Class II PI3Ks at the Intersection between Signal Transduction and Membrane Trafficking
Abstract
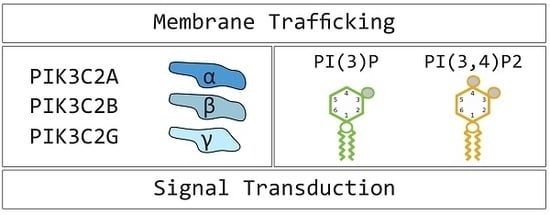
:1. Introduction
2. Class II PI3Ks Lipid Products
2.1. Class II Derived PI3P
2.2. Class II Derived PI(3,4)P2
3. PIK3C2A
3.1. PIK3C2A Structure
3.2. PIK3C2A in Vesicular Trafficking and Signaling
3.2.1. Endocytosis through Dynamin-Dependent and -Independent Mechanisms
3.2.2. Recycling and Exocytosis
3.2.3. Scaffolding Effect on Mitotic Spindle Assembly
4. PIK3C2B
4.1. PIK3C2B Structure
4.2. PIK3C2B Signaling in Vesicular Trafficking
5. PIK3C2G
5.1. PIK3C2G Structure
5.2. PIK3C2G in Metabolic Signaling and Vesicular Trafficking
6. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Vanhaesebroeck, B.; Guillermet-Guibert, J.; Graupera, M.; Bilanges, B. The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 2010, 11, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Backer, J.M. The intricate regulation and complex functions of the Class III phosphoinositide 3-kinase Vps34. Biochem. J. 2016, 473, 2251–2271. [Google Scholar] [CrossRef]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef]
- Kriplani, N.; Hermida, M.A.; Brown, E.R.; Leslie, N.R. Class I PI 3-kinases: Function and evolution. Adv. Biol. Regul. 2015, 59, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Soler, A.; Angulo-Urarte, A.; Graupera, M. PI3K at the crossroads of tumor angiogenesis signaling pathways. Mol. Cell. Oncol. 2015, 2, e975624. [Google Scholar] [CrossRef]
- Vadas, O.; Burke, J.E.; Zhang, X.; Berndt, A.; Williams, R.L. Structural basis for activation and inhibition of class I phosphoinositide 3-kinases. Sci. Signal. 2011, 4, re2. [Google Scholar] [CrossRef]
- Zou, W.; Lu, Q.; Zhao, D.; Li, W.; Mapes, J.; Xie, Y.; Wang, X. Caenorhabditis elegans myotubularin MTM-1 negatively regulates the engulfment of apoptotic cells. PLoS Genet. 2009, 5, e1000679. [Google Scholar] [CrossRef]
- Xue, Y.; Fares, H.; Grant, B.; Li, Z.; Rose, A.M.; Clark, S.G.; Skolnik, E.Y. Genetic analysis of the myotubularin family of phosphatases in Caenorhabditis elegans. J. Biol. Chem. 2003, 278, 34380–34386. [Google Scholar] [CrossRef]
- MacDougall, L.K.; Domin, J.; Waterfield, M.D. A family of phosphoinositide 3-kinases in Drosophila identifies a new mediator of signal transduction. Curr. Biol. 1995, 5, 1404–1415. [Google Scholar] [CrossRef]
- Virbasius, J.V.; Guilherme, A.; Czech, M.P. Mouse p170 is a novel phosphatidylinositol 3-kinase containing a C2 domain. J. Biol. Chem. 1996, 271, 13304–13307. [Google Scholar] [CrossRef] [PubMed]
- Domin, J.; Pages, F.; Volinia, S.; Rittenhouse, S.E.; Zvelebil, M.J.; Stein, R.C.; Waterfield, M.D. Cloning of a human phosphoinositide 3-kinase with a C2 domain that displays reduced sensitivity to the inhibitor wortmannin. Biochem. J. 1997, 326 (Pt 1), 139–147. [Google Scholar] [CrossRef]
- Gaidarov, I.; Smith, M.E.; Domin, J.; Keen, J.H. The class II phosphoinositide 3-kinase C2alpha is activated by clathrin and regulates clathrin-mediated membrane trafficking. Mol. Cell 2001, 7, 443–449. [Google Scholar] [CrossRef]
- Schu, P.V.; Takegawa, K.; Fry, M.J.; Stack, J.H.; Waterfield, M.D.; Emr, S.D. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science 1993, 260, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Franco, I.; Gulluni, F.; Campa, C.C.; Costa, C.; Margaria, J.P.; Ciraolo, E.; Martini, M.; Monteyne, D.; De Luca, E.; Germena, G.; et al. PI3K class II α controls spatially restricted endosomal PtdIns3P and Rab11 activation to promote primary cilium function. Dev. Cell 2014, 28, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Marat, A.L.; Wallroth, A.; Lo, W.T.; Müller, R.; Norata, G.D.; Falasca, M.; Schultz, C.; Haucke, V. mTORC1 activity repression by late endosomal phosphatidylinositol 3,4-bisphosphate. Science 2017, 356, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Posor, Y.; Eichhorn-Gruenig, M.; Puchkov, D.; Schöneberg, J.; Ullrich, A.; Lampe, A.; Müller, R.; Zarbakhsh, S.; Gulluni, F.; Hirsch, E.; et al. Spatiotemporal control of endocytosis by phosphatidylinositol-3,4-bisphosphate. Nature 2013, 499, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, K.; Yoshida, K.; Cui, H.; Wakayama, T.; Takuwa, N.; Okamoto, Y.; Du, W.; Qi, X.; Asanuma, K.; Sugihara, K.; et al. Endothelial PI3K-C2α, a class II PI3K, has an essential role in angiogenesis and vascular barrier function. Nat. Med. 2012, 18, 1560–1569. [Google Scholar] [CrossRef]
- Alliouachene, S.; Bilanges, B.; Chicanne, G.; Anderson, K.E.; Pearce, W.; Ali, K.; Valet, C.; Posor, Y.; Low, P.C.; Chaussade, C.; et al. Inactivation of the Class II PI3K-C2β Potentiates Insulin Signaling and Sensitivity. Cell Rep. 2015, 13, 1881–1894. [Google Scholar] [CrossRef] [PubMed]
- Braccini, L.; Ciraolo, E.; Campa, C.C.; Perino, A.; Longo, D.L.; Tibolla, G.; Pregnolato, M.; Cao, Y.; Tassone, B.; Damilano, F.; et al. PI3K-C2γ is a Rab5 effector selectively controlling endosomal Akt2 activation downstream of insulin signalling. Nat. Commun. 2015, 6, 7400. [Google Scholar] [CrossRef] [PubMed]
- Boller, D.; Doepfner, K.T.; De Laurentiis, A.; Guerreiro, A.S.; Marinov, M.; Shalaby, T.; Depledge, P.; Robson, A.; Saghir, N.; Hayakawa, M.; et al. Targeting PI3KC2β impairs proliferation and survival in acute leukemia, brain tumours and neuroendocrine tumours. Anticancer Res. 2012, 32, 3015–3027. [Google Scholar] [PubMed]
- Freitag, A.; Prajwal, P.; Shymanets, A.; Harteneck, C.; Nürnberg, B.; Schächtele, C.; Kubbutat, M.; Totzke, F.; Laufer, S.A. Development of first lead structures for phosphoinositide 3-kinase-C2γ inhibitors. J. Med. Chem. 2015, 58, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Arcaro, A.; Volinia, S.; Zvelebil, M.J.; Stein, R.; Watton, S.J.; Layton, M.J.; Gout, I.; Ahmadi, K.; Downward, J.; Waterfield, M.D. Human phosphoinositide 3-kinase C2beta, the role of calcium and the C2 domain in enzyme activity. J. Biol. Chem. 1998, 273, 33082–33090. [Google Scholar] [CrossRef] [PubMed]
- Misawa, H.; Ohtsubo, M.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; Yoshimura, A. Cloning and characterization of a novel class II phosphoinositide 3-kinase containing C2 domain. Biochem. Biophys. Res. Commun. 1998, 244, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Franco, I.; Margaria, J.P.; De Santis, M.C.; Ranghino, A.; Monteyne, D.; Chiaravalli, M.; Pema, M.; Campa, C.C.; Ratto, E.; Gulluni, F.; et al. Phosphoinositide 3-Kinase-C2α Regulates Polycystin-2 Ciliary Entry and Protects against Kidney Cyst Formation. J. Am. Soc. Nephrol. 2016, 27, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.P.; Vogel, P.; Wims, M.; Moberg, K.; Humphries, J.; Jhaver, K.G.; DaCosta, C.M.; Shadoan, M.K.; Xu, N.; Hansen, G.M.; et al. Requirement for class II phosphoinositide 3-kinase C2alpha in maintenance of glomerular structure and function. Mol. Cell. Biol. 2011, 31, 63–80. [Google Scholar] [CrossRef]
- Aki, S.; Yoshioka, K.; Okamoto, Y.; Takuwa, N.; Takuwa, Y. Phosphatidylinositol 3-kinase class II α-isoform PI3K-C2α is required for transforming growth factor β-induced Smad signaling in endothelial cells. J. Biol. Chem. 2015, 290, 6086–6105. [Google Scholar] [CrossRef] [PubMed]
- Maffucci, T.; Falasca, M. New insight into the intracellular roles of class II phosphoinositide 3-kinases. Biochem. Soc. Trans. 2014, 42, 1378–1382. [Google Scholar] [CrossRef] [PubMed]
- Falasca, M.; Hughes, W.E.; Dominguez, V.; Sala, G.; Fostira, F.; Fang, M.Q.; Cazzolli, R.; Shepherd, P.R.; James, D.E.; Maffucci, T. The role of phosphoinositide 3-kinase C2alpha in insulin signaling. J. Biol. Chem. 2007, 282, 28226–28236. [Google Scholar] [CrossRef] [PubMed]
- Domin, J.; Harper, L.; Aubyn, D.; Wheeler, M.; Florey, O.; Haskard, D.; Yuan, M.; Zicha, D. The class II phosphoinositide 3-kinase PI3K-C2beta regulates cell migration by a PtdIns3P dependent mechanism. J. Cell. Physiol. 2005, 205, 452–462. [Google Scholar] [CrossRef]
- Biswas, K.; Yoshioka, K.; Asanuma, K.; Okamoto, Y.; Takuwa, N.; Sasaki, T.; Takuwa, Y. Essential role of class II phosphatidylinositol-3-kinase-C2α in sphingosine 1-phosphate receptor-1-mediated signaling and migration in endothelial cells. J. Biol. Chem. 2013, 288, 2325–2339. [Google Scholar] [CrossRef]
- Banfic, H.; Visnjic, D.; Mise, N.; Balakrishnan, S.; Deplano, S.; Korchev, Y.E.; Domin, J. Epidermal growth factor stimulates translocation of the class II phosphoinositide 3-kinase PI3K-C2beta to the nucleus. Biochem. J. 2009, 422, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Campa, C.C.; Margaria, J.P.; Derle, A.; Del Giudice, M.; De Santis, M.C.; Gozzelino, L.; Copperi, F.; Bosia, C.; Hirsch, E. Rab11 activity and PtdIns(3)P turnover removes recycling cargo from endosomes. Nat. Chem. Biol. 2018, 14, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Bridges, D.; Ma, J.T.; Park, S.; Inoki, K.; Weisman, L.S.; Saltiel, A.R. Phosphatidylinositol 3,5-bisphosphate plays a role in the activation and subcellular localization of mechanistic target of rapamycin 1. Mol. Biol. Cell 2012, 23, 2955–2962. [Google Scholar] [CrossRef] [PubMed]
- Tiosano, D.; Feldman, H.B.; Chen, A.; Hitzert, M.M.; Schueler, M.; Gulluni, F.; Wiesener, A.; Bergua, A.; Mory, A.; Copeland, B.; et al. Mutations in PIK3C2A Cause Syndromic Short Stature, Skeletal Abnormalities, and Cataracts Associated With Ciliary Dysfunction. bioRxiv 2018. [Google Scholar] [CrossRef]
- Visnjić, D.; Curić, J.; Crljen, V.; Batinić, D.; Volinia, S.; Banfić, H. Nuclear phosphoinositide 3-kinase C2beta activation during G2/M phase of the cell cycle in HL-60 cells. Biochim. Biophys. Acta 2003, 1631, 61–71. [Google Scholar] [CrossRef]
- Srivastava, S.; Di, L.; Zhdanova, O.; Li, Z.; Vardhana, S.; Wan, Q.; Yan, Y.; Varma, R.; Backer, J.; Wulff, H.; et al. The class II phosphatidylinositol 3 kinase C2beta is required for the activation of the K+ channel KCa3.1 and CD4 T-cells. Mol. Biol. Cell 2009, 20, 3783–3791. [Google Scholar] [CrossRef] [PubMed]
- Ono, F.; Nakagawa, T.; Saito, S.; Owada, Y.; Sakagami, H.; Goto, K.; Suzuki, M.; Matsuno, S.; Kondo, H. A novel class II phosphoinositide 3-kinase predominantly expressed in the liver and its enhanced expression during liver regeneration. J. Biol. Chem. 1998, 273, 7731–7736. [Google Scholar] [CrossRef] [PubMed]
- Leibiger, B.; Moede, T.; Uhles, S.; Barker, C.J.; Creveaux, M.; Domin, J.; Berggren, P.O.; Leibiger, I.B. Insulin-feedback via PI3K-C2alpha activated PKBalpha/Akt1 is required for glucose-stimulated insulin secretion. FASEB J. 2010, 24, 1824–1837. [Google Scholar] [CrossRef]
- Blondeau, F.; Ritter, B.; Allaire, P.D.; Wasiak, S.; Girard, M.; Hussain, N.K.; Angers, A.; Legendre-Guillemin, V.; Roy, L.; Boismenu, D.; et al. Tandem MS analysis of brain clathrin-coated vesicles reveals their critical involvement in synaptic vesicle recycling. Proc. Natl. Acad. Sci. USA 2004, 101, 3833–3838. [Google Scholar] [CrossRef]
- Brown, R.A.; Domin, J.; Arcaro, A.; Waterfield, M.D.; Shepherd, P.R. Insulin activates the alpha isoform of class II phosphoinositide 3-kinase. J. Biol. Chem. 1999, 274, 14529–14532. [Google Scholar] [CrossRef]
- Arcaro, A.; Zvelebil, M.J.; Wallasch, C.; Ullrich, A.; Waterfield, M.D.; Domin, J. Class II phosphoinositide 3-kinases are downstream targets of activated polypeptide growth factor receptors. Mol. Cell. Biol. 2000, 20, 3817–3830. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.J.; Domin, J.; Waterfield, M.D.; Ward, S.G.; Westwick, J. The CC chemokine monocyte chemotactic peptide-1 activates both the class I p85/p110 phosphatidylinositol 3-kinase and the class II PI3K-C2alpha. J. Biol. Chem. 1998, 273, 25987–25995. [Google Scholar] [CrossRef] [PubMed]
- Stahelin, R.V.; Karathanassis, D.; Bruzik, K.S.; Waterfield, M.D.; Bravo, J.; Williams, R.L.; Cho, W. Structural and membrane binding analysis of the Phox homology domain of phosphoinositide 3-kinase-C2alpha. J. Biol. Chem. 2006, 281, 39396–39406. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lo, W.T.; Vujičić Žagar, A.; Gulluni, F.; Lehmann, M.; Scapozza, L.; Haucke, V.; Vadas, O. Autoregulation of Class II Alpha PI3K Activity by Its Lipid-Binding PX-C2 Domain Module. Mol. Cell 2018, 71, 343–351.e4. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.E.; Tillu, V.A.; Chandra, M.; Collins, B.M. Molecular Basis for Membrane Recruitment by the PX and C2 Domains of Class II Phosphoinositide 3-Kinase-C2α. Structure 2018, 26, 1612–1625.e4. [Google Scholar] [CrossRef] [PubMed]
- Irino, Y.; Tokuda, E.; Hasegawa, J.; Itoh, T.; Takenawa, T. Quantification and visualization of phosphoinositides by quantum dot-labeled specific binding-domain probes. J. Lipid Res. 2012, 53, 810–819. [Google Scholar] [CrossRef]
- Aung, K.T.; Yoshioka, K.; Aki, S.; Ishimaru, K.; Takuwa, N.; Takuwa, Y. The class II phosphoinositide 3-kinases PI3K-C2α and PI3K-C2β differentially regulate clathrin-dependent pinocytosis in human vascular endothelial cells. J. Physiol. Sci. 2018. [Google Scholar] [CrossRef]
- Wang, Y.; Yoshioka, K.; Azam, M.A.; Takuwa, N.; Sakurada, S.; Kayaba, Y.; Sugimoto, N.; Inoki, I.; Kimura, T.; Kuwaki, T.; et al. Class II phosphoinositide 3-kinase alpha-isoform regulates Rho, myosin phosphatase and contraction in vascular smooth muscle. Biochem. J. 2006, 394, 581–592. [Google Scholar] [CrossRef]
- Krag, C.; Malmberg, E.K.; Salcini, A.E. PI3KC2α, a class II PI3K, is required for dynamin-independent internalization pathways. J. Cell Sci. 2010, 123, 4240–4250. [Google Scholar] [CrossRef]
- Campa, C.C.; Franco, I.; Hirsch, E. PI3K-C2α: One enzyme for two products coupling vesicle trafficking and signal transduction. FEBS Lett. 2015, 589, 1552–1558. [Google Scholar] [CrossRef]
- Jean, S.; Cox, S.; Schmidt, E.J.; Robinson, F.L.; Kiger, A. Sbf/MTMR13 coordinates PI(3)P and Rab21 regulation in endocytic control of cellular remodeling. Mol. Biol. Cell 2012, 23, 2723–2740. [Google Scholar] [CrossRef] [PubMed]
- Welz, T.; Wellbourne-Wood, J.; Kerkhoff, E. Orchestration of cell surface proteins by Rab11. Trends Cell Biol. 2014, 24, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Horgan, C.P.; Hanscom, S.R.; Jolly, R.S.; Futter, C.E.; McCaffrey, M.W. Rab11-FIP3 links the Rab11 GTPase and cytoplasmic dynein to mediate transport to the endosomal-recycling compartment. J. Cell Sci. 2010, 123, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Horgan, C.P.; Hanscom, S.R.; Jolly, R.S.; Futter, C.E.; McCaffrey, M.W. Rab11-FIP3 binds dynein light intermediate chain 2 and its overexpression fragments the Golgi complex. Biochem. Biophys. Res. Commun. 2010, 394, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Velichkova, M.; Juan, J.; Kadandale, P.; Jean, S.; Ribeiro, I.; Raman, V.; Stefan, C.; Kiger, A.A. Drosophila Mtm and class II PI3K coregulate a PI(3)P pool with cortical and endolysosomal functions. J. Cell Biol. 2010, 190, 407–425. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, I.; Yuan, L.; Tanentzapf, G.; Dowling, J.J.; Kiger, A. Phosphoinositide regulation of integrin trafficking required for muscle attachment and maintenance. PLoS Genet. 2011, 7, e1001295. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, K.; Zou, W.; Miao, R.; Huang, Y.; Wang, H.; Wang, X. PtdIns(4,5)P₂ and PtdIns3P coordinate to regulate phagosomal sealing for apoptotic cell clearance. J. Cell Biol. 2015, 210, 485–502. [Google Scholar] [CrossRef]
- Lu, N.; Shen, Q.; Mahoney, T.R.; Neukomm, L.J.; Wang, Y.; Zhou, Z. Two PI 3-kinases and one PI 3-phosphatase together establish the cyclic waves of phagosomal PtdIns(3)P critical for the degradation of apoptotic cells. PLoS Biol. 2012, 10, e1001245. [Google Scholar] [CrossRef]
- Zhang, S.X.; Duan, L.H.; He, S.J.; Zhuang, G.F.; Yu, X. Phosphatidylinositol 3,4-bisphosphate regulates neurite initiation and dendrite morphogenesis via actin aggregation. Cell Res. 2017, 27, 253–273. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, G. Mechanisms of the anterograde trafficking of GPCRs: Regulation of AT1R transport by interacting proteins and motifs. Traffic 2019, 20, 110–120. [Google Scholar] [CrossRef]
- Shiwarski, D.J.; Darr, M.; Telmer, C.A.; Bruchez, M.P.; Puthenveedu, M.A. PI3K class II α regulates δ-opioid receptor export from the trans-Golgi network. Mol. Biol. Cell 2017, 28, 2202–2219. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, I.; Salazar, J.; Arqueros, C.; Andrés, M.; Sebio, A.; Majem, M.; Szafranska, J.; Martínez, E.; Páez, D.; López-Pousa, A.; et al. KRAS genetic variant as a prognostic factor for recurrence in resectable non-small cell lung cancer. Clin. Transl. Oncol. 2017, 19, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Lu, Y.; Wang, J.; Yang, L.; Han, Y.; Wang, Y.; Yan, D.; Ruan, Q.; Wang, S. A four-gene signature predicts survival in clear-cell renal-cell carcinoma. Oncotarget 2016, 7, 82712–82726. [Google Scholar] [CrossRef] [PubMed]
- Gulluni, F.; Martini, M.; De Santis, M.C.; Campa, C.C.; Ghigo, A.; Margaria, J.P.; Ciraolo, E.; Franco, I.; Ala, U.; Annaratone, L.; et al. Mitotic Spindle Assembly and Genomic Stability in Breast Cancer Require PI3K-C2α Scaffolding Function. Cancer Cell 2017, 32, 444–459.e7. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Tarbutton, E.; Wilson, G.; Prekeris, R. Rab11-FIP3 is a Rab11-binding protein that regulates breast cancer cell motility by modulating the actin cytoskeleton. Eur. J. Cell Biol. 2009, 88, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Didichenko, S.A.; Thelen, M. Phosphatidylinositol 3-kinase c2alpha contains a nuclear localization sequence and associates with nuclear speckles. J. Biol. Chem. 2001, 276, 48135–48142. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.; Domin, J. Recruitment of the class II phosphoinositide 3-kinase C2beta to the epidermal growth factor receptor: role of Grb2. Mol. Cell. Biol. 2001, 21, 6660–6667. [Google Scholar] [CrossRef]
- Wheeler, M.; Domin, J. The N-terminus of phosphoinositide 3-kinase-C2beta regulates lipid kinase activity and binding to clathrin. J. Cell. Physiol. 2006, 206, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Zoncu, R.; Perera, R.M.; Balkin, D.M.; Pirruccello, M.; Toomre, D.; De Camilli, P. A phosphoinositide switch controls the maturation and signaling properties of APPL endosomes. Cell 2009, 136, 1110–1121. [Google Scholar] [CrossRef]
- Das, M.; Scappini, E.; Martin, N.P.; Wong, K.A.; Dunn, S.; Chen, Y.J.; Miller, S.L.; Domin, J.; O’Bryan, J.P. Regulation of neuron survival through an intersectin-phosphoinositide 3′-kinase C2beta-AKT pathway. Mol. Cell. Biol. 2007, 27, 7906–7917. [Google Scholar] [CrossRef]
- Maffucci, T.; Cooke, F.T.; Foster, F.M.; Traer, C.J.; Fry, M.J.; Falasca, M. Class II phosphoinositide 3-kinase defines a novel signaling pathway in cell migration. J. Cell Biol. 2005, 169, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Mavrommati, I.; Cisse, O.; Falasca, M.; Maffucci, T. Novel roles for class II Phosphoinositide 3-Kinase C2β in signalling pathways involved in prostate cancer cell invasion. Sci. Rep. 2016, 6, 23277. [Google Scholar] [CrossRef] [PubMed]
- Tania, M.; Khan, M.A.; Fu, J. Epithelial to mesenchymal transition inducing transcription factors and metastatic cancer. Tumour Biol. 2014, 35, 7335–7342. [Google Scholar] [CrossRef]
- Kitatani, K.; Usui, T.; Sriraman, S.K.; Toyoshima, M.; Ishibashi, M.; Shigeta, S.; Nagase, S.; Sakamoto, M.; Ogiso, H.; Okazaki, T.; et al. Ceramide limits phosphatidylinositol-3-kinase C2β-controlled cell motility in ovarian cancer: potential of ceramide as a metastasis-suppressor lipid. Oncogene 2016, 35, 2801–2812. [Google Scholar] [CrossRef]
- Sarker, M.A.K.; Aki, S.; Yoshioka, K.; Kuno, K.; Okamoto, Y.; Ishimaru, K.; Takuwa, N.; Takuwa, Y. Class II PI3Ks α and β Are Required for Rho-Dependent Uterine Smooth Muscle Contraction and Parturition in Mice. Endocrinology 2019, 160, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Rozycka, M.; Lu, Y.J.; Brown, R.A.; Lau, M.R.; Shipley, J.M.; Fry, M.J. cDNA cloning of a third human C2-domain-containing class II phosphoinositide 3-kinase, PI3K-C2gamma, and chromosomal assignment of this gene (PIK3C2G) to 12p12. Genomics 1998, 54, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Daimon, M.; Sato, H.; Oizumi, T.; Toriyama, S.; Saito, T.; Karasawa, S.; Jimbu, Y.; Wada, K.; Kameda, W.; Susa, S.; et al. Association of the PIK3C2G gene polymorphisms with type 2 DM in a Japanese population. Biochem. Biophys. Res. Commun. 2008, 365, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M. Locus and gene-based GWAS meta-analysis identifies new diabetic nephropathy genes. Immunogenetics 2018, 70, 347–353. [Google Scholar] [CrossRef]
- Shia, W.C.; Ku, T.H.; Tsao, Y.M.; Hsia, C.H.; Chang, Y.M.; Huang, C.H.; Chung, Y.C.; Hsu, S.L.; Liang, K.W.; Hsu, F.R. Genetic copy number variants in myocardial infarction patients with hyperlipidemia. BMC Genom. 2011, 12 (Suppl. 3), S23. [Google Scholar] [CrossRef]
- Anderson, D.; Cordell, H.J.; Fakiola, M.; Francis, R.W.; Syn, G.; Scaman, E.S.; Davis, E.; Miles, S.J.; McLeay, T.; Jamieson, S.E.; et al. First genome-wide association study in an Australian aboriginal population provides insights into genetic risk factors for body mass index and type 2 diabetes. PLoS ONE 2015, 10, e0119333. [Google Scholar] [CrossRef]
- Ruzzenente, A.; Fassan, M.; Conci, S.; Simbolo, M.; Lawlor, R.T.; Pedrazzani, C.; Capelli, P.; D’Onofrio, M.; Iacono, C.; Scarpa, A.; et al. Cholangiocarcinoma Heterogeneity Revealed by Multigene Mutational Profiling: Clinical and Prognostic Relevance in Surgically Resected Patients. Ann. Surg. Oncol. 2016, 23, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Chen, H.; Lin, M.; Zhang, C.; Tang, E.; Peng, J.; Wei, Q.; Li, H.; Yin, L. PIK3C2G copy number is associated with clinical outcomes of colorectal cancer patients treated with oxaliplatin. Int. J. Clin. Exp. Med. 2015, 8, 1137–1143. [Google Scholar] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Margaria, J.P.; Ratto, E.; Gozzelino, L.; Li, H.; Hirsch, E. Class II PI3Ks at the Intersection between Signal Transduction and Membrane Trafficking. Biomolecules 2019, 9, 104. https://doi.org/10.3390/biom9030104
Margaria JP, Ratto E, Gozzelino L, Li H, Hirsch E. Class II PI3Ks at the Intersection between Signal Transduction and Membrane Trafficking. Biomolecules. 2019; 9(3):104. https://doi.org/10.3390/biom9030104
Chicago/Turabian StyleMargaria, Jean Piero, Edoardo Ratto, Luca Gozzelino, Huayi Li, and Emilio Hirsch. 2019. "Class II PI3Ks at the Intersection between Signal Transduction and Membrane Trafficking" Biomolecules 9, no. 3: 104. https://doi.org/10.3390/biom9030104