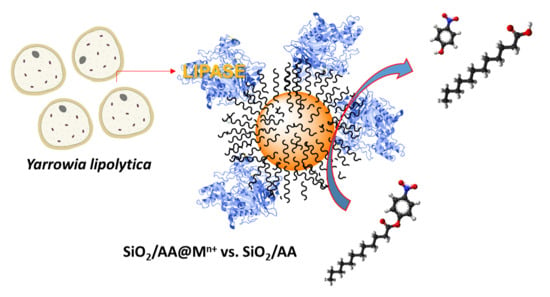
Noncovalent Immobilization of Yarrowia lipolytica Lipase on Dendritic-Like Amino Acid-Functionalized Silica Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Characterization
2.2. Lipase Production and Detection
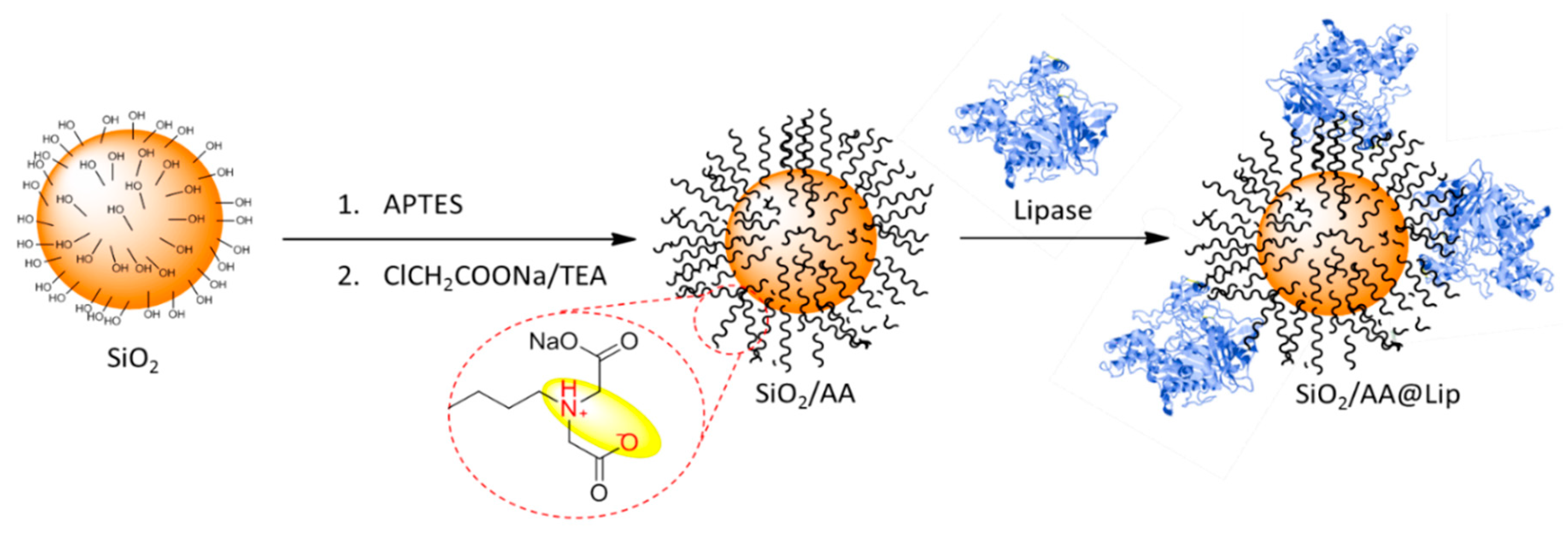
2.3. Synthesis of SiO2/AA
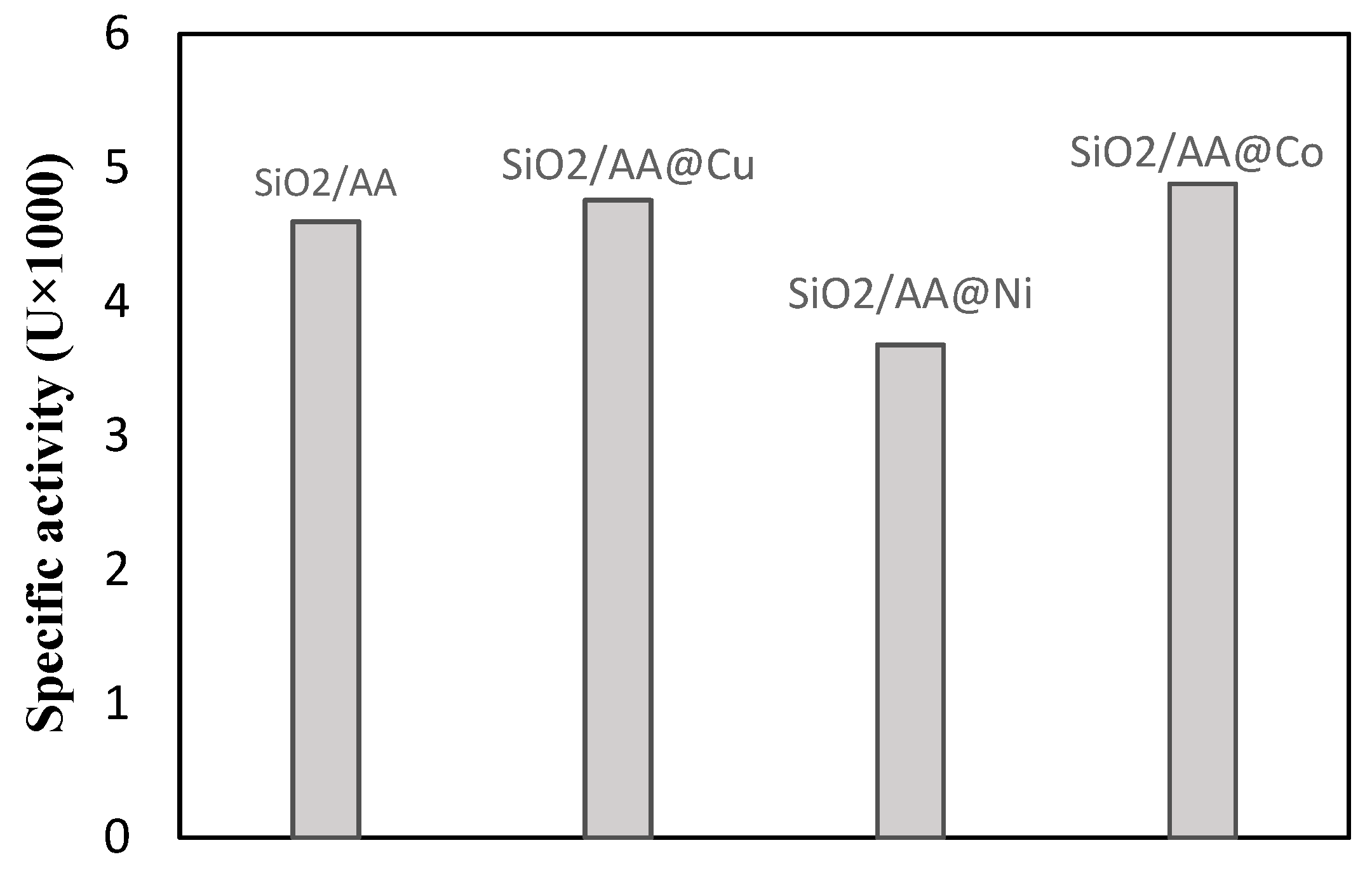
2.4. Metal Ion Chelation on SiO2/AA
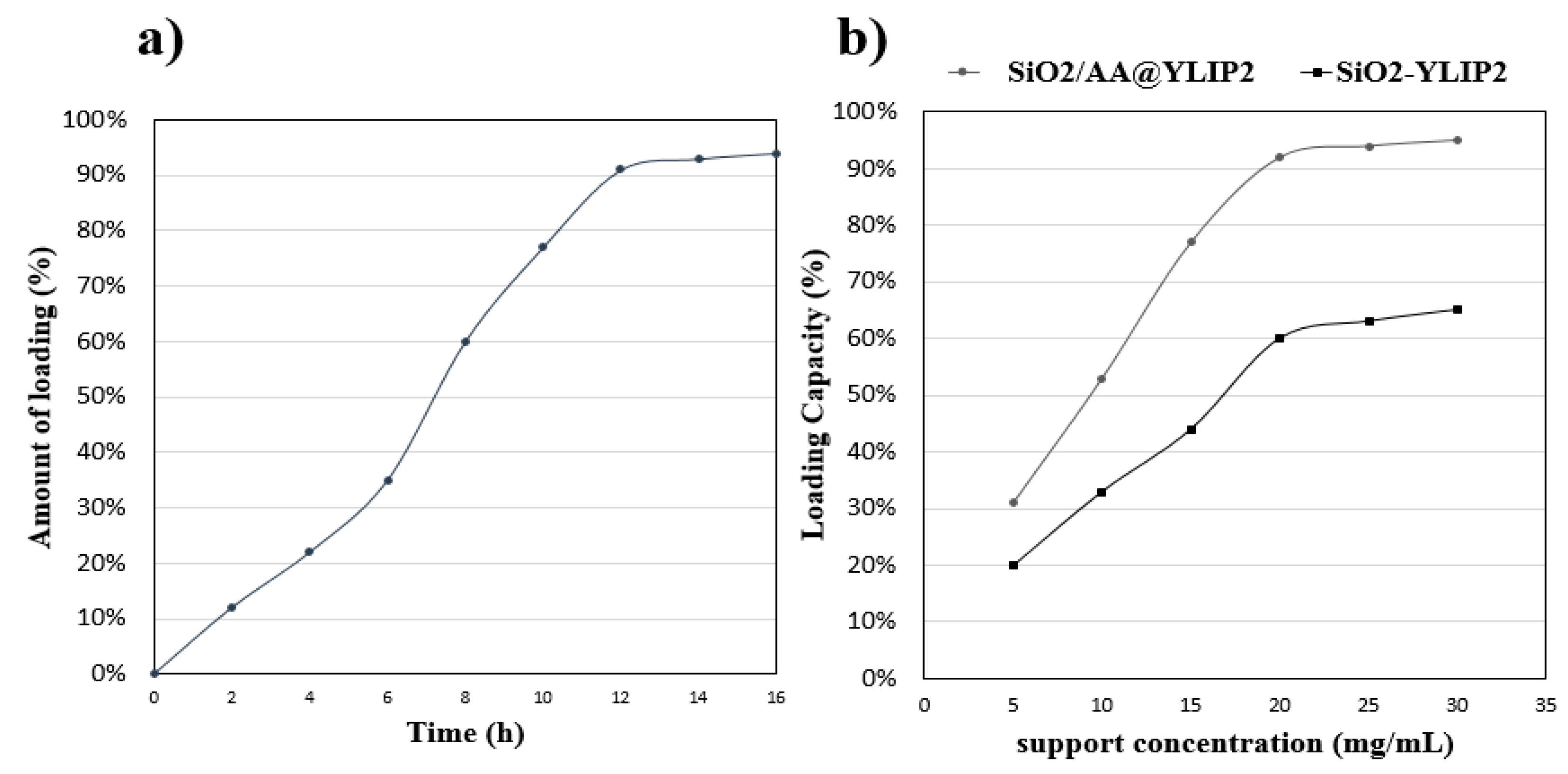
2.5. Lipase Immobilization
2.6. Reusability of Immobilized Lipase
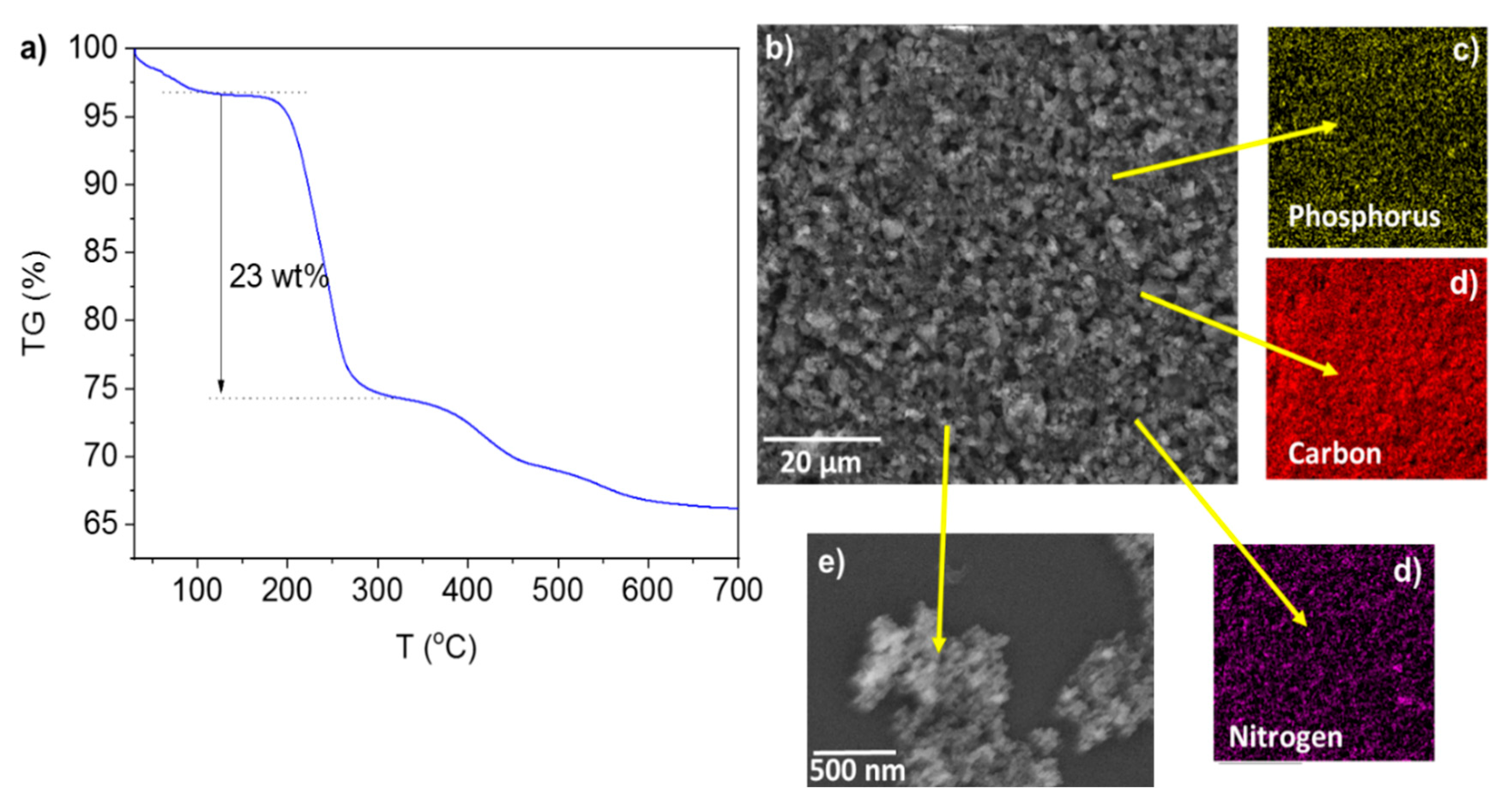
3. Results
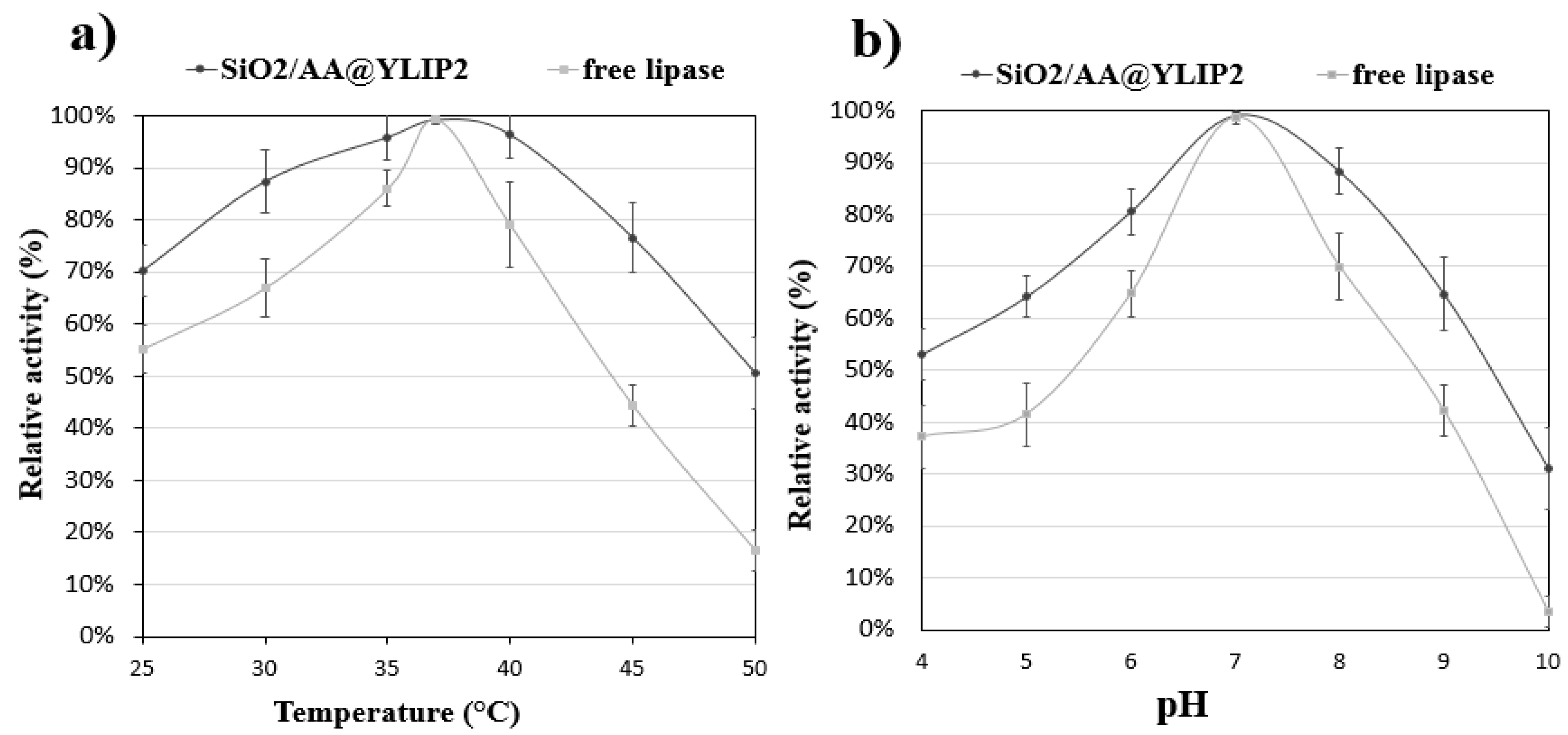
3.1. The Hydrolytic Activity of Immobilized Lipase
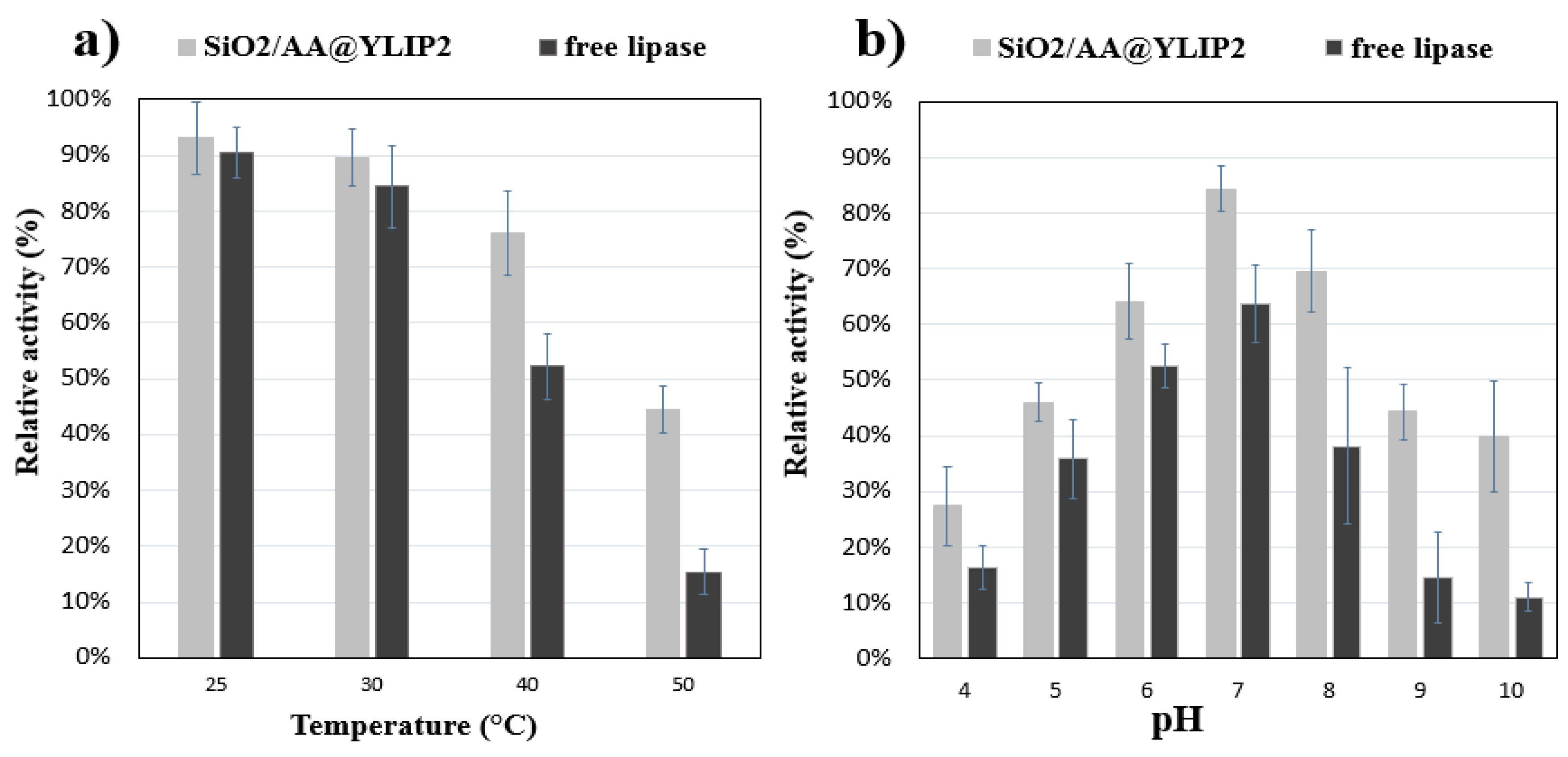
3.2. Reusability of SiO2/AA@YLIP2
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hasan, F.; Shah, A.A.; Hameed, A. Influence of culture conditions on lipase production by Bacillus sp. FH5. Ann. Microbiol. 2006, 56, 247–252. [Google Scholar] [CrossRef]
- Dizge, N.; Aydiner, C.; Imer, D.Y.; Bayramoglu, M.; Tanriseven, A.; Keskinler, B. Biodiesel production from sunflower, soybean, and waste cooking oils by transesterification using lipase immobilized onto a novel microporous polymer. Bioresour. Technol. 2009, 100, 1983–1991. [Google Scholar] [CrossRef] [PubMed]
- Aravindan, R.; Anbumathi, P.; Viruthagiri, T. Lipase applications in food industry. Indian J. Biotechnol. 2007, 6, 141–158. [Google Scholar]
- Solano, D.M.; Hoyos, P.; Hernáiz, M.; Alcántara, A.; Sánchez-Montero, J. Industrial bio-transformations in the synthesis of building blocks leading to enantiopure drugs. Bioresour. Technol. 2012, 115, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Ma, Q.; Zhu, H. Distribution, industrial applications, and enzymatic synthesis of D-amino acids. Appl. Microbiol. Biotechnol. 2015, 99, 3341–3349. [Google Scholar] [CrossRef] [PubMed]
- Pignède, G.; Wang, H.-J.; Fudalej, F.; Seman, M.; Gaillardin, C.; Nicaud, J.-M. Autocloning and amplification of LIP2 in Yarrowia lipolytica. Appl. Environ. Microbiol. 2000, 66, 3283–3289. [Google Scholar] [CrossRef]
- Pignède, G.; Wang, H.; Fudalej, F.; Gaillardin, C.; Seman, M.; Nicaud, J.-M. Characterization of an extracellular lipase encoded by LIP2 in Yarrowia lipolytica. J. Bacteriol. 2000, 182, 2802–2810. [Google Scholar] [CrossRef]
- Fickers, P.; Marty, A.; Nicaud, J.M. The lipases from Yarrowia lipolytica: genetics, production, regulation, biochemical characterization and biotechnological applications. Biotechnol. Adv. 2011, 29, 632–644. [Google Scholar] [CrossRef]
- Sheldon, R.A. Enzyme immobilization: the quest for optimum performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Tan, T.; Lu, J.; Nie, K.; Deng, L.; Wang, F. Biodiesel production with immobilized lipase: A review. Biotechnol. Adv. 2010, 28, 628–634. [Google Scholar] [CrossRef]
- Reetz, M.T.; Zonta, A.; Simpelkamp, J. Efficient immobilization of lipases by entrapment in hydrophobic sol-gel materials. Biotechnol. Bioeng. 1996, 49, 527–534. [Google Scholar] [CrossRef]
- Rostamnia, S.; Doustkhah, E. Nanoporous silica-supported organocatalyst: a heterogeneous and green hybrid catalyst for organic transformations. RSC Adv. 2014, 4, 28238–28248. [Google Scholar] [CrossRef]
- Alam, M.N.; Roy, N.; Mandal, D.; Begum, N.A. Green chemistry for nanochemistry: exploring medicinal plants for the biogenic synthesis of metal NPs with fine-tuned properties. RSC Adv. 2013, 3, 11935–11956. [Google Scholar] [CrossRef]
- Carril, M.; SanMartin, R.; Dominguez, E.; Tellitu, I. A highly advantageous metal-free approach to diaryl disulfides in water. Green Chem. 2007, 9, 315–317. [Google Scholar] [CrossRef]
- Elwahy, A.H.M.; Shaaban, M.R. Synthesis of heterocycles and fused heterocycles catalyzed by nanomaterials. RSC Adv. 2015, 5, 75659–75710. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, B.; Zhang, Q.; Deng, W.; Wang, Y.; Yang, Y. Recent advances in heterogeneous selective oxidation catalysis for sustainable chemistry. Chem. Soc. Rev. 2014, 43, 3480–3524. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.M.; Rice, J.K.; Pietron, J.J.; Stroud, R.M.; Long, J.W.; Rolison, D.R. Silica nanoarchitectures incorporating self-organized protein superstructures with gas-phase bioactivity. Nano Lett. 2003, 3, 1463–1467. [Google Scholar] [CrossRef]
- Heidarizadeh, M.; Doustkhah, E.; Saberi, F.; Rostamnia, S.; Hassankhani, A.; Rezaei, P.F.; Ide, Y. Silica Nanostructures, a Heterogeneous Surface for Dendrimer Functionalization. ChemestrySelect 2018, 3, 7137–7151. [Google Scholar] [CrossRef]
- Yamamoto, E.; Uchida, S.; Shimojima, A.; Wada, H.; Kuroda, K. Transformation of mesostructured silica nanoparticles into colloidal hollow nanoparticles in the presence of a bridged-organosiloxane shell. Chem. Mater. 2018, 30, 540–548. [Google Scholar] [CrossRef]
- Nabavi Zadeh, P.S.; Åkerman, B. Immobilization of enzymes in mesoporous silica particles: protein concentration and rotational mobility in the pores. J. Phys. Chem. B 2017, 121, 2575–2583. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kazlauskas, R.J. Biocatalysis in ionic liquids-advantages beyond green technology. Curr. Opin. Biotechnol. 2003, 14, 432–437. [Google Scholar] [CrossRef]
- Sun, L.B.; Liu, X.Q.; Zhou, H.C. Design and fabrication of mesoporous heterogeneous basic catalysts. Chem. Soc. Rev. 2015, 44, 5092–5147. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, R.; Ko, C.H.; Kruk, M.; Antochshuk, V.; Jaroniec, M. Block-copolymer-templated ordered mesoporous silica: Array of uniform mesopores or mesopore-micropore network? J. Phys. Chem. B 2000, 104, 11465–11471. [Google Scholar] [CrossRef]
- Zhang, C.H.; Liu, Y.; Sun, Y. Lipase immobilized to a short alkyl chain-containing zwitterionic polymer grafted on silica nanoparticles: Moderate activation and significant increase of thermal stability. Biochem. Eng. J. 2019, 146, 124–131. [Google Scholar] [CrossRef]
- Zhao, D.; Sun, J.; Li, Q.; Stucky, G.D. Morphological control of highly ordered mesoporous silica SBA-15. Chem. Mater. 2000, 12, 275–279. [Google Scholar] [CrossRef]
- Castanheiro, J.; Fonseca, I.; Ramos, A.; Vital, J. Tungstophosphoric acid immobilised in SBA-15 as an efficient heterogeneous acid catalyst for the conversion of terpenes and free fatty acids. Microporous Mesoporous Mater. 2017, 249, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Rostamnia, S.; Doustkhah, E.; Zeynizadeh, B. Cationic modification of SBA-15 pore walls for Pd supporting: Pd@SBA-15/ILDABCO as a catalyst for Suzuki coupling in water medium. Microporous Mesoporous Mater. 2016, 222, 87–93. [Google Scholar] [CrossRef]
- Rostamnia, S.; Doustkhah, E. Increased SBA-15-SO3H catalytic activity through hydrophilic/hydrophobic fluoroalkyl-chained alcohols (RFOH/SBA-15–Pr-SO3H). Synlett 2015, 26, 1345–1347. [Google Scholar] [CrossRef]
- Ferré, M.; Pleixats, R.; Wong Chi Man, M.; Cattoën, X. Recyclable organocatalysts based on hybrid silicas. Green Chem. 2016, 18, 881–922. [Google Scholar] [CrossRef] [Green Version]
- Darvishi, F.; Destain, J.; Nahvi, I.; Thonart, P.; Zarkesh-Esfahani, H. Effect of additives on freeze-drying and storage of Yarrowia lipolytica lipase. Appl. Biochem. Biotechnol. 2012, 168, 1101–1107. [Google Scholar] [CrossRef]
- Darvishi, F.; Destain, J.; Nahvi, I.; Thonart, P.; Zarkesh-Esfahani, H. High-level production of extracellular lipase by Yarrowia lipolytica mutants from methyl oleate. New Biotechnol. 2011, 28, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Fickers, P.; Nicaud, J.M.; Destain, J.; Thonart, P. Overproduction of lipase by Yarrowia lipolytica mutants. Appl. Microbiol. Biotechnol. 2003, 63, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Heidarizadeh, M.; Doustkhah, E.; Rostamnia, S.; Rezaei, P.F.; Harzevili, F.D.; Zeynizadeh, B. Dithiocarbamate to modify magnetic graphene oxide nanocomposite (Fe3O4-GO): A new strategy for covalent enzyme (lipase) immobilization to fabrication a new nanobiocatalyst for enzymatic hydrolysis of PNPD. Int. J. Biol. Macromol. 2017, 101, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Doustkhah, E.; Baghban, A.; Assadi, M.H.N.; Luque, R.; Rostamnia, S. Mesoporous SBA-15/PIDA as a dendrimer zwitterionic amino acid-type organocatalyst for three-component indazolophtalazine synthesis. Catal. Lett. 2019, 149, 591–600. [Google Scholar] [CrossRef]
- Kennedy, J.F.; Kalogerakis, B. Immobilization of glucoamylase on gelatin by transition-metal chelation. Biochimie 1980, 62, 549–561. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fathi, Z.; Doustkhah, E.; Ebrahimipour, G.; Darvishi, F. Noncovalent Immobilization of Yarrowia lipolytica Lipase on Dendritic-Like Amino Acid-Functionalized Silica Nanoparticles. Biomolecules 2019, 9, 502. https://doi.org/10.3390/biom9090502
Fathi Z, Doustkhah E, Ebrahimipour G, Darvishi F. Noncovalent Immobilization of Yarrowia lipolytica Lipase on Dendritic-Like Amino Acid-Functionalized Silica Nanoparticles. Biomolecules. 2019; 9(9):502. https://doi.org/10.3390/biom9090502
Chicago/Turabian StyleFathi, Zahra, Esmail Doustkhah, Golamhossein Ebrahimipour, and Farshad Darvishi. 2019. "Noncovalent Immobilization of Yarrowia lipolytica Lipase on Dendritic-Like Amino Acid-Functionalized Silica Nanoparticles" Biomolecules 9, no. 9: 502. https://doi.org/10.3390/biom9090502
APA StyleFathi, Z., Doustkhah, E., Ebrahimipour, G., & Darvishi, F. (2019). Noncovalent Immobilization of Yarrowia lipolytica Lipase on Dendritic-Like Amino Acid-Functionalized Silica Nanoparticles. Biomolecules, 9(9), 502. https://doi.org/10.3390/biom9090502