Lignin and Cellulose Blends as Pharmaceutical Excipient for Tablet Manufacturing via Direct Compression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Powder Characterisation
2.3. Tablet Manufacture
2.4. Tablet Characterisation
2.5. Dissolution Testing
2.6. LIG-Microcrystalline Cellulose Antioxidant Activity
2.7. Statistical Analysis
3. Results
3.1. Powder Characterisation
3.2. Tablet Morphology and Characterisation
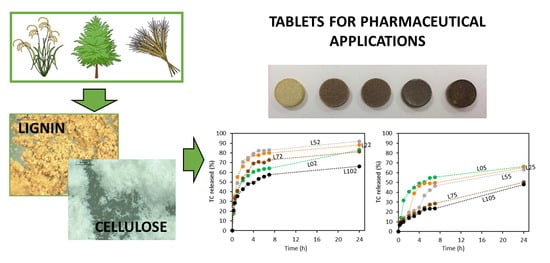
3.3. Tetracycline Release from LIG/MCC Tablets
3.4. Antioxidant Capabilities of LIG and MCC Blends
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mirani, A.G.; Patankar, S.P.; Borole, V.S.; Pawar, A.S.; Kadam, V.J. Direct Compression High Functionality Excipient using Coprocessing Technique: A Brief Review. Curr. Drug Deliv. 2011, 8, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.R.; Ibrahim, M.I.; Al-Haddad, M.S. The Influence of Consumers’ Preferences and Perceptions of Oral Solid Dosage Forms on their Treatment. Int. J. Clin. Pharm. 2012, 34, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Thoorens, G.; Krier, F.; Leclercq, B.; Carlin, B.; Evrard, B. Microcrystalline Cellulose, a Direct Compression Binder in a Quality by Design Environment—A Review. Int. J. Pharm. 2014, 473, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Jivraj, I.I.; Martini, L.G.; Thomson, C.M. An Overview of the Different Excipients Useful for the Direct Compression of Tablets. Pharm. Sci. Technol. Today 2000, 3, 58–63. [Google Scholar] [CrossRef]
- Beneke, C.E.; Viljoen, A.M.; Hamman, J.H. Polymeric Plant-Derived Excipients in Drug Delivery. Molecules 2009, 14, 2602–2620. [Google Scholar] [CrossRef]
- Larrañeta, E.; Martínez-Ohárriz, C.; Vélaz, I.; Zornoza, A.; Machín, R.; Isasi, J.R. In Vitro Release from Reverse Poloxamine/A-Cyclodextrin Matrices: Modelling and Comparison of Dissolution Profiles. J. Pharm. Sci. 2014, 103, 197–206. [Google Scholar] [CrossRef]
- Hsein, H.; Garrait, G.; Tamani, F.; Beyssac, E.; Hoffart, V. Denatured Whey Protein Powder as a New Matrix Excipient: Design and Evaluation of Mucoadhesive Tablets for Sustained Drug Release Applications. Pharm. Res. 2017, 34, 365–377. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Pandele, A.M.; Comanici, F.E.; Carp, C.A.; Miculescu, F.; Voicu, S.I.; Thakur, V.K.; Serban, B.C. Synthesis and Characterization of Cellulose Acetate-Hydroxyapatite Micro and Nano Composites Membranes for Water Purification and Biomedical Applications. Vacuum 2017, 146, 599–605. [Google Scholar] [CrossRef]
- Debotton, N.; Dahan, A. Applications of Polymers as Pharmaceutical Excipients in Solid Oral Dosage Forms. Med. Res. Rev. 2017, 37, 52–97. [Google Scholar] [CrossRef]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards Lignin-Based Functional Materials in a Sustainable World. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Savy, D.; Mazzei, P.; Drosos, M.; Cozzolino, V.; Lama, L.; Piccolo, A. Molecular Characterization of Extracts from Biorefinery Wastes and Evaluation of their Plant Biostimulation. ACS Sustain. Chem. Eng. 2017, 5, 9023–9031. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Karimi, S.; Iravani, S.; Varma, R.S. Plant-Derived Nanostructures: Types and Applications. Green Chem. 2016, 18, 20–52. [Google Scholar] [CrossRef]
- Turner, M.K. Pharmaceuticals from Agriculture: Manufacture of Discovery? Ind. Crops Prod. 1992, 1, 125–131. [Google Scholar] [CrossRef]
- Daudt, R.M.; Külkamp-Guerreiro, I.C.; Cladera-Olivera, F.; Thys, R.C.S.; Marczak, L.D.F. Determination of Properties of Pinhão Starch: Analysis of its Applicability as Pharmaceutical Excipient. Ind. Crops Prod. 2014, 52, 420–429. [Google Scholar] [CrossRef]
- Sharma, V.K.; Mazumdar, B. Feasibility and Characterization of Gummy Exudate of Cochlospermum Religiosum as Pharmaceutical Excipient. Ind. Crops Prod. 2013, 50, 776–786. [Google Scholar] [CrossRef]
- Wroblewska-Krepsztul, J.; Rydzkowski, T.; Michalska-Pozoga, I.; Thakur, V.K. Biopolymers for Biomedical and Pharmaceutical Applications: Recent Advances and Overview of Alginate Electrospinning. Nanomaterials 2019, 9, 404. [Google Scholar] [CrossRef]
- marketsandmarkets.com. Pharmaceutical Excipients Market by Type (Organic Chemical (Carbohydrate, Petrochemical), Inorganic Chemical), Functionality (Filler, Coating, Disintegrant, Binder), Formulation (Tablet, Capsule, Topical, Parenteral)—Global Forecast to 2023. Research and Markets, 19 April 2018. [Google Scholar]
- Penkina, A.; Antikainen, O.; Hakola, M.; Vuorinen, S.; Repo, T.; Yliruusi, J.; Veski, P.; Kogermann, K.; Heinamaki, J. Direct Compression of Cellulose and Lignin Isolated by a New Catalytic Treatment. AAPS PharmSciTech 2013, 14, 1129–1136. [Google Scholar] [CrossRef] [Green Version]
- Saha, S.; Shahiwala, A.F. Multifunctional Coprocessed Excipients for Improved Tabletting Performance. Expert Opin. Drug Deliv. 2009, 6, 197–208. [Google Scholar] [CrossRef]
- Santos, R.B.; Capanema, E.A.; Balakshin, M.Y.; Chang, H.M.; Jameel, H. Lignin Structural Variation in Hardwood Species. J. Agric. Food Chem. 2012, 60, 4923–4930. [Google Scholar] [CrossRef]
- Gellerstedt, G. Softwood Kraft Lignin: Raw Material for the Future. Ind. Crops Prod. 2015, 77, 845–854. [Google Scholar] [CrossRef]
- Costa, S.; Rugiero, I.; Larenas Uria, C.; Pedrini, P.; Tamburini, E. Lignin Degradation Efficiency of Chemical Pre-Treatments on Banana Rachis Destined to Bioethanol Production. Biomolecules 2018, 8, 141. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Thakur, M.K. Recent Advances in Green Hydrogels from Lignin: A Review. Int. J. Biol. Macromol. 2015, 72, 834–847. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Espinosa, E.; Savy, D.; Rosal, A.; Rodríguez, A. Biorefinery Process Combining Specel® Process and Selective Lignin Precipitation using Mineral Acids. Bioresources 2016, 11, 7061–7077. [Google Scholar] [CrossRef]
- Liu, D.; Li, Y.; Qian, Y.; Xiao, Y.; Du, S.; Qiu, X. Synergistic Antioxidant Performance of Lignin and Quercetin Mixtures. ACS Sustain. Chem. Eng. 2017, 5, 8424–8428. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Tarrés, Q.; Delgado-Aguilar, M.; Rodríguez, A.; Espinach, F.X.; Mutjé, P. Approaching a New Generation of Fiberboards Taking Advantage of Self Lignin as Green Adhesive. Int. J. Biol. Macromol. 2018, 108, 927–935. [Google Scholar] [CrossRef]
- Greco, L.; Ullo, S.; Rigano, L.; Fontana, M.; Berardesca, E. Evaluation of the Soothing and Protective Properties of a Lignin Hydrolyzate. Cosmetics 2019, 6, 38. [Google Scholar] [CrossRef]
- Oliviero, M.; Verdolotti, L.; Di Maio, E.; Aurilia, M.; Iannace, S. Effect of Supramolecular Structures on Thermoplastic Zein-Lignin Bionanocomposites. J. Agric. Food Chem. 2011, 59, 10062–10070. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in Green Polymer Composites from Lignin for Multifunctional Applications: A Review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Nandiwale, K.Y.; Danby, A.M.; Ramanathan, A.; Chaudhari, R.V.; Subramaniam, B. Zirconium-Incorporated Mesoporous Silicates show Remarkable Lignin Depolymerization Activity. ACS Sustain. Chem. Eng. 2017, 5, 7155–7164. [Google Scholar] [CrossRef]
- Stewart, D. Lignin as a Base Material for Materials Applications: Chemistry, Application and Economics. Ind. Crops Prod. 2008, 27, 202–207. [Google Scholar] [CrossRef]
- Rencoret, J.; Prinsen, P.; Gutierrez, A.; Martinez, A.T.; Del Rio, J.C. Isolation and Structural Characterization of the Milled Wood Lignin, Dioxane Lignin, and Cellulolytic Lignin Preparations from Brewer’s Spent Grain. J. Agric. Food Chem. 2015, 63, 603–613. [Google Scholar] [CrossRef]
- Kai, D.; Low, Z.W.; Liow, S.; Abdul Karim, A.; Ye, H.; Jin, G.; Li, K.; Loh, X.J. Development of Lignin Supramolecular Hydrogels with Mechanically Responsive and Self-Healing Properties. ACS Sustain. Chem. Eng. 2015, 3, 2160–2169. [Google Scholar] [CrossRef]
- Dominguez-Robles, J.; Martin, N.K.; Fong, M.L.; Stewart, S.A.; Irwin, N.J.; Rial-Hermida, M.I.; Donnelly, R.F.; Larraneta, E. Antioxidant PLA Composites Containing Lignin for 3D Printing Applications: A Potential Material for Healthcare Applications. Pharmaceutics 2019, 11, 165. [Google Scholar] [CrossRef]
- Kai, D.; Zhang, K.; Jiang, L.; Wong, H.Z.; Li, Z.; Zhang, Z.; Loh, X.J. Sustainable and Antioxidant Lignin-Polyester Copolymers and Nanofibers for Potential Healthcare Applications. ACS Sustain. Chem. Eng. 2017, 5, 6016–6025. [Google Scholar] [CrossRef]
- Thakur, S.; Govender, P.P.; Mamo, M.A.; Tamulevicius, S.; Mishra, Y.K.; Thakur, V.K. Progress in Lignin Hydrogels and Nanocomposites for Water Purification: Future Perspectives. Vacuum 2017, 146, 342–355. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Peresin, M.S.; Tamminen, T.; Rodríguez, A.; Larrañeta, E.; Jääskeläinen, A.S. Lignin-Based Hydrogels with “super-Swelling” Capacities for Dye Removal. Int. J. Biol. Macromol. 2018, 115, 1249–1259. [Google Scholar] [CrossRef]
- Larrañeta, E.; Imízcoz, M.; Toh, J.X.; Irwin, N.J.; Ripolin, A.; Perminova, A.; Domíguez-Robles, J.; Rodríguez, A.; Donnelly, R.F. Synthesis and Characterization of Lignin Hydrogels for Potential Applications as Drug Eluting Antimicrobial Coatings for Medical Materials. ACS Sustain. Chem. Eng. 2018, 6, 9037–9046. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Kiriazis, A.; Hynninen, V.; Liu, Z.; Bauleth-Ramos, T.; Rahikkala, A.; Correia, A.; Kohout, T.; Sarmento, B.; et al. In Vitro Evaluation of Biodegradable Lignin-Based Nanoparticles for Drug Delivery and Enhanced Antiproliferation Effect in Cancer Cells. Biomaterials 2017, 121, 97–108. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Sánchez, R.; Díaz-Carrasco, P.; Espinosa, E.; García-Domínguez, M.T.; Rodríguez, A. Isolation and Characterization of Lignins from Wheat Straw: Application as Binder in Lithium Batteries. Int. J. Biol. Macromol. 2017, 104, 909–918. [Google Scholar] [CrossRef]
- Pishnamazi, M.; Casilagan, S.; Clancy, C.; Shirazian, S.; Iqbal, J.; Egan, D.; Edlin, C.; Croker, D.M.; Walker, G.M.; Collins, M.N. Microcrystalline Cellulose, Lactose and Lignin Blends: Process Mapping of Dry Granulation Via Roll Compaction. Powder Technol. 2019, 341, 38–50. [Google Scholar] [CrossRef]
- Pishnamazi, M.; Iqbal, J.; Shirazian, S.; Walker, G.M.; Collins, M.N. Effect of Lignin on the Release Rate of Acetylsalicylic Acid Tablets. Int. J. Biol. Macromol. 2019, 124, 354–359. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Tamminen, T.; Liitiä, T.; Peresin, M.S.; Rodríguez, A.; Jääskeläinen, A.S. Aqueous Acetone Fractionation of Kraft, Organosolv and Soda Lignins. Int. J. Biol. Macromol. 2018, 106, 979–987. [Google Scholar] [CrossRef]
- Ahuja, S.; Scypinski, S. Handbook of Modern Pharmaceutical Analysis; Elsevier Science: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Khomane, K.S.; More, P.K.; Raghavendra, G.; Bansal, A.K. Molecular Understanding of the Compaction Behavior of Indomethacin Polymorphs. Mol. Pharm. 2013, 10, 631–639. [Google Scholar] [CrossRef]
- Nordstrom, J.; Klevan, I.; Alderborn, G. A Particle Rearrangement Index Based on the Kawakita Powder Compression Equation. J. Pharm. Sci. 2009, 98, 1053–1063. [Google Scholar] [CrossRef] [Green Version]
- Newton, J.M.; Rowley, G.; Fell, J.T.; Peacock, D.G.; Ridgway, K. Computer Analysis of the Relation between Tablet Strength and Compaction Pressure. J. Pharm. Pharmacol. 1971, 23, 195S–201S. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Wells, T.; Le, R.K.; Sadeghifar, F.; Yuan, J.S.; Ragauskas, A.J. Fractionation of Organosolv Lignin using Acetone:Water and Properties of the obtained Fractions. ACS Sustain. Chem. Eng. 2017, 5, 580–587. [Google Scholar] [CrossRef]
- Santl, M.; Ilic, I.; Vrecer, F.; Baumgartner, S. A Compressibility and Compactibility Study of Real Tableting Mixtures: The Effect of Granule Particle Size. Acta Pharm. 2012, 62, 325–340. [Google Scholar] [CrossRef]
- Rojas, J.; Lopez, A.; Guisao, S.; Ortiz, C. Evaluation of several Microcrystalline Celluloses obtained from Agricultural by-Products. J. Adv. Pharm. Technol. Res. 2011, 2, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; de la Luz Reus-Medina, M.; Yang, D. Preparation, Characterization, and Tabletting Properties of a New Cellulose-Based Pharmaceutical Aid. Int. J. Pharm. 2002, 235, 129–140. [Google Scholar] [CrossRef]
- Shah, R.B.; Tawakkul, M.A.; Khan, M.A. Comparative Evaluation of Flow for Pharmaceutical Powders and Granules. AAPS PharmSciTech 2008, 9, 250–258. [Google Scholar] [CrossRef] [Green Version]
- Ardizzone, S.; Dioguardi, F.S.; Mussini, T.; Mussini, P.R.; Rondinini, S.; Vercelli, B.; Vertova, A. Microcrystalline Cellulose Powders: Structure, Surface Features and Water Sorption Capability. Cellulose 1999, 6, 57–69. [Google Scholar] [CrossRef]
- Hentzschel, C.M.; Sakmann, A.; Leopold, C.S. Comparison of Traditional and Novel Tableting Excipients: Physical and Compaction Properties. Pharm. Dev. Technol. 2012, 17, 649–653. [Google Scholar] [CrossRef]
- Asnaashari, S.; Khoei, N.S.; Zarrintan, M.H.; Adibkia, K.; Javadzadeh, Y. Preparation and Evaluation of Novel Metronidazole Sustained Release and Floating Matrix Tablets. Pharm. Dev. Technol. 2011, 16, 400–407. [Google Scholar] [CrossRef]
- Kitazawa, S.; Johno, I.; Ito, Y.; Teramura, S.; Okado, J. Effects of Hardness on the Disintegration Time and the Dissolution Rate of Uncoated Caffeine Tablets. J. Pharm. Pharmacol. 1975, 27, 765–770. [Google Scholar] [CrossRef]
- Notley, S.M.; Norgren, M. Surface Energy and Wettability of Spin-Coated Thin Films of Lignin Isolated from Wood. Langmuir 2010, 26, 5484–5490. [Google Scholar] [CrossRef]
- Tagami, A.; Gioia, C.; Lauberts, M.; Budnyak, T.; Moriana, R.; Lindström, M.E.; Sevastyanova, O. Solvent Fractionation of Softwood and Hardwood Kraft Lignins for More Efficient Uses: Compositional, Structural, Thermal, Antioxidant and Adsorption Properties. Ind. Crops Prod. 2019, 129, 123–134. [Google Scholar] [CrossRef]
- An, L.; Si, C.; Wang, G.; Sui, W.; Tao, Z. Enhancing the Solubility and Antioxidant Activity of High-Molecular-Weight Lignin by Moderate Depolymerization Via in Situ Ethanol/Acid Catalysis. Ind. Crops Prod. 2019, 128, 177–185. [Google Scholar] [CrossRef]
- Zhao, L.; Ouyang, X.; Ma, G.; Qian, Y.; Qiu, X.; Ruan, T. Improving Antioxidant Activity of Lignin by Hydrogenolysis. Ind. Crops Prod. 2018, 125, 228–235. [Google Scholar] [CrossRef]
- Kalasz, H.; Antal, I. Drug Excipients. Curr. Med. Chem. 2006, 13, 2535–2563. [Google Scholar] [CrossRef]
- Vinardell, P.M.; Mitjans, M. Lignins and their Derivatives with Beneficial Effects on Human Health. Int. J. Mol. Sci. 2017, 18, 1219. [Google Scholar] [CrossRef] [PubMed]






| Material | Bulk Density (g/mL) | Tapped Density (g/mL) | Hausner Ratio | Carr Index | BET Specific Surface Area (m2/g) | Pore Size (Å) | Porosity |
|---|---|---|---|---|---|---|---|
| MCC | 0.306 ± 0.002 | 0.348 ± 0.008 | 1.13 ± 0.02 | 12 ± 2 | 1.53 | 294 | 0.80 ± 0.02 |
| LIG | 0.354 ± 0.005 | 0.405 ± 0.005 | 1.14 ± 0.03 | 12 ± 2 | 5.45 | 238 | 0.74 ± 0.01 |
| Kawakita | Heckel | |||
|---|---|---|---|---|
| a | 1/b (MPa) | Pγ (MPa) | Da | |
| MCC | 0.80 ± 0.01 | 2.3 ± 0.4 | 242 ± 32 | 0.88 ± 0.01 |
| LIG | 0.73 ± 0.01 | 7.9 ± 1.0 | 361 ± 77 | 0.78 ± 0.03 |
| Formulation | Compression (Tonnes/MPa) | Excipient Composition (%) | Thickness (mm) | Mass Uniformity (%) | |
|---|---|---|---|---|---|
| LIG | MCC | ||||
| L02 | 2.0/147.8 | 0.0 | 100.0 | 2.70 ± 0.02 | 0.5 ± 0.7 |
| L22 | 25.0 | 75.0 | 2.74 ± 0.01 | 0.7 ± 0.4 | |
| L52 | 50.0 | 50.0 | 2.76 ± 0.01 | 1.1 ± 0.4 | |
| L72 | 75.0 | 25.0 | 2.82 ± 0.01 | 1.2 ± 0.3 | |
| L102 | 100.0 | 0.0 | 2.85 ± 0.02 | 6.5 ± 1.3 | |
| L05 | 5.0/369.4 | 0.0 | 100.0 | 2.56 ± 0.01 | 0.8 ± 0.3 |
| L25 | 25.0 | 75.0 | 2.58 ± 0.02 | 1.1 ± 0.3 | |
| L55 | 50.0 | 50.0 | 2.61 ± 0.01 | 1.3 ± 0.3 | |
| L75 | 75.0 | 25.0 | 2.69 ± 0.01 | 1.9 ± 0.6 | |
| L105 | 100.0 | 0.0 | 2.72 ± 0.01 | 5.6 ± 2.7 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez-Robles, J.; Stewart, S.A.; Rendl, A.; González, Z.; Donnelly, R.F.; Larrañeta, E. Lignin and Cellulose Blends as Pharmaceutical Excipient for Tablet Manufacturing via Direct Compression. Biomolecules 2019, 9, 423. https://doi.org/10.3390/biom9090423
Domínguez-Robles J, Stewart SA, Rendl A, González Z, Donnelly RF, Larrañeta E. Lignin and Cellulose Blends as Pharmaceutical Excipient for Tablet Manufacturing via Direct Compression. Biomolecules. 2019; 9(9):423. https://doi.org/10.3390/biom9090423
Chicago/Turabian StyleDomínguez-Robles, Juan, Sarah A. Stewart, Andreas Rendl, Zoilo González, Ryan F. Donnelly, and Eneko Larrañeta. 2019. "Lignin and Cellulose Blends as Pharmaceutical Excipient for Tablet Manufacturing via Direct Compression" Biomolecules 9, no. 9: 423. https://doi.org/10.3390/biom9090423