Biomimetic Spider Leg Joints: A Review from Biomechanical Research to Compliant Robotic Actuators
Abstract
:1. Introduction
2. Biological Research
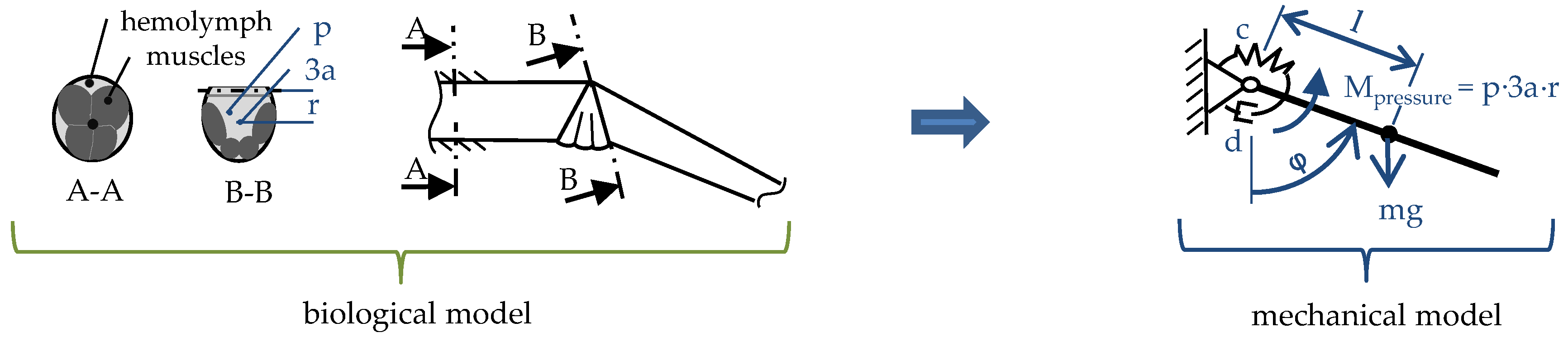
2.1. Hydraulic Leg Extension
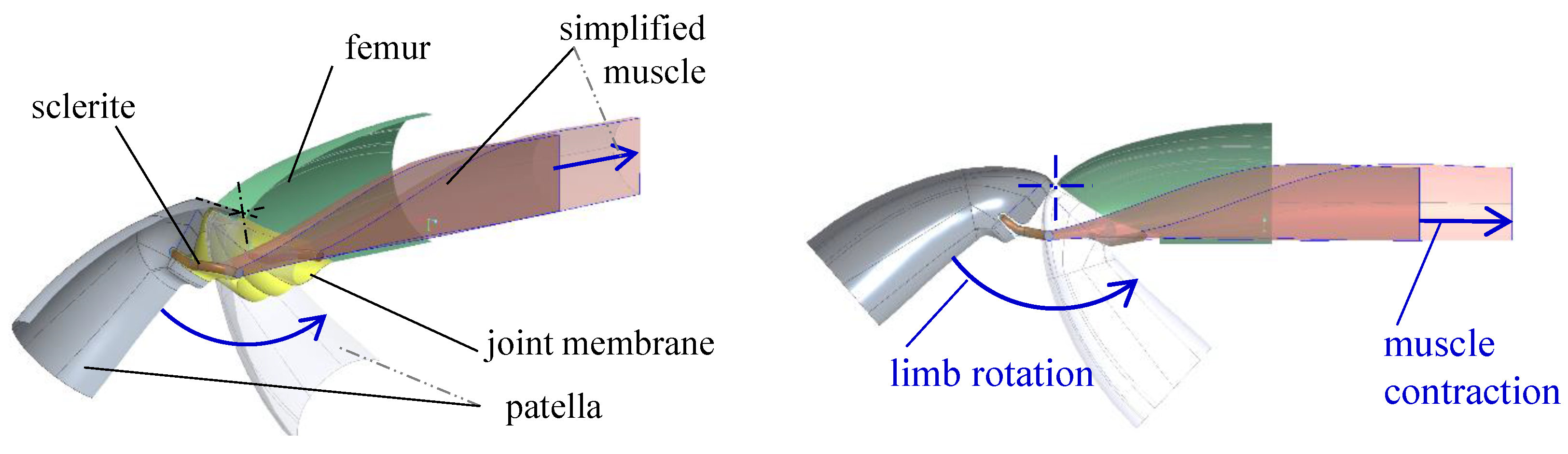
2.2. Muscular Flexion and Its Biomechanical Aspects
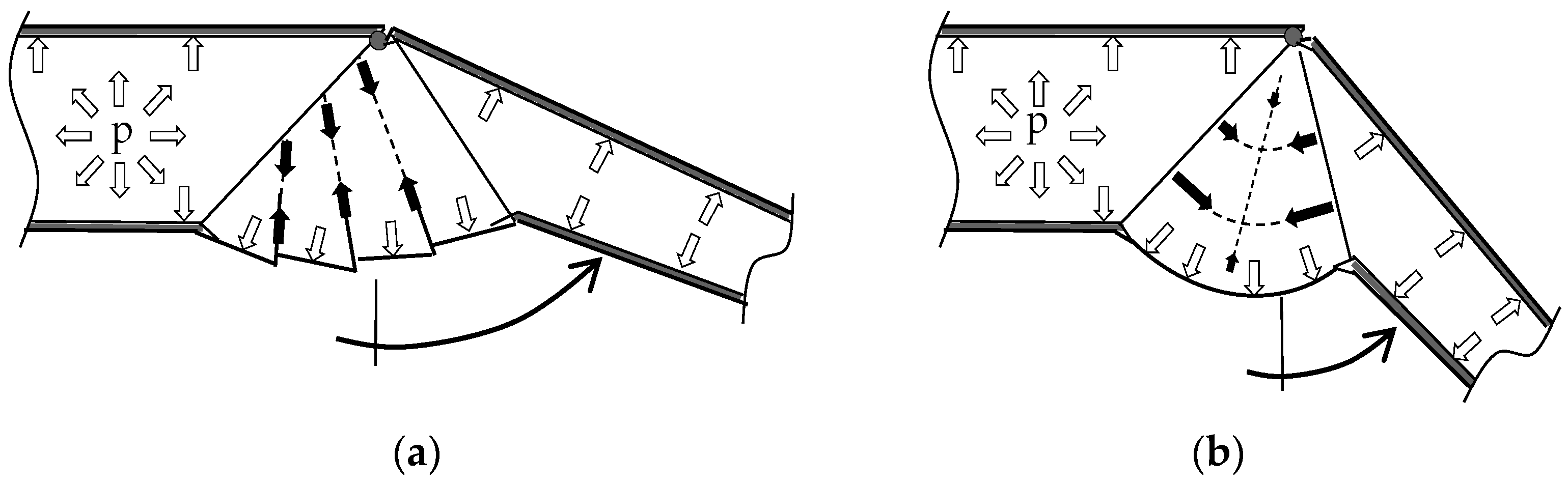
2.3. Interaction of Hydraulic Extension and Muscular Flexion
3. Technical Applications
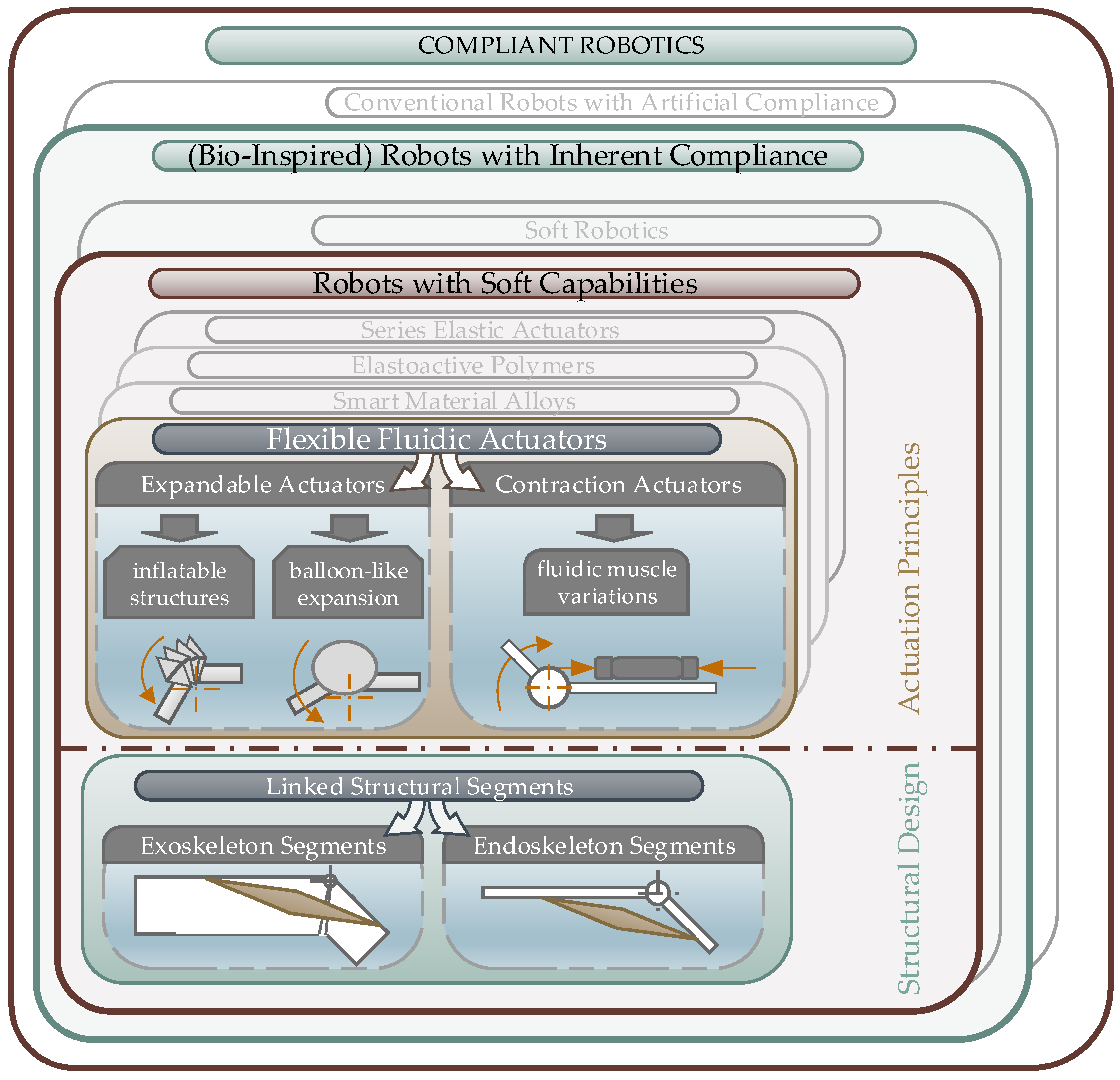
3.1. Classification of Biomimetic Spider Leg Actuators
- A biological model,
- An abstraction of the biological model,
- An implementation into a technical application, at least as a prototype.
3.1.1. Bio-Inspired Robots with Soft Capabilities
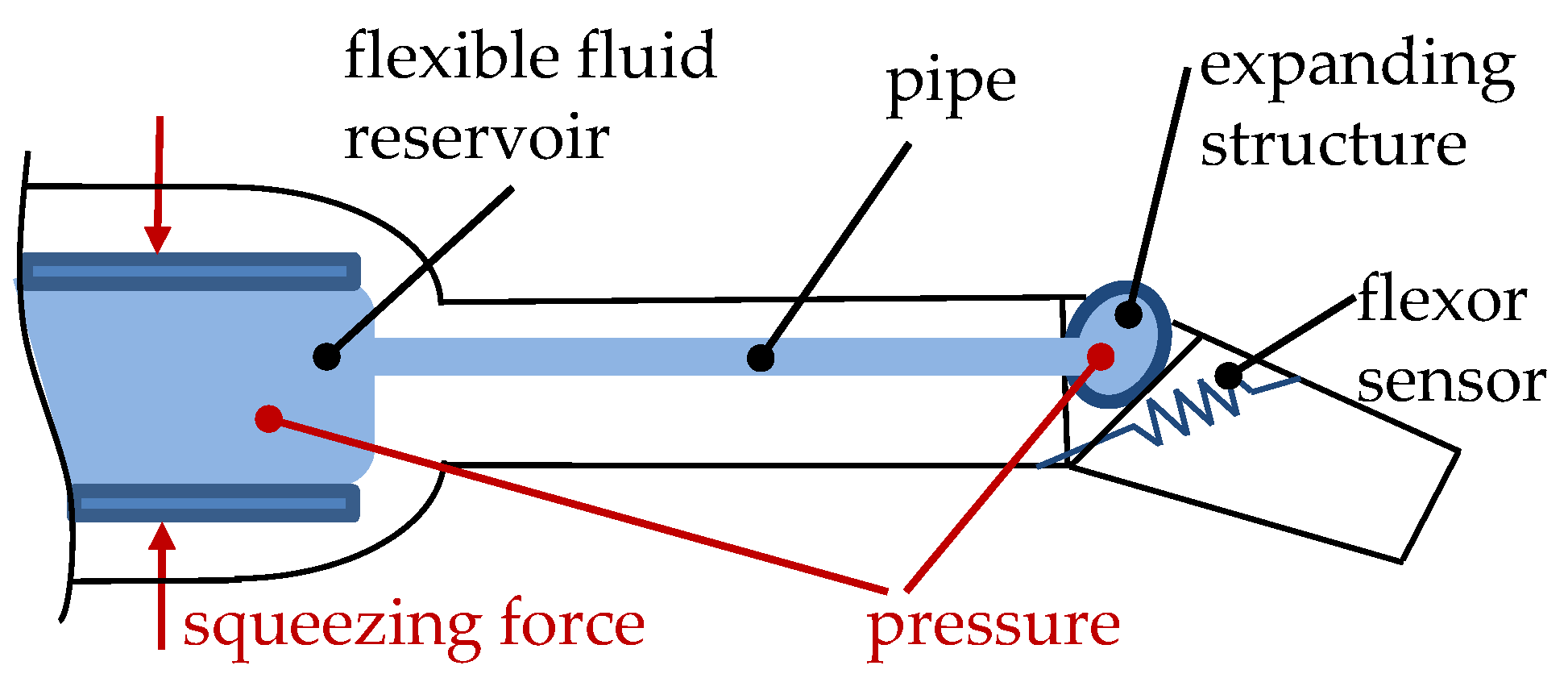
3.1.2. Flexible Fluidic Actuators
- The availability of mediums (air/water),
- Standardized periphery components like valves, pressure regulators and connections,
- Natural compressibility of air,
- No or low additional energy consumption for position holding with stop valves,
- High energy efficiency [68],
- Large deformation of the pressurized materials.
- Structural expansion to push the linked segments apart to generate a movement,
- Structural contraction, which can be used to apply a retraction force between the linked segments.
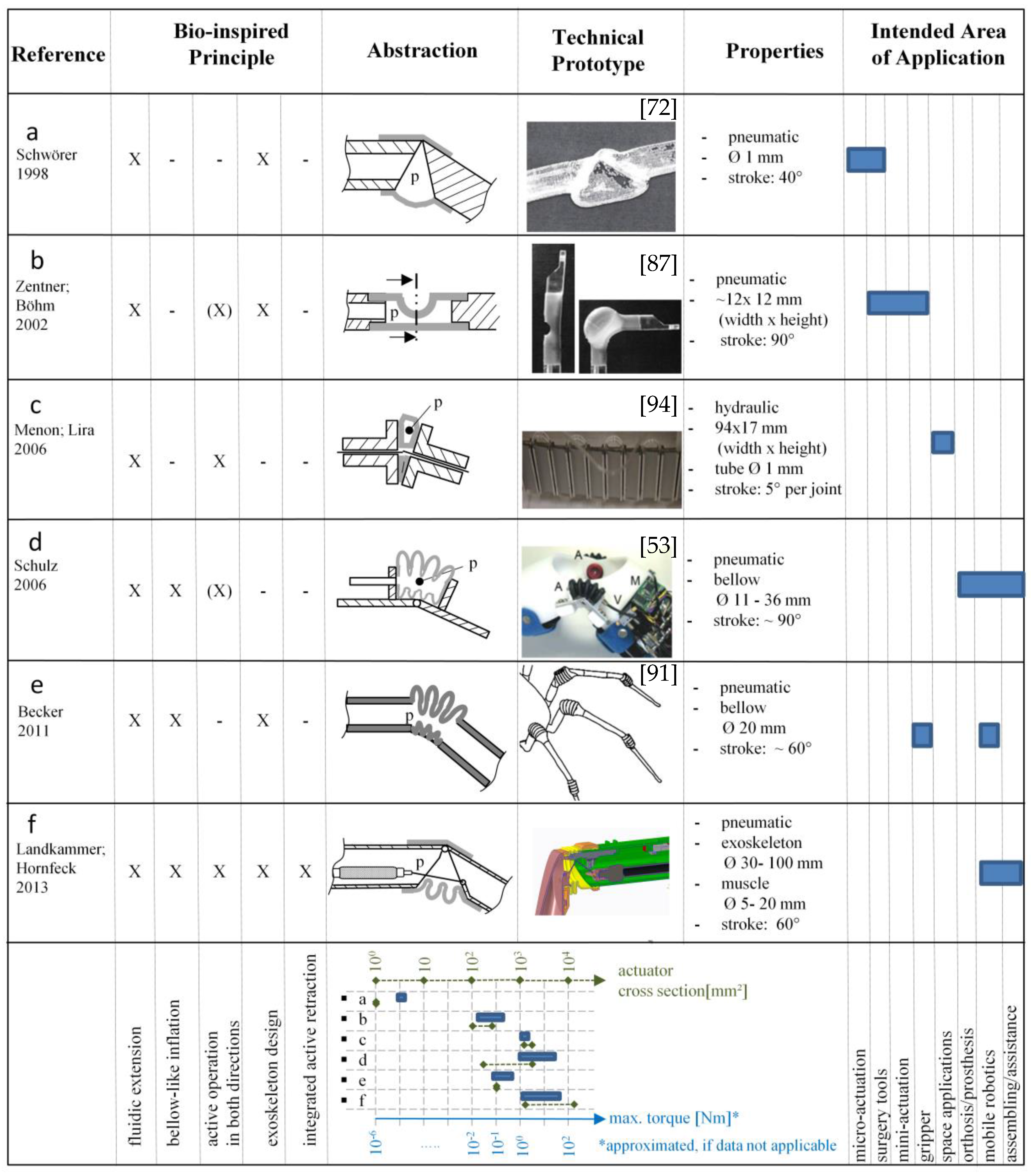
3.2. Bio-Inspired Actuators with Reference to the Spider Principle
4. Conclusions and Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Finkemeyer, B. Moderne Zeiten Werden Modern; Kieler Prozessmanagementforum: Kiel, Germany, 2014. [Google Scholar]
- De Greef, A.; Lambert, P.; Delchambre, A. Towards flexible medical instruments: Review of flexible fluidic actuators. Precis. Eng. 2009, 33, 311–321. [Google Scholar] [CrossRef]
- Belforte, G.; Eula, G.; Ivanov, A.; Sirolli, S. Soft Pneumatic actuators for rehabilitation. Actuators 2014, 3, 84–105. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO). Robots and Robotic Devices—Safety Requirements for Industrial Robots—Collaborative Operation; ISO/TS 15066; ISO: Geneva, Switzerland, 2011. [Google Scholar]
- O’Neill, J.; Lu, J.; Dockter, R.; Kowalewski, T. Design of a stretchable, flexible smart skin sensor for robotic applications. In Proceedings of the ICRA2015, International Conference on Robotics and Automation, Seattle, WA, USA, 26–30 May 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 624–629. [Google Scholar]
- Corrales, J.A. Safe Human-Robot Interaction Based on Multi-Sensor Fusion and Dexterous Manipulation Planning. Ph.D. Thesis, Universidad de Alicante, Alicante, Spain, 2011. [Google Scholar]
- Pedrocchi, N.; Vicentini, F.; Matteo, M.; Molinari, L. Safe human-robot cooperation in an industrial environment. Int. J. Adv. Robot. Syst. 2013, 27. [Google Scholar] [CrossRef]
- Iida, F.; Laschi, C. Soft robotics: Challenges and perspectives. Procedia Comput. Sci. 2011, 7, 99–102. [Google Scholar] [CrossRef]
- Gracia, E.; Arevalo, J.; Cestari, M.; Sanz-Merodio, D. On the Technological instantiation of a biomimetic leg concept for agile quadrupedal locomotion. J. Mech. Robot. 2015, 7, 031005. [Google Scholar] [CrossRef]
- Pescovitz, D. Robots That Show Their Softer Side. Bloomberg Buisnessweek, 2014. Available online: http://www.businessweek.com/articles/2014-08-21/soft-robotics-research-snuggle-against-the-machine (accessed on 1 March 2016).
- Kim, S.; Laschi, C.; Trimmer, B. Soft robotics: A bioinspired evolution in robotics. Trends Biotechnol. 2013, 31, 287–294. [Google Scholar] [PubMed]
- Gaiser, I.; Weigand, O.; Ivlev, O.; Andres, A.; Breitwieser, H.; Schulz, S.; Bretthauer, G. Compliant robotics and automation with flexible fluidic actuators and inflatable structures. In Smart Actuation and Sensing Systems—Recent Advances and Future Challenges; Berselli, G., Vertechy, R., Vassura, G., Eds.; INTECH Open Science: Morn Hill, UK, 2012; pp. 567–608. [Google Scholar]
- Toth, T.I.; Knops, S.; Daun-Gruhn, S. A neuromechanical model explaining forward and backward stepping in the stick insect. J. Neurophysiol. 2012, 107, 3267–3280. [Google Scholar] [PubMed]
- Schultz, J. Evolution of locomotion in Anarchida: The hydraulic pressure pump of the Giant Whipscorpion. J. Morphol. 1991, 210, 13–31. [Google Scholar] [CrossRef]
- Suter, R.B.; Wildman, H. Constraints on rowing locomotion on the water surface. J. Exp. Biol. 1999, 202, 2771–2785. [Google Scholar] [PubMed]
- Foelix, R. Biology of Spiders, 3rd ed.; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Eggs, B.; Wolff, J.O.; Kuhn-Nentwig, L.; Gorb, S.N.; Nentwig, W.; Wright, J. Hunting without a web: How lycosoid spiders subdue their prey. Int. J. Behav. Biol. Ethol. 2015, 121, 1166–1177. [Google Scholar]
- Gasparetto, A.; Vidoni, R.; Seidl, T. Kinematic study of the spider locomotor system in a biomimetic perspective. In Proceedings of the IROS 2008, International Conference on Intelligent Robots and Systems (IROS), Nice, France, 22–26 September 2008; IEEE: Piscataway, NJ, USA, 2008. [Google Scholar]
- Weihmann, T.; Gunther, M.; Blickhan, R. Hydraulic leg extension is not necessarily the main drive in large spiders. J. Exp. Biol. 2012, 215, 578–583. [Google Scholar] [PubMed]
- Sens, J. Funktionelle Anatomie und Biomechanik der Laufbeine Einer Vogelspinne (Grammostola Spatulata F.O. Pickard-Cambridge). Ph.D. Thesis, Universität des Saarlandes, Saarbrücken, Germany, 2006. [Google Scholar]
- Bögelsack, G. On technomorphic modelling and classification of biological joints. Theory Biosci. 2000, 119, 104–121. [Google Scholar] [CrossRef]
- Blickhan, R.; Barth, F.G. Strains in the exoskeleton of spiders. J. Comp. Physiol. A 1985, 157, 115–147. [Google Scholar] [CrossRef]
- Huckstorf, K.; Kosok, G.; Seyfarth, E.-A.; Wirkner, C.S. The hemolymph vascular system in Cupiennius salei (Araneae: Ctenidae). Zool. Anz. J. Comp. Zool. 2013, 252, 76–87. [Google Scholar] [CrossRef]
- Barth, F.G. A Spider’s World—Senses and Behavior; Springer: Berlin, Germany; New York, NY, USA, 2002. [Google Scholar]
- Petrunkevitch, A. Contributions to our knowledge of the anatomy and relationships of spiders. Ann. Entomol. Soc. Am. 1909, 2, 11–20. [Google Scholar] [CrossRef]
- Brown, R.B. The musculature of Angelina Naevia. J. Morphol. 1939, 64, 115–166. [Google Scholar] [CrossRef]
- Ellis, C. The mechanism of extension in the legs of spiders. Biol. Bull. 1944, 86, 41–50. [Google Scholar] [CrossRef]
- Parry, A.; Brown, H. The hydraulic mechanism of the spider leg. J. Exp. Biol. 1959, 36, 423–433. [Google Scholar]
- Wilson, R.S. Some comments on the hydrostatic system of spiders (Chelicerata, Araneae). Z. Morphol. Tiere 1970, 68, 308–322. [Google Scholar] [CrossRef]
- Stewart, D.; Martin, A. Blood pressure in the tarantula, Dugesiella hentzi. J. Comp. Physiol. 1974, 88, 141–172. [Google Scholar] [CrossRef]
- Anderson, J.; Prestwich, K. The Fluid pressure pumps of spiders (Chelicerata, Araneae). Z. Morphol. Tiere 1975, 81, 257–277. [Google Scholar] [CrossRef]
- Bohmann, L.; Blickhan, R. Der hydraulische Mechanismus des Spinnenbeines und seine anwendung für technische probleme. Z. Angew. Math. Mech. 1996, 78, 87–96. [Google Scholar] [CrossRef]
- Zentner, L.; Petkun, S.; Blickhan, R. From the spider leg to a hydraulic device. Tech. Mech. 1999, 20, 21–29. [Google Scholar]
- Zentner, L. Untersuchung und Entwicklung Nachgiebiger Strukturen Basierend auf Innendruckbelasteten Röhren mit Stoffschlüssigen Gelenken, Habilitation; Technische Universität: Ilmenau, Germany, 2003. [Google Scholar]
- Zentner, L. Modelling and application of the hydraulic spider leg mechanism. In Spider Ecophysiology; Nentwig, W., Ed.; Springer: Heidelberg, Germany, 2013; pp. 451–462. [Google Scholar]
- Ruhland, M.; Rathmayer, W. Die Beinmuskulatur und ihre Innervation bei der Vogelspinne (Araneae, Aviculariidae). Zoomorphologie 1978, 89, 33–46. [Google Scholar] [CrossRef]
- Clarke, J. The comparative functional morphology of the leg joints and muscles of five spiders. Bull. Br. Arachnol. Soc. 1986, 7, 37–47. [Google Scholar]
- Siebert, T.; Weihmann, T.; Rode, C.; Blickhan, R. Cupiennius salei: Biomechanical properties of the tibia-metatarsus joint and its flexing muscles. J. Comp. Physiol. B 2010, 180, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Runge, J.; Landkammer, S.; Valek, R.; Wirkner, C.S. 3D functional morphology of the femur-patella joint in Cupiennius salei (Araneae; Ctenidae). In Proceedings of the 28th European Congress of Arachnology, Turin, Italy, 24–29 August 2014.
- Manton, S.M.; Harding, J.P. Hydrostatic pressure and leg extension in arthropods, with special reference to arachnids. Ann. Mag. Nat. Hist. 1958, 1, 161–182. [Google Scholar] [CrossRef]
- Kropf, C. Hydraulic system of locomotion. In Spider Ecophysiology; Nentwig, W., Ed.; Springer: Heidelberg, Germany, 2013; pp. 43–56. [Google Scholar]
- Prestwich, K.N. The constraints on maximal activity in spiders. J. Comp. Physiol. B 1988, 158, 437–447. [Google Scholar] [CrossRef]
- Sensenig, A.T. Mechanics of cuticular elastic energy storage in leg joints lacking extensor muscles in arachnids. J. Exp. Biol. 2003, 206, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, D.; Rahn, C.D.; Kier, W.M.; Walker, I.D. Soft robotics: Biological inspiration, state of the art, and future research. Appl. Bionics Biomech. 2008, 5, 99–117. [Google Scholar] [CrossRef]
- VDI—Gesellschaft Technologies of Life Science (TLS). Biomimetics—Conception and Strategy, Difference between Biomimetic and Conventional Methods/Products; VDI 6220, 07.080; Beuth: Düsseldorf, Germany, 2012. [Google Scholar]
- Rus, D.; Tolley, M.T. Design, fabrication and control of soft robots. Nature 2015, 521, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.D.; Katzschmann, R.K.; Rus, D. A recipe for soft fluidic elastomer robots. Soft Robot. 2015, 2, 7–25. [Google Scholar] [CrossRef]
- Cianchetti, M.; Arienti, A.; Follador, M.; Mazzolai, B.; Dario, P.; Laschi, C. Design concept and validation of a robotic arm inspired by the octopus. Mater. Sci. Eng. C 2011, 31, 1230–1239. [Google Scholar] [CrossRef]
- Mao, S.; Dong, E.; Xu, M.; Jin, H.; Li, F.; Yang, J. Design and development of starfisch-like robot: Soft bionic platform with multi-motion using SMA actuators. In Proceedings of the ROBIO 2013, International Conference on Robotics and Biomimetics, Shenzhen, China, 12–14 December 2013; IEEE: Piscataway, NJ, USA, 2013. [Google Scholar]
- Haegele, M.; Maufroy, C.; Kraus, W.; Siee, M.; Breuninger, J. Musculoskeletal robots and wearable devices on the basis of cable-driven actuators. In Soft Robotics; Verl, A., Albu-Schäffer, A., Brock, O., Raatz, A., Eds.; Springer Verlag: Heidelberg, Germany, 2015; pp. 42–53. [Google Scholar]
- Rosendo, A.; Liu, X.; Shimizu, M.; Hosoda, K. Stretch reflex improves rolling stability during hopping of a decerebrate biped system. Bioinspir. Biomim. 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Ivlev, O. Soft fluidic actuators of rotary type for safe physical human-machine interaction. In Proceedings of the ICORR2009, IEEE 11th International Conference on Rehabilitation Robotics (ICORR), Kyoto, Japan, 23–26 June 2009; IEEE: Piscataway, NJ, USA, 2009. [Google Scholar]
- Pylatiuk, C.; Kargov, A.; Gaiser, I.; Schulz, S.; Bretthauer, G. Design of a flexible fluidic actuation system for a hybrid elbow orthosis. In Proceedings of the ICORR2009, IEEE 11th International Conference on Rehabilitation Robotics (ICORR), Kyoto, Japan, 23–26 June 2009; IEEE: Piscataway, NJ, USA, 2009. [Google Scholar]
- Van der Linde, R.; Caarls, J.; Menon, C.; Verhoef, J. Biological Inspired Joints for Innovative Articulation Concepts; ESA Report; TU Delft: Delft, The Netherlands, 2006. [Google Scholar]
- Yatsko, J.S.; Yatsko, D.E. Fluid-Powerd Mechanical Actuator and Method for Controlling. U.S. Patent 7,100,491, 5 September 2006. [Google Scholar]
- Guo, Z.; Pan, Y.; Wee, L.B.; Yu, H. Design and control of a novel compliant differential shape memory alloy actuator. Sens. Actuators A Phys. 2015, 225, 71–80. [Google Scholar] [CrossRef]
- Coral, W.; Rossi, C.; Martin, I. Bio-inspired morphing caudal fin using shape memory alloy composites for a fish-like robot. In Proceedings of the ICINCO 2015, 12th International Conference on Informatics in Control, Automation and Robotics, Colmar, France, 21–23 July 2015; pp. 336–343.
- Rodrigue, H.; Bhandari, B.; Han, M.-W.; Ahn, S.-H. A shape memory alloy-based soft morphing actuator capable of pure twisting motion. J. Intell. Mater. Syst. Struct. 2015, 26, 1071–1078. [Google Scholar] [CrossRef]
- Brochu, P.; Pei, Q. Dielectric Elastomers for actuators and artificial muscles. In Electroactivity in Polymeric Materials; Rasmussen, L., Ed.; Springer: New York, NY, USA, 2012; pp. 1–56. [Google Scholar]
- Hager, M.D.; Bode, S.; Weber, C.; Schubert, U.S. Shape memory polymers—Past, present and future developments. Prog. Polym. Sci. 2015, 49, 3–33. [Google Scholar] [CrossRef]
- Vallery, H.; Veneman, J.; van Asseldonk, E.; Ekkelenkamp, R.; Buss, M.; van der Kooij, H. Compliant actuation of rehabilitation robots. IEEE Robot. Autom. Mag. 2008, 15, 60–69. [Google Scholar] [CrossRef]
- Baiden, D.; Wilkening, A.; Ivlev, O. Safety and handling concept for assistive robotic devices with pneumatic rotary soft-actuators. In Proceedings of the AIM 2011, International Conference on Advanced Intelligent Mechatronics, Budapest, Hungary, 3–7 July 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 754–759. [Google Scholar]
- Taghia, J.; Wilkening, A.; Ivlev, O. Position control of soft-robots with rotary-type pneumatic actuators. In Proceedings of the ROBOTIK, 7th German Conference on Robotics, Munich, Germany, 21–22 May 2012; pp. 399–404.
- Andrikopoulos, G.; Nikolakopoulos, G.; Manesis, S. A survey on applications of pneumatic artificial muscles. In Proceedings of the 19th Mediterranean Conference on Control & Automation (MED), Corfu, Greece, 20–23 June 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 1439–1446. [Google Scholar]
- De Volder, M.; Reynaerts, D. Pneumatic and hydraulic microactuators: A review. J. Micromech. Microeng. 2010, 20. [Google Scholar] [CrossRef]
- Blanes, C.; Mellado, M.; Beltran, P. Novel additive manufacturing pneumatic actuators and mechanisms for food handling grippers. Actuators 2014, 3, 205–225. [Google Scholar] [CrossRef]
- Rost, A.; Schadle, S. The SLS-generated soft robotic hand—An integrated approach using additive manufacturing and reinforcement learning. In Proceedings of the 12th International Conference on Machine Learning and Applications (ICMLA), Miami, FL, USA, 4–7 December 2013; IEEE: Piscataway, NJ, USA, 2013; pp. 215–220. [Google Scholar]
- Jani, J.M.; Leary, M.; Subic, A.; Gibson, M.A. A review of shape memory alloy research, applications and opportunities. Mater. Des. 2014, 56, 1078–1113. [Google Scholar] [CrossRef]
- Sawicki, G.S.; Ferris, D.P. A pneumatically powered knee-ankle-foot orthosis (KAFO) with myoelectric activation and inhibition. J. Neuro. Eng. Rehabil. 2009, 6. [Google Scholar] [CrossRef] [PubMed]
- Schulz, S.; Pylatiuk, C.; Kargov, A.; Oberle, R.; Klosek, H.; Bretthauer, G. Biologically inspired fluidically driven robots. In Proceedings of the CLAWAR 2005—8th International Conference on Climbing and Walking Robots, London, UK; Springer: Berlin/Heidelberg, Germany, 2006; pp. 97–104. [Google Scholar]
- Kargov, A.; Werner, T.; Pylatiuk, C.; Schulz, S. Development of a miniaturised hydraulic actuation system for artificial hands. Sens. Actuators A Phys. 2008, 141, 548–557. [Google Scholar] [CrossRef]
- Schwörer, M.; Kohl, M.; Menz, W. Fluidic microjoints based on spider legs. In Proceedings of the Actuator 98, 6th International Conference on New Actuators, Bremen, Germany, 17–19 June 1998; Messe Bremen GmbH: Bremen, Germany, 1998; pp. 103–106. [Google Scholar]
- Daerden, F.; Lefeber, D. Pneumatic artificial muscles: Actuators for robotics and automation. Eur. J. Mech. Environ. Eng. 2000, 47, 10–21. [Google Scholar]
- Chou, C.-P.; Hannaford, B. Static and dynamic characteristics of McKibben pneumatic artificial muscles. In Proceedings of the ICRA 1994, IEEE International Conference on Robotics and Automation (ICRA), San Diego, CA, USA, 8–13 May 1994; IEEE Computer Society Press: Los Alamitos, CA, USA, 1994; pp. 281–286. [Google Scholar]
- Tondu, B.; Lopez, P. Modeling and control of McKibben artificial muscle robot actuators. IEEE Control Syst. Mag. 2000, 20, 15–38. [Google Scholar] [CrossRef]
- Tondu, B. Modelling of the McKibben artificial muscle: A review. J. Intell. Mater. Syst. Struct. 2012, 23, 225–253. [Google Scholar] [CrossRef]
- Chou, C.-P.; Hannaford, M. Measurement and modeling of McKibben pneumatic artificial muscles. IEEE Trans. Robot. Autom. 1996, 12, 90–102. [Google Scholar] [CrossRef]
- Immega, G.; Kukolj, M. Axially Contractable Actuator. U.S. Patent 4,939,982, 10 July 1990. [Google Scholar]
- Daerden, F.; Lefeber, D.; Verrelst, R. Pleated pneumatic artificial muscles: Compliant robotic actuators. In Proceedings of the ICIRS 2011, International Conference on Intelligent Robots and Systems, Maui, HI, USA, 29 September–3 October 2001; IEEE: Piscataway, NJ, USA, 2001; pp. 1958–1963. [Google Scholar]
- Waycaster, G. Design of a Powered Above Knee Prothesis Using Pneumatic Artificial Muscles. Master’s Thesis, University of Alabama, Tuscaloosa, AL, USA, 2010. [Google Scholar]
- Tsagarakis, N.G.; Caldwell, D.G. Improved modelling and assessment of pneumatic muscle actuators. In Proceedings of the ICRA 2000, International Conference on Robotics and Automation (ICRA), San Francisco, CA, USA, 24–28 April 2000; IEEE Robotics and Automation Society: Piscataway, NJ, USA, 2000; pp. 3641–3646. [Google Scholar]
- Jordan, M.; Pietrusky, D.; Mihajlov, M.; Ivlev, O. Precise Position and trajectory control of pneumatic soft-actuators for assistance robots and motion therapy devices. In Proceedings of the ICORR2009, IEEE 11th International Conference on Rehabilitation Robotics (ICORR), Kyoto, Japan, 23–26 June 2009; IEEE: Piscataway, NJ, USA, 2009; pp. 663–668. [Google Scholar]
- Fluidic Muscle DMSP/MAS-Product-Catalogue, Festo GmbH. Available online: http://www.festo.com/cat/de_de/data/doc_de/PDF/DE/DMSP-MAS_DE.PDF (accessed on 18 February 2014).
- Shadow Air Muscles-Product-Catalogue, Shadow Robot Company. Available online: http://www.shadowrobot.com/products/air-muscles/ (accessed on 18 February 2014).
- Böhm, V.; Zentner, L. Stoffschlüssige Gelenke nach biologischem Vorbild. In Proceedings of the Wissenschaftliche Mitteilungen der 14. Frühlingsakademie, München, Germany, 30 April–5 May 2002; pp. 19–27.
- Zentner, L. Fluidisch Angetriebenes Stoffschlüssiges Gelenkelement. Deutsches Patent TU Ilmenau, DE000010316959A1, 12 April 2003. [Google Scholar]
- Zentner, L.; Böhm, V. On the mechanical compliance of technical systems. In Mechanical Engineering; Gokcek, M., Ed.; INTECH Open Science: Morn Hill, UK, 2012; pp. 341–352. [Google Scholar]
- Menon, C.; Lira, C. Active articulation for future space applications inspired by the hydraulic system of spiders. Bioinspir. Biomim. 2006, 1, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Schulz, S. Fluid Actuator. Forschungszentrum Karlsruhe. European Patent EP 1,519,055B1, 8 September 2004. [Google Scholar]
- Breuninger, J.; Becker, R.; Wolf, A.; Rommel, S.; Verl, A. Generative Fertigung mit Kunststoffen-Konzeption und Konstruktion für Selektives Lasersintern; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Becker, R.; Ondratschek, K. International Patent—Self-Propelling Platform. World Patent WO002012159737A1, 29 November 2012. [Google Scholar]
- Landkammer, S.; Hornfeck, R. A novel bio-inspired fluidic actuator for robotic applications. In Proceedings of the ICAST2014, 25th International Conference on Adaptive Structures and Technologies, The Hague, The Netherlands, 6–8 October 2014.
- Landkammer, S.; Hornfeck, R. Drehantrieb. Deutsches Patent, Technische Hochschule Nürnberg—Georg Simon Ohm. German Patent DE 10,2013,114,660B4, 20 December 2013. [Google Scholar]
- Menon, C.; Lira, C. Spider-Inspired embedded actuator for space applications. In Proceedings of the AISB 06, Adaptation in Artificial and Biological Systems, Bristol, UK, 3–6 April 2006.
- Schulz, S.; Pylatiuk, C.; Bretthauer, G. A new ultralight anthropomorphic hand. In Proceedings of the ICRA2001, International Conference on Robotics and Automation, Seoul, Korea, 21–26 May 2001; IEEE: Piscataway, NJ, USA, 2001; pp. 2437–2441. [Google Scholar]
- Hornfeck, R.; Landkammer, S. Drehantrieb. European Patent Application, Technische Hochschule Nürnberg Georg Simon Ohm. European Patent EP 2,902,642,A1, 12 December 2014. [Google Scholar]
- Landkammer, S.; Schneider, D.; Winter, F.; Heß, P.; Hornfeck, R. Static modeling of an antagonistic pneumatic actuator for robotic applications. In Proceedings of the ECMSM2015, IEEE Electronics, Control, Measurement, Signals and their Application to Mechatronics (ECMSM2015), Liberec, Czech Republic, 22–24 June 2015; IEEE: Piscataway, NJ, USA, 2015. [Google Scholar]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landkammer, S.; Winter, F.; Schneider, D.; Hornfeck, R. Biomimetic Spider Leg Joints: A Review from Biomechanical Research to Compliant Robotic Actuators. Robotics 2016, 5, 15. https://doi.org/10.3390/robotics5030015
Landkammer S, Winter F, Schneider D, Hornfeck R. Biomimetic Spider Leg Joints: A Review from Biomechanical Research to Compliant Robotic Actuators. Robotics. 2016; 5(3):15. https://doi.org/10.3390/robotics5030015
Chicago/Turabian StyleLandkammer, Stefan, Florian Winter, Daniel Schneider, and Rüdiger Hornfeck. 2016. "Biomimetic Spider Leg Joints: A Review from Biomechanical Research to Compliant Robotic Actuators" Robotics 5, no. 3: 15. https://doi.org/10.3390/robotics5030015
APA StyleLandkammer, S., Winter, F., Schneider, D., & Hornfeck, R. (2016). Biomimetic Spider Leg Joints: A Review from Biomechanical Research to Compliant Robotic Actuators. Robotics, 5(3), 15. https://doi.org/10.3390/robotics5030015