Growth, Yield, and Bunch Quality of “Superior Seedless” Vines Grown on Different Rootstocks Change in Response to Salt Stress
Abstract
:1. Introduction
2. Results
2.1. Salinity Injury Index (SI-Index)
2.2. Chlorophyll Fluorescence Parameters
2.3. Photosynthetic Pigment (Chlorophyll and Carotene Content)
2.4. Leaf Area, Shoot Carbohydrate, Proline, and Glycine Accumulation
2.5. Malondialdehyde (MDA), and Electrolyte Leakage Percentage (EL%)
2.6. Na+, K+, and K+/Na+ Content of Leaves
2.7. Growth, Yield, and Bunch Quality
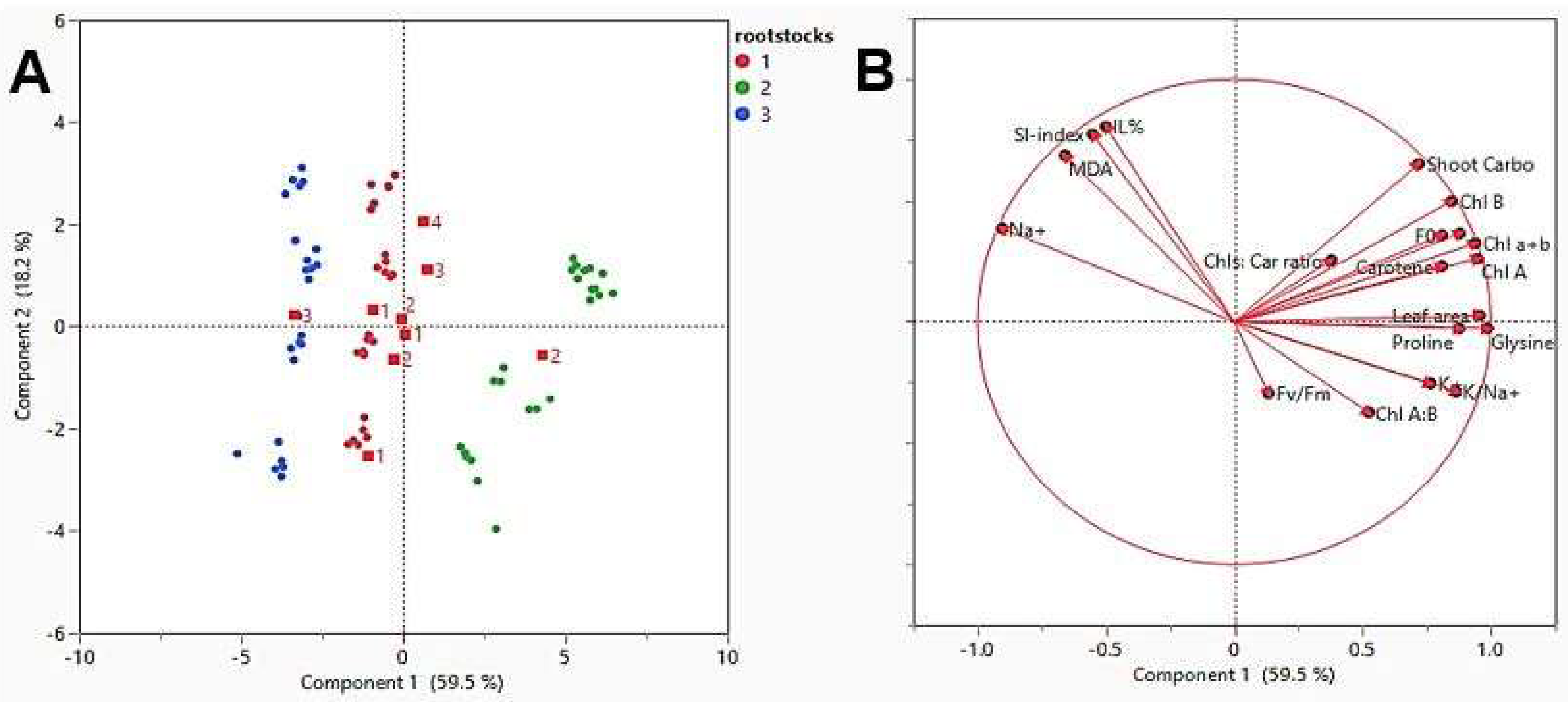
2.8. Data Correlation
3. Discussion
4. Materials and Methods
4.1. Vine and Field Experimental Setup
4.2. Salt Injury Index (SS-Index)
4.3. Chlorophyll Fluorescence (CF) and Photosynthetic Pigment Analysis
4.4. Leaf Area and Shoot Carbohydrate Accumulation
4.5. Leaf Proline and Glycine Content
4.6. Leaf Mineral Content
4.7. Malondialdehyde (MDA) and Ion Leakage%
4.8. Growth, Yield and Bunch Quality
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shalaby, M.Y.; Al-Zahrani, K.H.; Baig, M.B.; Straquadine, G.S.; Aldosari, F. Threats and challenges to sustainable agriculture and rural development in Egypt: Implications for agricultural extension. J. Anim. Plant Sci. 2011, 21, 581–588. [Google Scholar]
- FAO. Source FAOSTAT. 2019. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 26 December 2019).
- Grieve, C.M.; Grattan, S.R.; Maas, E.V. Plant salt tolerance in agricultural salinity assessment and management. Am. Soc. Civ. Eng. (ASCE) 2012, 71, 405–459. [Google Scholar]
- Li, C.; Srivastava, R.K.; Athar, M. The influence of grapevine rootstocks on scion growth and drought resistance. Ann. N. Y. Acad. Sci. 2016, 28, 143–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Z.-X.; Sun, T.-Y.; Sun, H.; Yue, Q.-Y.; Yao, Y.-X. Modifications of ‘Summer Black’ grape berry quality as affected by the different rootstocks. Sci. Hortic. 2016, 210, 130–137. [Google Scholar] [CrossRef]
- Linderman, R.G.; Davis, E.A. Comparative response of selected grapevine rootstocks and cultivars to inoculation with different mycorrhizal fungi. Am. J. Enol. Vitic. 2001, 52, 8–11. [Google Scholar]
- Téliz, D.; Landa, B.B.; Rapoport, H.; Camacho, F.P.; Jiménez-Díaz, R.M.; Castillo, P. Plant-parasitic nematodes infecting grapevine in southern spain and susceptible reaction to root-knot nematodes of rootstocks reported as moderately resistant. Plant Dis. 2007, 91, 1147–1154. [Google Scholar] [CrossRef] [Green Version]
- Hepaksoy, S.; Ben-Asher, J.; De Malach, Y.; David, I.; Sagih, M.; Bravdo, B. Grapevine irrigation with saline water: Effect of rootstocks on quality and yield of cabernet sauvignon. J. Plant Nutr. Soil Sci. 2006, 29, 783–795. [Google Scholar] [CrossRef]
- Jogaiah, S.; Ramteke, S.D.; Sharma, J.; Upadhyay, A.K. Moisture and salinity stress induced changes in biochemical constituents and water relations of different grape rootstock cultivars. Int. J. Agron. 2014, 2014, 789087. [Google Scholar] [CrossRef] [Green Version]
- Koundouras, S.; Tsialtas, I.T.; Zioziou, E.; Nikolaou, N. Rootstock effects on the adaptive strategies of grapevine (Vitis vinifera L. cv. Cabernet–Sauvignon) under contrasting water status: Leaf physiological and structural responses. Agric. Ecosyst. Environ. 2008, 128, 86–96. [Google Scholar] [CrossRef]
- Reynolds, A.G.; Wardle, D.A. Rootstocks impact vine performance and fruit composition of grapes in British Columbia. HortTechnology 2001, 11, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Southey, J.; Jooste, J. The effect of grapevine rootstock on the performance of vitis vinifera L. (cv. Colombard) on a relatively saline soil. S. Afr. J. Enol. Vitic. 2017, 12, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Chou, M.Y.; Li, K.T. Rootstock and seasonal variations affect Anthocyanin accumulation and quality traits of ’Kyoho’ grape berries in subtropical double cropping system. Vitis. J. Grapevine Res. 2014, 53, 193–199. [Google Scholar]
- Lo’Ay, A.; El-Khateeb, A. Evaluation the effect of rootstocks on postharvest berries quality of ‘Flame Seedless’ grapes. Sci. Hortic. 2017, 220, 299–302. [Google Scholar] [CrossRef]
- Verma, S.K.; Singh, S.K.; Krishna, H. The effect of certain rootstocks on the grape cultivar ‘Pusa Urvashi’ (Vitis viniferal.). Int. J. Fruit Sci. 2010, 10, 16–28. [Google Scholar] [CrossRef]
- Lovisolo, C.; Lavoie-Lamoureux, A.; Tramontini, S.; Ferrandino, A. Grapevine adaptations to water stress: New perspectives about soil/plant interactions. Theor. Exp. Plant Physiol. 2016, 28, 53–66. [Google Scholar] [CrossRef]
- Aroca, R.; Porcel, R.; Ruiz-Lozano, J.M. Regulation of root water uptake under abiotic stress conditions. J. Exp. Bot. 2011, 63, 43–57. [Google Scholar] [CrossRef]
- Bechtold, U.; Field, B. Molecular mechanisms controlling plant growth during abiotic stress. J. Exp. Bot. 2018, 69, 2753–2758. [Google Scholar] [CrossRef] [Green Version]
- Cookson, S.J.; Ollat, N. Grafting with rootstocks induces extensive transcriptional re-programming in the shoot apical meristem of grapevine. BMC Plant Biol. 2013, 13, 147. [Google Scholar] [CrossRef]
- Gautier, A.T.; Chambaud, C.; Brocard, L.; Ollat, N.; Gambetta, G.A.; Delrot, S.; Cookson, S.J. Merging genotypes: Graft union formation and scion–rootstock interactions. J. Exp. Bot. 2018, 70, 747–755. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Vimal, S.R.; Singh, J.S.; Arora, N.K.; Singh, S. Soil-plant-microbe interactions in stressed agriculture management: A review. Pedosphere 2017, 27, 177–192. [Google Scholar] [CrossRef]
- Rashid, M.I.; Mujawar, L.H.; Shahzad, T.; Almeelbi, T.; Ismail, I.M.; Oves, M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 2016, 183, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Baneh, H.D.; Hassani, A.; Shaieste, F.G. Effects of salinity on leaf mineral composition and salt injury symptoms of some Iranian wild grapevine (Vitis vinifera L. ssp. sylvestris) genotypes. J. Int. Sci. Vigne. Vin. 2014, 48, 231–235. [Google Scholar] [CrossRef]
- Gramaje, D.; Armengol, J. Fungal trunk pathogens in the grapevine propagation process: Potential inoculum sources, detection, identification, and management strategies. Plant Dis. 2011, 95, 1040–1055. [Google Scholar] [CrossRef] [Green Version]
- Jacobi, K.K.; MacRae, E.A.; Hetherington, S.E. Early detection of abnormal skin ripening characteristics of’ Kensington’ mango (Mangifera indica Linn). Sci. Horti. 1998, 72, 2015–2225. [Google Scholar] [CrossRef]
- DeEll, J.R.; Toivonen, P.M.A. Chlorophyll fluorescence as a nondestructive indicator of broccoli quality during storage in modified-atmosphere packaging. HortScience 2000, 35, 256–259. [Google Scholar] [CrossRef] [Green Version]
- Rosenqvist, E.; Kooten, O. Chlorophyll fluorescence: A general description and nomenclature. In Practical Applications of Chlorophyll Fluorescence in Plant Biology; DeEll, J.R., Tiovonen, P.M.A., Eds.; Kluwer Academic Publishers: Boston, MA, USA, 2003; pp. 31–77. [Google Scholar]
- Jiang, C.D.; Shi, L.; Gao, H.Y.; Schansker, G.; Tóth, S.Z.; Strasser, R.J. Development of photosystems 2 and 1 during leaf growth in grapevine seedlings probed by chlorophyll a fluorescence transient and 820 nm transmission in vivo. Photosynthetica 2006, 44, 454–463. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Hüner, P.A.N. Introduction to Plant Physiology, 4th ed.; John Wiley & Sons: New York, NY, USA, 2009. [Google Scholar]
- Cogdell, R.J. Carotenoids in photosynthesis. Pure Appl. Chem. 1985, 57, 723–728. [Google Scholar] [CrossRef]
- Burzo, I.; Dejeu, L.; Şerdinescu, A.; Bädulescu, L. Grapevine Physiology, Volume 3; Elisavaros: Bucharest, Romania, 2005. (In Romanian) [Google Scholar]
- Bertamini, M.; Nedunchezhian, N. Decline of photosynthetic pigments, ribulose-1,5-bisphosphate carboxylase and soluble protein contents, nitrate reductase and photosynthetic activities, and changes in thylakoid membrane protein pattern in canopy shade grapevine (V vinifera cv. Moscato Giallo) leaves. Photosynthetica 2001, 39, 529–537. [Google Scholar]
- Young, A.J. Occurrence and distribution of carotenoids in photosynthetic systems. In Carotenoids in Photosynthesis; Springer: London, UK, 1993; pp. 16–71. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Patakas, A.; Stavrakas, D.; Fisarakis, I. Relationship between CO2 assimilation and leaf anatomical characteristics of two grapevine cultivars. Agronomie 2003, 23, 293–296. [Google Scholar] [CrossRef] [Green Version]
- Gomez-del-Campo, M.; Ruiz, C.; Lissarrague, J.R. Effect of water stress on leaf area development, photosynthesis and productivity in Chardonnay and Airen grapevines. Am. J. Enol. Vitic. 2002, 53, 138–143. [Google Scholar]
- Sivritepe, N.; Sivritepe, H.O.; Celik, H.; Katkat, A.V. Salinity responses of grafted grapevines: Effects of scion and rootstock genotypes. Not. Bot. Horti Agrobot. Cluj 2010, 38, 193–201. [Google Scholar]
- Delauney, A.J.; Verma, D.P.S. Proline biosynthesis and osmoregulation in plants. Plant J. 1993, 4, 215–223. [Google Scholar] [CrossRef]
- Walker, R.R.; Blackmore, D.H.; Clingeleffer, P.R.; Correll, R.L. Rootstock effects on salt tolerance of irrigated field grown grapevines (Vitis vinifera L. cv. Sultana) 2. Ion concentrations in leaves and juice. Aust. J. Grape Wine Res. 2004, 10, 90–99. [Google Scholar] [CrossRef]
- Shani, U.; Ben-Gal, A. Long-term response of grapevine to salinity: Osmatic effects and ion toxicity. Am. J. Enol. Vitic. 2005, 56, 148–154. [Google Scholar]
- Troncoso de Arce, A.; Matte, C.; Cantos, M.; Lavee, S. Evaluation of salt tolerance of in vitro-grown grapevine rootstock varieties. Vitis 1999, 38, 55–60. [Google Scholar]
- Foyer, C.H.; Noctor, G. Oxidant and antioxidant signalling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Foyer, C.H.; Ruban, A.V.; Noctor, G. Viewing oxidative stress through the lens of oxidative signalling rather than damage. Biochem. J. 2017, 474, 877–883. [Google Scholar] [CrossRef] [Green Version]
- Fattahi, M.; Mohammadkhani, A.; Shiran, B.; Baninasab, B.; Ravash, R.; Gogorcena, Y. Beneficial effect of mycorrhiza on nutritional uptake and oxidative balance in pistachio (Pistacia spp.) rootstocks submitted to drought and salinity stress. Sci. Hortic. 2021, 281, 109937. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Sugimoto, Y. Effect of protein modification by malondialdehyde on the interaction between the oxygen-evolving complex 33 kDa protein and photosystem II core proteins. Planta 2010, 231, 1077–1088. [Google Scholar] [CrossRef]
- Khoyerdi, F.F.; Shamshiri, M.H.; Estaji, A. Changes in some physiological and osmotic parameters of several pistachio genotypes under drought stress. Sci. Hortic. 2016, 198, 44–51. [Google Scholar] [CrossRef]
- Goharrizi, K.J.; Baghizadeh, A.; Kalantar, M.; Fatehi, F. Combined effects of salinity and drought on physiological and biochemical characteristics of pistachio rootstocks. Sci. Hortic. 2020, 261, 108970. [Google Scholar] [CrossRef]
- Li, J.; Lv, Y.; Li, N.; Wu, R.; Xing, M.; Shen, H.; Li, L.S.; Chen, X. Robust synthesis of bright multiple quantum dot-embedded nanobeads and its application to quantitative immunoassay. Chem. Eng. J. 2018, 361, 499–507. [Google Scholar] [CrossRef]
- Turóczy, Z.; Kis, P.; Török, K.; Cserháti, M.; Lendvai, Á.; Dudits, D.; Horváth, G.V. Overproduction of a rice aldo–keto reductase increases oxidative and heat stress tolerance by malondialdehyde and methylglyoxal detoxification. Plant Mol. Biol. 2011, 75, 399–412. [Google Scholar] [CrossRef]
- Dettori, S. Leaf water potential, stomatal resistance and transpiration response to different watering in almond, peach and “pixy” plum. Acta Hortic. 1985, 171, 181–186. [Google Scholar] [CrossRef]
- Flowers, T.; García, A.; Koyama, M.; Yeo, A.R. Breeding for salt tolerance in crop plants—The role of molecular biology. Acta Physiol. Plant. 1997, 19, 427–433. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Gorham, J.; Wyn Jones, R.G. Utilization of triticeae for improved salt tolerance in wheat. In Towards the Rational use of High Salinity Tolerant Plants; Leih, H., Massoum, A.A., Eds.; Kluwer: Amsterdam, The Netherlands, 1993; ISBN 9789401118583. [Google Scholar]
- Tong, Y.; Wenjuan, M.; Yimin, G.; Shulan, Z. Characteristics of nutrient uptake by grape. Better Crop. 2010, 94, 29–31. [Google Scholar]
- Jayaganesh, S.; Venkatesan, S.; Senthurpan, V. Impact of different sources and doses of magnesium fertilizer on biochemical constituents and quality parameters of black tea. Asian J. Biochem. 2011, 6, 273–281. [Google Scholar] [CrossRef]
- Delfani, M.; Firouzabadi, M.B.; Farrokhi, N.; Makarian, H. Some physiological responses of black-eyed pea to iron and magnesium nanofertilizers. Commun. Soil Sci. Plant Anal. 2014, 45, 530–540. [Google Scholar] [CrossRef]
- Bianchi, D.; Grossi, D.; Simone Di Lorenzo, G.; Zi Ying, Y.; Rustioni, L.; Brancadoro, L. Phenotyping of the “G series” vitis hybrids: First screening of the mineral composition. Sci. Hortic. 2020, 264, 109155. [Google Scholar] [CrossRef]
- He, H.; Jin, X.; Ma, H.; Deng, Y.; Huang, J.; Yin, L. Changes of plant biomass partitioning, tissue nutrients and carbohydrates status in magnesium-deficient banana seedlings and remedy potential by foliar application of magnesium. Sci. Hortic. 2020, 268, 109377. [Google Scholar] [CrossRef]
- Wolpert, J.A.; Walker, E.; Weber, L.; Bettiga, R.; Smith, P. Rootstocks and Phylloxera. Viticultural Notes; NUMBER 6; University of California Cooperative Extension Napa County: Napa, CA, USA, 1994. [Google Scholar]
- Lo’ay, A.A.; El-Ezz, S.F.A. Performance of ‘Flame seedless’ grapevines grown on different rootstocks in response to soil salinity stress. Sci. Hortic. 2021, 275, 109704. [Google Scholar] [CrossRef]
- Black, C.A. Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties, 2nd ed.; American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis, 1st ed.; Prentice Hall Ltd.: New Delhi, India, 1973; p. 498. [Google Scholar]
- Wilde, A.A.; Corey, R.B.; Lyer, J.G.; Voigt, G.K. Soil and Plant Analysis for Tree Culture, 3rd ed.; Oxford IBH Publishing Co.: New Delhi, India, 1985; pp. 64–115. [Google Scholar]
- Toivonen, P.M.; DeEll, J.R. Chlorophyll fluorescence, fermentation product accumulation, and quality of stored broccoli in modified atmosphere packages and subsequent air storage. Postharvest Biol. Technol. 2001, 23, 61–69. [Google Scholar] [CrossRef]
- Lo’ay, A.A. Chilling Injury in Mangoes. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2005; pp. 1–224. [Google Scholar]
- Porra, R.; Thompson, W.; Kriedemann, P. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Et Biophys. Acta (BBA) Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Miller, D.P.; Howell, G.S.; Flore, J.A. Influence of shoot number and crop Ioad on potted Chambourcin grapevines. II: Whole-vine vs. single-leafphotosynthesis. Vitis 1997, 36, 109–114. [Google Scholar]
- Zufferey, V.; Murisier, F.; Belcher, S.; Lorenzini, F.; Vivin, P.; Spring, J.; Viret, O. Carbohydrate reserves in the grapevine (Vitis vinifera L. “Chasselas”): The influence of the leaf to fruit ratio. VITIS J. Grapevine Res. 2015, 54, 183–188. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; El-Ezz, S.F.A.; Awadeen, A.A. Effect of different foliar potassium fertilization forms on vegetative growth, yield, and fruit quality of kaki trees grown in sandy soil. Sci. Hortic. 2021, 288, 110420. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S. Rapid assay for determination of water soluble quaternary ammonium compounts. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Iturbe-Ormaetxe, I.; Escuredo, P.R.; Arrese-Igor, C.; Becana, M. Oxidative damage in pea plants exposed to water deficit or paraquat. Plant Physiol. 1998, 116, 173–181. [Google Scholar] [CrossRef] [Green Version]
- A.O.A.C. Association of Official of Analytical Chemist, 15th ed.; A.O.A.C.: Washington, DC, USA, 1995. [Google Scholar]

| Variables | Rootstocks | Flowering | Fruit Set | Veraison | At Harvesting | ||||
|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | ||
| Fv/Fm | SO4 | 0.806 ± 0.002 b A * | 0.805 ± 0.000 b A | 0.815 ± 0.002 b A | 0.808 ± 0.000 b A | 0.791 ± 0.004 b B | 0.782 ± 0.001 b B | 0.728 ± 0.001 b C | 0.729 ± 0.000 b C |
| 1103 P | 0.837 ± 0.004 a A | 0.838 ± 0.000 a A | 0.858 ± 0.000 a A | 0.839 ± 0.000 a A | 0.842 ± 0.000 a A | 0.826 ± 0.001 a A | 0.805 ± 0.002 a A | 0.802 ± 0.001 a A | |
| Own root | 0.798 ± 0.001 b A | 0.799 ± 0.000 c A | 0.803 ± 0.001 c A | 0.798 ± 0.000 c A | 0.785 ± 0.002 b B | 0.779 ± 0.000 b B | 0.628 ± 0.001 c C | 0.634 ± 0.000 c C | |
| Fm | SO4 | 1885.00 ± 2.081 b D | 1881.00 ± 0.577 b D | 2115.33 ± 2.603 b C | 2109.00 ± 0.577 b C | 2326.66 ± 2.603 b A | 2319.00 ± 0.577 b A | 2274.33 ± 1.763 b B | 2271.00 ± 1.000 b B |
| 1103 P | 2136.00 ± 3.214 a D | 2133.66 ± 1.201 a D | 2344.33 ± 2.603 a C | 2336.00 ± 1.527 a C | 2564.33 ± 2.185 a A | 2565.67 ± 2.603 a A | 2483.00 ± 1.154 a B | 2490.00 ± 0.577 a B | |
| Own root | 1640.00 ± 1.527 c E | 1649.67 ± 0.881 c D | 1872.66 ± 2.185 c C | 1865.33 ± 1.763 c C | 1996.67 ± 1.527 c A | 1992.33 ± 1.201 c A | 1894.33 ± 2.403 c B | 1893.33 ± 1.201 c B | |
| F0 | SO4 | 402.66 ± 1.111 b D | 408.00 ± 0.577 b D | 441.00 ± 0557 b C | 443.00 ± 0.577 b C | 514.33 ± 1.763 b A | 517.66 ± 0.881 b A | 476.00 ± 1.855 b B | 477.00 ± 0.577 c B |
| 1103 P | 421.00 ± 1.728 a D | 423.00 ± 1.154 a D | 536.66 ± 2.333 a C | 536.00 ± 0.577 a C | 881.33 ± 0.881 a A | 882.00 ± 0.577 a A | 765.66 ± 1.763 a B | 770.00 ± 0.577 a B | |
| Own root | 385.00 ± 1.081 c D | 385.00 ± 0.577 c D | 427.33 ± 1.855 c C | 428.66 ± 0.881 c C | 483.33 ± 2.027 c A | 485.00 ± 0.577 c A | 439.66 ± 1.855 c B | 482.00 ± 1.154 b A | |
| Variables | Rootstocks | Flowering | Fruit Set | Veraison | At Harvesting | ||||
|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | ||
| Chl A (mg 100 g−1 FW) | SO4 | 1.57 ± 0.014 b D * | 1.64 ± 0.008 ab C | 1.68 ± 0.008 b C | 1.68 ± 0.008 b C | 1.80 ± 0.014 b AB | 1.76 ± 0.005 b B | 1.84 ± 0.005 b A | 1.85 ± 0.008 b A |
| 1103 P | 1.86 ± 0.008 a E | 1.88 ± 0.011 a E | 2.14 ± 0.020 a C | 1.96 ± 0.008 a D | 2.25 ± 0.029 a B | 2.19 ± 0.008 a BC | 2.35 ± 0.011 a A | 2.35 ± 0.005 a A | |
| Own root | 1.47 ± 0.012 c C | 1.38 ± 0.118 c BC | 1.58 ± 0.014 c ABC | 1.56 ± 0.005 c ABC | 1.66 ± 0.005 c AB | 1.63 ± 0.017 c AB | 1.73 ± 0.008 c A | 1.71 ± 0.005 c A | |
| Chl B (mg 100 g−1 FW) | SO4 | 0.61 ± 0.003 b F | 0.63 ± 0.005 b F | 0.66 ± 0.005 b E | 0.67 ± 0.008 b DE | 0.70 ± 0.005 b CD | 0.71 ± 0.005 b BC | 0.73 ± 0.005 b AB | 0.75 ± 0.005 b A |
| 1103 P | 0.67 ± 0.008 a D | 0.67 ± 0.005 a D | 0.72 ± 0.005 a C | 0.75 ± 0.005 a C | 0.84 ± 0.008 a AB | 0.86 ± 0.008 a A | 0.82 ± 0.005 a B | 0.82 ± 0.003 a B | |
| Own root | 0.54 ± 0.005 c D | 0.53 ± 0.005 c D | 0.61 ± 0.005 c C | 0.66 ± 0.005 c AB | 0.64 ± 0.005 c B | 0.66 ± 0.006 c AB | 0.66 ± 0.005 c AB | 0.68 ± 0.005 c A | |
| Chl A+B (mg 100 g−1 FW) | SO4 | 2.18 ± 0.017 b F | 2.27 ± 0.008 a E | 2.34 ± 0.013 b D | 2.36 ± 0.017 b D | 2.50 ± 0.018 b BC | 2.47 ± 0.001 b C | 2.57 ± 0.011 b AB | 2.60 ± 0.012 b A |
| 1103 P | 2.54 ± 0.011 a E | 2.55 ± 0.015 a E | 2.86 ± 0.026 a C | 2.71 ± 0.008 a D | 3.09 ± 0.037 a AB | 3.06 ± 0.017 a B | 3.17 ± 0.017 a A | 3.17 ± 0.008 a A | |
| Own root | 2.01 ± 0.012 c BC | 1.91 ± 0.113 b C | 2.19 ± 0.008 c AB | 2.22 ± 0.011 c A | 2.31 ± 0.005 c A | 2.29 ± 0.012 c A | 2.39 ± 0.013 c A | 2.39 ± 0.010 c A | |
| Chl A:B ratio | SO4 | 2.56 ± 0.012 b ABC | 2.61 ± 0.031 a ABC | 2.55 ± 0.018 b A | 2.48 ± 0.021 b BC | 2.58 ± 0.017 b AB | 2.48 ± 0.028 a BC | 2.52 ± 0.011 c ABC | 2.47 ± 0.020 b C |
| 1103 P | 2.77 ± 0.040 a B | 2.80 ± 0.024 a B | 2.98 ± 0.006 a A | 2.62 ± 0.028 a CD | 2.67 ± 0.011 a C | 2.53 ± 0.015 a D | 2.87 ± 0.005 a B | 2.84 ± 0.005 a B | |
| Own root | 2.73 ± 0.041 a A | 2.62 ± 0.245 a A | 2.59 ± 0.046 b A | 2.36 ± 0.011 c A | 2.55 ± 0.027 b A | 2.47 ± 0.046 a A | 2.62 ± 0.016 c A | 2.51 ± 0.018 b A | |
| Caroten (mg 100 g−1 FW) | SO4 | 2.37 ± 0.012 b C | 2.47 ± 0.065 a BC | 2.46 ± 0.005 b BC | 2.46 ± 0.005 b BC | 2.63 ± 0.012 b A | 2.66 ± 0.005 b A | 2.51 ± 0.005 b B | 2.50 ± 0.005 b B |
| 1103 P | 2.58 ± 0.014 a D | 2.57 ± 0.005 a D | 2.91 ± 0.017 a C | 2.91 ± 0.008 a C | 3.05 ± 0.029 a B | 3.05 ± 0.005 a B | 2.91 ± 0.008 a C | 3.93 ± 0.006 a A | |
| Own root | 2.25 ± 0.020 c F | 2.23 ± 0.014 b F | 2.37 ± 0.012 c E | 2.40 ± 0.005 c DE | 2.53 ± 0.012 c AB | 2.58 ± 0.008 c A | 2.44 ± 0.008 c CD | 2.49 ± 0.005 b BC | |
| Chls: Caro ratio | SO4 | 0.92 ± 0.008 b B | 0.92 ± 0.026 a B | 0.95 ± 0.006 b B | 0.96 ± 0.005 a B | 0.94 ± 0.003 b B | 0.93 ± 0.002 b B | 1.02 ± 0.003 b A | 1.04 ± 0.006 a A |
| 1103 P | 0.98 ± 0.006 a C | 0.99 ± 0.006 a BC | 0.98 ± 0.003 a C | 0.93 ± 0.001 b D | 1.01 ± 0.003 a B | 1.00 ± 0.006 a BC | 1.08 ± 0.008 a A | 0.80 ± 0.003 c E | |
| Own root | 0.89 ± 0.008 b AB | 0.85 ± 0.053 a B | 0.92 ± 0.003 c AB | 0.92 ± 0.006 b AB | 0.91 ± 0.003 c AB | 0.88 ± 0.008 c AB | 0.98 ± 0.005 c A | 0.96 ± 0.005 b A | |
| Variables | Rootstocks | Flowering | Fruit Set | Veraison | At Harvesting | ||||
|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | ||
| Proline% | SO4 | 0.33 ± 0.048 a AB * | 0.37 ± 0.005 a A | 0.31 ± 0.005 b B | 0.31 ± 0.005 b B | 0.32 ± 0.005 b AB | 0.33 ± 0.005 b AB | 0.31 ± 0.005 b B | 0.32 ± 0.005 b AB |
| 1103 P | 0.33 ± 0.003 a D | 0.35 ± 0.003 a CD | 0.36 ± 0.005 a CD | 0.36 ± 0.005 a CD | 0.37 ± 0.005 a BC | 0.39 ± 0.005 a AB | 0.39 ± 0.008 a AB | 0.40 ± 0.004 a A | |
| Own root | 0.28 ± 0.005 a B | 0.28 ± 0.003 b AB | 0.29 ± 0.005 b AB | 0.30 ± 0.005 a AB | 0.30 ± 0.003 b AB | 0.30 ± 0.005 c A | 0.29 ± 0.005 b AB | 0.31 ± 0.005 b A | |
| Glycine% | SO4 | 1.47 ± 0.017 b E | 1.48 ± 0.011 b E | 1.59 ± 0.005 b CD | 1.61 ± 0.003 b BC | 1.65 ± 0.005 b AB | 1.66 ± 0.008 b A | 1.56 ± 0.005 b D | 1.58 ± 0.005 b CD |
| 1103 P | 1.96 ± 0.012 a E | 1.97 ± 0.005 a E | 2.14 ± 0.020 a CD | 2.13 ± 0.003 a D | 2.39 ± 0.017 a A | 2.40 ± 0.005 a A | 2.20 ± 0.011 a BC | 2.23 ± 0.005 a B | |
| Own root | 1.24 ± 0.008 c C | 1.24 ± 0.005 c C | 1.27 ± 0.015 c BC | 1.27 ± 0.008 c BC | 1.34 ± 0.005 c A | 1.35 ± 0.005 c A | 1.29 ± 0.005 c B | 1.30 ± 0.005 c B | |
| Shoot carboh. % | SO4 | 19.56 ± 0.594 b E | 19.45 ± 0.583 b E | 23.17 ± 0.551 b D | 24.24 ± 0.586 b CD | 26.36 ± 0.591 b C | 26.43 ± 0.557 b C | 29.44 ± 0.568 b B | 32.85 ± 0.577 a A |
| 1103 P | 22.84 ± 0.565 a C | 22.74 ± 0.580 a C | 25.75 ± 0.589 a B | 27.44 ± 0.591 a B | 31.84 ± 0.560 a A | 32.22 ± 0.671 a A | 33.55 ± 0.571 a A | 34.36 ± 0.591 a A | |
| Own root | 17.66 ± 0.594 b C | 16.67 ± 0.571 c C | 20.64 ± 0.591 c B | 21.14 ± 0.597 c B | 22.94 ± 0.589 c AB | 25.33 ± 0.586 c A | 24.64 ± 0.560 c A | 25.64 ± 0.588 b A | |
| Leaf area(Cm2) | SO4 | 108.12 ± 0.571 b E | 109.92 ± 0.328 b DE | 111.58 ± 0.864 b CD | 112.35 ± 0.586 b CD | 114.36 ± 0.574 b BC | 116.67 ± 0.600 b AB | 116.73 ± 0.568 b AB | 117.37 ± 0.583 b A |
| 1103 P | 127.62 ± 0.439 a D | 128.01 ± 0.678 a D | 129.31 ± 0.877 a CD | 131.74 ± 0.594 a ABC | 130.46 ± 0.586 a BC | 133.67 ± 0.577 a AB | 131.59 ± 0.562 a BC | 134.73 ± 0.583 a A | |
| Own root | 96.88 ± 0.548 c D | 96.22 ± 0.887 c D | 97.82 ± 0.583 c CD | 97.31 ± 0.868 c D | 102.69 ± 0.910 c AB | 101.14 ± 0.571 c BC | 103.79 ± 0897 c AB | 105.27 ± 0.585 c A | |
| Variables | Rootstocks | Flowering | Fruit Set | Veraison | At Harvesting | ||||
|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | ||
| MDA (nm 100 g−1 FW) | SO4 | 0.17 ± 0.005 b D * | 0.18 ± 0.008 a CD | 0.20 ± 0.005 a BCD | 0.21 ± 0.005 a BC | 0.23 ± 0.005 b AB | 0.23 ± 0.005 b AB | 0.26 ± 0.006 b A | 0.26 ± 0.005 b A |
| 1103 P | 0.14 ± 0.005 c AB | 0.13 ± 0.006 b B | 0.15 ± 0.008 b AB | 0.14 ± 0.005 b AB | 0.17 ± 0.005 c A | 0.15 ± 0.005 c AB | 0.17 ± 0.006 c A | 0.16 ± 0.005 c AB | |
| Own root | 0.20 ± 0.005 a C | 0.20 ± 0.006 a C | 0.22 ± 0.005 a C | 0.22 ± 0.005 a C | 0.26 ± 0.004 a B | 0.26 ± 0.003 a B | 0.31 ± 0.008 a A | 0.33 ± 0.008 a A | |
| EL% | SO4 | 10.76 ± 0.574 a E | 11.25 ± 0.580 a 3 | 14.84 ± 0.574 a CD | 12.34 ± 0.577 b DE | 17.64 ± 0.580 b BC | 19.65 ± 0.568 b AB | 21.34 ± 0.565 b A | 22.31 ± 0.568 b A |
| 1103 P | 7.17 ± 0.598 b C | 7.15 ± 0.571 b C | 11.41 ± 0.859 b AB | 9.44 ± 0.583 c BC | 12.94 ± 0.580 c A | 11.46 ± 0.583 c AB | 13.23 ± 0.571 c A | 13.54 ± 0.580 c A | |
| Own root | 12.34 ± 0.580 a D | 12.49 ± 0.588 a D | 18.12 ± 0.864 a C | 17.56 ± 0.588 a C | 22.54 ± 0.589 a B | 23.84 ± 0.597 a B | 27.14 ± 0.831 a A | 27.95 ± 0.583 a A | |
| Variables | Rootstocks | Flowering | Fruit set | Veraison | At harvesting | ||||
|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | ||
| K% | SO4 | 1.62 ± 0.038 b F * | 1.73 ± 0.015 b BC | 1.67 ± 0.008 b DE | 1.74 ± 0.012 b ABC | 1.70 ± 0.014 b CD | 1.76 ± 0.005 b AB | 1.64 ± 0.005 b EF | 1.78 ± 0.005 b A |
| 1103 P | 1.80 ± 0.029 a D | 1.83 ± 0.012 a CD | 1.80 ± 0.015 a D | 1.87 ± 0.005 a AB | 1.86 ± 0.011 a BC | 1.89 ± 0.005 a AB | 1.81 ± 0.026 a D | 1.90 ± 0.005 a A | |
| Own root | 1.53 ± 0.020 c C | 1.45 ± 0.015 c D | 1.59 ± 0.005 c AB | 1.56 ± 0.008 c ABC | 1.60 ± 0.008 c A | 1.59 ± 0.005 c AB | 1.54 ± 0.014 c BC | 1.56 ± 0.004 cABC | |
| Na% | SO4 | 0.85 ± 0.012 b D | 0.89 ± 0.011 b CD | 0.93 ± 0.012 b C | 0.91 ± 0.005 b C | 1.09 ± 0.023 b B | 1.07 ± 0.005 b B | 1.16 ± 0.008 b A | 1.16 ± 0.005 b A |
| 1103 P | 0.28 ± 0.005 c CD | 0.27 ± 0.005 c D | 0.30 ± 0.005 c BCD | 0.30 ± 0.008 c BC | 0.32 ± 0.005 c AB | 0.33 ± 0.005 c AB | 0.33 ± 0.008 c A | 0.35 ± 0.005 c A | |
| Own root | 1.22 ± 0.005 a D | 1.24 ± 0.005 a D | 1.39 ± 0.018 a C | 1.36 ± 0.005 a C | 1.47 ± 0.020 a B | 1.41 ± 0.005 a C | 1.62 ± 0.011 a A | 1.65 ± 0.005 a A | |
| K/Na ratio | SO4 | 1.89 ± 0.051 b A | 1.94 ± 0.013 b B | 1.78 ± 0.021 b A | 1.91 ± 0.008 b A | 1.56 ± 0.034 b CD | 1.64 ± 0.012 b C | 1.41 ± 0.008 b E | 1.53 ± 0.014 b D |
| 1103 P | 6.43 ± 0.010 a AB | 6.79 ± 0.101 a A | 6.00 ± 0.026 a BCD | 6.17 ± 0.185 a ABC | 5.82 ± 0.187 a BCD | 5.73 ± 0.083 a CD | 5.38 ± 0.343 a D | 5.43 ± 0.099 a D | |
| Own root | 1.25 ± 0.020 c A | 1.16 ± 0.017 c B | 1.13 ± 0.008 c B | 1.15 ± 0.008 c B | 1.09 ± 0.006 c C | 1.13 ± 0.001 c BC | 0.93 ± 0.012 c D | 0.94 ± 0.008 c D | |
| Rootstocks | Internode Length (cm) | Internode Thickness (cm) | Leaf Area (cm2) | Bunch Weight (gm) | Bunch Number Vine−1 | Yield Vine−1 (Kg) | Wood Pruning Weight (Kg Vine−1) |
|---|---|---|---|---|---|---|---|
| SO4 | 9.09 ± 0.011 ab | 1.29 ± 0.008 b | 121.36 ± 0.574 b | 321.15 ± 0.100 b | 19.33 ± 0.881 b | 6.21 ± 0.324 b | 15.88 ± 0.012 b |
| 1103 Paulson | 10.13 ± 0.580 a | 1.55 ± 0.011 a | 136.22 ± 2.612 a | 424.68 ± 0.115 a | 26.01 ± 0.577 a | 11.04 ± 0.289 a | 17.39 ± 0.291 a |
| Own root | 7.78 ± 0.015 b | 0.86 ± 0.012 c | 113.56 ± 0.586 c | 301.77 ± 0.164 c | 16.00 ± 0.574 c | 4.83 ± 0.202 c | 15.05 ± 0.037 c |
| Rootstocks | SSC% | TA% | SSC:TA Ratio |
|---|---|---|---|
| SO4 | 16.17 ± 0.014 b | 0.760 ± 0.001 b | 20.99 ± 0.013 b |
| 1103 Paulson | 14.28 ± 0.014 c | 0.805 ± 0.002 a | 18.77 ± 0.060 c |
| Own root | 16.89 ± 0.012 a | 0.694 ± 0.001 c | 23.29 ± 0.020 a |
| Variables | Chl A | Chl B | Chl a + b | Chl A: B | SI-index | Carotene | Chls: Car Ratio | Proline | Shoot Carbo | MDA | EL% | Fv/Fm | Fm | F0 | Glycine | Leaf Area | K+ | Na+ | K+/Na+ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chl A | *1.0000 | ||||||||||||||||||
| Chl B | 0.9064 | 1.0000 | |||||||||||||||||
| Chl a+b | 0.9946 | 0.9453 | 1.0000 | ||||||||||||||||
| Chl A: B | 0.5334 | 0.1305 | 0.4439 | 1.0000 | |||||||||||||||
| SI-index | −0.2962 | −0.1084 | −0.2552 | −0.4673 | 1.0000 | ||||||||||||||
| Carotene | 0.8506 | 0.7905 | 0.8507 | 0.3878 | −0.3108 | 1.0000 | |||||||||||||
| Chls: Car ratio | 0.4353 | 0.4386 | 0.4439 | 0.1950 | 0.1017 | −0.0869 | 1.0000 | ||||||||||||
| Proline | 0.8047 | 0.7368 | 0.8021 | 0.3990 | −0.5428 | 0.7566 | 0.2117 | 1.0000 | |||||||||||
| Shoot Carbo | 0.8382 | 0.9315 | 0.8758 | 0.1035 | 0.1071 | 0.7382 | 0.4145 | 0.5873 | 1.0000 | ||||||||||
| MDA | −0.4190 | −0.2517 | −0.3852 | −0.4694 | 0.9471 | −0.4033 | −0.0003 | −0.6027 | −0.0478 | 1.0000 | |||||||||
| EL% | −0.2486 | −0.0487 | −0.2038 | −0.4835 | 0.9128 | −0.2055 | −0.0036 | −0.4523 | 0.1519 | 0.9194 | 1.0000 | ||||||||
| Fv/Fm | 0.0400 | 0.0044 | 0.0319 | 0.0858 | −0.1774 | −0.0026 | 0.0659 | 0.0574 | −0.0382 | −0.2246 | −0.2240 | 1.0000 | |||||||
| Fm | 0.8972 | 0.9382 | 0.9230 | 0.2352 | −0.2110 | 0.7684 | 0.4303 | 0.7259 | 0.8758 | −0.3710 | −0.1767 | 0.0257 | 1.0000 | ||||||
| F0 | 0.8466 | 0.8815 | 0.8700 | 0.2209 | −0.2657 | 0.7923 | 0.2564 | 0.7045 | 0.7894 | −0.3143 | −0.1303 | −0.0647 | 0.8072 | 1.0000 | |||||
| Glycine | 0.9098 | 0.8309 | 0.9064 | 0.4742 | −0.5731 | 0.7843 | 0.3523 | 0.8420 | 0.6852 | −0.6844 | −0.5335 | 0.1282 | 0.8812 | 0.7956 | 1.0000 | ||||
| Leaf area | 0.8960 | 0.8213 | 0.8933 | 0.4609 | −0.4696 | 0.7601 | 0.3937 | 0.8371 | 0.7162 | −0.6160 | −0.4801 | 0.1505 | 0.8857 | 0.6864 | 0.9612 | 1.0000 | |||
| K+ | 0.6498 | 0.4959 | 0.6234 | 0.5067 | −0.5791 | 0.4190 | 0.4240 | 0.6313 | 0.3756 | −0.5848 | −0.4905 | 0.2292 | 0.5315 | 0.5213 | 0.7151 | 0.6521 | 1.0000 | ||
| Na+ | −0.7384 | −0.5839 | −0.7134 | −0.5589 | 0.7939 | −0.6265 | −0.2588 | −0.7985 | −0.4113 | 0.8808 | 0.7912 | −0.2065 | −0.6815 | −0.5538 | −0.9193 | −0.8943 | −0.7421 | 1.0000 | |
| K+/Na+ | 0.7558 | 0.5601 | 0.7210 | 0.6359 | −0.6286 | 0.5338 | 0.4178 | 0.6875 | 0.4142 | −0.6854 | −0.5879 | 0.2848 | 0.6105 | 0.5594 | 0.8423 | 0.7924 | 0.9210 | −0.8643 | 1.0000 |
| Physical Properties | Soluble Anions (meq L−1) | Soluble Cations (meq L−1) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sand% | Clay% | Silt% | Texture | EC dsm−1 | pH | HCO3− | Cl− | SO4 | Na+ | K+ | Mg++ | Ca++ | SAR | |
| 86.7 | 7.7 | 11.93 | Sandy | 4.8 | 7.89 | 3.00 | 15.20 | 13.11 | 26 | 2.99 | 3.77 | 11.01 | 9.59 | |
| Cations (meq L−1) | ||||||||||||||
| pH | EC (dS m−1) 0.86 | CO3− | HCO3− | Cl− | SO4− | Ca++ | Mg++ | Na+ | K+ | |||||
| 7.28 | 563 ppm | 0.21 | 2.64 | 0.92 | 1.21 | 1.78 | 0.73 | 25 | 0.17 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
A. A., L.; Ghazi, D.A.; Al-Harbi, N.A.; Al-Qahtani, S.M.; Hassan, S.; Abdein, M.A. Growth, Yield, and Bunch Quality of “Superior Seedless” Vines Grown on Different Rootstocks Change in Response to Salt Stress. Plants 2021, 10, 2215. https://doi.org/10.3390/plants10102215
A. A. L, Ghazi DA, Al-Harbi NA, Al-Qahtani SM, Hassan S, Abdein MA. Growth, Yield, and Bunch Quality of “Superior Seedless” Vines Grown on Different Rootstocks Change in Response to Salt Stress. Plants. 2021; 10(10):2215. https://doi.org/10.3390/plants10102215
Chicago/Turabian StyleA. A., Lo’ay, Dina A. Ghazi, Nadi Awad Al-Harbi, Salem Mesfir Al-Qahtani, Sabry Hassan, and Mohamed A. Abdein. 2021. "Growth, Yield, and Bunch Quality of “Superior Seedless” Vines Grown on Different Rootstocks Change in Response to Salt Stress" Plants 10, no. 10: 2215. https://doi.org/10.3390/plants10102215









