Gibberellic Acid and Jasmonic Acid Improve Salt Tolerance in Summer Squash by Modulating Some Physiological Parameters Symptomatic for Oxidative Stress and Mineral Nutrition
Abstract
:1. Introduction
2. Results
2.1. Biomass and Water Content Percentage
2.2. Plant Pigments
2.3. Mineral Ions
2.4. Proline
2.5. Thiobarbituric Acid Reactive Substances (TBARS)
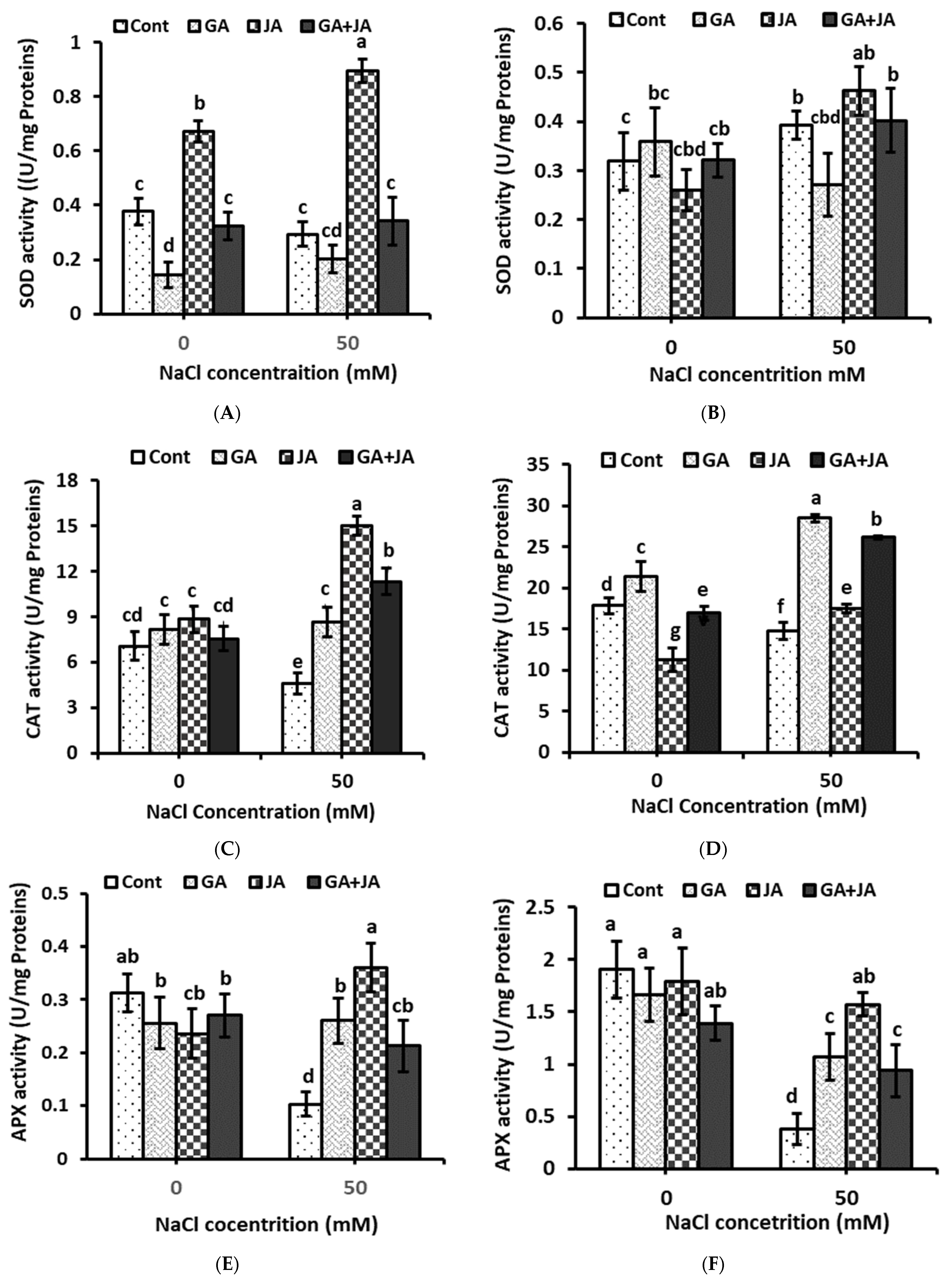
2.6. Antioxidant Enzymes
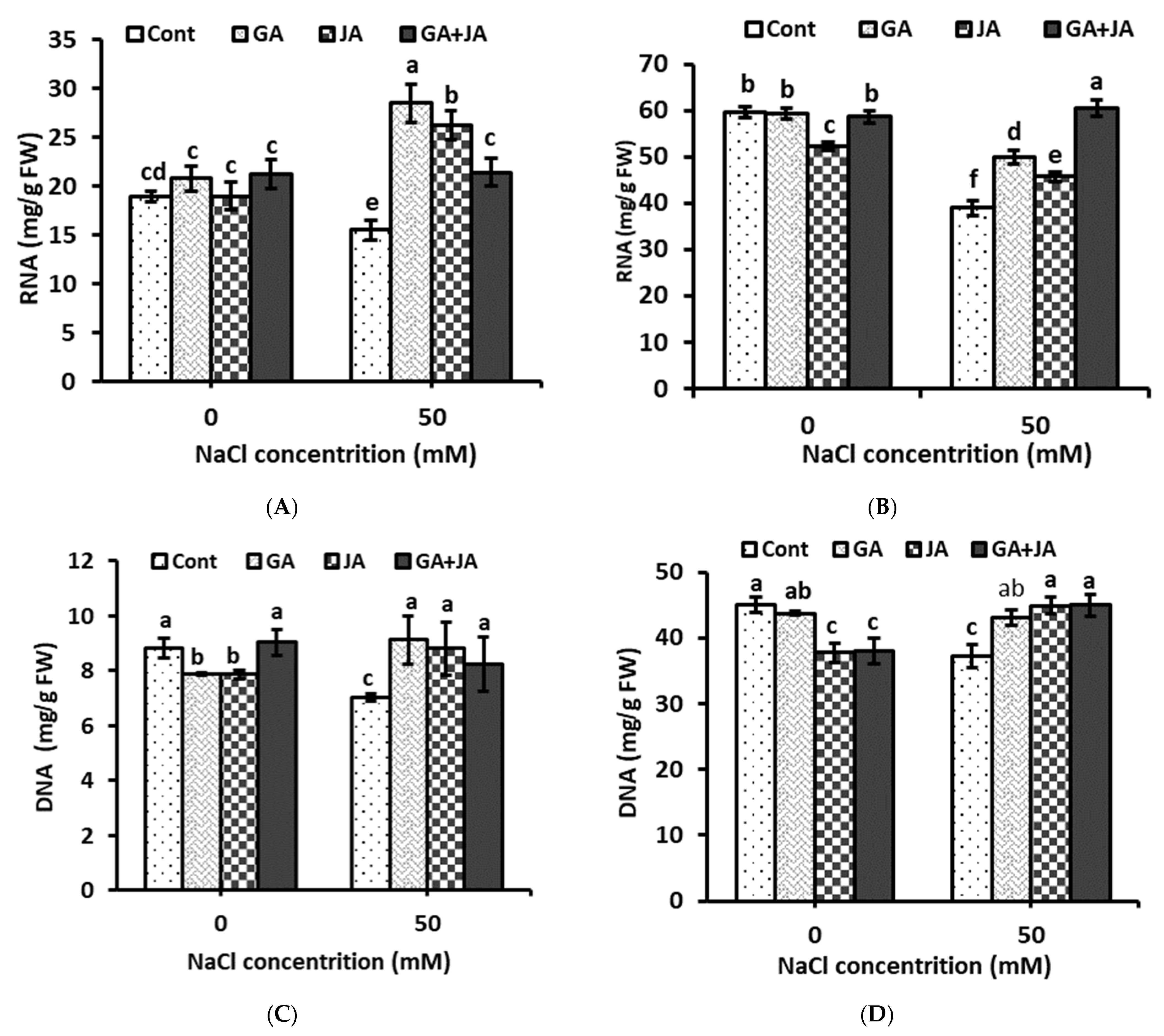
2.7. Nucleic Acids
3. Discussion
4. Materials and Methods
4.1. Plant Cultivation and Hormonal Treatments
4.2. Plant Biomass and Water Content
4.3. Chlorophyll and Carotenoid Determination
4.4. Mineral Ion Determination
4.5. Proline Determination
4.6. Lipid Peroxidation Products Determination
4.7. Antioxidant Enzymes Activity Determination
4.8. Nuclein Acids Determination
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evelin, H.; Devi, T.S.; Gupta, S.; Kapoor, R. Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: Current understanding and new challenges. Front. Plant Sci. 2019, 10, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo-Campohermoso, M.A.; Broetto, F.; Rodríguez-Hernández, A.M.; Soriano-Melgar, L.D.A.A.; Mounzer, O.; Sánchez-Blanco, M.J. Plant-available water, stem diameter variations, chlorophyll fluorescence, and ion content in Pistacia lentiscus under salinity stress. Terra Latinoam. 2020, 38, 103–111. [Google Scholar] [CrossRef]
- Abdel-Hamid, A.M. Physiological and molecular markers for salt tolerance in four barley cultivars. Eur. Sci. J. 2014, 10, 252–272. [Google Scholar]
- Sahin, O.; Karlik, E.; Meric, S.; Ari, S.; Gozukirmizi, N. Genome organization changes in GM and non-GM soybean [Glycine max (L.) Merr.] under salinity stress by retro-transposition events. Genet. Resour. Crop Evol. 2020, 67, 1551–1566. [Google Scholar] [CrossRef]
- Augé, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular mycorrhizal symbiosis and osmotic adjustment in response to NaCl stress: A meta-analysis. Front. Plant Sci. 2014, 5, 562. [Google Scholar] [PubMed] [Green Version]
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B. Function and mechanism of jasmonic acid in plant responses to abiotic and biotic stresses. Int. J. Mol. Sci. 2021, 22, 8568. [Google Scholar] [CrossRef]
- Kosakivska, I.V.; Vedenicheva, N.P.; Babenko, L.M.; Voytenko, L.V.; Romanenko, K.O.; Vasyuk, V.A. Exogenous phytohormones in the regulation of growth and development of cereals under abiotic stresses. Mol. Biol. Rep. 2021. [Google Scholar] [CrossRef]
- Teklić, T.; Parađiković, N.; Špoljarević, M.; Zeljković, S.; Lončarić, Z.; Lisjak, M. Linking abiotic stress, plant metabolites, biostimulants and functional food. Ann. Appl. Biol. 2021, 178, 169–191. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Ali, S.; Babar, M.A. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Miceli, A.; Moncada, A.; Sabatino, L.; Vetrano, F. Effect of gibberellic acid on growth, yield, and quality of leaf lettuce and rocket grown in a floating system. Agronomy 2019, 9, 382. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Ramírez, A.; Rodríguez, D.; Reyes, D.; Jiménez, J.A.; Nicolás, G.; López-Climent, M.; Nicolás, C. Evidence for a role of gibberellins in salicylic acid-modulated early plant responses to abiotic stress in Arabidopsis seeds. Plant Physiol. 2009, 150, 1335–1344. [Google Scholar] [CrossRef] [Green Version]
- Rady, M.M.; Boriek, S.H.; El-Mageed, A.; Taia, A.; Seif El-Yazal, M.A.; Ali, E.F.; Abdelkhalik, A. Exogenous gibberellic acid or dilute bee honey boosts drought stress tolerance in Vicia faba by rebalancing osmoprotectants, antioxidants, nutrients, and phytohormones. Plants 2021, 10, 748. [Google Scholar] [CrossRef] [PubMed]
- Emamverdian, A.; Ding, Y.; Xie, Y. The role of new members of phytohormones in plant amelioration under abiotic stress with an emphasis on heavy metals. Pol. J. Environ. 2020, 29, 1009–1020. [Google Scholar] [CrossRef]
- Schenk, P.M.; Kazan, K.; Wilson, I.; Anderson, J.P.; Richmond, T.; Somerville, S.C.; Manners, J.M. Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc. Natl. Acad. Sci. USA 2000, 97, 11655–11660. [Google Scholar] [CrossRef] [Green Version]
- Walling, L.L. The myriad plant responses to herbivores. J. Plant Growth Regul. 2000, 19, 195–216. [Google Scholar] [CrossRef]
- Browse, J. Jasmonate passes muster: A receptor and targets for the defense hormone. Annu. Rev. Plant Biol. 2009, 60, 183–205. [Google Scholar] [CrossRef] [PubMed]
- Achard, P.; Gong, F.; Cheminant, S.; Alioua, M.; Hedden, P.; Genschik, P. The cold-inducible CBF1 factor–dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell 2008, 20, 2117–2129. [Google Scholar] [CrossRef] [Green Version]
- Wingler, A.; Tijero, V.; Müller, M.; Yuan, B.; Munné-Bosch, S. Interactions between sucrose and jasmonate signalling in the response to cold stress. BMC Plant Biol. 2020, 20, 176. [Google Scholar] [CrossRef]
- Soto, A.; Ruiz, K.B.; Ziosi, V.; Costa, G.; Torrigiani, P. Ethylene and auxin biosynthesis and signaling are impaired by methyl jasmonate leading to a transient slowing down of ripening in peach fruit. J. Plant Physiol. 2012, 169, 1858–1865. [Google Scholar] [CrossRef]
- Paris, H.S. Summer squash. In Handbook of Plant Breeding, Vegetables I; Prohens, J., Nuez, F., Eds.; Springer: New York, NY, USA, 2008; pp. 351–379. [Google Scholar]
- Adepoju, G.K.A.; Adepanjo, A.A. Effect of consumption of Cucurbita pepo seeds on hematological and biochemical parameters. Afr. J. Pharm. Pharmacol. 2011, 5, 18–22. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; NeSmith, D.S.; Bridges, D.C.; Johnson, J.W. Responses of squash to salinity, waterlogging, and subsequent drainage: II. Root and shoot growth. J. Plant Nutr. 1995, 18, 141–152. [Google Scholar] [CrossRef]
- Mantri, N.; Patade, V.; Penna, S.; Ford, R.; Pang, E. Abiotic stress responses in plants: Present and future. In Abiotic Stress Responses in Plants; Springer: New York, NY, USA, 2012; pp. 1–19. [Google Scholar]
- Rhaman, M.S.; Imran, S.; Rauf, F.; Baskin, C.C. Seed priming with phytohormones: An effective approach for the mitigation of abiotic stress. Plants 2021, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Moore, S.; Chen, C.; Lindsey, K. Crosstalk complexities between auxin, cytokinin and ethylene in Arabidopsis root development: From experiments to systems modeling and back again. Mol. Plant 2017, 10, 1480–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muniandi, S.K.M.; Hossain, M.A.; Abdullah, M.P.; Ab Shukor, N.A. Gibberellic acid (GA3) affects growth and development of some selected kenaf (Hibiscus cannabinus L.) cultivars. Ind. Crops Prod. 2018, 118, 180–187. [Google Scholar] [CrossRef]
- Kaur, H.; Sirhindi, G.; Sharma, P. Effect of jasmonic acid on some biochemical and physiological parameters in salt-stressed Brassica napus seedlings. Plant Physiol. Biochem. 2017, 9, 36–42. [Google Scholar]
- Saeedipour, S.; Alipoor, R.; Farhoudi, R. Feasibility of Seed Priming for Improving Salt Tolerance of Summer Squash (Cucurbita pepo) Seedling. Int. J. Appl. Agric. Res. 2010, 5, 205–213. [Google Scholar]
- Ashraf, M.; Karim, F.; Rasul, E. Interactive effects of gibberellic acid (GA 3) and salt stress on growth, ion accumulation and photosynthetic capacity of two spring wheat (Triticum aestivum L.) cultivars differing in salt tolerance. Plant Growth Regul. 2002, 36, 49–59. [Google Scholar] [CrossRef]
- Seo, H.S.; Kim, S.K.; Jang, S.W.; Choo, Y.S.; Sohn, E.Y.; Lee, I.J. Effect of jasmonic acid on endogenous gibberellins and abscisic acid in rice under NaCl stress. Biol. Plant. 2005, 49, 447–450. [Google Scholar] [CrossRef]
- Avalbaev, A.; Yuldashev, R.; Fedorova, K.; Somov, K.; Vysotskaya, L.; Allagulova, C.; Shakirova, F. Exogenous methyl jasmonate regulates cytokinin content by modulating cytokinin oxidase activity in wheat seedlings under salinity. J. Plant Physiol. 2016, 191, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Djanaguiraman, M.; Sheeba, J.A.; Shanker, A.K.; Devi, D.D.; Bangarusamy, U. Rice can acclimate to lethal level of salinity by pretreatment with sublethal level of salinity through osmotic adjustment. Plant Soil 2006, 284, 363–373. [Google Scholar] [CrossRef]
- Misratia, K.M.; Ismail, M.R.; Hakim, M.A.; Musa, M.H.; Puteh, A. Effect of salinity and alleviating role of gibberellic acid (GA3) for improving the morphological, physiological and yield traits of rice varieties. Aust. J. Crop Sci. 2013, 7, 1682–1692. [Google Scholar]
- Qiu, Z.; Guo, J.; Zhu, A.; Zhang, L.; Zhang, M. Exogenous jasmonic acid can enhance tolerance of wheat seedlings to salt stress. Ecotoxicol. Environ. Saf. 2014, 104, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Mauchamp, A.; Méthy, M. Submergence-induced damage of photosynthetic apparatus in Phragmites australis. Environ. Exp. Bot. 2004, 51, 227–235. [Google Scholar] [CrossRef]
- Zrig, A.; Tounekti, T.; Ben-Mohamed, H.; AbdElgawad, H.; Vadel, A.M.; Valero, D.; Khemira, H. Differential response of two almond rootstocks to chloride salt mixtures in the growing medium. Russ. J. Plant Physiol. 2016, 63, 143–151. [Google Scholar] [CrossRef]
- Terletskaya, N.; Zobova, N.; Stupko, V.; Shuyskaya, E. Growth and photosynthetic reactions of different species of wheat seedlings under drought and salt stress. Period. Biol. 2017, 119, 37–45. [Google Scholar] [CrossRef]
- Yan, Y.; Hou, P.; Duan, F.; Niu, L.; Dai, T.; Wang, K.; Zhou, W. Improving photosynthesis to increase grain yield potential: An analysis of maize hybrids released in different years in China. Photosynth. Res. 2021, 150, 295–311. [Google Scholar] [CrossRef]
- Keutgen, A.J.; Pawelzik, E. Impacts of NaCl stress on plant growth and mineral nutrient assimilation in two cultivars of strawberry. Environ. Exp. Bot. 2009, 65, 170–176. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cakmak, I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant. Nutr. Soil Sci. 2005, 168, 521–530. [Google Scholar] [CrossRef]
- Mohammed, A.H.M.A. Physiological aspects of mungbean plant (Vigna radiata L. Wilczek) in response to salt stress and gibberellic acid treatment. Res. J. Biol. Sci. 2007, 3, 200–213. [Google Scholar]
- Chakrabarti, N.; Mukherji, S. Effect of phytohormone pretreatment on nitrogen metabolism in Vigna radiata under salt stress. Biol. Plant. 2003, 46, 63–66. [Google Scholar] [CrossRef]
- Kang, D.J.; Seo, Y.J.; Lee, J.D.; Ishii, R.; Kim, K.U.; Shin, D.H.; Lee, I.J. Jasmonic acid differentially affects growth, ion uptake and abscisic acid concentration in salt-tolerant and salt-sensitive rice cultivars. J. Agron. Crop Sci. 2005, 191, 273–282. [Google Scholar] [CrossRef]
- Balkaya, A. Effects of salt stress on vegetative growth parameters and ion accumulations in cucurbit rootstock genotypes. Ekin J. Crop Breed. Genet. 2016, 2, 11–24. [Google Scholar]
- Qian, Y.L.; Fu, J.M. Response of creeping bentgrass to salinity and mowing management: Carbohydrate availability and ion accumulation. HortScience 2005, 40, 2170–2174. [Google Scholar] [CrossRef] [Green Version]
- Abdelgawad, Z.A.; Khalafaallah, A.A.; Abdallah, M.M. Impact of methyl jasmonate on antioxidant activity and some biochemical aspects of maize plant grown under water stress condition. J. Agric. Sci. 2014, 5, 1077. [Google Scholar] [CrossRef] [Green Version]
- Onanuga, A.O.; Jiang, P.; Adl, S. Effect of phytohormones, phosphorus and potassium on cotton varieties (Gossypium hirsutum) root growth and root activity grown in hydroponic nutrient solution. J. Agric. Sci. 2012, 4, 93–110. [Google Scholar] [CrossRef]
- Benková, E.; Hejátko, J. Hormone interactions at the root apical meristem. Plant Mol. Biol. 2009, 69, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Ivanchenko, M.G.; Muday, G.K.; Dubrovsky, J.G. Ethylene–auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana. Plant J. 2008, 55, 335–347. [Google Scholar] [CrossRef]
- Khan, M.A.; Gul, B.; Weber, D.J. Improving seed germination of Salicornia rubra (Chenopodiaceae) under saline conditions using germination regulating chemicals. West. N. Am. Nat. 2002, 62, 101–105. [Google Scholar]
- de Castro, R.D.; van Lammeren, A.A.; Groot, S.P.; Bino, R.J.; Hilhorst, H.W. Cell division and subsequent radicle protrusion in tomato seeds are inhibited by osmotic stress but DNA synthesis and formation of microtubular cytoskeleton are not. Plant Physiol. 2000, 122, 327–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, P.; Alarcon, J.J. Effect of salinity on growth, shoot water relation and root hydraulic conductivity in tomato plants. J. Agric. Sci. 1997, 128, 439–444. [Google Scholar] [CrossRef]
- Heidari, M. Effects of salinity stress on growth, chlorophyll content and osmotic components of two basil (Ocimum basilicum L.) genotypes. Afr. J. Biotechnol. 2012, 11, 379–384. [Google Scholar]
- Singh, S.P.; Singh, B.B.; Singh, M. Effect of kinetin on chlorophyll, nitrogen and proline in mungbean (Vigna radiata) under saline condition. Indian J. Plant Physiol. 1991, 37, 37–39. [Google Scholar]
- Huguet-Robert, V.; Sulpice, R.; Lefort, C.; Maerskalck, V.; Emery, N.; Larher, F.R. The suppression of osmoinduced proline response of Brassica napus L. var oleifera leaf discs by polyunsaturated fatty acids and methyl-jasmonate. Plant Sci. 2003, 164, 119–127. [Google Scholar] [CrossRef]
- Maslenkova, L.T.; Miteva, T.S.; Popova, L.P. Changes in the polypeptide patterns of barley seedlings exposed to jasmonic acid and salinity. Plant Physiol. 1992, 98, 700–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dheeba, B.; Selvakumar, S.; Kannan, M.; Kannan, K. Effect of gibberellic acid on black gram (Vigna mungo) irrigated with different levels of saline water. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 709–720. [Google Scholar]
- Meloni, D.A.; Oliva, M.A.; Martinez, C.A.; Cambraia, J. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ. Exp. Bot. 2003, 49, 69–76. [Google Scholar] [CrossRef]
- Saeidi-Sar, S.; Abbaspour, H.; Afshari, H.; Yaghoobi, S.R. Effects of ascorbic acid and gibberellin A 3 on alleviation of salt stress in common bean (Phaseolus vulgaris L.) seedlings. Acta Physiol. Plant. 2013, 35, 667–677. [Google Scholar] [CrossRef]
- Bandurska, H.; Stroiński, A.; Kubiś, J. The effect of jasmonic acid on the accumulation of ABA, proline and spermidine and its influence on membrane injury under water deficit in two barley genotypes. Acta Physiol. Plant. 2003, 25, 279–285. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Mir, R.A.; Alyemeni, M.N.; Ahmad, P. Combined effects of brassinosteroid and kinetin mitigates salinity stress in tomato through the modulation of antioxidant and osmolyte metabolism. Plant Physiol. Biochem. 2020, 147, 31–42. [Google Scholar] [CrossRef]
- Shah, T.; Latif, S.; Saeed, F.; Ali, I.; Ullah, S.; Alsahli, A.A.; Ahmad, P. Seed priming with titanium dioxide nanoparticles enhances seed vigor, leaf water status, and antioxidant enzyme activities in maize (Zea mays L.) under salinity stress. J. King Saud. Univ. Sci. 2021, 33, 101207. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Esfandiari, E.; Shakiba, M.R.; Mahboob, S.A.; Alyari, H.; Toorchi, M. Water stress, antioxidant enzyme activity and lipid peroxidation in wheat seedling. J. Food Agric. Environ. 2007, 5, 149. [Google Scholar]
- Gunes, A.; Pilbeam, D.J.; Inal, A.; Coban, S. Influence of silicon on sunflower cultivars under drought stress, I: Growth, antioxidant mechanisms, and lipid peroxidation. Commun. Soil Sci. Plant Anal. 2008, 39, 1885–1903. [Google Scholar] [CrossRef]
- Rouhi, H.R.; Aboutalebian, M.A.; Moosavi, S.A.; Karimi, F.A.; Karimi, F.; Saman, M.; Samadi, M. Change in several antioxidant enzymes activity of berseem clover (Trifolium alexandrinum L.) by priming. Int. J. Agric. Sci. 2012, 2, 237–243. [Google Scholar]
- Shalata, A.; Neumann, P.M. Exogenous ascorbic acid (vitamin C) increases resistance to salt stress and reduces lipid peroxidation. J. Exp. Bot. 2001, 52, 2207–2211. [Google Scholar] [CrossRef] [PubMed]
- Yupsanis, T.; Kefalas, P.S.; Eleftheriou, P.; Kotinis, K. RNase and DNase activities in the alfalfa and lentil grown in iso-osmotic solutions of NaCl and mannitol. J. Plant Physiol. 2001, 158, 921–927. [Google Scholar] [CrossRef]
- Abo-kassem, E.E. Effects of salinity: Calcium interaction on growth and nucleic acid metabolism in five species of Chenopodiaceae. Turk. J. Bot. 2007, 31, 125–134. [Google Scholar]
- Ismail, A.M. Physiological studies on the influence of cytokinin or GA3 in the alleviation of salt stress in sorghum plants. Acta Agron. Hung. 2003, 51, 371–380. [Google Scholar] [CrossRef]
- Fujii, H.; Shimada, T.; Sugiyama, A.; Endo, T.; Nishikawa, F.; Nakano, M.; Omura, M. Profiling gibberellin (GA3)-responsive genes in mature mandarin fruit using a citrus 22K oligoarray. Sci. Hortic. 2008, 116, 291–298. [Google Scholar] [CrossRef]
- Sumithra, K.; Jutur, P.P.; Carmel, D.B.; Reddy, A.R. Salinity-induced changes in two cultivars of Vigna radiata: Responses of antioxidative and proline metabolism. Plant Growth Regul. 2006, 50, 11–22. [Google Scholar] [CrossRef]
- Su, X.; Howell, A.B.; D’Souza, D.H. Antiviral effects of cranberry juice and cranberry proanthocyanidins on foodborne viral surrogates–a time dependence study in vitro. Food Microbiol. 2010, 27, 985–991. [Google Scholar] [CrossRef]
- Hseu, Z.Y. Evaluating heavy metal contents in nine composts using four digestion methods. Bioresour. Technol. 2004, 95, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Zhang, J.; Kirkham, M.B. Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytol. 1996, 132, 361–373. [Google Scholar] [CrossRef]
- Schmidt, G.; Thannhauser, S.J. A method for the determination of desoxyribonucleic acid, ribonucleic acid, and phosphoproteins in animal tissues. J. Biol. Chem. 1945, 161, 83–89. [Google Scholar] [CrossRef]
- Morse, M.L.; Carter, C.E. The synthesis of nucleic acids in cultures of Escherichia coli, strains B and B/r. J. Bacteriol. 1949, 58, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Wahab, N.H. Hormonal and Metabolic Effects of Coumarin on Germination of Sorghum. Master’s Thesis, Faculty of Science, Ain Shams University, Cairo, Egypt, 1982. [Google Scholar]
- Burton, K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem. J. 1956, 62, 315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
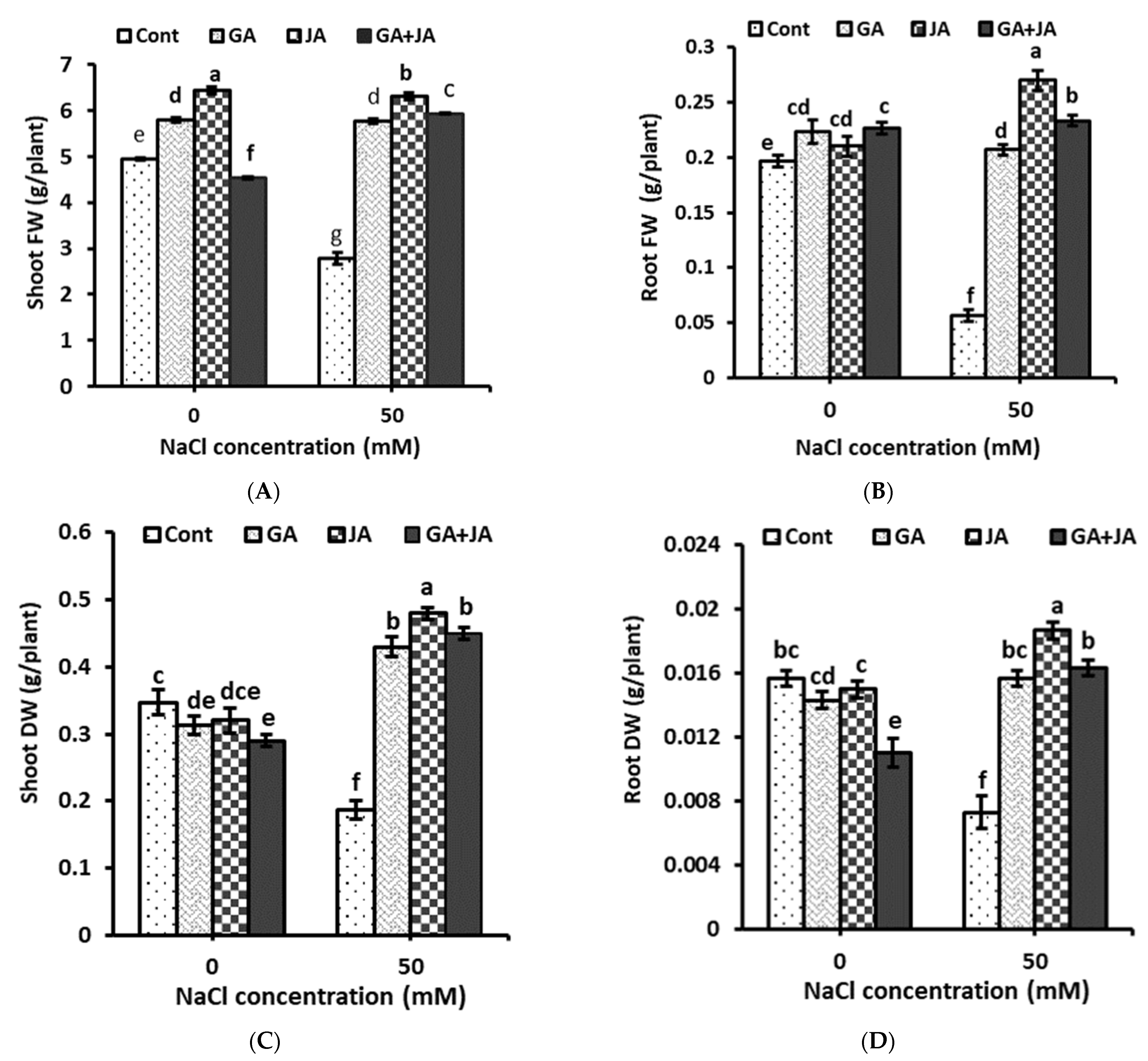
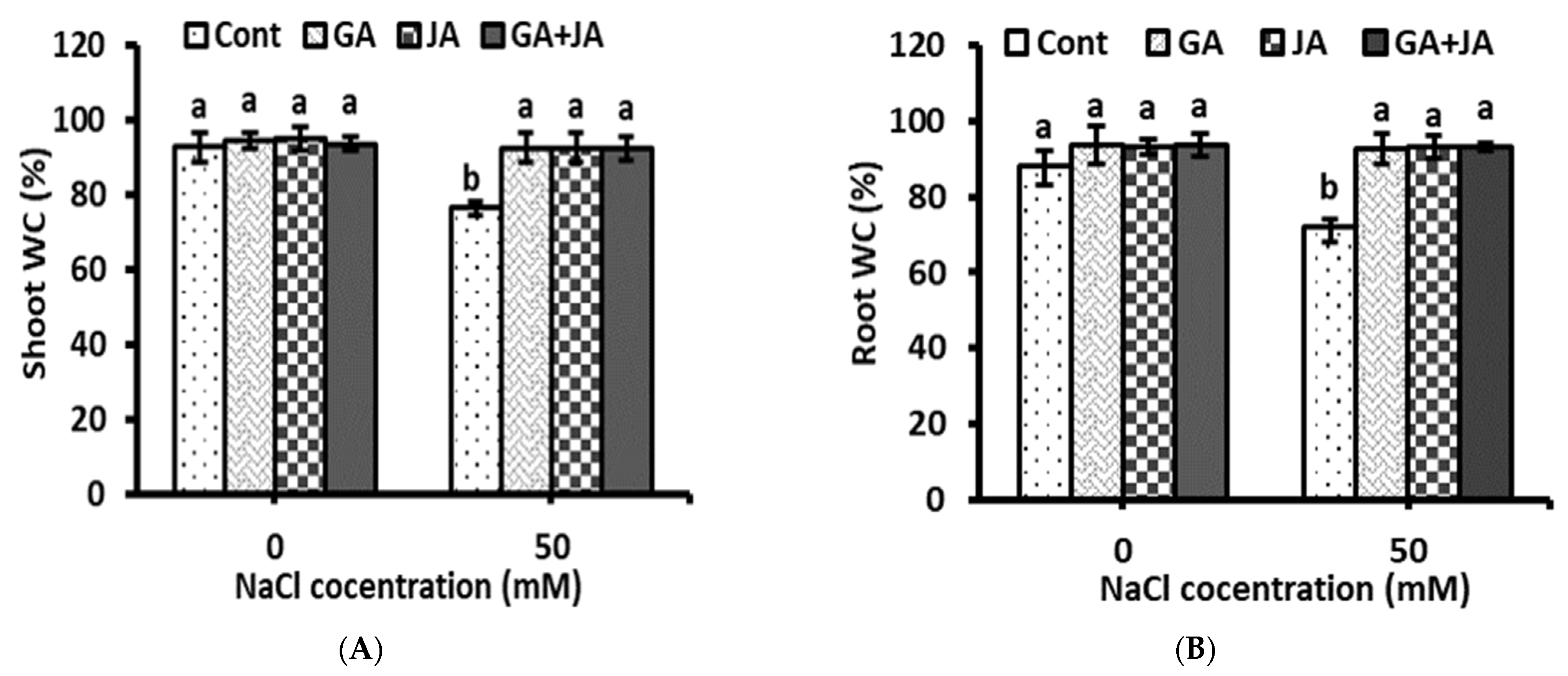
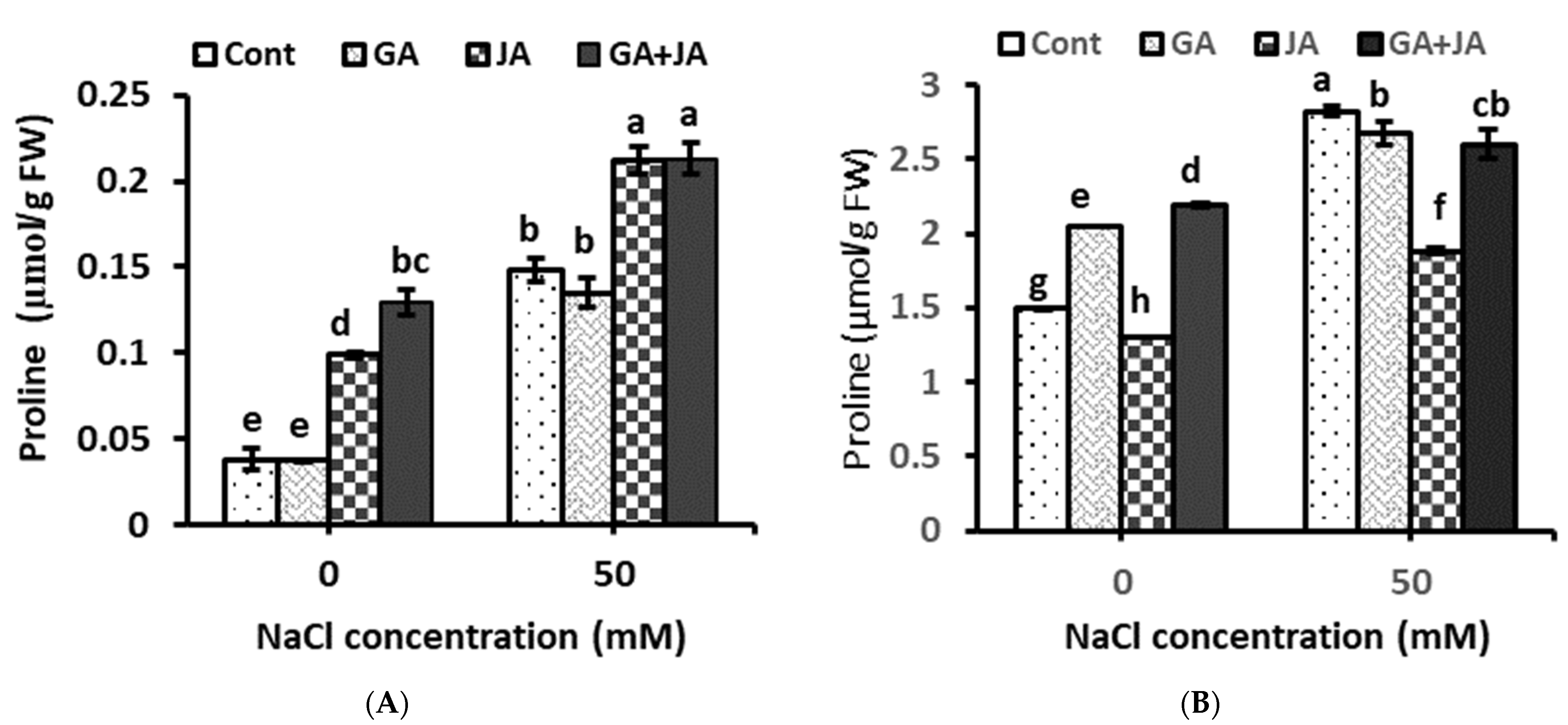
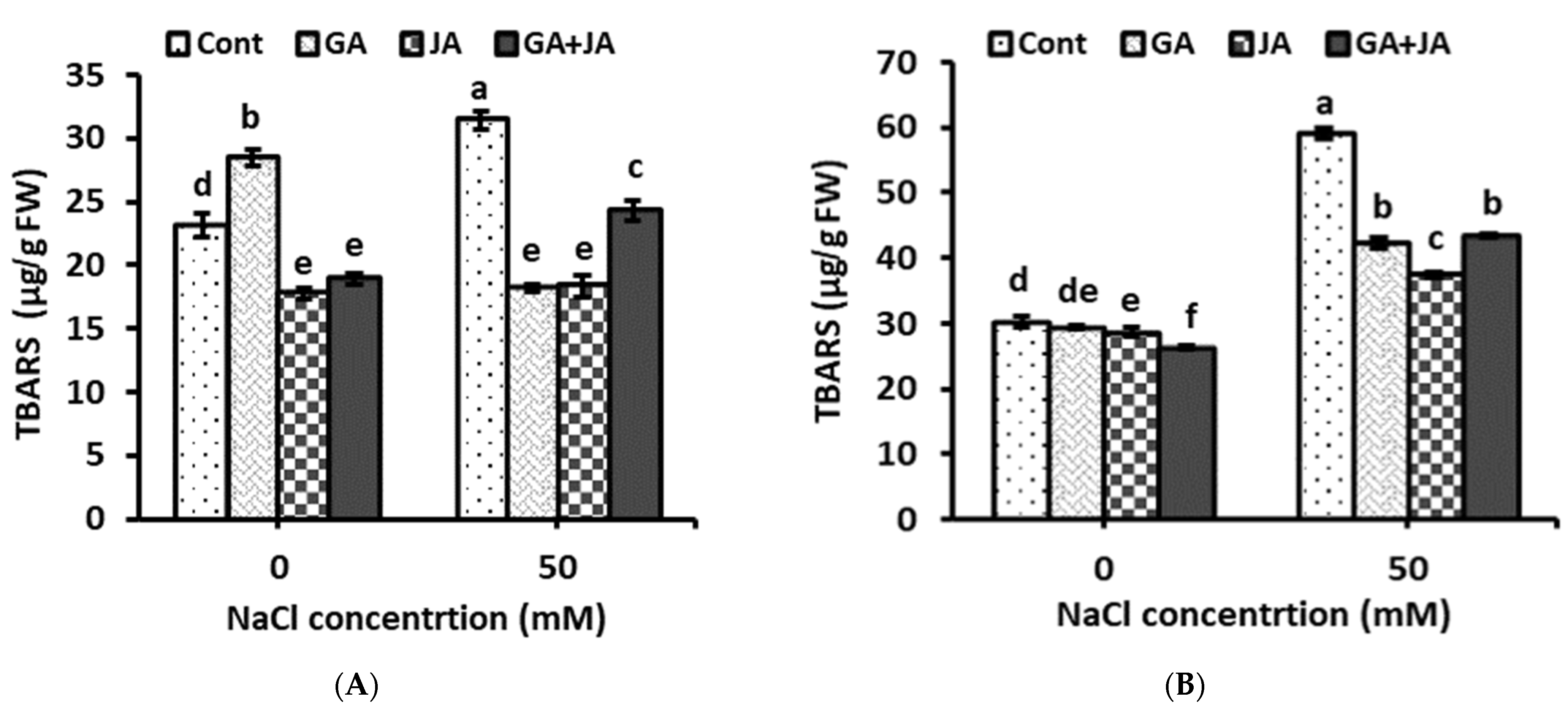
| Parameters | Significance of Sources of Variation | ||
|---|---|---|---|
| Salinity (S) | Hormones (H) | S × H | |
| Shoot FW | *** | ** | *** |
| Root FW | *** | * | *** |
| Shoot DW | *** | ns | *** |
| Root DW | *** | ns | *** |
| Shoot WC | * | ns | ** |
| Root WC | * | ns | ** |
| Proline in shoot | *** | ** | ** |
| Proline in root | *** | ** | * |
| TBARS in shoot | *** | * | * |
| TBARS in root | *** | *** | *** |
| SOD activity in shoot | ns | *** | ns |
| SOD activity in root | * | *** | ns |
| CAT activity in shoot | *** | ns | *** |
| CAT activity in root | *** | * | *** |
| APX activity in shoot | *** | ns | *** |
| APX activity in root | *** | ns | *** |
| RNA in shoot | *** | ns | *** |
| RNA in root | *** | ns | *** |
| DNA in shoot | *** | * | *** |
| DNA in root | *** | * | *** |
| NaCl (mM) | Hormones | Chl a (mg/g DW) | Chl b (mg/g DW) | Chl a/b Ratio | Carotenoid (mg/g DW) |
|---|---|---|---|---|---|
| 0 | Non | 11.60 ± 0.08 a | 6.24 ± 0.11 f | 1.9 ± 0.03 b | 1.56 ± 0.07 f |
| GA | 5.07 ± 0.52 ef | 30.27 ± 0.38 c | 0.17 ± 0.02 d | 1.92 ± 0.04 d | |
| JA | 5.61 ± 0.16 e | 39.79 ± 0.75 a | 0.15 ± 0.01 d | 2.48 ± 0.08 a | |
| GA + JA | 5.74 ± 0.52 e | 32.34 ± 0.88 c | 0.17 ± 0.02 d | 2.06 ± 0.05 c | |
| 50 | Non | 11.62 ± 0.03 a | 16.96 ± 0.11 e | 0.69 ± 0.01 a | 1.80 ± 0.02 e |
| GA | 7.65 ± 0.06 c | 29.37 ± 0.44 c | 0.28 ± 0.02 c | 2.14 ± 0.04 c | |
| JA | 9.07 ± 0.58 b | 35.82 ± 0.50 b | 0.25 ± 0.02 c | 2.25 ± 0.07 b | |
| GA + JA | 6.24 ± 0.39 d | 29.84 ± 0.92 cd | 0.25 ± 0.02 c | 2.07 ± 0.06 c | |
| ANOVA | |||||
| Salinity (S) | ns | *** | ** | * | |
| Hormones (H) | *** | *** | *** | *** | |
| S × H | *** | *** | *** | *** | |
| NaCl (mM) | Hormones | Shoot | Root | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | K+/Na+ Ratio | Mg++ | Cl− | Na+ | K+ | K+/Na+ Ratio | Mg++ | Cl− | ||
| 0 | Non | 2.50 ± 0.10 c | 16.33 ± 0.37 a | 6.53 ± 0.15 e | 8.17 ± 0.47 b | 36.41 ± 2.38 c | 12.25 ± 0.27 f | 54.67 ± 7.60 d | 4.46 ± 0.02 b | 13.24 ± 2.30 b | 118.40 ± 10.70 e |
| GA | 2.20 ± 0.11 d | 11.14 ± 0.38 d | 5.06 ± 0.49 f | 8.95 ± 1.23 a | 35.24 ± 2.90 c | 12.61 ± 0.15 e | 44.43 ± 3.64 e | 3.52 ± 0.03 d | 15.46 ± 3.88 b | 115.10 ± 4.15 e | |
| JA | 1.07 ± 0.04 g | 11.37 ± 0.43 d | 10.63 ± 0.12 b | 7.80 ± 0.35 c | 25.00 ± 1.85 d | 10.37 ± 0.25 g | 73.29 ± 6.40 b | 7.07 ± 0.05 a | 10.80 ± 1.30 c | 143.50 ± 16.30 c | |
| GA + JA | 1.04 ± 0.01 g | 14.19 ± 0.27 c | 13.64 ± 0.14 a | 7.44 ± 0.53 c | 33.24 ± 5.57 c | 13.79 ± 0.56 e | 53.67 ± 6.20 d | 3.89 ± 0.01 c | 11.90 ± 2.57 c | 120.70 ± 8.32 d | |
| 50 | Non | 3.54 ± 0.11 a | 13.09 ± 0.56 c | 3.69 ± 0.02 g | 6.65 ± 0.50 d | 54.29 ± 5.31 a | 54.25 ± 4.51 a | 44.06 ± 3.10 e | 0.81 ± 0.01 f | 11.73 ± 1.52 c | 251.10 ± 18.40 a |
| GA | 2.77 ± 0.08 b | 15.05 ± 0.15 b | 5.43 ± 0.06 f | 6.20 ± 0.40 d | 44.71 ± 2.57 b | 23.76 ± 2.82 d | 59.96 ± 6.90 d | 2.52 ± 0.41 e | 13.56 ± 1.93 b | 198.30 ± 20.90 b | |
| JA | 1.37 ± 0.03 f | 11.26 ± 0.78 d | 8.22 ± 0.02 d | 7.61 ± 0.27 c | 55.98 ± 3.16 a | 32.68 ± 2.81 b | 83.54 ± 7.27 a | 2.56 ± 0.32 e | 21.14 ± 1.69 a | 185.30 ± 6.81 b | |
| GA + JA | 1.45 ± 0.01 e | 13.70 ± 0.10 c | 9.45 ± 0.12 c | 7.47 ± 0.67 c | 55.68 ± 3.54 a | 24.81 ± 3.27 c | 70.38 ± 3.37 c | 2.84 ± 0.31 e | 18.23 ± 1.98 a | 217.51 ± 11.63 b | |
| ANOVA | |||||||||||
| Salinity (S) | ** | ** | *** | ** | *** | *** | *** | *** | ** | *** | |
| Hormones (H) | ** | ** | ** | ns | ns | ** | ** | ** | ns | ** | |
| S × H | *** | * | *** | ns | ns | *** | *** | *** | *** | *** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-harthi, M.M.; Bafeel, S.O.; El-Zohri, M. Gibberellic Acid and Jasmonic Acid Improve Salt Tolerance in Summer Squash by Modulating Some Physiological Parameters Symptomatic for Oxidative Stress and Mineral Nutrition. Plants 2021, 10, 2768. https://doi.org/10.3390/plants10122768
Al-harthi MM, Bafeel SO, El-Zohri M. Gibberellic Acid and Jasmonic Acid Improve Salt Tolerance in Summer Squash by Modulating Some Physiological Parameters Symptomatic for Oxidative Stress and Mineral Nutrition. Plants. 2021; 10(12):2768. https://doi.org/10.3390/plants10122768
Chicago/Turabian StyleAl-harthi, Mashael M., Sameera O. Bafeel, and Manal El-Zohri. 2021. "Gibberellic Acid and Jasmonic Acid Improve Salt Tolerance in Summer Squash by Modulating Some Physiological Parameters Symptomatic for Oxidative Stress and Mineral Nutrition" Plants 10, no. 12: 2768. https://doi.org/10.3390/plants10122768






