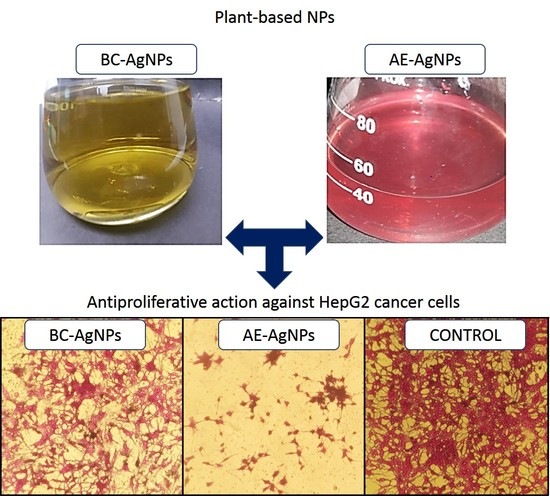
A Comparative Evaluation of the Antiproliferative Activity against HepG2 Liver Carcinoma Cells of Plant-Derived Silver Nanoparticles from Basil Extracts with Contrasting Anthocyanin Contents
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Biological Synthesis of AgNPs
2.3. Structural and Optical Characterization of Green Synthesized AgNPs
2.4. Solid Phase Extraction Procedure
2.5. HPLC Analysis
2.6. In Vitro Cytotoxicity of AgNPs against HepG2 Cell Line
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Biosynthesized AgNPs
3.1.1. UV-Vis Spectrophotometric Analysis
3.1.2. XRD Analysis
3.1.3. FTIR Analysis
3.1.4. SEM and EDX Analysis
3.2. SPE and HPLC
3.3. Anticancer Potential Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Muhammad, W.; Khan, M.A.; Nazir, M.; Siddiquah, A.; Mushtaq, S.; Hashmi, S.S.; Abbasi, B.H. Papaver somniferum L. mediated novel bioinspired lead oxide (PbO) and iron oxide (Fe2O3) nanoparticles: In-vitro biological applications, biocompatibility and their potential towards HepG2 cell line. Mater. Sci. Eng. C 2019, 103, 109740. [Google Scholar] [CrossRef] [PubMed]
- Zia, M.; Gul, S.; Akhtar, J.; ul Haq, I.; Abbasi, B.H.; Hussain, A.; Naz, S.; Chaudhary, M.F. Green synthesis of silver nanoparticles from grape and tomato juices and evaluation of biological activities. IET nanobiotechnol. 2016, 11, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Farias, C.B.B.; Silva, A.F.; Rufino, R.D.; Luna, J.M.; Souza, J.E.G.; Sarubbo, L.A. Synthesis of silver nanoparticles using a biosurfactant produced in low-cost medium as stabilizing agent. Electron. J. Biotechnol. 2014, 17, 122–125. [Google Scholar] [CrossRef] [Green Version]
- Elgorban, A.M.; Al-Rahmah, A.N.; Sayed, S.R.; Hirad, A.; Mostafa, A.A.-F.; Bahkali, A.H. Antimicrobial activity and green synthesis of silver nanoparticles using Trichoderma viride. Biotechnol. Biotechnol. Equip. 2016, 30, 299–304. [Google Scholar] [CrossRef]
- Mashwani, Z.R.; Khan, T.; Khan, M.A.; Nadhman, A. Synthesis in plants and plant extracts of silver nanoparticles with potent antimicrobial properties: Current status and future prospects. Appl. Microbiol. Biotechnol. 2015, 99, 9923–9934. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Abbasi, B.H.; Younas, M.; Ahmad, W.; Khan, T. A review of the green syntheses and anti-microbial applications of gold nanoparticles. Green Chem. Lett. Rev. 2017, 10, 216–227. [Google Scholar] [CrossRef] [Green Version]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.K.; Poinern, G.E.J. Green Synthesis of Metallic Nanoparticles via Biological Entities. Materials 2015, 8, 7278–7308. [Google Scholar] [CrossRef] [Green Version]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chemistry 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Gowda, R.; Jones, N.R.; Banerjee, S.; Robertson, G.P. Use of Nanotechnology to Develop Multi-Drug Inhibitors For Cancer Therapy. J. Nanomed. Nanotechnol. 2014, 4, 6. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Zaka, M.; Hashmi, S.S.; Khan, Z. Biogenic synthesis of Au, Ag and Au–Ag alloy nanoparticles using Cannabis sativa leaf extract. IET Nanobiotechnol. 2017, 12, 277–284. [Google Scholar] [CrossRef]
- Ankamwar, B.; Damle, C.; Ahmad, A.; Sastry, M. Biosynthesis of gold and silver nanoparticles using Emblics officinalis fruit extract and their phase transfer and transmetallation in an organic solution. J. Nanosci. Nanotechnol. 2005, 5, 1665–1671. [Google Scholar] [CrossRef]
- Siddiquah, A.; Hashmi, S.S.; Mushtaq, S.; Renouard, S.; Blondeau, J.P.; Abbasi, R.; Hano, C.; Abbasi, B.H. Exploiting in vitro potential and characterization of surface modified Zinc Oxide nanoparticles of Isodon rugosus extract: Their clinical potential towards HepG2 cell line and human pathogenic bacteria. EXCLI J. 2018, 17, 671–687. [Google Scholar]
- Pedro, A.C.; Moreira, F.; Granato, D.; Rosso, N.D. Extraction of bioactive compounds and free radical scavenging activity of purple basil (Ocimum basilicum L.) leaf extracts as affected by temperature and time. Ann. Braz. Acad. Sci. 2017, 88, 1055–1068. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Sherazi, S.T.H.; Przybylski, R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef]
- Phippen, W.B.; Simon, J.E. Anthocyanins in basil (Ocimum basilicum L.). J. Agric. Food Chem. 1998, 46, 1734–1738. [Google Scholar] [CrossRef]
- Grotewold, E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant. Biol. 2006, 57, 761–780. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Anthocyanins and other flavonoids. Nat. Prod. Rep. 1998, 15, 631–652. [Google Scholar] [CrossRef]
- Stafford, H.A. Flavonoid evolution: An enzymic approach. Plant. Physiol. 1991, 96, 680–685. [Google Scholar] [CrossRef]
- Bowen-Forbes, C.S.; Zhang, Y.; Nair, M.G. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J. Food Compos. Anal. 2010, 23, 554–560. [Google Scholar] [CrossRef]
- Nazir, M.; Tungmunnithum, D.; Bose, S.; Drouet, S.; Garros, L.; Giglioli-Guivarc’h, N.; Abbasi, B.H.; Hano, C. Differential production of phenylpropanoid metabolites in callus cultures of Ocimum basilicum L. with distinct in vitro antioxidant activities and in vivo protective effects against UV stress. J. Agric. Food Chem. 2019, 67, 7. [Google Scholar] [CrossRef]
- Rodriguez-Saona, L.E.; Wrolstad, R.E. Extraction, isolation, and purification of anthocyanins. Curr. Protoc. Food Anal. Chem. 2001. [Google Scholar] [CrossRef]
- Strack, D.; Wray, V. Anthocyanins. In Methods in plant Biochemistry; Harborne, J.B., Ed.; Academic Press: Amsterdam, Netherlands, 1989; Volume 1, pp. 325–356. [Google Scholar]
- Lopez, R.; Aznar, M.; Cacho, J.; Ferreira, V. Determination of minor and trace volatile compounds in wine by solid-phase extraction and gas chromatography with mass spectrometric detection. J. Chromatogr. A 2002, 966, 167–177. [Google Scholar] [CrossRef]
- Mozetič, B.; Trebše, P.; Hribar, J. Determination and quantitation of anthocyanins and hydroxycinnamic acids in different cultivars of sweet cherries (Prunus avium L.) from Nova Gorica region (Slovenia). Food Technol. Biotechnol. 2002, 40, 207–212. [Google Scholar]
- Priyadharshini, R.I.; Prasannaraj, G.; Geetha, N.; Venkatachalam, P. Microwave-mediated extracellular synthesis of metallic silver and zinc oxide nanoparticles using macro-algae (Gracilaria edulis) extracts and its anticancer activity against human PC3 Cell Lines. Appl. Biochem. Biotechnol. 2014, 174, 2777–2790. [Google Scholar] [CrossRef]
- Riaz, H.R.; Hashmi, S.S.; Khan, T.; Hano, C.; Giglioli-Guivarc’h, N.; Abbasi, B.H. Melatonin-stimulated biosynthesis of anti-microbial ZnONPs by enhancing bio-reductive prospective in callus cultures of Catharanthus roseus var. Alba. Artif. Cells Nanomed. biotechnol. 2018, 46. [Google Scholar] [CrossRef]
- Philip, D.; Unni, C.; Aromal, S.A.; Vidhu, V.K. Murraya koenigii leaf-assisted rapid green synthesis of silver and gold nanoparticles. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2011, 78, 899–904. [Google Scholar] [CrossRef]
- Banerjee, P.; Satapathy, M.; Mukhopahayay, A.; Das, P. Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: Synthesis, characterization, antimicrobial property and toxicity analysis. Bioresour. Bioprocess. 2014, 1, 3. [Google Scholar] [CrossRef]
- Siddiqui, B.S.; Afshan, F.; Faizi, S.; Naqvi, S.N.H.; Tariq, R.M. Two insecticidal tetranortriterpenoids from Azadirachta indica. Phytochemistry 2000, 53, 371–376. [Google Scholar] [CrossRef]
- Huang, J.; Li, Q.; Sun, D.; Lu, Y.; Su, Y.; Yang, X.; Wang, H.; Wang, Y.; Shao, W.; He, N.; et al. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology 2007, 18, 105104. [Google Scholar]
- Rajski, Ł.; Lozano, A.; Uclés, A.; Ferrer, C.; Fernández-Alba, A.R. Determination of pesticide residues in high oil vegetal commodities by using various multi-residue methods and clean-ups followed by liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2013, 1304, 109–120. [Google Scholar] [CrossRef]
- Molins-Delgado, D.; García-Sillero, D.; Díaz-Cruz, M.S.; Barceló, D. On-line solid phase extraction-liquid chromatography-tandem mass spectrometry for insect repellent residue analysis in surface waters using atmospheric pressure photoionization. J. Chromatogr. A 2018, 1544, 33–40. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Wang, Y.; Wei, Y. Synthesis and application of surface-imprinted activated carbon sorbent for solid-phase extraction and determination of copper (II). Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2014, 117, 422–427. [Google Scholar] [CrossRef]
- Khorasani, R.; DindarlooInaloo, K.; Heidari, M.; Behbahani, M.; Rahmanian, O. Application of solvent-assisted dispersive solid phase extraction combined with flame atomic absorption spectroscopy for the determination of trace amounts of Cadmium. Hormozgan Med. J. 2017, 20, 355–364. [Google Scholar] [CrossRef]
- Dehaghi, S.M.; Rahmanifar, B.; Moradi, A.M.; Azar, P.A. Removal of permethrin pesticide from water by chitosan–zinc oxide nanoparticles composite as an adsorbent. J. Saudi Chem. Soc. 2014, 18, 348–355. [Google Scholar] [CrossRef]
- Badawy, M.E.; El-Nouby, M.A.; Marei, A.E.S.M. Development of a Solid-Phase Extraction (SPE) Cartridge Based on Chitosan-Metal Oxide Nanoparticles (Ch-MO NPs) for Extraction of Pesticides from Water and Determination by HPLC. Int. J. Anal. Chem. 2018. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.S.; Shaheen, M.S.; El-Nekeety, A.A.; Abdel-Wahhab, M.A. Antioxidant and antibacterial activity of silver nanoparticles biosynthesized using Chenopodium murale leaf extract. J. Saudi Chem. Soc. 2014, 18, 356–363. [Google Scholar] [CrossRef]
- Demirbas, A.; Yilmaz, V.; Ildiz, N.; Baldemir, A.; Ocsoy, I. Anthocyanins-rich berry extracts directed formation of Ag NPs with the investigation of their antioxidant and antimicrobial activities. J. Mol. Liq. 2017, 248, 1044–1049. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Anthocyanins and other flavonoids. Nat. Prod. Rep. 1995, 7, 639–657. [Google Scholar] [CrossRef]
- Massarweh, N.N.; El-Serag, H.B. Epidemiology of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Cancer Control. 2017. [CrossRef]
- Sriram, M.I.; Kanth, S.B.M.; Kalishwaralal, K.; Gurunathan, S. Antitumor activity of silver nanoparticles in Dalton’s lymphoma ascites tumor model. Int. J. Nanomed. 2017, 5, 753. [Google Scholar]
- Jacob, S.J.P.; Finub, J.S.; Narayanan, A. Synthesis of silver nanoparticles using Piper longum leaf extracts and its cytotoxic activity against Hep-2 cell line. Colloids Surf. B Biointerfaces 2012, 91, 212–214. [Google Scholar] [CrossRef]
- Krishnan, V.; Bupesh, G.; Manikandan, E.; Thanigai, A.K.; Magesh, S.; Kalyanaraman, R.; Maaza, M. Green synthesis of silver nanoparticles using Piper nigrum concoction and its anticancer activity against MCF-7 and Hep-2 cell lines. J. Antimicrob. Agents 2016, 2, 123–128. [Google Scholar]
- Berrington, D.; Lall, N. Anticancer activity of certain herbs and spices on the cervical epithelial carcinoma (HeLa) cell line. Evid. Based Complement. Altern. Med. 2012. [Google Scholar] [CrossRef]





| Phenylpropanoid Metabolites (µg/g) | BC-AgNPs | AE-AgNPs | CM-AgNPs |
|---|---|---|---|
| Caffeic acid | 3.31 ± 0.01 | nd | nd |
| Chicoric acid | 68.64 ± 2.05 | nd | nd |
| Rosmarinic acid | 235.35 ± 4.55 | nd | nd |
| Cyanidin | 0.47 ± 0.006 | 1.56 ± 0.074 | nd |
| Caffeic acid | 3.31 ± 0.01 | 0.85 ± 0.0019 | nd |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbasi, B.H.; Nazir, M.; Muhammad, W.; Hashmi, S.S.; Abbasi, R.; Rahman, L.; Hano, C. A Comparative Evaluation of the Antiproliferative Activity against HepG2 Liver Carcinoma Cells of Plant-Derived Silver Nanoparticles from Basil Extracts with Contrasting Anthocyanin Contents. Biomolecules 2019, 9, 320. https://doi.org/10.3390/biom9080320
Abbasi BH, Nazir M, Muhammad W, Hashmi SS, Abbasi R, Rahman L, Hano C. A Comparative Evaluation of the Antiproliferative Activity against HepG2 Liver Carcinoma Cells of Plant-Derived Silver Nanoparticles from Basil Extracts with Contrasting Anthocyanin Contents. Biomolecules. 2019; 9(8):320. https://doi.org/10.3390/biom9080320
Chicago/Turabian StyleAbbasi, Bilal Haider, Munazza Nazir, Wali Muhammad, Syed Salman Hashmi, Rashda Abbasi, Lubna Rahman, and Christophe Hano. 2019. "A Comparative Evaluation of the Antiproliferative Activity against HepG2 Liver Carcinoma Cells of Plant-Derived Silver Nanoparticles from Basil Extracts with Contrasting Anthocyanin Contents" Biomolecules 9, no. 8: 320. https://doi.org/10.3390/biom9080320
APA StyleAbbasi, B. H., Nazir, M., Muhammad, W., Hashmi, S. S., Abbasi, R., Rahman, L., & Hano, C. (2019). A Comparative Evaluation of the Antiproliferative Activity against HepG2 Liver Carcinoma Cells of Plant-Derived Silver Nanoparticles from Basil Extracts with Contrasting Anthocyanin Contents. Biomolecules, 9(8), 320. https://doi.org/10.3390/biom9080320