen, W.; Lean, M.E.; Edwards, C.A.; Crozier, A. Green Tea Flavan-3-ols: Colonic Degradation and Urinary Excretion of Catabolites by Humans. J. Agric. Food Chem. 2010, 58, 1296–1304. [Google Scholar] [CrossRef]
- Carrie, E.P.; Steven, M.W.; Allen, C.E. Isolation of Subsets of Immune Cells. Methods Mol. Biol. 2005, 302, 95–115. [Google Scholar]
- Cuevas, A.; Saavedra, N.; Salazar, L.A.; Abdalla, D.S. Modulation of immune function by polyphenols: Possible contribution of epigenetic factors. Nutrients 2013, 5, 2314–2332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conti, B.J.; Búfalo, M.C.; Golim, M.D.A.; Bankova, V.; Sforcin, J.M. Cinnamic Acid Is Partially Involved in Propolis Immunomodulatory Action on Human Monocytes. Evid. Based Complementary Altern. Med. 2013, 2013, 109864. [Google Scholar] [CrossRef] [PubMed]
- David, M.P.; Patrícia, V.; José, A.P.; Paula, B.A. Phenolics: From Chemistry to Biology. Molecules 2009, 14, 2202–2211. [Google Scholar]
- Parr, A.J.; Bolwell, J.P. Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J. Sci. Food Agric. 2002, 80, 985–1012. [Google Scholar] [CrossRef]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; De las Rivas, B.; De Felipe, F.L.; Gómez-Cordovés, C.; Muñoz, R. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, N.P. Studies on flavonoid metabolism: Absorption and metabolism of (+)-catechin in man. Biochem. Pharmacol. 1971, 20, 3435–3445. [Google Scholar] [CrossRef]
- Ward, N.C.; Croft, K.D.; Puddey, I.B.; Hodgson, J.M. Supplementation with grape seed polyphenols results in increased urinary excretion of 3-hydroxyphenylpropionic acid, an important metabolite of proanthocyanidins in humans. J. Agric. Food Chem. 2004, 52, 5545–5549. [Google Scholar] [CrossRef] [PubMed]
- Rios, L.Y.; Gonthier, M.P.; Rémésy, C.; Mila, I.; Lapierre, C.; Lazarus, S.A.; Williamson, G.; Scalbert, A. Chocolate intake increases urinary excretion of polyphenol-derived phenolic acids in healthy human subjects. Am. J. Clin. Nutr. 2003, 77, 912–918. [Google Scholar] [CrossRef] [Green Version]
- Gonthier, M.P.; Remesy, C.; Scalbert, A.; Cheynier, V.; Souquet, J.M.; Poutanen, K.; Aura, A.M. Microbial metabolism of caffeic acid and its esters chlorogenic and caftaric acids by human faecal microbiota in vitro. Biomed. Pharmacother. 2006, 60, 536–540. [Google Scholar] [CrossRef]
- Sánchez-Patán, F.; Cueva, C.; Monagas, M.; Walton, G.E.; Gibson, G.R.; Martín-Álvarez, P.J.; Victoria Moreno-Arribas, M.; Bartolomé, B. Gut microbial catabolism of grape seed flavan-3-ols by human faecal microbiota. Targeted analysis of precursor compounds, intermediate metabolites andend-products. Food Chem. 2012, 131, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Sipahi, H.; Gostner, J.M.; Becker, K.; Charehsaz, M.; Kirmizibekmez, H.; Schennach, H.; Aydin, A.; Fuchs, D. Bioactivities of two common polyphenolic compounds: Verbascoside and catechin. Pharm. Biol. 2015, 54, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Saleh, F.; Raghupathy, R.; Asfar, S.; Oteifa, M.; Al-Saleh, N. Analysis of the effect of the active compound of green tea (EGCG) on the proliferation of peripheral blood mononuclear cells. BMC Complementary Altern. Med. 2014, 14, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Guo, Z.; Ren, Z.; Guo, W.; Meydani, S.N. Green tea EGCG suppresses T cell proliferation through impairment of IL-2/IL-2 receptor signaling. Free Radic. Biol. Med. 2009, 47, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Joray, M.B.; Trucco, L.D.; González, M.L.; Napal, G.N.; Palacios, S.M.; Bocco, J.L.; Carpinella, M.C. Antibacterial and Cytotoxic Activity of Compounds Isolated from Flourensia oolepis. Evid. Based Complementary Altern. Med. 2015, 21, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Awale, S.; Tezuka, Y.; Kadota, S. Cytotoxic constituents from Brazilian red propolis and their structure-activity relationship. Bioorg. Med. Chem. 2008, 16, 5434–5440. [Google Scholar] [CrossRef] [PubMed]
- Kawai, Y.; Nishikawa, T.; Shiba, Y.; Saito, S.; Murota, K.; Shibata, N.; Kobayashi, M.; Kanayama, M.; Uchida, K.; Terao, J. Macrophage as a target of quercetin glucuronides in human atherosclerotic arteries: Implication in the anti-atherosclerotic mechanism of dietary flavonoids. J. Biol. Chem. 2008, 283, 9424–9434. [Google Scholar] [CrossRef] [Green Version]
- Neyestani, T.R.; Gharavi, A.; Kalayi, A. Selective effects of tea extract and its phenolic compounds on human peripheral blood mononuclear cell cytokine secretions. Int. J. Food Sci. Nutr. 2009, 60, 79–88. [Google Scholar] [CrossRef]
- Crouvezier, S.; Powell, B.; Keir, D.; Yaqoob, P. The effects of phenolic components of Tea on the production of pro- and anti-inflammatory cytokines by human leukocytes in vitro. Cytokine 2001, 13, 280–286. [Google Scholar] [CrossRef]
- Schirmer, M.; Smeekens, S.P.; Vlamakis, H.; Jaeger, M.; Oosting, M.; Franzosa, E.A.; ter Horst, R.; Jansen, T.; Jacobs, L.; Bonder, M.J. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell 2016, 167, 1125–1136. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Mazza, G. Effects of anthocyanins and other phenolic compounds on the production of tumour necrosis factor alpha in LPS/IFN-gamma-activated RAW 264.7 macrophages. J. Agric. Food Chem. 2002, 50, 4183–4189. [Google Scholar] [CrossRef]
- Nair, M.P.; Kandaswami, C.; Mahajan, S.; Chadha, K.C.; Chawda, R.; Nair, H.; Schwartz, S.A. The flavonoid, quercetin, differentially regulates Th-1 (IFNgamma) and Th-2 (IL4) cytokine gene expression by normal peripheral blood mononuclear cells. Biochim. Biophys. Acta 2002, 1593, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.; Jiang, H.; Fang, J. Regulation of Immune Function by Polyphenols. J. Immunol. Res. 2018, 3, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akdis, M. Healthy immune response to allergens: T regulatory cells and more. Curr. Opin. Immunol. 2006, 18, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Seltmann, J.; Roesner, L.M.; von Hesler, F.W.; Wittmann, M.; Werfel, T. IL-33 impacts on the skin barrier by downregulating the expression of filaggrin. J. Allergy Clin. Immunol. 2015, 135, 1659–1661. [Google Scholar] [CrossRef] [PubMed]
- Soyka, M.B.; Wawrzyniak, P.; Eiwegger, T.; Holzmann, D.; Treis, A.; Wanke, K.; Kast, J.I.; Akdis, C.A. Defective epithelial barrier in chronic rhinosinusitis: The regulation of tight junctions by IFN-gamma and IL-4. J. Allergy Clin. Immunol. 2012, 130, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Terao, J.; Murota, K.; Kawai, Y. Conjugated quercetin glucuronides as bioactive metabolites and precursors of aglycone in vivo. Food Funct. 2011, 2, 11–17. [Google Scholar] [CrossRef]
- Li, L.; Davie, J.R. The role of Sp1 and Sp3 in normal and cancer cell biology. Ann. Anat. 2010, 192, 275–283. [Google Scholar] [CrossRef]
- Luo, X.; Yu, X.; Liu, S.; Deng, Q.; Liu, X.; Peng, S.; Li, H.; Liu, J.; Cao, Y. The role of targeting kinase activity by natural products in cancer chemoprevention and chemotherapy (Review). Oncol. Rep. 2015, 34, 547–554. [Google Scholar] [CrossRef] [Green Version]
- Engelhardt, K.R.; Shah, N.; Faizura-Yeop, I.; Kocacik Uygun, D.F.; Frede, N.; Muise, A.M.; Shteyer, E.; Filiz, S.; Chee, R.; Elawad, M.; et al. Clinical outcome in IL-10- and IL-10 receptor-deficient patients with or without hematopoietic stem cell transplantation. J. Allergy Clin. Immunol. 2013, 131, 825–830. [Google Scholar] [CrossRef]
- Hsieh, C.S.; Macatonia, S.E.; Tripp, C.S.; Wolf, S.F.; O’Garra, A.; Murphy, K.M. Development of TH1 CD41 T cells through IL-12 produced by Listeria-induced macrophages. Science 1993, 260, 547–549. [Google Scholar] [CrossRef]
- Richmond, J.; Tuzova, M.; Cruikshank, W.; Center, D. Regulation of cellular processes by interleukin-16 in homeostasis and cancer. J. Cell Physiol. 2014, 229, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Haabeth, O.A.; Kristina, B.L.; Clara, H.; Ian, M.D.; Guttorm, H.; Bjarne, B.; Alexandre, C. Inflammation driven by tumour-specific Th1 cells protects against B-cell cancer. Nat. Commun. 2011, 2, 240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mellor-Heineke, S.; Villanueva, J.; Jordan, M.B.; Marsh, R.; Zhang, K.; Bleesing, J.J.; Filipovich, A.H.; Risma, K.A. Elevated Granzyme B in Cytotoxic Lymphocytes is a Signature of Immune Activation in Hemophagocytic Lymphohistiocytosis. Front. Immunol. 2013, 4, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krishnamoorthy, R.; Adisa, A.R.; Periasamy, V.S.; Athinarayanan, J.; Pandurangan, S.-B.; Alshatwi, A.A. Colonic Bacteria-Transformed Catechin Metabolite Response to Cytokine Production by Human Peripheral Blood Mononuclear Cells. Biomolecules 2019, 9, 830. https://doi.org/10.3390/biom9120830
Krishnamoorthy R, Adisa AR, Periasamy VS, Athinarayanan J, Pandurangan S-B, Alshatwi AA. Colonic Bacteria-Transformed Catechin Metabolite Response to Cytokine Production by Human Peripheral Blood Mononuclear Cells. Biomolecules. 2019; 9(12):830. https://doi.org/10.3390/biom9120830
Chicago/Turabian StyleKrishnamoorthy, Rajapandiyan, Abdulraheem R. Adisa, Vaiyapuri Subbarayan Periasamy, Jegan Athinarayanan, Subash-Babu Pandurangan, and Ali A. Alshatwi. 2019. "Colonic Bacteria-Transformed Catechin Metabolite Response to Cytokine Production by Human Peripheral Blood Mononuclear Cells" Biomolecules 9, no. 12: 830. https://doi.org/10.3390/biom9120830




