Perspectives on Intra- and Intercellular Trafficking of Hedgehog for Tissue Patterning
Abstract
:1. Introduction
2. Hedgehog Synthesis, Lipid Modification and Shedding
3. Apicobasal Polarity of Hh Gradient and Trafficking Regulators
4. Hedgehog Loaded in Exovesicles
5. Cytoneme-Mediated Hh Transport
6. Vesicular Trafficking for Hh Reception
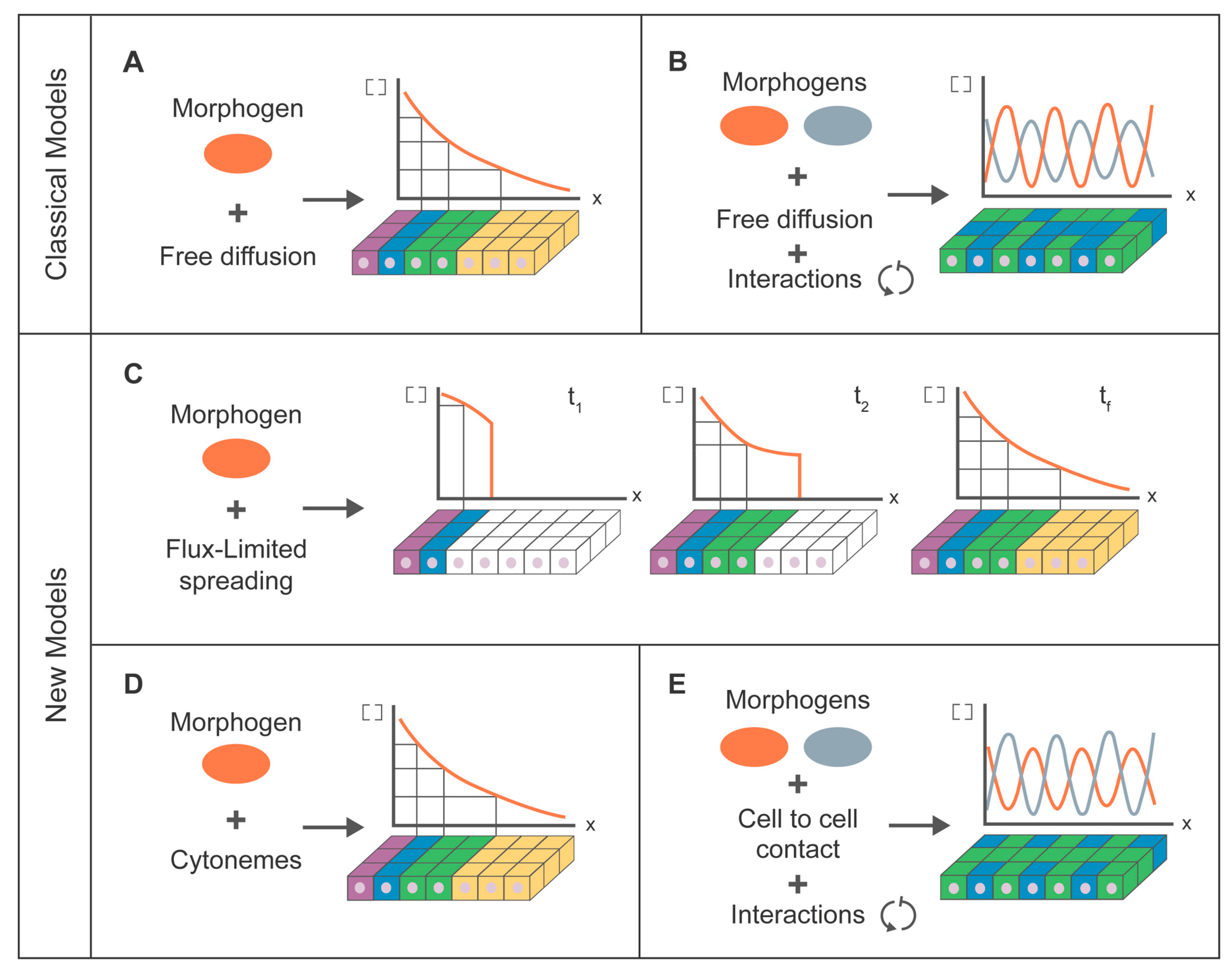
7. Modeling for Morphogen Dispersion
7.1. Classical Modeling Approach
7.2. Cytoneme Modeling Approach
8. Outlook on Cytonemes
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nusslein-Volhard, C.; Wieschaus, E. Mutations affecting segment number and polarity in Drosophila. Nature 1980, 287, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, J.; Therond, P.P. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 2013, 14, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Li, Y.J.; Kawakami, T.; Xu, S.M.; Chuang, P.T. Palmitoylation is required for the production of a soluble multimeric Hedgehog protein complex and long-range signaling in vertebrates. Genes Dev. 2004, 18, 641–659. [Google Scholar] [CrossRef] [PubMed]
- Panakova, D.; Sprong, H.; Marois, E.; Thiele, C.; Eaton, S. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature 2005, 435, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Matusek, T.; Wendler, F.; Poles, S.; Pizette, S.; D’Angelo, G.; Furthauer, M.; Therond, P.P. The ESCRT machinery regulates the secretion and long-range activity of Hedgehog. Nature 2014, 516, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Gradilla, A.C.; Gonzalez, E.; Seijo, I.; Andres, G.; Bischoff, M.; Gonzalez-Mendez, L.; Sanchez, V.; Callejo, A.; Ibanez, C.; Guerra, M.; et al. Exosomes as Hedgehog carriers in cytoneme-mediated transport and secretion. Nat. Commun. 2014, 5, 5649. [Google Scholar] [CrossRef] [PubMed]
- Bilioni, A.; Sanchez-Hernandez, D.; Callejo, A.; Gradilla, A.C.; Ibanez, C.; Mollica, E.; Carmen Rodriguez-Navas, M.; Simon, E.; Guerrero, I. Balancing Hedgehog, a retention and release equilibrium given by Dally, Ihog, Boi and shifted/DmWif. Dev. Biol. 2013, 376, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, M.; Gradilla, A.C.; Seijo, I.; Andres, G.; Rodriguez-Navas, C.; Gonzalez-Mendez, L.; Guerrero, I. Cytonemes are required for the establishment of a normal Hedgehog morphogen gradient in Drosophila epithelia. Nat. Cell Biol. 2013, 15, 1269–1281. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Rios, P.; Guerrero, I.; Gonzalez-Reyes, A. Cytoneme-mediated delivery of Hedgehog regulates the expression of bone morphogenetic proteins to maintain germline stem cells in Drosophila. PLoS Biol. 2012, 10, e1001298. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.A.; Llagostera, E.; Barna, M. Specialized filopodia direct long-range transport of SHH during vertebrate tissue patterning. Nature 2013, 497, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, M.J.; Sanchez-Arrones, L.; Sandonis, A.; Sanchez-Camacho, C.; Gestri, G.; Wilson, S.W.; Guerrero, I.; Bovolenta, P. Cdon acts as a Hedgehog decoy receptor during proximal-distal patterning of the optic vesicle. Nat. Commun. 2014, 5, 4272. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Guerrero, I.; Hidalgo, A.; Taylor, A.; Whittle, J.R.; Ingham, P.W. A protein with several possible membrane-spanning domains encoded by the Drosophila segment polarity gene patched. Nature 1989, 341, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Lum, L.; Yao, S.; Mozer, B.; Rovescalli, A.; von Kessler, D.; Nirenberg, M.; Beachy, P.A. Identification of Hedgehog pathway components by RNAi in Drosophila cultured cells. Science 2003, 299, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.L.; Song, J.Y.; Izzi, L.; Althaus, I.W.; Kang, J.S.; Charron, F.; Krauss, R.S.; McMahon, A.P. Overlapping roles and collective requirement for the coreceptors GAS1, CDO, and BOC in SHH pathway function. Dev. Cell 2011, 20, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tukachinsky, H.; Huang, C.H.; Jao, C.; Chu, Y.R.; Tang, H.Y.; Mueller, B.; Schulman, S.; Rapoport, T.A.; Salic, A. Processing and turnover of the Hedgehog protein in the endoplasmic reticulum. J. Cell Biol. 2011, 192, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Hsiao, H.T.; Chu, Y.R.; Ye, Y.; Chen, X. Derlin2 protein facilitates HRD1-mediated retro-translocation of sonic hedgehog at the endoplasmic reticulum. J. Biol. Chem. 2013, 288, 25330–25339. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.A.; Ekker, S.C.; Park, W.J.; von Kessler, D.P.; Young, K.E.; Chen, C.H.; Ma, Y.; Woods, A.S.; Cotter, R.J.; Koonin, E.V.; et al. Hedgehog patterning activity: Role of a lipophilic modification mediated by the carboxy-terminal autoprocessing domain. Cell 1996, 86, 21–34. [Google Scholar] [CrossRef]
- Lee, J.J.; Ekker, S.C.; von Kessler, D.P.; Porter, J.A.; Sun, B.I.; Beachy, P.A. Autoproteolysis in Hedgehog protein biogenesis. Science 1994, 266, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- Pepinsky, R.B.; Zeng, C.; Wen, D.; Rayhorn, P.; Baker, D.P.; Williams, K.P.; Bixler, S.A.; Ambrose, C.M.; Garber, E.A.; Miatkowski, K.; et al. Identification of a palmitic acid-modified form of human Sonic hedgehog. J. Biol. Chem. 1998, 273, 14037–14045. [Google Scholar] [CrossRef] [PubMed]
- Chamoun, Z.; Mann, R.K.; Nellen, D.; von Kessler, D.P.; Bellotto, M.; Beachy, P.A.; Basler, K. Skinny hedgehog, an acyltransferase required for palmitoylation and activity of the hedgehog signal. Science 2001, 293, 2080–2084. [Google Scholar] [CrossRef] [PubMed]
- Callejo, A.; Torroja, C.; Quijada, L.; Guerrero, I. Hedgehog lipid modifications are required for Hedgehog stabilization in the extracellular matrix. Development 2006, 133, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Tokhunts, R.; Singh, S.; Chu, T.; D’Angelo, G.; Baubet, V.; Goetz, J.A.; Huang, Z.; Yuan, Z.; Ascano, M.; Zavros, Y.; et al. The full-length unprocessed Hedgehog protein is an active signaling molecule. J. Biol. Chem. 2010, 285, 2562–2568. [Google Scholar] [CrossRef] [PubMed]
- Palm, W.; Swierczynska, M.M.; Kumari, V.; Ehrhart-Bornstein, M.; Bornstein, S.R.; Eaton, S. Secretion and signaling activities of lipoprotein-associated Hedgehog and non-sterol-modified Hedgehog in flies and mammals. PLoS Biol. 2013, 11, e1001505. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Wolf, A.; Wagner, M.; Kuhlmann, J.; Waldmann, H. The cholesterol membrane anchor of the Hedgehog protein confers stable membrane association to lipid-modified proteins. Proc. Natl. Acad. Sci. USA 2004, 101, 8531–8536. [Google Scholar] [CrossRef] [PubMed]
- Gallet, A.; Ruel, L.; Staccini-Lavenant, L.; Therond, P.P. Cholesterol modification is necessary for controlled planar long-range activity of Hedgehog in Drosophila epithelia. Development 2006, 133, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Rietveld, A.; Neutz, S.; Simons, K.; Eaton, S. Association of sterol- and glycosylphosphatidylinositol-linked proteins with Drosophila raft lipid microdomains. J. Biol. Chem. 1999, 274, 12049–12054. [Google Scholar] [CrossRef] [PubMed]
- Resh, M.D. Palmitoylation of ligands, receptors, and intracellular signaling molecules. Sci. STKE 2006, 2006, re14. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, I.; Chiang, C. A conserved mechanism of Hedgehog gradient formation by lipid modifications. Trends Cell Biol. 2007, 17, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.; Nellen, D.; Bellotto, M.; Hafen, E.; Senti, K.A.; Dickson, B.J.; Basler, K. Dispatched, a novel sterol-sensing domain protein dedicated to the release of cholesterol-modified Hedgehog from signaling cells. Cell 1999, 99, 803–815. [Google Scholar] [CrossRef]
- Amanai, K.; Jiang, J. Distinct roles of Central missing and Dispatched in sending the Hedgehog signal. Development 2001, 128, 5119–5127. [Google Scholar] [PubMed]
- Ma, Y.; Erkner, A.; Gong, R.; Yao, S.; Taipale, J.; Basler, K.; Beachy, P.A. Hedgehog-mediated patterning of the mammalian embryo requires transporter-like function of Dispatched. Cell 2002, 111, 63–75. [Google Scholar] [CrossRef]
- Kawakami, T.; Kawcak, T.; Li, Y.J.; Zhang, W.; Hu, Y.; Chuang, P.T. Mouse Dispatched mutants fail to distribute Hedgehog proteins and are defective in Hedgehog signaling. Development 2002, 129, 5753–5765. [Google Scholar] [CrossRef] [PubMed]
- Callejo, A.; Bilioni, A.; Mollica, E.; Gorfinkiel, N.; Andres, G.; Ibanez, C.; Torroja, C.; Doglio, L.; Sierra, J.; Guerrero, I. Dispatched mediates Hedgehog basolateral release to form the long-range morphogenetic gradient in the Drosophila wing disk epithelium. Proc. Natl. Acad. Sci. USA 2011, 108, 12591–12598. [Google Scholar] [CrossRef] [PubMed]
- Elkins, C.A.; Mullis, L.B. Mammalian steroid hormones are substrates for the major RND- and MFS-type tripartite multidrug efflux pumps of Escherichia coli. J. Bacteriol. 2006, 188, 1191–1195. [Google Scholar] [CrossRef] [PubMed]
- Etheridge, L.A.; Crawford, T.Q.; Zhang, S.; Roelink, H. Evidence for a role of vertebrate Disp1 in long-range Shh signaling. Development 2010, 137, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Tukachinsky, H.; Kuzmickas, R.P.; Jao, C.Y.; Liu, J.; Salic, A. Dispatched and Scube mediate the efficient secretion of the cholesterol-modified Hedgehog ligand. Cell Rep. 2012, 2, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Woods, I.G.; Talbot, W.S. The you gene encodes an EGF-CUB protein essential for Hedgehog signaling in zebrafish. PLoS Biol. 2005, 3, e66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollway, G.E.; Maule, J.; Gautier, P.; Evans, T.M.; Keenan, D.G.; Lohs, C.; Fischer, D.; Wicking, C.; Currie, P.D. Scube2 mediates Hedgehog signalling in the zebrafish embryo. Dev. Biol. 2006, 294, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Hall, T.E.; Dyson, J.M.; Sonntag, C.; Ayers, K.; Berger, S.; Gautier, P.; Mitchell, C.; Hollway, G.E.; Currie, P.D. Scube activity is necessary for Hedgehog signal transduction in vivo. Dev. Biol. 2012, 368, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Creanga, A.; Glenn, T.D.; Mann, R.K.; Saunders, A.M.; Talbot, W.S.; Beachy, P.A. Scube/You activity mediates release of dually lipid-modified Hedgehog signal in soluble form. Genes Dev. 2012, 26, 1312–1325. [Google Scholar] [CrossRef] [PubMed]
- Dierker, T.; Dreier, R.; Petersen, A.; Bordych, C.; Grobe, K. Heparan sulfate-modulated, metalloprotease-mediated Sonic Hedgehog release from producing cells. J. Biol. Chem. 2009, 284, 8013–8022. [Google Scholar] [CrossRef] [PubMed]
- Ohlig, S.; Farshi, P.; Pickhinke, U.; van den Boom, J.; Hoing, S.; Jakuschev, S.; Hoffmann, D.; Dreier, R.; Scholer, H.R.; Dierker, T.; et al. Sonic hedgehog shedding results in functional activation of the solubilized protein. Dev. Cell 2011, 20, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Ohlig, S.; Pickhinke, U.; Sirko, S.; Bandari, S.; Hoffmann, D.; Dreier, R.; Farshi, P.; Gotz, M.; Grobe, K. An emerging role of Sonic hedgehog shedding as a modulator of heparan sulfate interactions. J. Biol. Chem. 2012, 287, 43708–43719. [Google Scholar] [CrossRef] [PubMed]
- Jakobs, P.; Exner, S.; Schurmann, S.; Pickhinke, U.; Bandari, S.; Ortmann, C.; Kupich, S.; Schulz, P.; Hansen, U.; Seidler, D.G.; et al. Scube2 enhances proteolytic Shh processing from the surface of Shh-producing cells. J. Cell Sci. 2014, 127, 1726–1737. [Google Scholar] [CrossRef] [PubMed]
- Glise, B.; Miller, C.A.; Crozatier, M.; Halbisen, M.A.; Wise, S.; Olson, D.J.; Vincent, A.; Blair, S.S. Shifted, the Drosophila ortholog of Wnt inhibitory factor-1, controls the distribution and movement of Hedgehog. Dev. Cell 2005, 8, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Gorfinkiel, N.; Sierra, J.; Callejo, A.; Ibanez, C.; Guerrero, I. The Drosophila ortholog of the human Wnt inhibitor factor shifted controls the diffusion of lipid-modified Hedgehog. Dev. Cell 2005, 8, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Avanesov, A.; Honeyager, S.M.; Malicki, J.; Blair, S.S. The role of glypicans in Wnt inhibitory factor-1 activity and the structural basis of Wif1’s effects on Wnt and Hedgehog signaling. PLoS Genet. 2012, 8, e1002503. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Hernandez, D.; Sierra, J.; Ortigao-Farias, J.R.; Guerrero, I. The Wif domain of the human and Drosophila Wif-1 secreted factors confers specificity for Wnt or Hedgehog. Development 2012, 139, 3849–3858. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, G.; Zhou, K.; Wan, J.Y.; Friedrich, J.; Jourjine, N.; Smith, D.; Schedl, P. The hedgehog pathway gene shifted functions together with the hmgcr-dependent isoprenoid biosynthetic pathway to orchestrate germ cell migration. PLoS Genet. 2013, 9, e1003720. [Google Scholar] [CrossRef] [PubMed]
- Ayers, K.L.; Gallet, A.; Staccini-Lavenant, L.; Therond, P.P. The long-range activity of Hedgehog is regulated in the apical extracellular space by the glypican Dally and the hydrolase Notum. Dev. Cell 2010, 18, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.L.; Milenkovic, L.; Scott, M.P. In vivo functions of the Patched protein: Requirement of the C terminus for target gene inactivation but not Hedgehog sequestration. Mol. Cell 2000, 6, 467–478. [Google Scholar] [CrossRef]
- Lu, X.; Liu, S.; Kornberg, T.B. The C-terminal tail of the Hedgehog receptor Patched regulates both localization and turnover. Genes Dev. 2006, 20, 2539–2551. [Google Scholar] [CrossRef] [PubMed]
- Tabata, T.; Kornberg, T.B. Hedgehog is a signaling protein with a key role in patterning Drosophila imaginal discs. Cell 1994, 76, 89–102. [Google Scholar] [CrossRef]
- D’Angelo, G.; Matusek, T.; Pizette, S.; Therond, P.P. Endocytosis of Hedgehog through Dispatched regulates long-range signaling. Dev. Cell 2015, 32, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Gallet, A.; Rodriguez, R.; Ruel, L.; Therond, P.P. Cholesterol modification of Hedgehog is required for trafficking and movement, revealing an asymmetric cellular response to Hedgehog. Dev. Cell 2003, 4, 191–204. [Google Scholar] [CrossRef]
- Mertens, G.; van der Schueren, B.; van den Berghe, H.; David, G. Heparan sulfate expression in polarized epithelial cells: The apical sorting of glypican (GPI-anchored proteoglycan) is inversely related to its heparan sulfate content. J. Cell Biol. 1996, 132, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Gallet, A.; Staccini-Lavenant, L.; Therond, P.P. Cellular trafficking of the glypican Dally-like is required for full-strength Hedgehog signaling and Wingless transcytosis. Dev. Cell 2008, 14, 712–725. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Palmer, L.; Alexandre, C.; Kakugawa, S.; Beckett, K.; Gaugue, I.; Palmer, R.H.; Vincent, J.P. Godzilla-dependent transcytosis promotes Wingless signalling in Drosophila wing imaginal discs. Nat. Cell Biol. 2016, 18, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Steinhauer, J.; Liu, H.H.; Miller, E.; Treisman, J.E. Trafficking of the EGFR ligand Spitz regulates its signaling activity in polarized tissues. J. Cell Sci. 2013, 126, 4469–4478. [Google Scholar] [CrossRef] [PubMed]
- Takeo, S.; Akiyama, T.; Firkus, C.; Aigaki, T.; Nakato, H. Expression of a secreted form of Dally, a Drosophila glypican, induces overgrowth phenotype by affecting action range of Hedgehog. Dev. Biol. 2005, 284, 204–218. [Google Scholar] [CrossRef] [PubMed]
- Ayers, K.L.; Mteirek, R.; Cervantes, A.; Lavenant-Staccini, L.; Therond, P.P.; Gallet, A. Dally and Notum regulate the switch between low and high level Hedgehog pathway signalling. Development 2012, 139, 3168–3179. [Google Scholar] [CrossRef] [PubMed]
- Kakugawa, S.; Langton, P.F.; Zebisch, M.; Howell, S.A.; Chang, T.H.; Liu, Y.; Feizi, T.; Bineva, G.; O’Reilly, N.; Snijders, A.P.; et al. Notum deacylates Wnt proteins to suppress signalling activity. Nature 2015, 519, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Parchure, A.; Vyas, N.; Ferguson, C.; Parton, R.G.; Mayor, S. Oligomerization and endocytosis of Hedgehog is necessary for its efficient exovesicular secretion. Mol. Biol. Cell 2015, 26, 4700–4717. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Goetz, J.A.; Suber, L.M.; Scott, W.J., Jr.; Schreiner, C.M.; Robbins, D.J. A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature 2001, 411, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Goetz, J.A.; Singh, S.; Suber, L.M.; Kull, F.J.; Robbins, D.J. A highly conserved amino-terminal region of Sonic hedgehog is required for the formation of its freely diffusible multimeric form. J. Biol. Chem. 2006, 281, 4087–4093. [Google Scholar] [CrossRef] [PubMed]
- Dierker, T.; Dreier, R.; Migone, M.; Hamer, S.; Grobe, K. Heparan sulfate and transglutaminase activity are required for the formation of covalently cross-linked Hedgehog oligomers. J. Biol. Chem. 2009, 284, 32562–32571. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; White, B.; Tyurina, O.V.; Guner, B.; Larson, T.; Lee, H.Y.; Karlstrom, R.O.; Kohtz, J.D. Synergistic and antagonistic roles of the Sonic hedgehog N- and C-terminal lipids. Development 2004, 131, 4357–4370. [Google Scholar] [CrossRef] [PubMed]
- Vyas, N.; Goswami, D.; Manonmani, A.; Sharma, P.; Ranganath, H.A.; VijayRaghavan, K.; Shashidhara, L.S.; Sowdhamini, R.; Mayor, S. Nanoscale organization of Hedgehog is essential for long-range signaling. Cell 2008, 133, 1214–1227. [Google Scholar] [CrossRef] [PubMed]
- Greco, V.; Hannus, M.; Eaton, S. Argosomes: A potential vehicle for the spread of morphogens through epithelia. Cell 2001, 106, 633–645. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Vyas, N.; Walvekar, A.; Tate, D.; Lakshmanan, V.; Bansal, D.; Lo Cicero, A.; Raposo, G.; Palakodeti, D.; Dhawan, J. Vertebrate Hedgehog is secreted on two types of extracellular vesicles with different signaling properties. Sci. Rep. 2014, 4, 7357. [Google Scholar] [CrossRef] [PubMed]
- Rusten, T.E.; Vaccari, T.; Stenmark, H. Shaping development with ESCRTs. Nat. Cell. Biol. 2012, 14, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brugger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Weber, F.A.; Kornberg, T.B. Cytonemes: Cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell 1999, 97, 599–607. [Google Scholar] [CrossRef]
- Hsiung, F.; Ramirez-Weber, F.A.; Iwaki, D.D.; Kornberg, T.B. Dependence of Drosophila wing imaginal disc cytonemes on Decapentaplegic. Nature 2005, 437, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Huang, H.; Liu, S.; Kornberg, T.B. Cytoneme-mediated contact-dependent transport of the Drosophila Decapentaplegic signaling protein. Science 2014, 343, 1244624. [Google Scholar] [CrossRef] [PubMed]
- Stanganello, E.; Hagemann, A.I.; Mattes, B.; Sinner, C.; Meyen, D.; Weber, S.; Schug, A.; Raz, E.; Scholpp, S. Filopodia-based Wnt transport during vertebrate tissue patterning. Nat. Commun. 2015, 6, 5846. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Kornberg, T.B. Myoblast cytonemes mediate Wg signaling from the wing imaginal disc and Delta-Notch signaling to the air sac primordium. eLife 2015, 4, e06114. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Hsiung, F.; Kornberg, T.B. Specificity of Drosophila cytonemes for distinct signaling pathways. Science 2011, 332, 354–358. [Google Scholar] [CrossRef] [PubMed]
- De Joussineau, C.; Soule, J.; Martin, M.; Anguille, C.; Montcourrier, P.; Alexandre, D. Delta-promoted filopodia mediate long-range lateral inhibition in Drosophila. Nature 2003, 426, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Georgiou, M.; Stevenson, N.L.; Miodownik, M.; Baum, B. Dynamic filopodia transmit intermittent Delta-Notch signaling to drive pattern refinement during lateral inhibition. Dev. Cell 2010, 19, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Hamada, H.; Watanabe, M.; Lau, H.E.; Nishida, T.; Hasegawa, T.; Parichy, D.M.; Kondo, S. Involvement of Delta/Notch signaling in zebrafish adult pigment stripe patterning. Development 2014, 141, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Eom, D.S.; Bain, E.J.; Patterson, L.B.; Grout, M.E.; Parichy, D.M. Long-distance communication by specialized cellular projections during pigment pattern development and evolution. eLife 2015, 4, e12401. [Google Scholar] [CrossRef] [PubMed]
- Seijo-Barandiaran, I.; Guerrero, I.; Bischoff, M. In vivo imaging of Hedgehog transport in Drosophila epithelia. Methods Mol. Biol. 2015, 1322, 9–18. [Google Scholar] [PubMed]
- Kerber, M.L.; Cheney, R.E. Myosin-X: A MyTH-FERM myosin at the tips of filopodia. J. Cell Sci. 2011, 124, 3733–3741. [Google Scholar] [CrossRef] [PubMed]
- Bellaiche, Y.; The, I.; Perrimon, N. Tout-velu is a Drosophila homologue of the putative tumour suppressor EXT-1 and is needed for Hh diffusion. Nature 1998, 394, 85–88. [Google Scholar] [PubMed]
- The, I.; Bellaiche, Y.; Perrimon, N. Hedgehog movement is regulated through tout velu-dependent synthesis of a heparan sulfate proteoglycan. Mol. Cell 1999, 4, 633–639. [Google Scholar] [CrossRef]
- Takei, Y.; Ozawa, Y.; Sato, M.; Watanabe, A.; Tabata, T. Three Drosophila EXT genes shape morphogen gradients through synthesis of heparan sulfate proteoglycans. Development 2004, 131, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Belenkaya, T.Y.; Khodoun, M.; Tauchi, M.; Lin, X. Distinct and collaborative roles of Drosophila E family proteins in morphogen signalling and gradient formation. Development 2004, 131, 1563–1575. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; Charrin, S.; Simoes, S.; Romao, M.; Rochin, L.; Saftig, P.; Marks, M.S.; Rubinstein, E.; Raposo, G. The tetraspanin cd63 regulates escrt-independent and -dependent endosomal sorting during melanogenesis. Dev. Cell 2011, 21, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Lidke, D.S.; Lidke, K.A.; Rieger, B.; Jovin, T.M.; Arndt-Jovin, D.J. Reaching out for signals: Filopodia sense EGF and respond by directed retrograde transport of activated receptors. J. Cell Biol. 2005, 170, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Heusermann, W.; Hean, J.; Trojer, D.; Steib, E.; von Bueren, S.; Graff-Meyer, A.; Genoud, C.; Martin, K.; Pizzato, N.; Voshol, J.; et al. Exosomes surf on filopodia to enter cells at endocytic hot spots, traffic within endosomes, and are targeted to the ER. J. Cell Biol. 2016, 213, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Torroja, C.; Gorfinkiel, N.; Guerrero, I. Patched controls the Hedgehog gradient by endocytosis in a dynamin-dependent manner, but this internalization does not play a major role in signal transduction. Development 2004, 131, 2395–2408. [Google Scholar] [CrossRef] [PubMed]
- Gallet, A.; Therond, P.P. Temporal modulation of the Hedgehog morphogen gradient by a Patched-dependent targeting to lysosomal compartment. Dev. Biol. 2005, 277, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Hooper, J.E.; Scott, M.P. The Drosophila patched gene encodes a putative membrane protein required for segmental patterning. Cell 1989, 59, 751–765. [Google Scholar] [CrossRef]
- Marigo, V.; Davey, R.A.; Zuo, Y.; Cunningham, J.M.; Tabin, C.J. Biochemical evidence that Patched is the Hedgehog receptor. Nature 1996, 384, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Stone, D.M.; Hynes, M.; Armanini, M.; Swanson, T.A.; Gu, Q.; Johnson, R.L.; Scott, M.P.; Pennica, D.; Goddard, A.; Phillips, H.; et al. The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature 1996, 384, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Fuse, N.; Maiti, T.; Wang, B.; Porter, J.A.; Hall, T.M.; Leahy, D.J.; Beachy, P.A. Sonic hedgehog protein signals not as a hydrolytic enzyme but as an apparent ligand for Patched. Proc. Natl. Acad. Sci. USA 1999, 96, 10992–10999. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.T.; Gratwick, K.S.; Kollman, J.; Park, D.; Nies, D.H.; Goffeau, A.; Saier, M.H., Jr. The RND permease superfamily: An ancient, ubiquitous and diverse family that includes human disease and development proteins. J. Mol. Microbiol. Biotechnol. 1999, 1, 107–125. [Google Scholar] [PubMed]
- Loftus, S.K.; Morris, J.A.; Carstea, E.D.; Gu, J.Z.; Cummings, C.; Brown, A.; Ellison, J.; Ohno, K.; Rosenfeld, M.A.; Tagle, D.A.; et al. Murine model of Niemann-Pick C disease: Mutation in a cholesterol homeostasis gene. Science 1997, 277, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Carstea, E.D.; Morris, J.A.; Coleman, K.G.; Loftus, S.K.; Zhang, D.; Cummings, C.; Gu, J.; Rosenfeld, M.A.; Pavan, W.J.; Krizman, D.B.; et al. Niemann-Pick C1 disease gene: Homology to mediators of cholesterol homeostasis. Science 1997, 277, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Carrillo, G.; Torroja, C.; Guerrero, I. The sterol-sensing domain of Patched protein seems to control Smoothened activity through Patched vesicular trafficking. Curr. Biol. 2001, 11, 601–607. [Google Scholar] [CrossRef]
- Strutt, H.; Thomas, C.; Nakano, Y.; Stark, D.; Neave, B.; Taylor, A.M.; Ingham, P.W. Mutations in the sterol-sensing domain of Patched suggest a role for vesicular trafficking in Smoothened regulation. Curr. Biol. 2001, 11, 608–613. [Google Scholar] [CrossRef]
- Karpen, H.E.; Bukowski, J.T.; Hughes, T.; Gratton, J.P.; Sessa, W.C.; Gailani, M.R. The Sonic hedgehog receptor Patched associates with Caveolin-1 in cholesterol-rich microdomains of the plasma membrane. J. Biol. Chem. 2001, 276, 19503–19511. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Mann, R.K.; Sever, N.; Beachy, P.A. Genetic and biochemical definition of the Hedgehog receptor. Genes Dev. 2010, 24, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Desbordes, S.C.; Sanson, B. The glypican Dally-like is required for Hedgehog signalling in the embryonic epidermis of Drosophila. Development 2003, 130, 6245–6255. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.H.; Pappano, W.N.; Saunders, A.M.; Kim, M.S.; Leahy, D.J.; Beachy, P.A. Dally-like core protein and its mammalian homologues mediate stimulatory and inhibitory effects on Hedgehog signal response. Proc. Natl. Acad. Sci. USA 2010, 107, 5869–5874. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Saunders, A.M.; Hamaoka, B.Y.; Beachy, P.A.; Leahy, D.J. Structure of the protein core of the glypican Dally-like and localization of a region important for Hedgehog signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 13112–13117. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, S.; Hervold, K.; Ramirez-Weber, F.A.; Kornberg, T.B. Two Patched protein subtypes and a conserved domain of group I proteins that regulates turnover. J. Biol. Chem. 2008, 283, 30964–30969. [Google Scholar] [CrossRef] [PubMed]
- Camp, D.; Currie, K.; Labbe, A.; van Meyel, D.J.; Charron, F. Ihog and Boi are essential for Hedgehog signaling in Drosophila. Neural Dev. 2010, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Wojcinski, A.; Nakato, H.; Soula, C.; Glise, B. Dsulfatase-1 fine-tunes Hedgehog patterning activity through a novel regulatory feedback loop. Dev. Biol. 2011, 358, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, D.; Liu, A.; Rakeman, A.S.; Murcia, N.S.; Niswander, L.; Anderson, K.V. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 2003, 426, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Corbit, K.C.; Aanstad, P.; Singla, V.; Norman, A.R.; Stainier, D.Y.; Reiter, J.F. Vertebrate Smoothened functions at the primary cilium. Nature 2005, 437, 1018–1021. [Google Scholar] [CrossRef] [PubMed]
- Haycraft, C.J.; Banizs, B.; Aydin-Son, Y.; Zhang, Q.; Michaud, E.J.; Yoder, B.K. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein Polaris for processing and function. PLoS Genet. 2005, 1, e53. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, R.; Milenkovic, L.; Scott, M.P. Patched1 regulates Hedgehog signaling at the primary cilium. Science 2007, 317, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, T.B. The contrasting roles of primary cilia and cytonemes in Hh signaling. Dev. Biol. 2014, 394, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Berg, H.C. Random Walks in Biology, New, Expanded Edition ed.; Princeton University Press: Princeton, NJ, USA, 1993. [Google Scholar]
- Wolpert, L. Positional information and the spatial pattern of cellular differentiation. J. Theor. Biol. 1969, 25, 1–47. [Google Scholar] [CrossRef]
- Turing, A.M. The chemical basis of morphogenesis. Philos. Trans. R. Soc. Lond. B 1952, 237, 37–72. [Google Scholar] [CrossRef]
- Marcon, L.; Sharpe, J. Turing patterns in development: What about the horse part? Curr. Opin. Genet. Dev. 2012, 22, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Miura, T. Reaction-diffusion model as a framework for understanding biological pattern formation. Science 2010, 329, 1616–1620. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Francis-West, P.H.; Widelitz, R.B.; Jiang, T.X.; Ting-Berreth, S.; Tickle, C.; Wolpert, L.; Chuong, C.M. Local inhibitory action of Bmps and their relationships with activators in feather formation: Implications for periodic patterning. Dev. Biol. 1998, 196, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Menshykau, D.; Kraemer, C.; Iber, D. Branch mode selection during early lung development. PLoS Comput. Biol. 2012, 8, e1002377. [Google Scholar] [CrossRef] [PubMed]
- Economou, A.D.; Ohazama, A.; Porntaveetus, T.; Sharpe, P.T.; Kondo, S.; Basson, M.A.; Gritli-Linde, A.; Cobourne, M.T.; Green, J.B. Periodic stripe formation by a turing mechanism operating at growth zones in the mammalian palate. Nat. Genet. 2012, 44, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Iber, D. Inter-dependent tissue growth and turing patterning in a model for long bone development. Phys. Biol. 2013, 10, 056009. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.; Rogers, K.W.; Yu, S.R.; Brand, M.; Schier, A.F. Morphogen transport. Development 2013, 140, 1621–1638. [Google Scholar] [CrossRef] [PubMed]
- Verbeni, M.; Sanchez, O.; Mollica, E.; Siegl-Cachedenier, I.; Carleton, A.; Guerrero, I.; Ruiz i Altaba, A.; Soler, J. Morphogenetic action through flux-limited spreading. Phys. Life Rev. 2013, 10, 457–475. [Google Scholar] [CrossRef] [PubMed]
- Teimouri, H.; Kolomeisky, A.B. New model for understanding mechanisms of biological signaling: Direct transport via cytonemes. J. Phys. Chem. Lett. 2016, 7, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Collier, J.R.; Monk, N.A.; Maini, P.K.; Lewis, J.H. Pattern formation by lateral inhibition with feedback: A mathematical model of Delta-Notch intercellular signalling. J. Theor. Biol. 1996, 183, 429–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasilopoulos, G.; Painter, K.J. Pattern formation in discrete cell tissues under long range filopodia-based direct cell to cell contact. Math. Biosci. 2016, 273, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.K.; Lappalainen, P. Filopodia: Molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 2008, 9, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Bornschlogl, T. How filopodia pull: What we know about the mechanics and dynamics of filopodia. Cytoskeleton (Hoboken) 2013, 70, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Mogilner, A.; Rubinstein, B. The physics of filopodial protrusion. Biophys. J. 2005, 89, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Erban, R.; Flegg, M.B.; Papoian, G.A. Multiscale stochastic reaction-diffusion modeling: Application to actin dynamics in filopodia. Bull. Math. Biol. 2014, 76, 799–818. [Google Scholar] [CrossRef] [PubMed]
- Leijnse, N.; Oddershede, L.B.; Bendix, P.M. Helical buckling of actin inside filopodia generates traction. Proc. Natl. Acad. Sci. USA 2015, 112, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Zhuravlev, P.I.; Lan, Y.; Minakova, M.S.; Papoian, G.A. Theory of active transport in filopodia and stereocilia. Proc. Natl. Acad. Sci. USA 2012, 109, 10849–10854. [Google Scholar] [CrossRef] [PubMed]
- Nahmad, M.; Stathopoulos, A. Dynamic interpretation of Hedgehog signaling in the Drosophila wing disc. PLoS Biol. 2009, 7, e1000202. [Google Scholar] [CrossRef] [PubMed]
- Dessaud, E.; Ribes, V.; Balaskas, N.; Yang, L.L.; Pierani, A.; Kicheva, A.; Novitch, B.G.; Briscoe, J.; Sasai, N. Dynamic assignment and maintenance of positional identity in the ventral neural tube by the morphogen Sonic hedgehog. PLoS Biol. 2010, 8, e1000382. [Google Scholar] [CrossRef] [PubMed]
- Rogers, K.W.; Schier, A.F. Morphogen gradients: From generation to interpretation. Annu. Rev. Cell. Dev. Biol. 2011, 27, 377–407. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Lo, W.C.; Suhalim, J.L.; Digman, M.A.; Gratton, E.; Nie, Q.; Lander, A.D. Free extracellular diffusion creates the Dpp morphogen gradient of the Drosophila wing disc. Curr. Biol. 2012, 22, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.; Rogers, K.W.; Jordan, B.M.; Lee, J.S.; Robson, D.; Ramanathan, S.; Schier, A.F. Differential diffusivity of Nodal and Lefty underlies a reaction-diffusion patterning system. Science 2012, 336, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, J.; Small, S. Morphogen rules: Design principles of gradient-mediated embryo patterning. Development 2015, 142, 3996–4009. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Kicheva, A.; Ribeiro, A.; Blassberg, R.; Page, K.M.; Barnes, C.P.; Briscoe, J. Ptch1 and Gli regulate Shh signalling dynamics via multiple mechanisms. Nat. Commun. 2015, 6, 6709. [Google Scholar] [CrossRef] [PubMed]
- Kicheva, A.; Pantazis, P.; Bollenbach, T.; Kalaidzidis, Y.; Bittig, T.; Julicher, F.; Gonzalez-Gaitan, M. Kinetics of morphogen gradient formation. Science 2007, 315, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Schwank, G.; Dalessi, S.; Yang, S.F.; Yagi, R.; de Lachapelle, A.M.; Affolter, M.; Bergmann, S.; Basler, K. Formation of the long range Dpp morphogen gradient. PLoS Biol. 2011, 9, e1001111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kornberg, T.B.; Roy, S. Cytonemes as specialized signaling filopodia. Development 2014, 141, 729–736. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simon, E.; Aguirre-Tamaral, A.; Aguilar, G.; Guerrero, I. Perspectives on Intra- and Intercellular Trafficking of Hedgehog for Tissue Patterning. J. Dev. Biol. 2016, 4, 34. https://doi.org/10.3390/jdb4040034
Simon E, Aguirre-Tamaral A, Aguilar G, Guerrero I. Perspectives on Intra- and Intercellular Trafficking of Hedgehog for Tissue Patterning. Journal of Developmental Biology. 2016; 4(4):34. https://doi.org/10.3390/jdb4040034
Chicago/Turabian StyleSimon, Eléanor, Adrián Aguirre-Tamaral, Gustavo Aguilar, and Isabel Guerrero. 2016. "Perspectives on Intra- and Intercellular Trafficking of Hedgehog for Tissue Patterning" Journal of Developmental Biology 4, no. 4: 34. https://doi.org/10.3390/jdb4040034




