Hydrophilic Interaction Liquid Chromatography Coupled to Mass Spectrometry and Multivariate Analysis of the De Novo Pyrimidine Pathway Metabolites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Apparatus
2.3. Stock Solution Preparation
2.4. Sample Preparation
2.4.1. Tomato Plants
2.4.2. Phytophthora infestans Strain
2.4.3. Plant Inoculation
2.5. Multivariate Design
2.6. Analytical Parameters
3. Results and Discussion
3.1. Factorial Fractional Designs
3.2. Box-Behnken Design and Central Composite Design
3.3. Linearity, Limits of Detection (LOD) and Limits of Quantification (LOQ)
3.4. Matrix Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Stasolla, C.; Katahira, R.; Thorpe, T.A.; Ashihara, H. Purine and pyrimidine nucleotide metabolism in higher plants. J. Plant Physiol. 2003, 160, 1271–1295. [Google Scholar] [CrossRef] [PubMed]
- García-Bayona, L.; Garavito, M.F.; Lozano, G.L.; Vasquez, J.J.; Myers, K.; Fry, W.E.; Bernal, A.; Zimmermann, B.H.; Restrepo, S. De novo pyrimidine biosynthesis in the oomycete plant pathogen Phytophthora infestans. Gene 2014, 537, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Jupe, J.; Stam, R.; Howden, A.J.; Morris, J.A.; Zhang, R.; Hedley, P.E.; Huitema, E. Phytophthora capsici-tomato interaction features dramatic shifts in gene expression associated with a hemi-biotrophic lifestyle. Genome Biol. 2013, 14. [Google Scholar] [CrossRef] [PubMed]
- Marrubini, G.; Mendoza, B.E.C.; Massolini, G. Separation of purine and pyrimidine bases and nucleosides by hydrophilic interaction chromatography. J. Sep. Sci. 2010, 33, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Seifar, R.M.; Ras, C.; Dam, J.C.; Gulik, W.M.; Heijnen, J.J.; Winden, W.A. Simultaneous quantification of free nucleotides in complex biological samples using ion pair reversed phase liquid chromatography isotope dilution tandem mass spectrometry. Anal. Biochem. 2009, 388, 213–219. [Google Scholar] [CrossRef]
- Mateos-Vivas, M.; Rodríguez-Gonzalo, E.; García-Gómez, D.; Carabias-Martínez, R. Hydrophilic interaction chromatography coupled to tandem mass spectrometry in the presence of hydrophilic ion-pairing reagents for the separation of nucleosides and nucleotide mono-, di- and triphosphates. J. Chromatogr. 2015, 1414, 129–137. [Google Scholar] [CrossRef]
- Vela, J.E.; Olson, L.Y.; Huang, A.; Fridland, A.; Ray, A.S. Simultaneous quantitation of the nucleotide analog adefovir, its phosphorylated anabolites and 2′-deoxyadenosine triphosphate by ion-pairing LC/MS/MS. J. Chromatogr. 2007, 848, 335–343. [Google Scholar] [CrossRef]
- Ikegami, T.; Tomomatsu, K.; Takubo, H.; Horie, K.; Tanaka, N. Separation efficiencies in hydrophilic interaction chromatography. J. Chromatogr. 2008, 1184, 474–503. [Google Scholar] [CrossRef]
- Buszewski, B.; Noga, S. Hydrophilic interaction liquid chromatography (HILIC)—A powerful separation technique. Anal. Bioanal. Chem. 2012, 402, 231–247. [Google Scholar] [CrossRef]
- Guo, Y.; Gaiki, S. Retention behavior of small polar compounds on polar stationary phases in hydrophilic interaction chromatography. J. Chromatogr. 2005, 1074, 71–80. [Google Scholar] [CrossRef]
- Li, R.; Huang, J. Chromatographic behavior of epirubicin and its analogues on high-purity silica in hydrophilic interaction chromatography. J. Chromatogr. 2004, 1041, 163–169. [Google Scholar] [CrossRef]
- Nguyen, H.P.; Schug, K.A. The advantages of ESI-MS detection in conjunction with HILIC mode separations: Fundamentals and applications. J. Sep. Sci. 2008, 31, 1465–1480. [Google Scholar] [CrossRef]
- Naidong, W. Bioanalytical liquid chromatography tandem mass spectrometry methods on underivatized silica columns with aqueous/organic mobile phases. J. Chromatogr. 2003, 796, 209–224. [Google Scholar] [CrossRef]
- Hemström, P.; Irgum, K. Hydrophilic interaction chromatography. J. Sep. Sci. 2006, 29, 1784–1821. [Google Scholar] [CrossRef]
- Alpert, A.J. Hydrophilic-interaction chromatography for the separation of peptides, nucleic acids and other polar compounds. J. Chromatogr. 1990, 499, 177–196. [Google Scholar] [CrossRef]
- McCalley, D.V. Is hydrophilic interaction chromatography with silica columns a viable alternative to reversed-phase liquid chromatography for the analysis of ionisable compounds? J. Chromatogr. 2007, 1171, 46–55. [Google Scholar] [CrossRef]
- García-Gómez, D.; Rodríguez-Gonzalo, E.; Carabias-Martínez, R. Stationary phases for separation of nucleosides and nucleotides by hydrophilic interaction liquid chromatography. TrAC Trends Anal. Chem. 2013, 47, 111–128. [Google Scholar] [CrossRef]
- Jandera, P.; Janás, P. Recent advances in stationary phases and understanding of retention in hydrophilic interaction chromatography. A review. Anal. Chim. Acta 2017, 967, 12–32. [Google Scholar] [CrossRef]
- Jandera, P.; Hájek, T. Mobile phase effects on the retention on polar columns with special attention to the dual hydrophilic interaction-reversed-phase liquid chromatography mechanism, a review. J. Sep. Sci. 2018, 41, 145–162. [Google Scholar] [CrossRef]
- Marrubini, G.; Appelblad, P.; Maietta, M.; Papetti, A. Hydrophilic interaction chromatography in food matrices analysis: An updated review. Food Chem. 2018, 257, 53–66. [Google Scholar] [CrossRef]
- Taraji, M.; Haddad, P.R.; Amos, R.I.J.; Talebi, M.; Szucs, R.; Dolan, J.W.; Pohl, C.A. Chemometric-assisted method development in hydrophilic interaction liquid chromatography: A review. Anal. Chim. Acta 2018, 1000, 20–40. [Google Scholar] [CrossRef]
- Hibbert, D.B. Experimental design in chromatography: A tutorial review. J. Chromatogr. 2012, 910, 2–13. [Google Scholar] [CrossRef]
- Lopes, W.A.; Rocha, G.O.; Pereira, P.A.; Oliveira, F.S.; Carvalho, L.S.; Bahia, N.; Conceição, L.; Andrade, J.B. Multivariate optimization of a GC–MS method for determination of sixteen priority polycyclic aromatic hydrocarbons in environmental samples. J. Sep. Sci. 2008, 31, 1787–1796. [Google Scholar] [CrossRef]
- Fry, W. Phytophthora infestans: The plant (and R gene) destroyer. Mol. Plant Pathol. 2008, 9, 385–402. [Google Scholar] [CrossRef]
- Vargas, A.M.; Ocampo, L.M.Q.; Céspedes, M.C.; Carreño, N.; González, A.; Rojas, A.; Zuluaga, A.P.; Myers, K.; Fry, W.E.; Jiménez, P.; et al. Characterization of Phytophthora infestans Populations in Colombia: First Report of the A2 Mating Type. Phytopathology 2009, 99, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Laurindo, B.S.; Laurindo, R.D.F.; Fontes, P.P.; Vital, C.E.; Delazari, F.T.; Baracat-Pereira, M.C.; Silva, D.J.H. Comparative analysis of constitutive proteome between resistant and susceptible tomato genotypes regarding to late blight. Funct. Int. Genom. 2018, 18, 11–21. [Google Scholar] [CrossRef]
- Nick, C.; Aguilera, J.G.; Laurindo, B.S.; Almeida, V.S.; Freitas, R.D.; Cruz, C.D.; Silva, D.J.H. Genetic Parameters and Correlation between Tomato Late Blight Field Resistance and Fruit Quality. Am. J. Plant Sci. 2014, 5, 2418–2425. [Google Scholar] [CrossRef] [Green Version]
- Ko, W. Hormonal Heterothallism and Homothallism in Phytophthora. Annu. Rev. Phytopathol. 1988, 26, 57–73. [Google Scholar] [CrossRef]
- Garcia, G.P.; Santos, F.; Zanotta, S.; Eberlin, M.; Carazzone, C. Metabolomics of Solanum lycopersicum Infected with Phytophthora infestans Leads to Early Detection of Late Blight in Asymptomatic Plants. Molecules 2018, 23, 3330. [Google Scholar] [CrossRef]
- Arase, S.; Kimura, S.; Ikegami, T. Method optimization of hydrophilic interaction chromatography separation of nucleotides using design of experiment approaches I: Comparison of several zwitterionic columns. J. Pharm. Biomed. Anal. 2018, 158, 307–316. [Google Scholar] [CrossRef]
- Padivitage, N.L.T.; Dissanayake, M.K.; Armstrong, D.W. Separation of nucleotides by hydrophilic interaction chromatography using the FRULIC-N column. Anal. Bioanal. Chem. 2013, 405, 8837–8848. [Google Scholar] [CrossRef]
- Wu, J.; Bicker, W.; Lindner, W. Separation properties of novel and commercial polar stationary phases in hydrophilic interaction and reversed-phase liquid chromatography mode. J. Sep. Sci. 2008, 31, 1492–1503. [Google Scholar] [CrossRef]
- Kawachi, Y.; Ikegami, T.; Takubo, H.; Ikegami, Y.; Miyamoto, M.; Tanaka, N. Chromatographic characterization of hydrophilic interaction liquid chromatography stationary phases: Hydrophilicity, charge effects, structural selectivity, and separation efficiency. J. Chromatogr. 2011, 1218, 5903–5919. [Google Scholar] [CrossRef]
- Johnsen, E.; Wilson, S.R.; Odsbu, I.; Krapp, A.; Malerod, H.; Skarstad, K.; Lundanes, E. Hydrophilic interaction chromatography of nucleoside triphosphates with temperature as a separation parameter. J. Chromatogr. 2011, 1218, 5981–5986. [Google Scholar] [CrossRef] [Green Version]
- Alpert, A.J. Electrostatic repulsion hydrophilic interaction chromatography for isocratic separation of charged solutes and selective isolation of phosphopeptides. Anal. Chem. 2008, 80, 62–76. [Google Scholar] [CrossRef]
- Louw, S.; Lynen, F.; Hanna-Brown, M.; Sandra, P. High-efficiency hydrophilic interaction chromatography by coupling 25 cm × 4.6mm ID × 5 microm silica columns and operation at 80 degrees C. J. Chromatogr. 2010, 1217, 514–521. [Google Scholar] [CrossRef]
- McNeff, C.V.; Yan, B.; Stoll, D.R.; Henry, R.A. Practice and theory of high temperature liquid chromatography. J. Sep. Sci. 2007, 30, 1672–1685. [Google Scholar] [CrossRef]
- Hatsis, P.; Lucy, C.A. Effect of temperature on retention and selectivity in ion chromatography of anions. J. Chromatogr. 2001, 920, 3–11. [Google Scholar] [CrossRef]
- Fountain, K.J.; Xu, J.; Diehl, D.M.; Morrison, D. Influence of stationary phase chemistry and mobile-phase composition on retention, selectivity, and MS response in hydrophilic interaction chromatography. J. Sep. Sci. 2010, 33, 740–751. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, C.; Ding, X.; Yuan, D.; Chen, K.; Gao, B.; Chen, Y.; Sun, A. Simultaneous determination of adenine nucleotides, creatine phosphate and creatine in rat liver by high performance liquid chromatography–electrospray ionization-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2012, 66, 258–263. [Google Scholar] [CrossRef]
- Bajad, S.U.; Lu, W.; Kimball, E.H.; Yuan, J.; Peterson, C.; Rabinowitz, J.D. Separation and quantitation of water soluble cellular metabolites by hydrophilic interaction chromatography-tandem mass spectrometry. J. Chromatogr. 2006, 1125, 76–88. [Google Scholar] [CrossRef]
- Teleki, A.; Sanchez-Kopper, A.; Takors, R. Alkaline conditions in hydrophilic interaction liquid chromatography for intracellular metabolite quantification using tandem mass spectrometry. Anal. Biochem. 2015, 475, 4–13. [Google Scholar] [CrossRef]
- Gika, H.G.; Theodoridis, G.A.; Vrhovsek, U.; Mattivi, F. Quantitative profiling of polar primary metabolites using hydrophilic interaction ultrahigh performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. 2012, 1259, 121–127. [Google Scholar] [CrossRef]
- Gallart-Ayala, H.; Konz, I.; Mehl, F.; Teav, T.; Oikonomidi, A.; Peyratout, G.; Velpen, V.; Popp, J.; Ivanisevic, J. Global HILIC-MS approach to measure polar human cerebrospinal fluid metabolome: Exploring gender-associated variation in a cohort of elderly cognitively healthy subjects. Anal. Chim. Acta 2018, 1037, 327–337. [Google Scholar] [CrossRef]


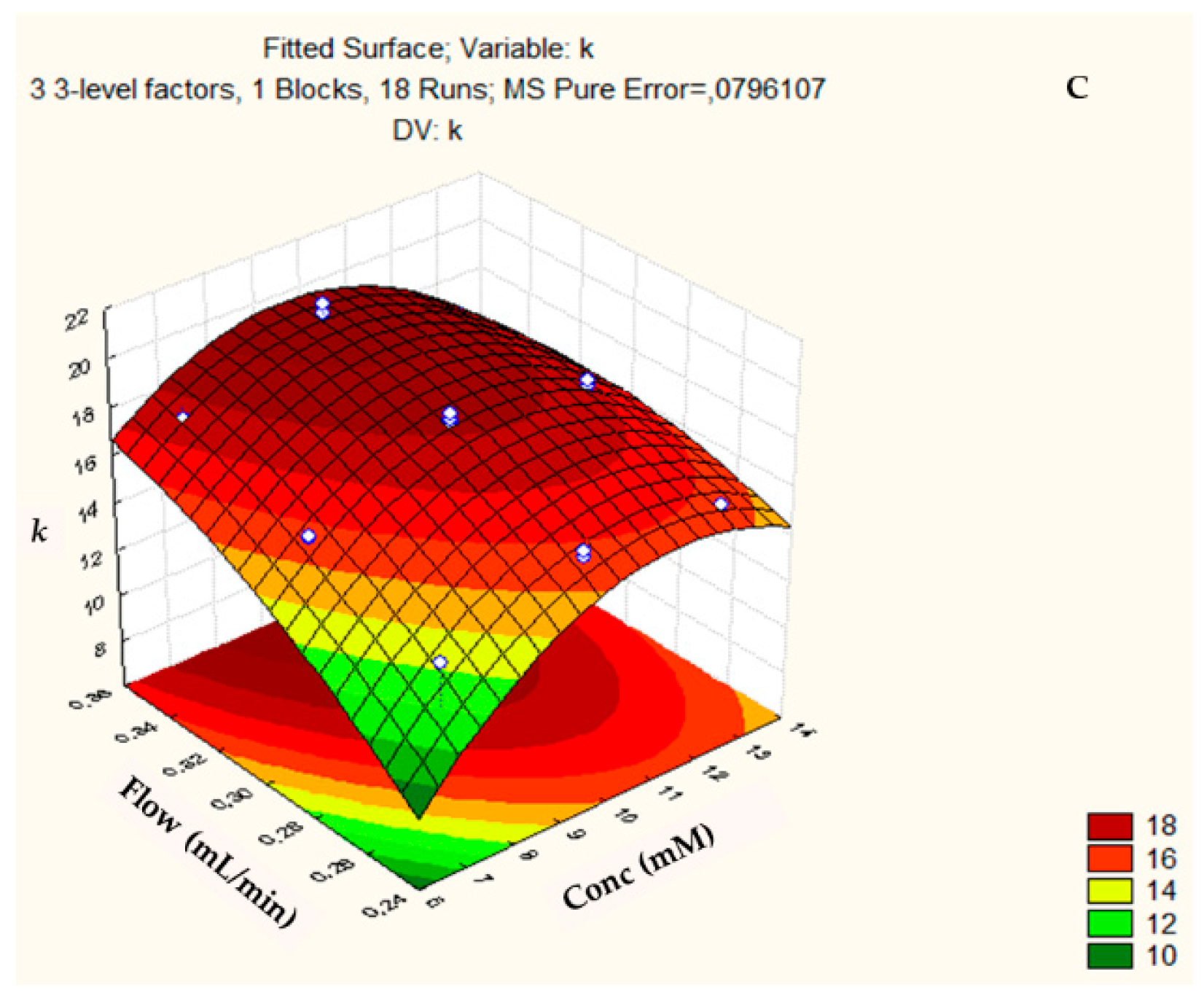


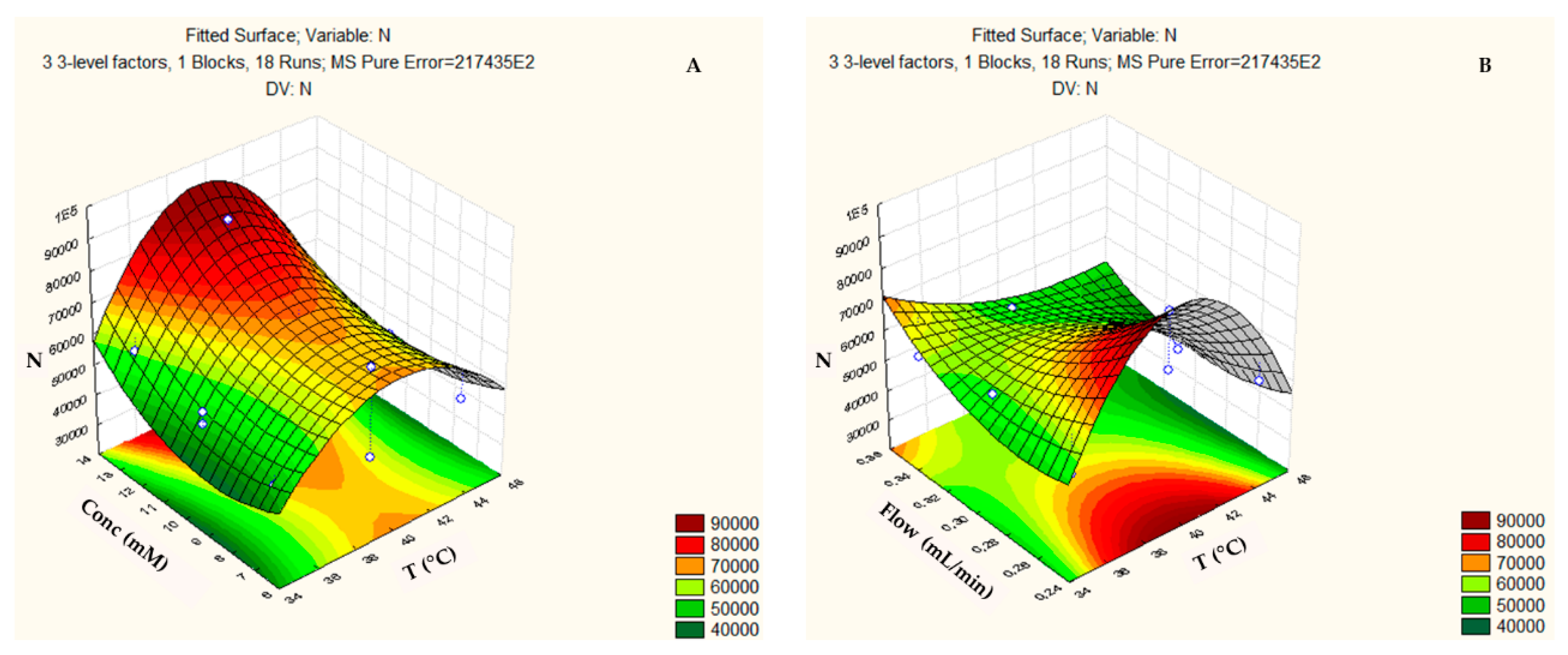

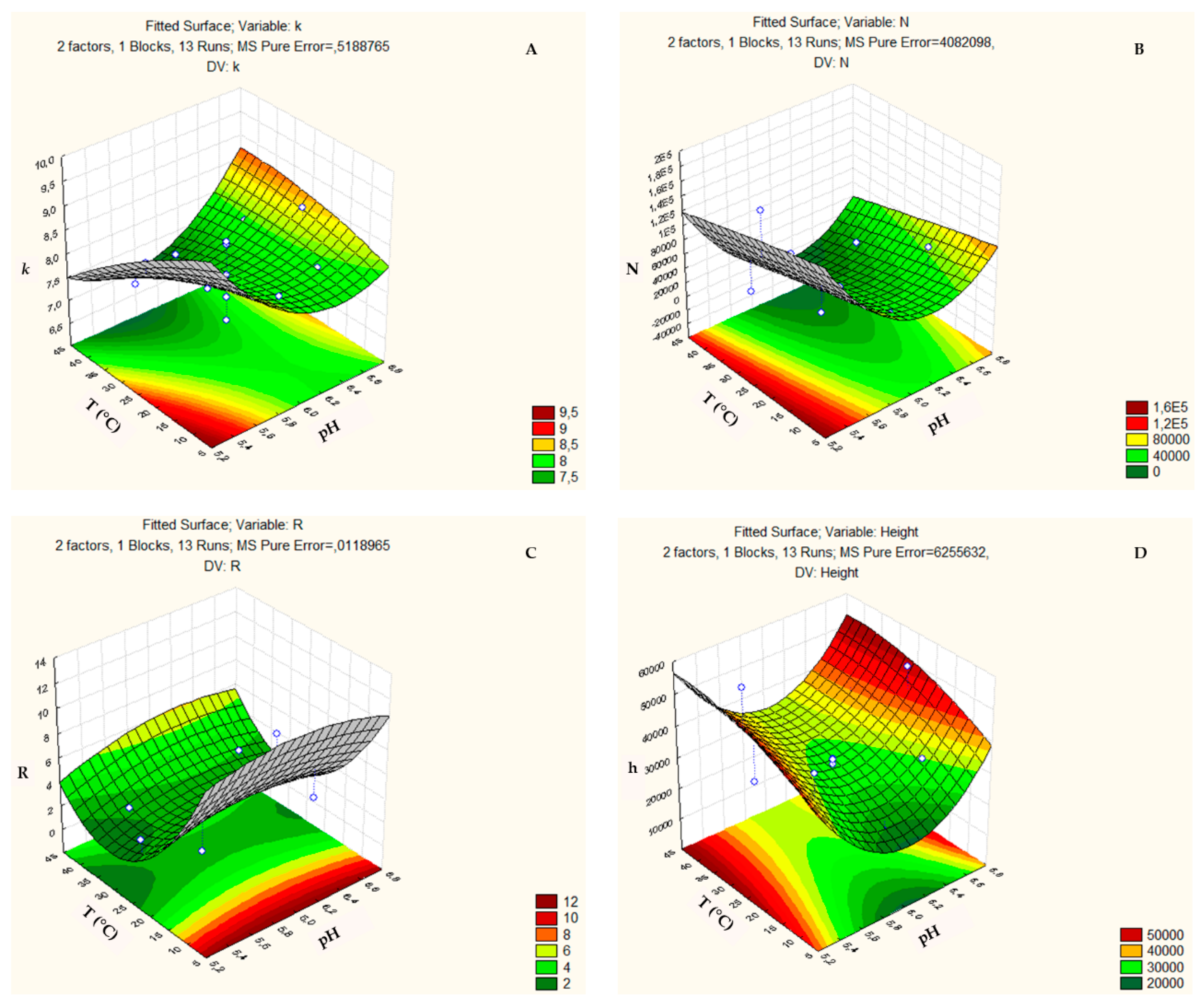
| COLUMN (Design) | FACTOR (Abbreviation) | Minimum Level (−) | Medium Level (0) | Maximum Level (+) |
|---|---|---|---|---|
| ZIC-p-HILIC (Factorial #1) | Temperature in °C (T) | 20 | 30 | 40 |
| Flow in mL min−1 (Flow) | 0.15 | 0.2 | 0.25 | |
| Additive concentration in mM (Conc) | 5.0 | 7.5 | 10 | |
| Gradient rate (Grad) | 2 | 3 | 4 | |
| pH | 4 | 5.5 | 7.0 | |
| ZIC-HILIC (Factorial #2) | Temperature in °C (T) | 25 | 40 | 55 |
| Flow in mL min−1 (Flow) | 0.15 | 0.2 | 0.25 | |
| Additive concentration in mM (Conc) | 5 | 7.5 | 10 | |
| Gradient rate (Grad) | 2 | 3 | 4 | |
| pH | 3.0 | 4.5 | 6.0 |
| COLUMN (Design) | FACTOR (Abbreviation) | Minimum Level (−) | Medium Level (0) | Maximum Level (+) |
|---|---|---|---|---|
| ZIC-p-HILIC (Box-Behnken) | Temperature in °C (T) | 35 | 40 | 45 |
| Flow in mL min−1 (Flow) | 0.25 | 0.3 | 0.35 | |
| Concentration in mM (Conc) | 7.0 | 10 | 13 | |
| ZIC-HILIC (Central composite) | Temperature in °C (T) | 15 | 20 | 35 |
| pH | 5.5 | 6.0 | 6.6 |
| Analyte Concentration “Medium” (ng mL−1) | ng Analyte/mg of Tomato Leave Extract a | ng Analyte/mg of Tomato Leave Extract b |
|---|---|---|
| Aspartic acid (9000) | 12,874.8 ± 219 | 11,265.5 ± 113 |
| Glutamine (9000) | 41,446.4 ± 630 | 40,882.4 ± 143 |
| Uracil (2000) | 11,598.6 ± 157 | 3202.3 ± 41 |
| Uridine (2000) | 24,078.3 ± 48 | 25,122.6 ± 201 |
| Cytidine (3000) | 8355.3 ± 74 | 4265.1 ± 189 |
| UMP (4500) | 2168.6 ± 53 | ND |
| UDP-Glu (3000) | 5720.7 ± 71 | ND |
| UTP (16,000) | ND | ND |
| ATP (16,000) | ND | ND |
| CTP (16,000) | ND | ND |
| Carbamoyl aspartic acid (9000) | 20,481.4 ± 190 | ND |
| Orotic acid (2000) | 387.1 ± 76 | ND |
| DHO (2000) | 4886.5 ± 84 | 1024.7 ± 18 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galeano Garcia, P.; Zimmermann, B.H.; Carazzone, C. Hydrophilic Interaction Liquid Chromatography Coupled to Mass Spectrometry and Multivariate Analysis of the De Novo Pyrimidine Pathway Metabolites. Biomolecules 2019, 9, 328. https://doi.org/10.3390/biom9080328
Galeano Garcia P, Zimmermann BH, Carazzone C. Hydrophilic Interaction Liquid Chromatography Coupled to Mass Spectrometry and Multivariate Analysis of the De Novo Pyrimidine Pathway Metabolites. Biomolecules. 2019; 9(8):328. https://doi.org/10.3390/biom9080328
Chicago/Turabian StyleGaleano Garcia, Paula, Barbara H. Zimmermann, and Chiara Carazzone. 2019. "Hydrophilic Interaction Liquid Chromatography Coupled to Mass Spectrometry and Multivariate Analysis of the De Novo Pyrimidine Pathway Metabolites" Biomolecules 9, no. 8: 328. https://doi.org/10.3390/biom9080328
APA StyleGaleano Garcia, P., Zimmermann, B. H., & Carazzone, C. (2019). Hydrophilic Interaction Liquid Chromatography Coupled to Mass Spectrometry and Multivariate Analysis of the De Novo Pyrimidine Pathway Metabolites. Biomolecules, 9(8), 328. https://doi.org/10.3390/biom9080328





