Heavy Metal Pre-Conditioning History Modulates Spartina patens Physiological Tolerance along a Salinity Gradient
Abstract
:1. Introduction
2. Results
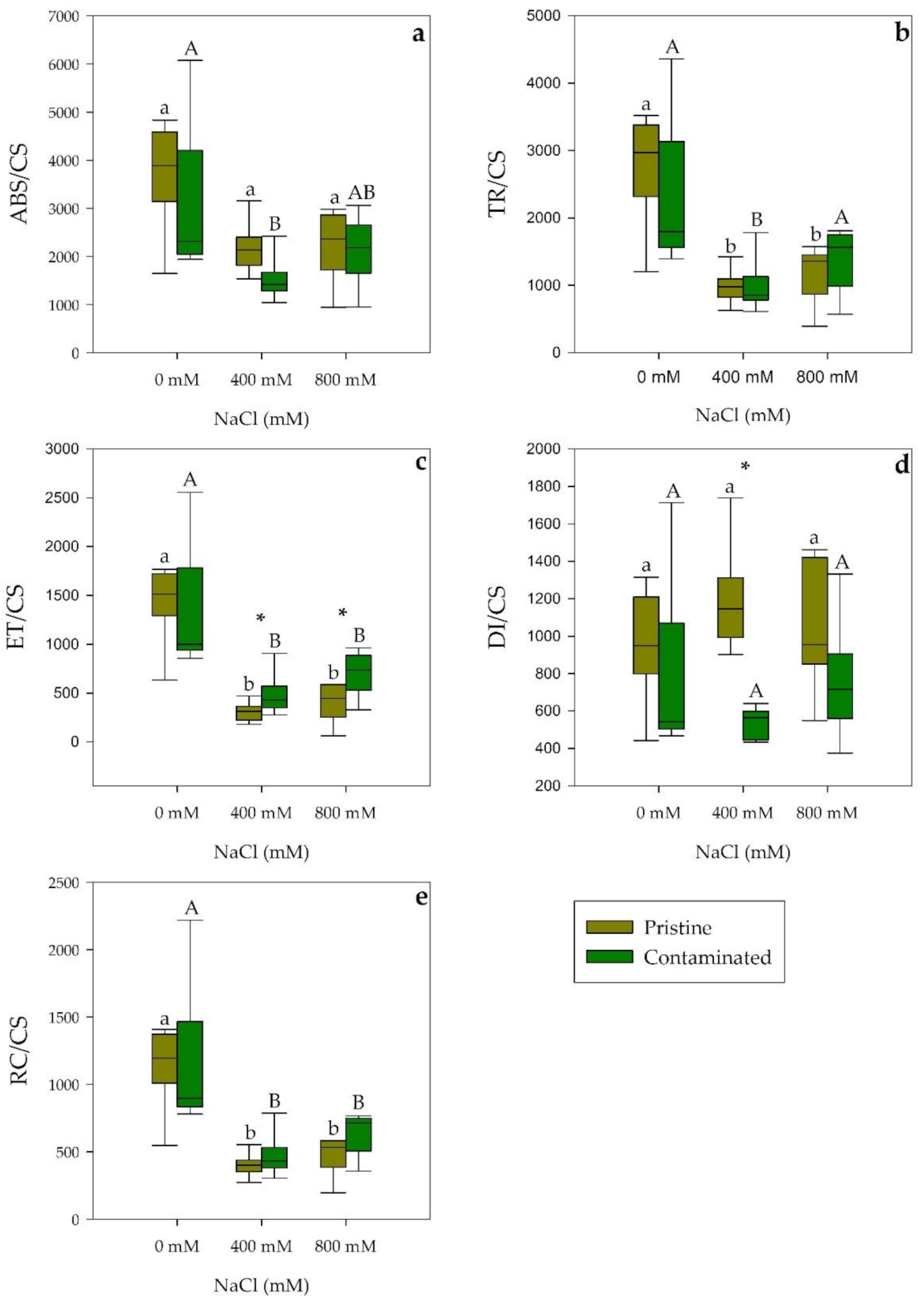
2.1. Photochemical Processes
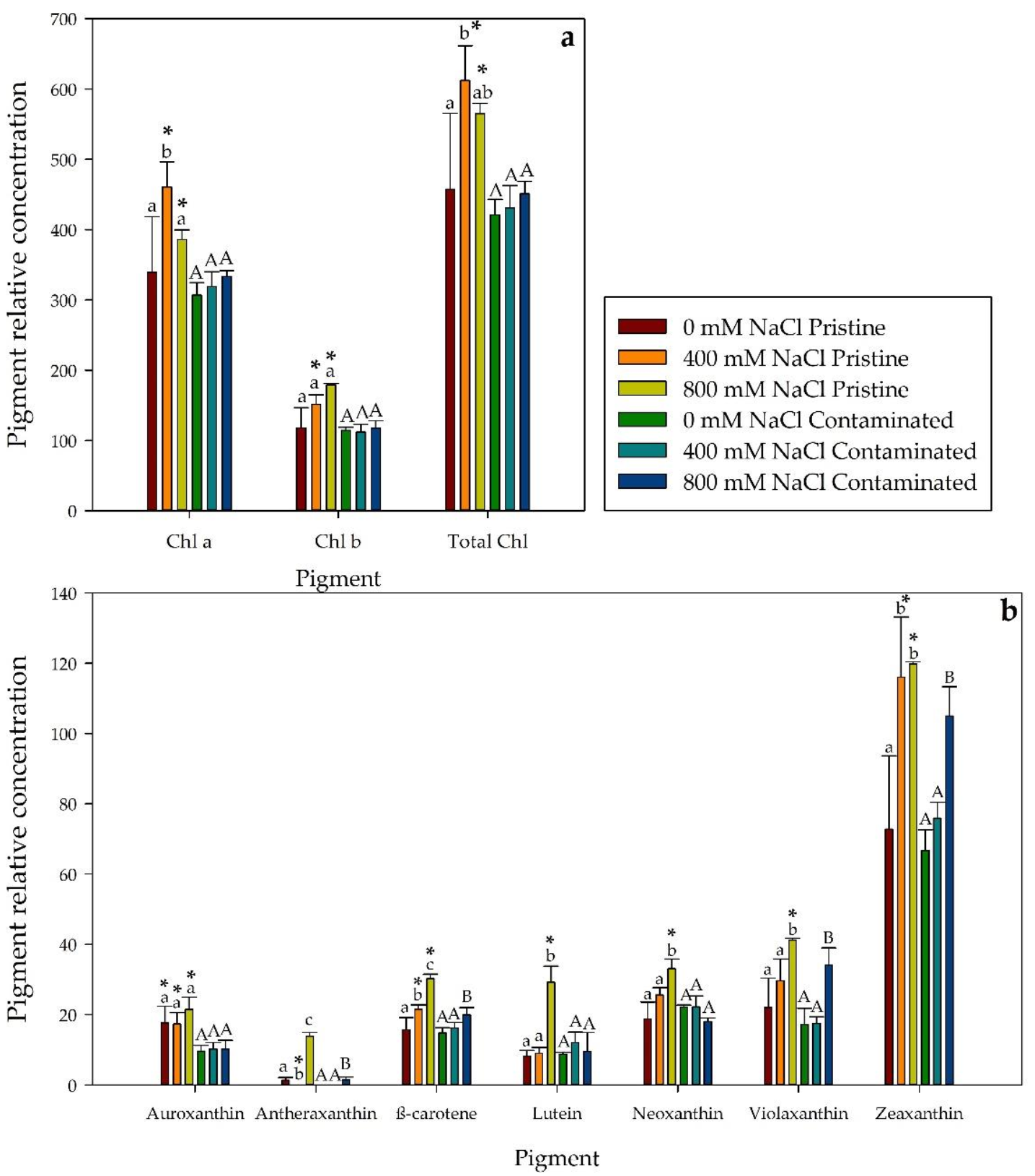
2.2. Photosynthetic Pigments Profile
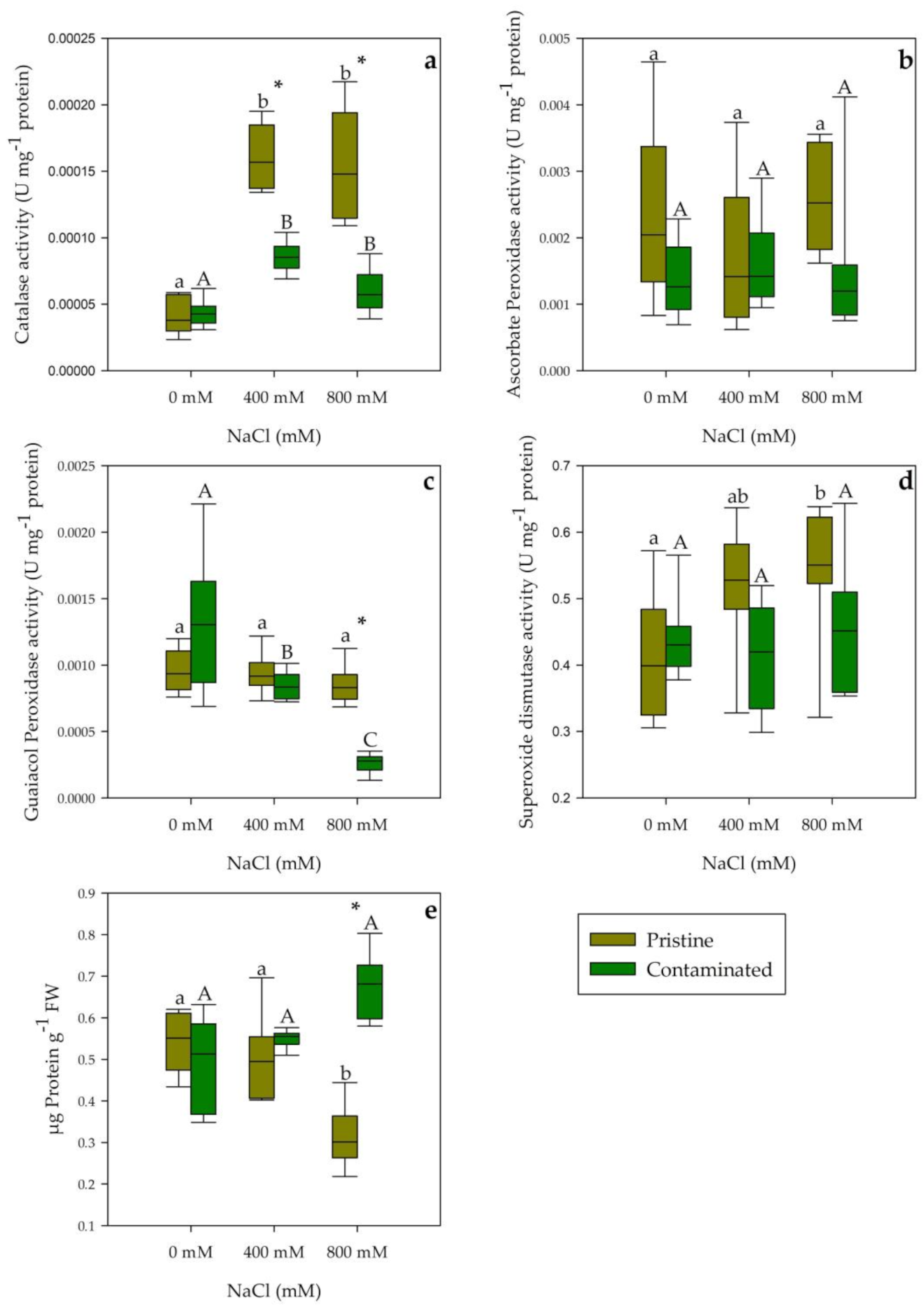
2.3. Antioxidant Enzymatic Activities
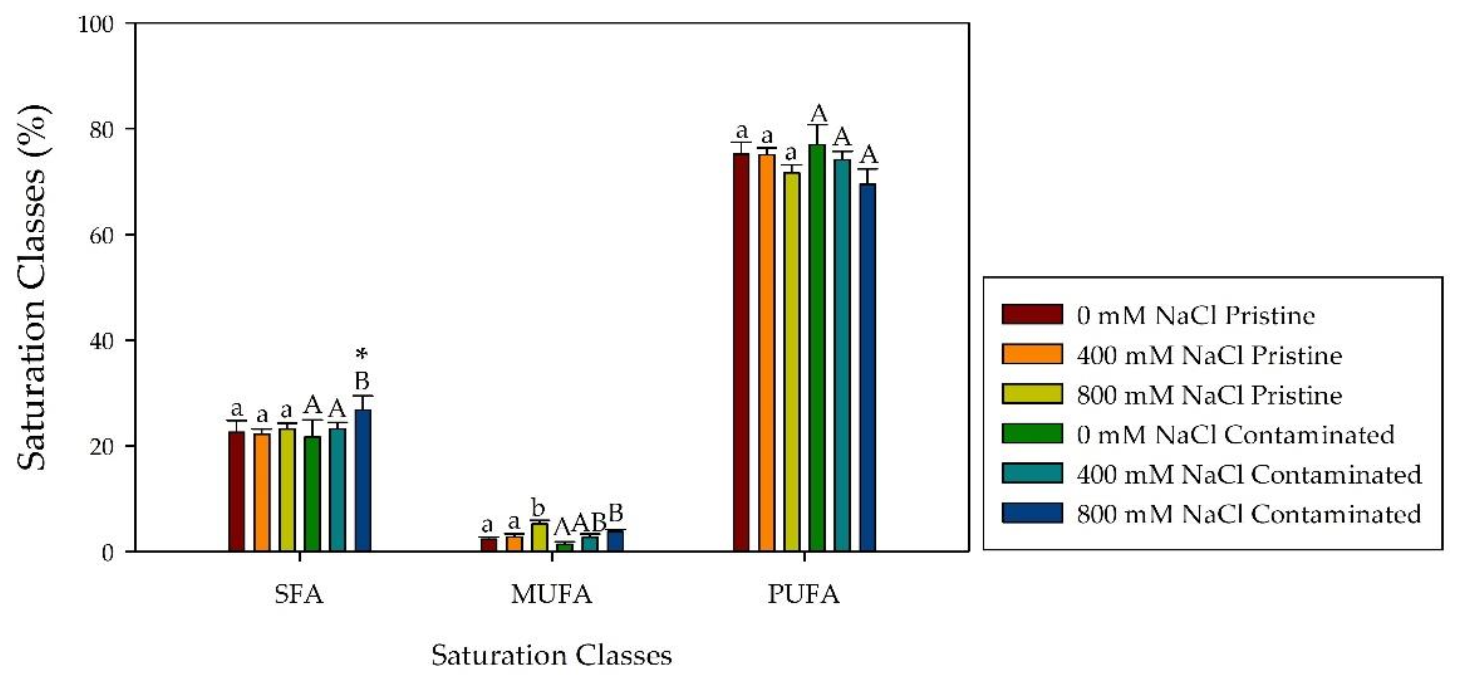
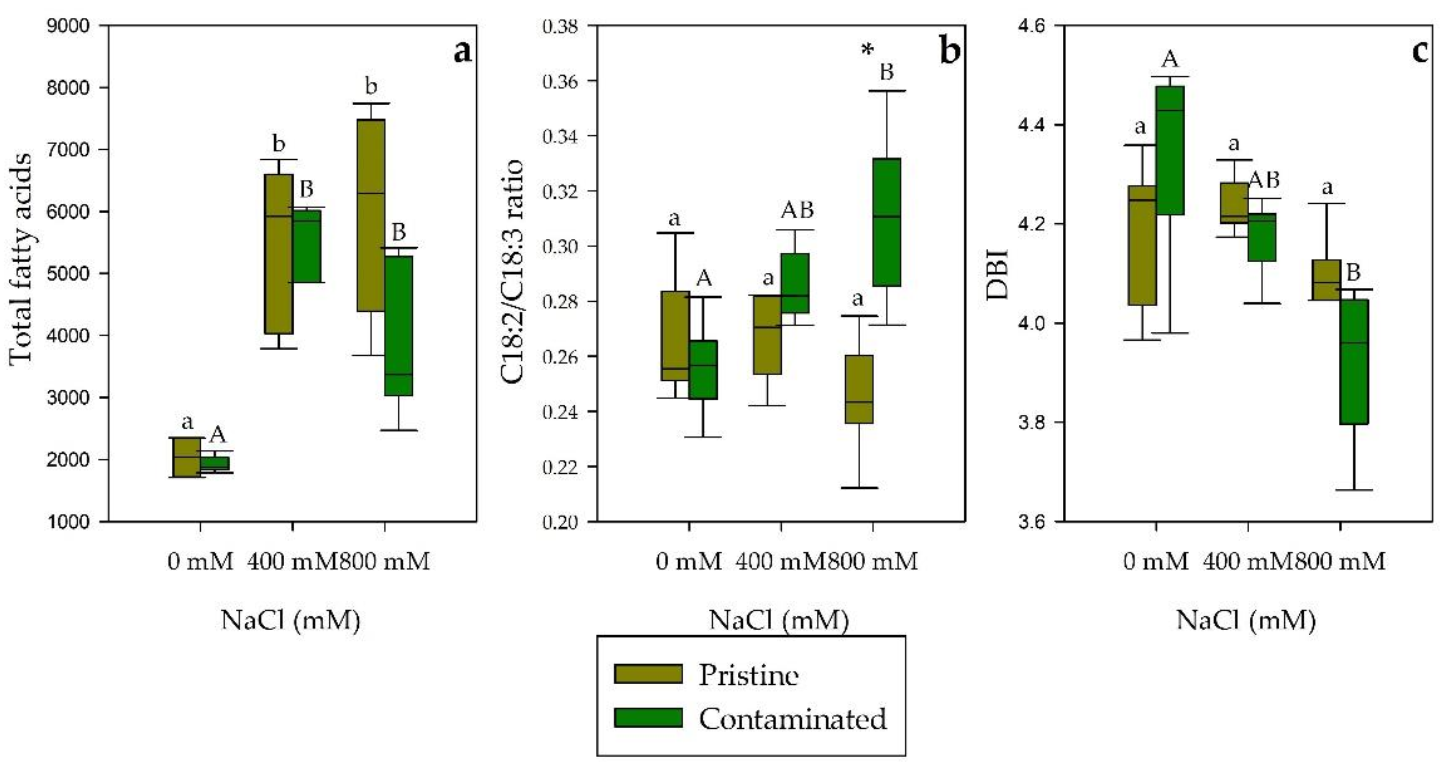
2.4. Fatty Acid Composition
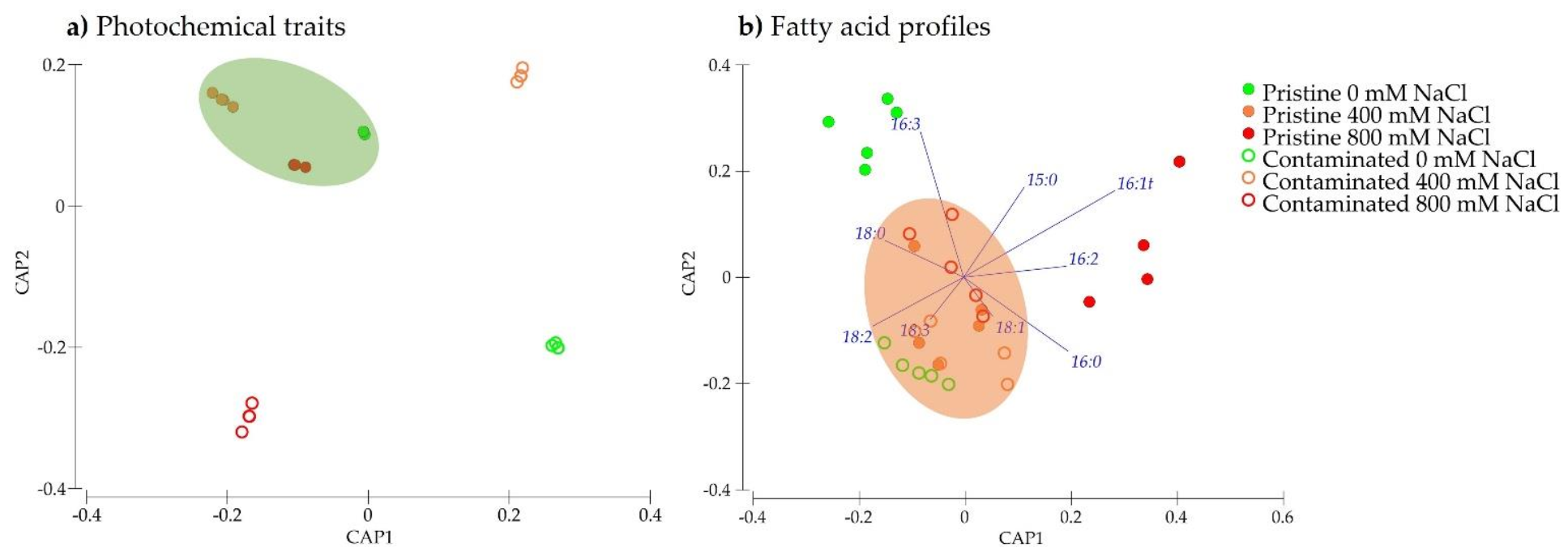
2.5. Multivariate Classification
3. Discussion
4. Material and Methods
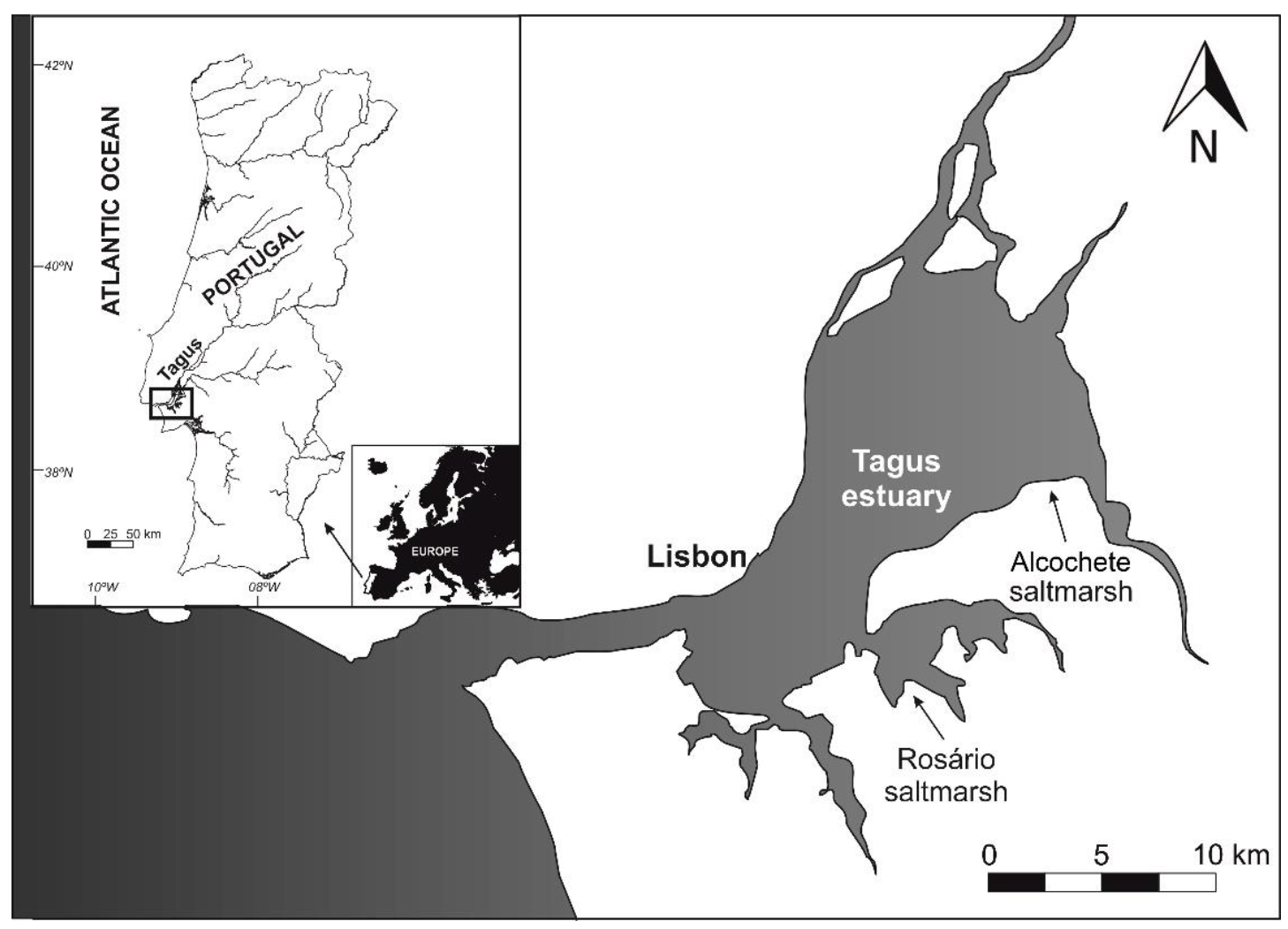
4.1. Sampling Sites and Plant Material Collection
4.2. Experimental Setup
4.3. Pulse Amplitude Modulated (PAM) Fluorometry
4.4. Pigment Profiling
4.5. Leaf Fatty Acid Composition
4.6. Oxidative Stress Biomarkers
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barros, V.R.; Field, C.B.; Dokken, D.J.; Mastrandrea, M.D.; Mach, K.J.; Bilir, T.E.; Chatterjee, M.; Ebi, K.L.; Estrada, Y.O.; Genova, R.C.; et al. Climate Change 2014 Impacts, Adaptation and Vulnerability Part B: Regional Aspects: Working Group Ii Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Barros, V.R., Field, C.B., Dokken, D.J., Mastrandrea, M.D., Mach, K.J., Eds.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar] [CrossRef] [Green Version]
- Shiklomanov, I.A. Appraisal and assessment of world water resources. Water Int. 2000, 25. [Google Scholar] [CrossRef]
- Glick, B.R.; Cheng, Z.; Czarny, J.; Duan, J. Promotion of Plant Growth by ACC Deaminase-Producing Soil Bacteria. Eur. J. Plant Pathol. 2007. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil Salinity: A Serious Environmental Issue and Plant Growth Promoting Bacteria as One of the Tools for Its Alleviation. Saudi J. Biol. Sci. 2015. [Google Scholar] [CrossRef] [Green Version]
- Bacilio, M.; Rodriguez, H.; Moreno, M.; Hernandez, J.P.; Bashan, Y. Mitigation of Salt Stress in Wheat Seedlings by a Gfp-Tagged Azospirillum Lipoferum. Biol. Fertil. Soils 2004, 40. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbarimoghaddam, H.; Galavi, M.; Ghanbari, A.; Panjehkeh, N. Salinity Effects on Seed Germination and Seedling Growth of Bread Wheat Cultivars. Trakia J. Sci. 2011, 9, 43–50. [Google Scholar]
- Jamil, A.; Riaz, S.; Ashraf, M.; Foolad, M.R. Gene Expression Profiling of Plants under Salt Stress. Crit. Rev. Plant Sci. 2011. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity Tolerance in Halophytes. New Phytol. 2008. [Google Scholar] [CrossRef] [PubMed]
- Duarte, B.; Santos, D.; Marques, J.C.; Caçador, I. Biophysical Probing of Spartina Maritima Photo-System II Changes during Prolonged Tidal Submersion Periods. Plant Physiol. Biochem. 2014, 77. [Google Scholar] [CrossRef] [Green Version]
- Adam, P. Saltmarshes in a Time of Change. Environ. Conserv. 2002, 29. [Google Scholar] [CrossRef]
- Caçador, I.; Tibério, S.; Cabral, H.N. Species zonation in corroios salt marsh in the tagus estuary (Portugal) and its dynamics in the past fifty years. Hydrobiologia 2007, 587, 205–211. [Google Scholar] [CrossRef]
- Jump, A.S.; Peñuelas, J. Running to Stand Still: Adaptation and the Response of Plants to Rapid Climate Change. Ecol. Lett. 2005, 8, 1010–1020. [Google Scholar] [CrossRef]
- Gratani, L.; Crescente, M.F. Phenology and Leaf Adaptive Strategies of Mediterranean Maquis Plants. Ecol. Mediterr. 1997, 23, 11–19. [Google Scholar] [CrossRef]
- Valladares, F.; Gianoli, E.; Gómez, J.M. Ecological Limits to Plant Phenotypic Plasticity. New Phytol. 2007, 176, 749–763. [Google Scholar] [CrossRef] [PubMed]
- Duarte, B.; Baeta, A.; Rousseau-Gueutin, M.; Ainouche, M.; Marques, J.C.; Caçador, I. A Tale of Two Spartinas: Climatic, Photobiological and Isotopic Insights on the Fitness of Non-Indigenous versus Native Species. Estuar. Coast. Shelf Sci. 2015, 167, 178–190. [Google Scholar] [CrossRef]
- Caçador, I.; Vale, C.; Catarino, F. The Influence of Plants on Concentration and Fractionation of Zn, Pb and Cu in Salt Marsh Sediments (Tagus Estuary, Portugal). J. Aquat. Ecosyst. Stress Recov. 1996, 5. [Google Scholar] [CrossRef]
- Flowers, T.J. Improving crop salt tolerance. J. Exp. Bot. 2004, 55. [Google Scholar] [CrossRef]
- Hester, M.W.; Mendelssohn, I.A.; Mckee, K.L. Intraspecific Variation in Salt Tolerance and Morphology in the Coastal Grass Spartina Patens (Poaceae). Am. J. Bot. 1996, 83. [Google Scholar] [CrossRef]
- Carreiras, J.; Alberto Pérez-Romero, J.; Mateos-Naranjo, E.; Redondo-Gómez, S.; Rita Matos, A.; Caçador, I.; Duarte, B. The Effect of Heavy Metal Contamination Pre-Conditioning in the Heat Stress Tolerance of Native and Invasive Mediterranean Halophytes. Ecol. Indic. 2020, 111. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Marotzke, J.; Nala, G.; Cao, L.; Corti, S.; Dunne, J.P.; Engelbrecht, F.; Fisher, E.; Fyfe, J.C.; Jones, C.; et al. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge Univ. Press 2021, No. In Press, 3949. [Google Scholar]
- Rodrigues, M.; Fortunato, A.B. Assessment of a Three-Dimensional Baroclinic Circulation Model of the Tagus Estuary (Portugal). AIMS Environ. Sci. 2017, 4, 763–787. [Google Scholar] [CrossRef]
- Dasgupta, S.; Hossain, M.M.; Huq, M.; Wheeler, D. Climate Change and Soil Salinity: The Case of Coastal Bangladesh. Ambio 2015, 44. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, D. Quantifying Vulnerability to Climate Change: Implications for Adaptation Assistance. SSRN Electron. J. 2012. [Google Scholar] [CrossRef] [Green Version]
- Duarte, B.; Sleimi, N.; Cagador, I. Biophysical and Biochemical Constraints Imposed by Salt Stress: Learning from Halophytes. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silvestri, S.; Defina, A.; Marani, M. Tidal Regime, Salinity and Salt Marsh Plant Zonation. Estuar. Coast. Shelf Sci. 2005, 62. [Google Scholar] [CrossRef]
- Duarte, B.; Santos, D.; Marques, J.C.; Caçador, I. Ecophysiological Constraints of Two Invasive Plant Species under a Saline Gradient: Halophytes versus Glycophytes. Estuar. Coast. Shelf Sci. 2015, 167, 154–165. [Google Scholar] [CrossRef]
- Duarte, B.; Matos, A.R.; Marques, J.C.; Caçador, I. Leaf Fatty Acid Remodeling in the Salt-Excreting Halophytic Grass Spartina Patens along a Salinity Gradient. Plant Physiol. Biochem. 2018, 124, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Govindjee; Bosa, K.; Kościelniak, J.; Zuk-Gołaszewska, K. Effects of Salt Stress on Photosystem II Efficiency and CO2 Assimilation of Two Syrian Barley Landraces. Environ. Exp. Bot. 2011, 73. [Google Scholar] [CrossRef]
- Duarte, B.; Cabrita, M.T.; Gameiro, C.; Matos, A.R.; Godinho, R.; Marques, J.C.; Caçador, I. Disentangling the Photochemical Salinity Tolerance in Aster Tripolium, L.: Connecting Biophysical Traits with Changes in Fatty Acid Composition. Plant Biol. 2017, 19. [Google Scholar] [CrossRef] [PubMed]
- Duarte, B.; Santos, D.; Marques, J.C.; Caçador, I. Ecophysiological Adaptations of Two Halophytes to Salt Stress: Photosynthesis, PS II Photochemistry and Anti-Oxidant Feedback-Implications for Resilience in Climate Change. Plant Physiol. Biochem. 2013, 67. [Google Scholar] [CrossRef] [PubMed]
- Wentworth, M.; Ruban, A.V.; Horton, P. Chlorophyll Fluorescence Quenching in Isolated Light Harvesting Complexes Induced by Zeaxanthin. FEBS Lett. 2000, 471. [Google Scholar] [CrossRef] [Green Version]
- Duarte, B.; Santos, D.; Caçador, I. Halophyte anti-oxidant feedback seasonality in two salt marshes with different degrees of metal contamination: Search for an efficient biomarker. Funct. Plant Biol. 2013, 40. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. The Role of Endogenous Nitric Oxide in Salicylic Acid-Induced up-Regulation of Ascorbate-Glutathione Cycle Involved in Salinity Tolerance of Pepper (Capsicum Annuum L.) Plants. Plant Physiol. Biochem. 2020, 147, 10–20. [Google Scholar] [CrossRef]
- Zhou, H.; Lin, H.; Chen, S.; Becker, K.; Yang, Y.; Zhao, J.; Kudla, J.; Schumaker, K.S.; Guo, Y. Inhibition of the Arabidopsis Salt Overly Sensitive Pathway by 14-3-3 Proteins. Plant Cell 2014, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheteiwy, M.S.; Shao, H.; Qi, W.; Hamoud, Y.A.; Shaghaleh, H.; Khan, N.U.; Yang, R.; Tang, B. GABA-Alleviated Oxidative Injury Induced by Salinity, Osmotic Stress and Their Combination by Regulating Cellular and Molecular Signals in Rice. Int. J. Mol. Sci. 2019, 20, 5709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahanger, M.A.; Aziz, U.; Alsahli, A.A.; Alyemeni, M.N.; Ahmad, P. Influence of Exogenous Salicylic Acid and Nitric Oxide on Growth, Photosynthesis and Ascorbate- Glutathione Cycle in Salt Stressed Vigna Angularis. Biomolecules 2020, 10, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, I.; Raza, M.A.; Awan, S.A.; Shah, G.A.; Rizwan, M.; Ali, B.; Tariq, R.; Hassan, M.J.; Alyemeni, M.N.; Brestic, M.; et al. Amelioration of Salt Induced Toxicity in Pearl Millet by Seed Priming with Silver Nanoparticles (AgNPs): The Oxidative Damage, Antioxidant Enzymes and Ions Uptake Are Major Determinants of Salt Tolerant Capacity. Plant Physiol. Biochem. 2020, 156, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Ben Hamed, K.; Ben Youssef, N.; Ranieri, A.; Zarrouk, M.; Abdelly, C. Changes in Content and Fatty Acid Profiles of Total Lipids and Sulfolipids in the Halophyte Crithmum Maritimum under Salt Stress. J. Plant Physiol. 2005, 162. [Google Scholar] [CrossRef]
- Mène-Saffrané, L.; Dubugnon, L.; Chételat, A.; Stolz, S.; Gouhier-Darimont, C.; Farmer, E.E. Nonenzymatic Oxidation of Trienoic Fatty Acids Contributes to Reactive Oxygen Species Management in Arabidopsis. J. Biol. Chem. 2009, 284. [Google Scholar] [CrossRef] [Green Version]
- Siefermann-Harms, D.; Angerhofer, A. Evidence for an O2-Barrier in the Light-Harvesting Chlorophyll-a/b-Protein Complex LHC II. Photosynth. Res. 1998, 55. [Google Scholar] [CrossRef]
- Trémolières, A.; Siegenthaler, P.-A. Reconstitution of photosynthetic structures and activities with lipids. Lipids Photosynth. Struct. Funct. Genet. 1998, 6. [Google Scholar] [CrossRef]
- Hobe, S.; Prytulla, S.; Kühlbrandt, W.; Paulsen, H. Trimerization and Crystallization of Reconstituted Light-Harvesting Chlorophyll a/b Complex. EMBO J. 1994, 13. [Google Scholar] [CrossRef]
- Hobe, S.; Förster, R.; Klingler, J.; Paulsen, H. N-Proximal Sequence Motif in Light-Harvesting Chlorophyll a/b-Binding Protein Is Essential for the Trimerization of Light-Harvesting Chlorophyll Alb Complex. Biochemistry 1995, 34. [Google Scholar] [CrossRef] [PubMed]
- Nußberger, S.; Dörr, K.; Wang, D.N.; Kühlbrandt, W. Lipid-Protein Interactions in Crystals of Plant Light-Harvesting Complex. J. Mol. Biol. 1993, 234. [Google Scholar] [CrossRef] [PubMed]
- Duarte, B.; Prata, D.; Matos, A.R.; Cabrita, M.T.; Caçador, I.; Marques, J.C.; Cabral, H.N.; Reis-Santos, P.; Fonseca, V.F. Ecotoxicity of the Lipid-Lowering Drug Bezafibrate on the Bioenergetics and Lipid Metabolism of the Diatom Phaeodactylum Tricornutum. Sci. Total Environ. 2019, 650. [Google Scholar] [CrossRef]
- Duarte, B.; Caçador, I.; Matos, A.R. Lipid Landscape Remodelling in Sarcocornia Fruticosa Green and Red Physiotypes. Plant Physiol. Biochem. 2020, 157. [Google Scholar] [CrossRef]
- Duarte, B.; Matos, A.R.; Caçador, I. Photobiological and Lipidic Responses Reveal the Drought Tolerance of Aster Tripolium Cultivated under Severe and Moderate Drought: Perspectives for Arid Agriculture in the Mediterranean. Plant Physiol. Biochem. 2020, 154. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. Preparing the Nutrient Solution. Water-Cult. Method Grow. Plants Soil 1950, 347, 2–33. [Google Scholar]
- Pedro, S.; Duarte, B.; Raposo de Almeida, P.; Caçador, I. Metal Speciation in Salt Marsh Sediments: Influence of Halophyte Vegetation in Salt Marshes with Different Morphology. Estuar. Coast. Shelf Sci. 2015, 167. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The Relationship between the Quantum Yield of Photosynthetic Electron Transport and Quenching of Chlorophyll Fluorescence. Biochim. Biophys. Acta-Gen. Subj. 1989, 990. [Google Scholar] [CrossRef]
- Beer, S.; Björk, M. Measuring Rates of Photosynthesis of Two Tropical Seagrasses by Pulse Amplitude Modulated (PAM) Fluorometry. Aquat. Bot. 2000, 66. [Google Scholar] [CrossRef]
- Beer, S.; Vilenkin, B.; Weil, A.; Veste, M.; Susel, L.; Eshel, A. Measuring Photosynthetic Rates in Seagrasses by Pulse Amplitude Modulated (PAM) Fluorometry. Mar. Ecol. Prog. Ser. 1998, 174. [Google Scholar] [CrossRef]
- Runcie, J.W.; Durako, M.J. Among-Shoot Variability and Leaf-Specific Absorptance Characteristics Affect Diel Estimates of in Situ Electron Transport of Posidonia Australis. Aquat. Bot. 2004, 80. [Google Scholar] [CrossRef]
- Marshall, H.L.; Geider, R.J.; Flynn, K.J. A Mechanistic Model of Photoinhibition. New Phytol. 2000, 145. [Google Scholar] [CrossRef] [Green Version]
- Platt, T.; Gallegos, C.L.; Harrison, W.G. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. Mar. Res. 1980, 38, 687–701. [Google Scholar]
- Zhu, X.G.; Govindjee; Baker, N.R.; DeSturler, E.; Ort, D.R.; Long, S.P. Chlorophyll a Fluorescence Induction Kinetics in Leaves Predicted from a Model Describing Each Discrete Step of Excitation Energy and Electron Transfer Associated with Photosystem II. Planta 2005, 223. [Google Scholar] [CrossRef] [PubMed]
- Küpper, H.; Seibert, S.; Parameswaran, A. Fast, Sensitive and Inexpensive Alternative to Analytical Pigment HPLC: Quantification of Chlorophylls and Carotenoids in Crude Extracts by Fitting with Gauss Peak Spectra. Anal. Chem. 2007, 79. [Google Scholar] [CrossRef] [PubMed]
- Duarte, B.; Pedro, S.; Marques, J.C.; Adão, H.; Caçador, I. Zostera Noltii Development Probing Using Chlorophyll a Transient Analysis (JIP-Test) under Field Conditions: Integrating Physiological Insights into a Photochemical Stress Index. Ecol. Indic. 2017, 76, 219–229. [Google Scholar] [CrossRef]
- Esquível, M.G.; Matos, A.R.; Marques Silva, J. Rubisco Mutants of Chlamydomonas Reinhardtii Display Divergent Photosynthetic Parameters and Lipid Allocation. Appl. Microbiol. Biotechnol. 2017, 101. [Google Scholar] [CrossRef] [PubMed]
- Tiryakioglu, M.; Eker, S.; Ozkutlu, F.; Husted, S.; Cakmak, I. Antioxidant Defense System and Cadmium Uptake in Barley Genotypes Differing in Cadmium Tolerance. J. Trace Elem. Med. Biol. 2006, 20. [Google Scholar] [CrossRef]
- Teranishi, Y.; Tanaka, A.; Osumi, M.; Fukui, S. Catalase Activities of Hydrocarbon-Utilizing Candida Yeasts. Agric. Biol. Chem. 1974, 38. [Google Scholar] [CrossRef]
- Bergmeyer, H.U.; Gawehn, K.; Grassl, M. Lactatedehydrogenase, UV-assay with pyruvate and NADH. Methods Enzym. Anal. 1974, 2, 574–579. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts. I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial., 1st ed.; PRIMER-E: Plymouth, UK, 2006; 192p. [Google Scholar]
- Cabrita, M.T.; Duarte, B.; Gameiro, C.; Godinho, R.M.; Caçador, I. Photochemical Features and Trace Element Substituted Chlorophylls as Early Detection Biomarkers of Metal Exposure in the Model Diatom Phaeodactylum Tricornutum. Ecol. Indic. 2018, 95. [Google Scholar] [CrossRef]



| Salinity (mM) | 15:0 | 16:0 | 16:1t | 16:3 | 18:0 | 18:1 | 18:2 | 18:3 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | Pristine | 4.16 ± 0.42 a | 12.12 ± 0.72 a | * | 1.34 ± 0.37 a | * | 1.13 ± 0.44 a | 6.26 ± 1.68 a | 0.97 ± 0.38 a | 15.55 ± 0.66 a | 58.46 ± 3.00 a |
| Contaminated | 2.82 ± 1.18 A | 15.34 ± 1.78 A | 0.42 ± 0.24 A | 3.46 ± 1.57 A | 0.94 ± 0.38 A | 0.00 ± 0.00 A | 15.64 ± 0.61 A | 61.37 ± 3.65 A | |||
| 400 | Pristine | 4.07 ± 0.85 a | 12.78 ± 0.59 a | * | 1.02 ± 0.41 a | 0.00 ± 0.00 a | 5.35 ± 0.87 a | 1.74 ± 0.27 a | 15.79 ± 0.96 a | 59.21 ± 1.01 a | |
| Contaminated | 3.20 ± 0.47 A | 14.92 ± 1.03 A | 0.96 ± 0.21 A | 5.08 ± 0.98 A | 1.71 ± 0.50 A | 0.00 ± 0.00 A | 16.49 ± 0.71 A | 57.65 ± 1.41 A | |||
| 800 | Pristine | 4.66 ± 1.20 a | 16.03 ± 1.28 b | * | 3.84 ± 0.86 b | * | 2.47 ± 0.47 a | 1.36 ± 0.18 a | 0.23 ± 0.29 a | 14.06 ± 1.25 a | 57.25 ± 1.34 a |
| Contaminated | 4.01 ± 0.39 B | 13.77 ± 0.93 A | 1.59 ± 0.37 A | 9.00 ± 3.05 A | 2.17 ± 0.74 A | 0.00 ± 0.00 A | 16.41 ± 1.31 A | 53.05 ± 2.87 A |
| JIP-Test | |
|---|---|
| Rapid Light Curves (RLCs) | |
| rETR | Relative electron transport rate at each light intensity (rETR = QY × PAR × 0.5). |
| ETRmax | Maximum ETR after which photo-inhibition can be observed. |
| α | Photosynthetic efficiency, obtained from the initial slope of the RLC. |
| Area | Corresponds to the oxidized quinone pool size available for reduction and is a function of the area above the Kautsky plot. |
| N | Reaction center turnover rate. |
| SM | Corresponds to the energy needed to close all reaction centers. |
| M0 | The net rate of PS II RC closure. |
| δR0 | PS I efficiency in reducing its electron acceptors. |
| PG | Grouping probability, directly related to PS II antennae connectivity. |
| ABS/CS | Absorbed energy flux per cross-section. |
| TR/CS | Trapped energy flux per cross-section |
| ET/CS | Electron transport energy flux per cross-section. |
| DI/CS | Dissipated energy flux per cross-section. |
| RC/CS | The number of available reaction centers per cross-section. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carreiras, J.; Pérez-Romero, J.A.; Mateos-Naranjo, E.; Redondo-Gómez, S.; Matos, A.R.; Caçador, I.; Duarte, B. Heavy Metal Pre-Conditioning History Modulates Spartina patens Physiological Tolerance along a Salinity Gradient. Plants 2021, 10, 2072. https://doi.org/10.3390/plants10102072
Carreiras J, Pérez-Romero JA, Mateos-Naranjo E, Redondo-Gómez S, Matos AR, Caçador I, Duarte B. Heavy Metal Pre-Conditioning History Modulates Spartina patens Physiological Tolerance along a Salinity Gradient. Plants. 2021; 10(10):2072. https://doi.org/10.3390/plants10102072
Chicago/Turabian StyleCarreiras, João, Jesús Alberto Pérez-Romero, Enrique Mateos-Naranjo, Susana Redondo-Gómez, Ana Rita Matos, Isabel Caçador, and Bernardo Duarte. 2021. "Heavy Metal Pre-Conditioning History Modulates Spartina patens Physiological Tolerance along a Salinity Gradient" Plants 10, no. 10: 2072. https://doi.org/10.3390/plants10102072







