Cassia fistula Leaves; UHPLC-QTOF-MS/MS Based Metabolite Profiling and Molecular Docking Insights to Explore Bioactives Role towards Inhibition of Pancreatic Lipase
Abstract
:1. Introduction
2. Results and Discussion
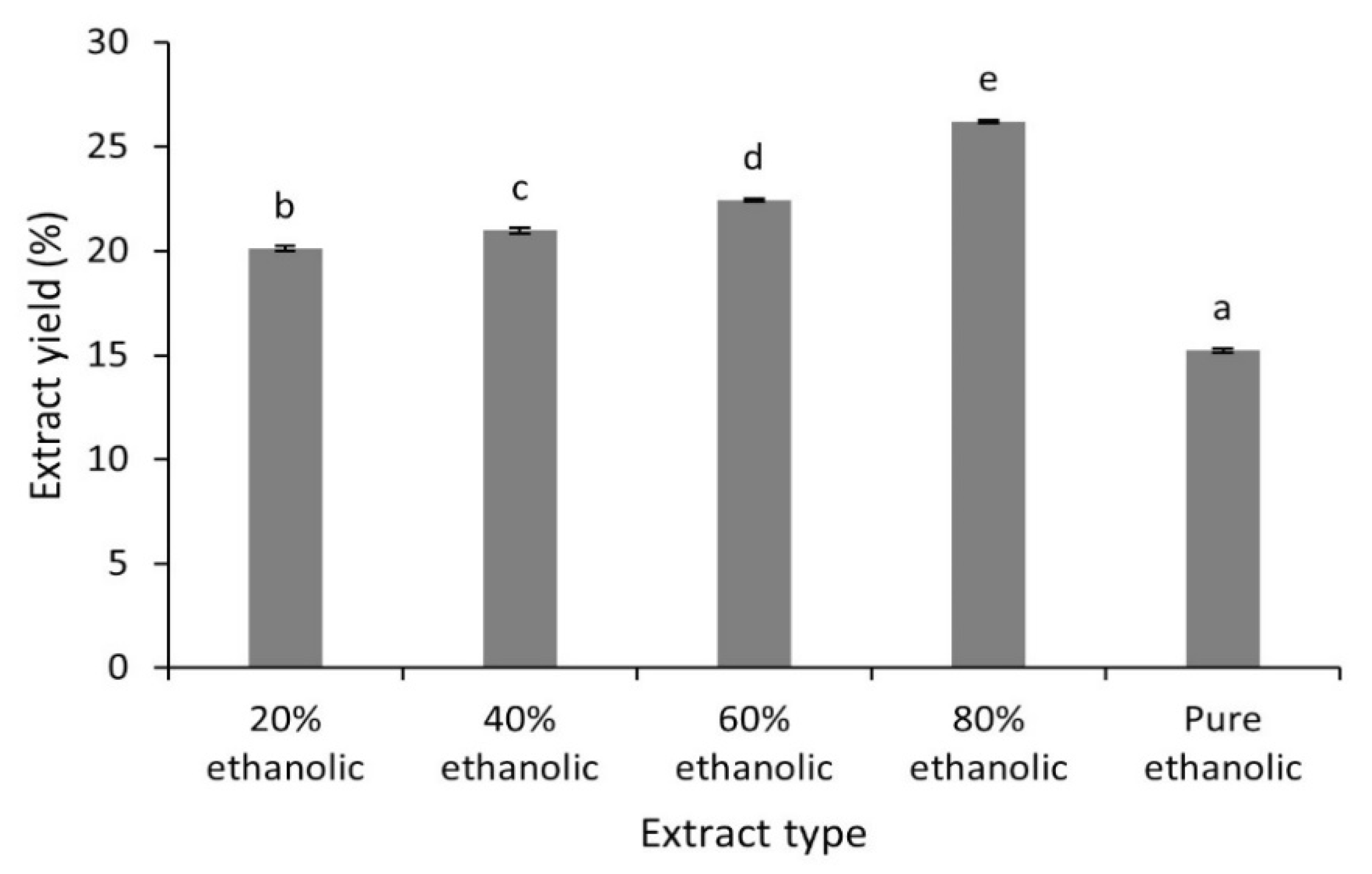
2.1. Extract Yield
2.2. Total Phenolic and Flavonoid Contents
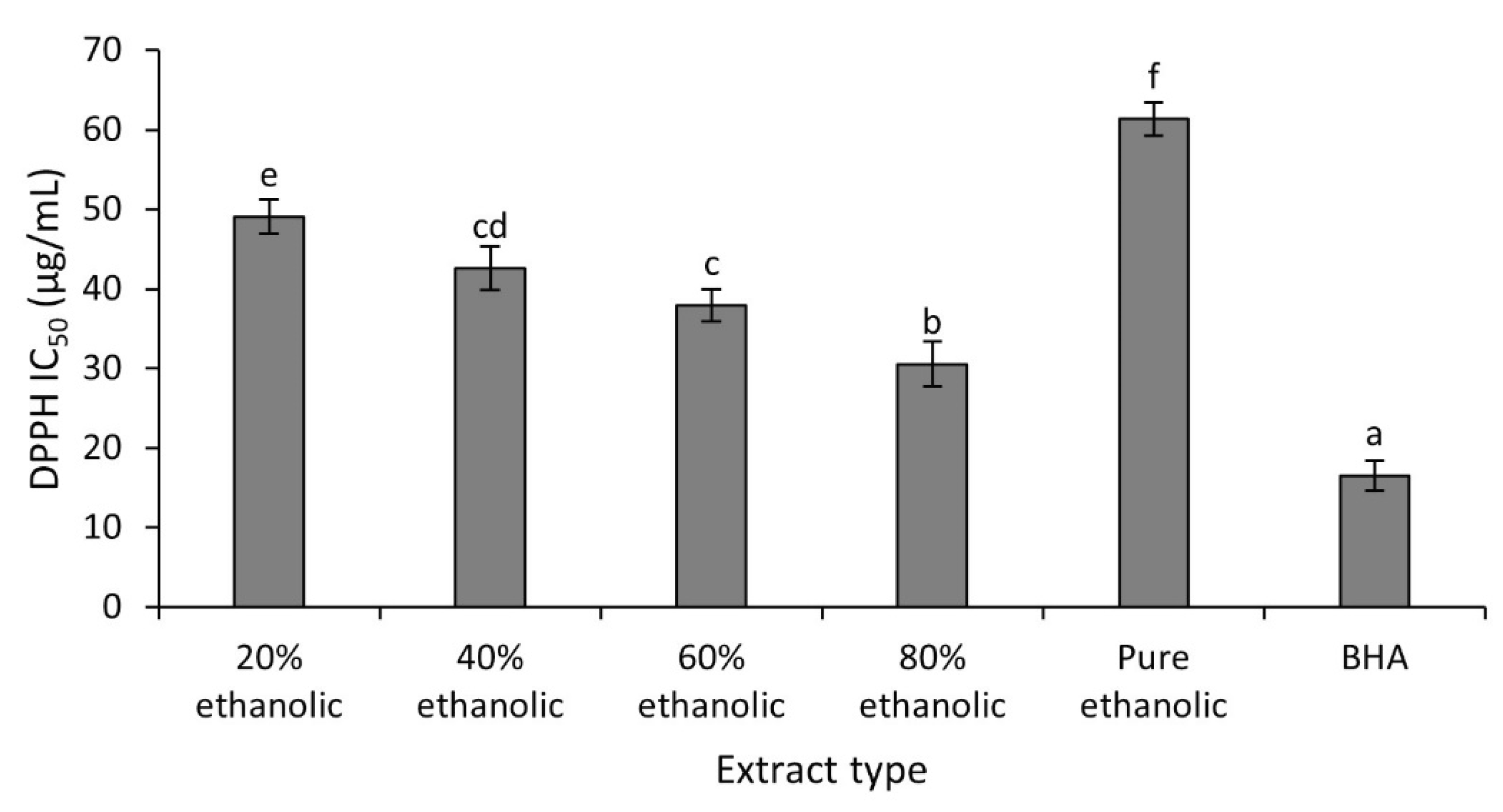
2.3. DPPH Radical Scavenging Activity In Vitro
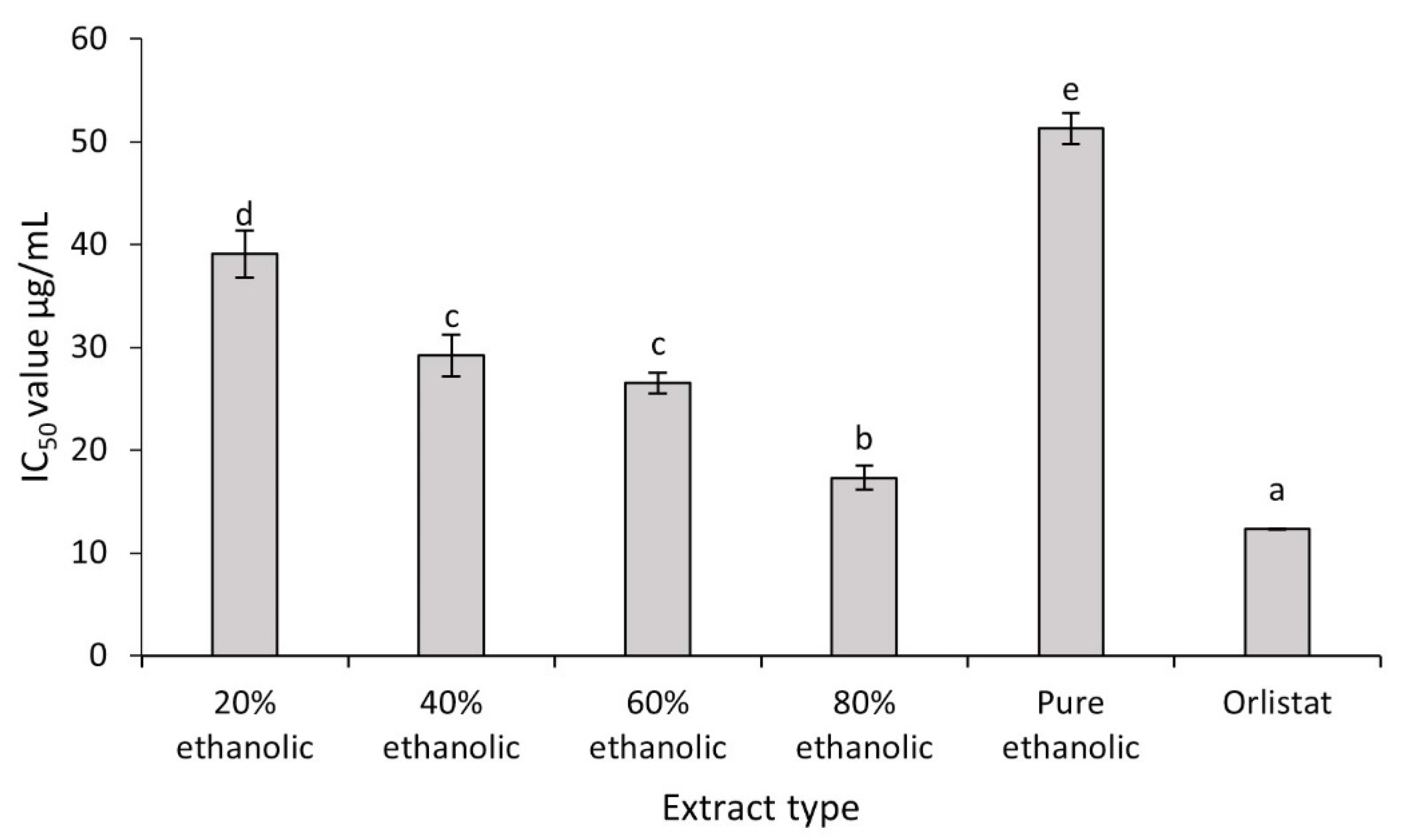
2.4. Antiobesity Activity of C. fistula
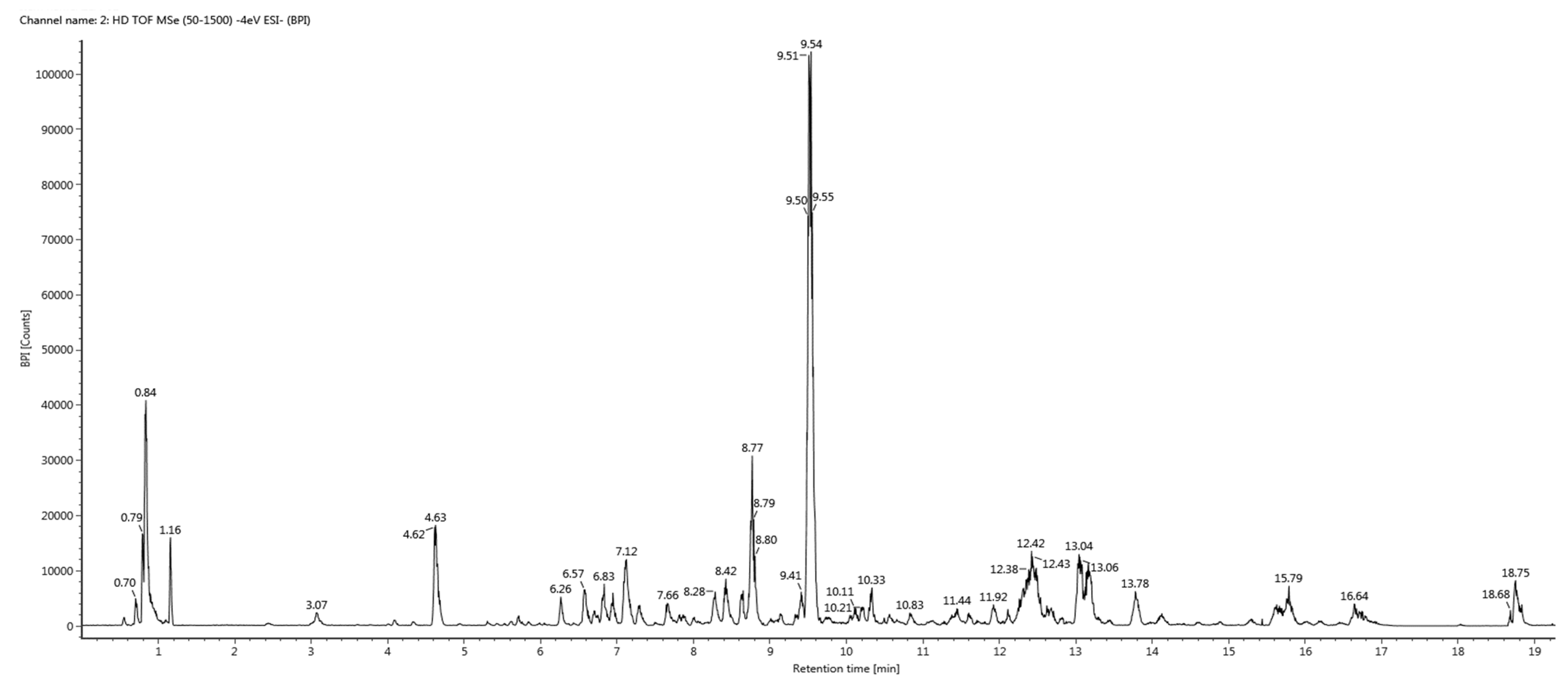
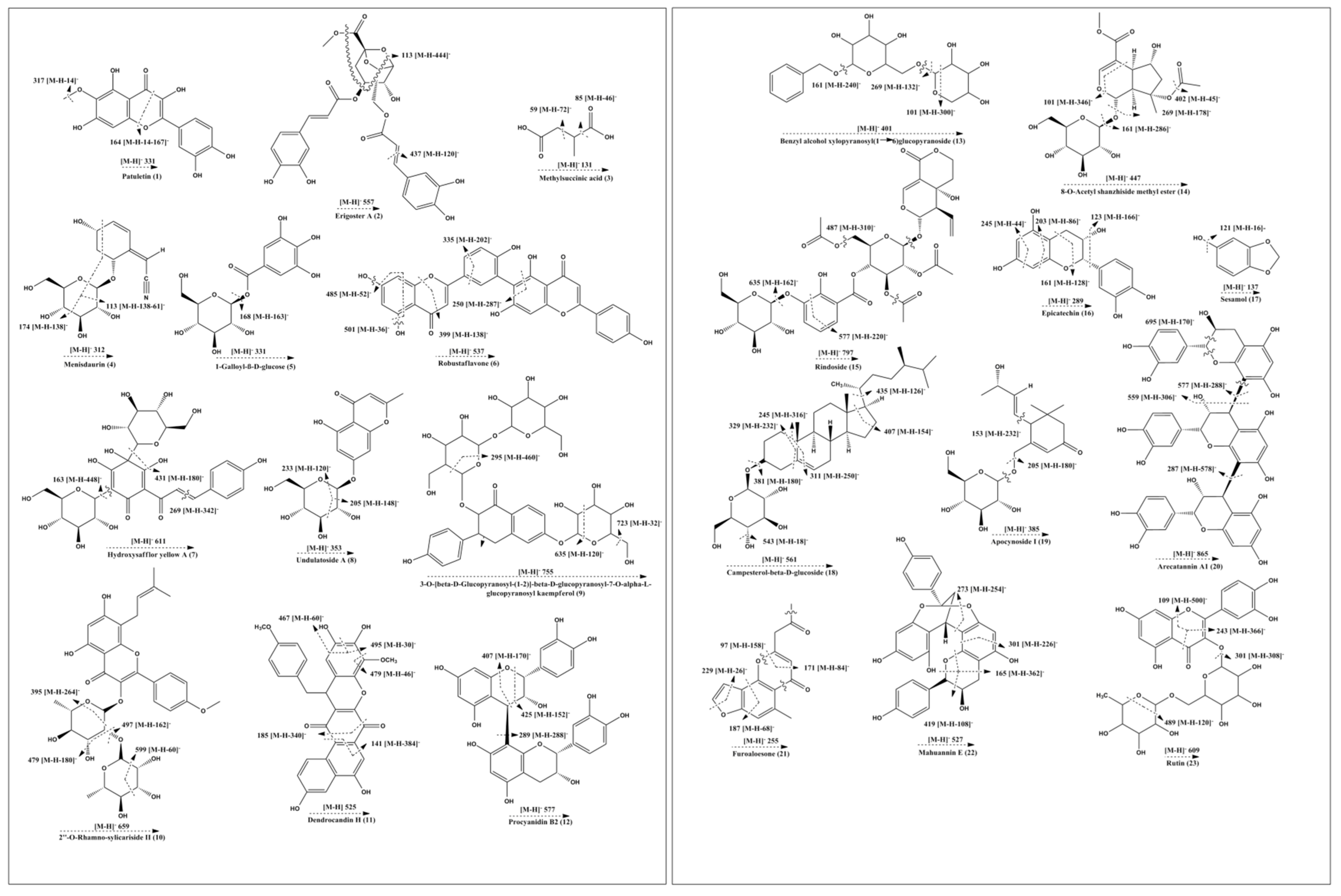
2.5. UHPLC-QTOF-MS/MS Based Metabolite Profiling
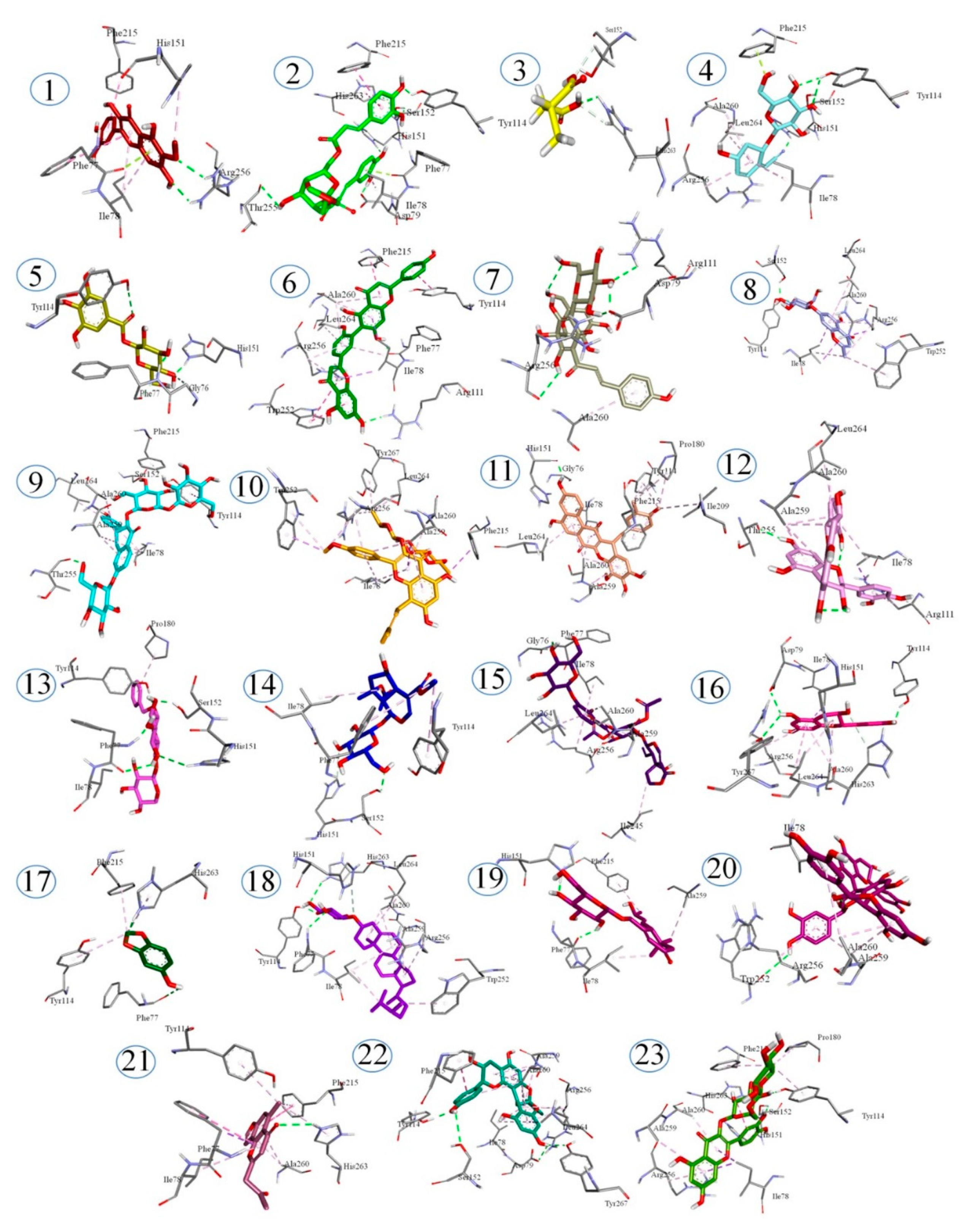
2.6. Docking Studies
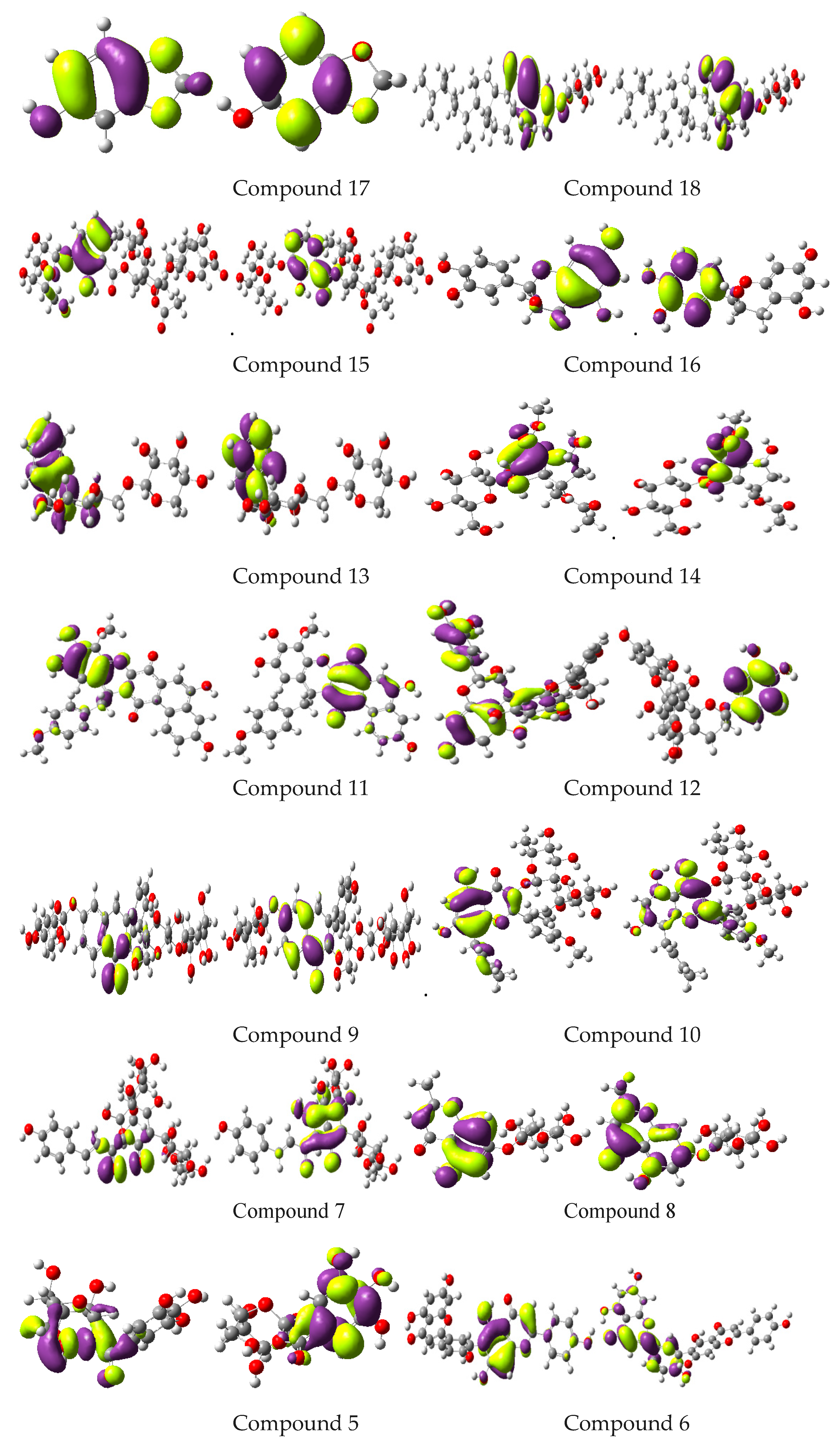
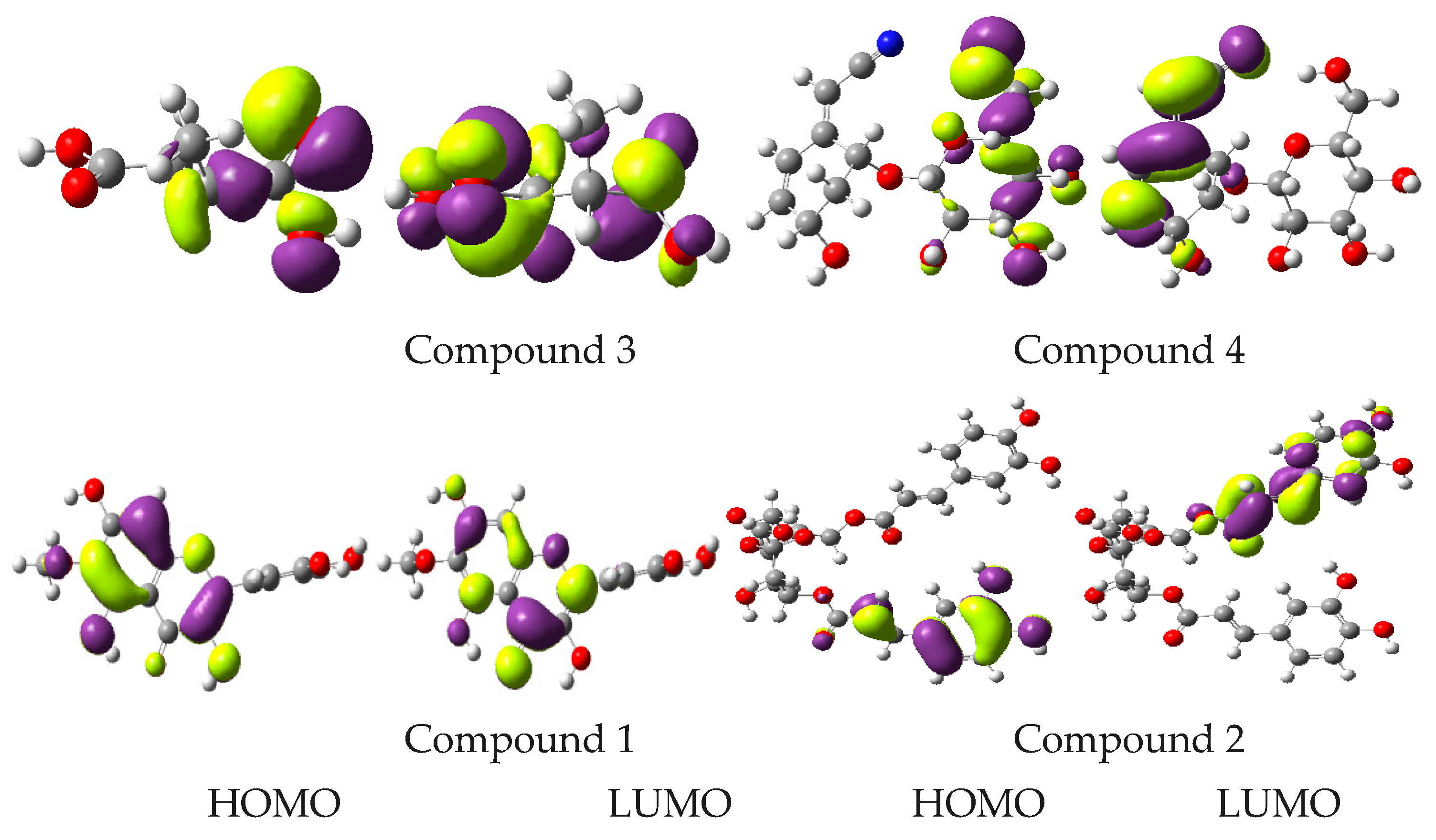
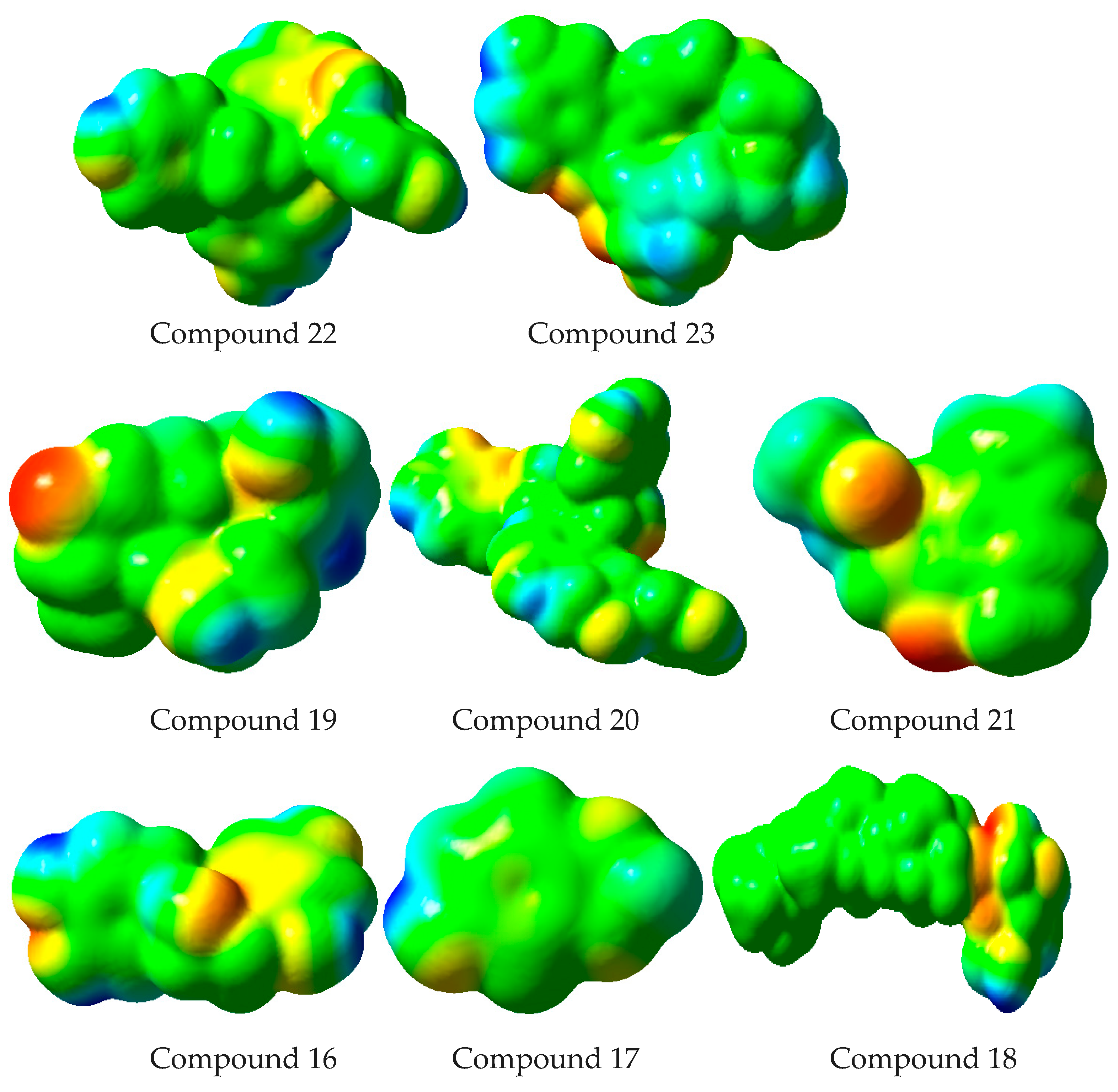
2.7. Computational Studies
3. Materials and Methods
3.1. Plant Collection and Identification
3.2. Extract Preparation
3.3. Phytochemical Screening
3.3.1. Total Phenolic Contents
3.3.2. Total Flavonoid Contents
3.4. Antioxidant Activity
3.5. Pancreatic Lipase Inhibitory Assay
3.6. UHPLC-QTOF-MS/MS-Based Metabolite Profiling
3.7. Molecular Docking Studies
3.8. Computational Studies
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Lu, Y. Inhibitory activities of extracts from Cleistocalyx operculatus flower buds on pancreatic lipase and α-amylase. Eur. Food Res. Technol. 2012, 235, 1133–1139. [Google Scholar] [CrossRef]
- Alias, N.; Leow, T.; Ali, M.; Tajudin, A.; Salleh, A. Anti-obesity potential of selected tropical plants via pancreatic lipase inhibition. Adv. Obes. Weight Manag. Control 2017, 6, 122–131. [Google Scholar] [CrossRef] [Green Version]
- Abdul Rahman, H.; Saari, N.; Abas, F.; Ismail, A.; Mumtaz, M.W.; Abdul Hamid, A. Anti-obesity and antioxidant activities of selected medicinal plants and phytochemical profiling of bioactive compounds. Int. J. Food Prop. 2017, 20, 2616–2629. [Google Scholar] [CrossRef]
- Jamous, R.M.; Abu-Zaitoun, S.Y.; Akkawi, R.J.; Ali-Shtayeh, M.S. Anti-obesity and antioxidant potentials of selected Palestinian medicinal plants. Evid. Based Complementary Altern. Med. 2018, 2018, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, R. Pharmacotherapy of obesity: Emerging drugs and targets. Expert Opin. Ther. Targets 2009, 13, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A.; Ibrahim, S.R.; Elkhayat, E.S.; El Dine, R.S. Natural anti-obesity agents. Bull. Fac. Pharm. Cairo Univ. 2014, 52, 269–284. [Google Scholar] [CrossRef] [Green Version]
- Moreno, D.A.; Ilic, N.; Poulev, A.; Brasaemle, D.L.; Fried, S.K.; Raskin, I. Inhibitory effects of grape seed extract on lipases. Nutrition 2003, 19, 876–879. [Google Scholar] [CrossRef]
- McDougall, G.J.; Kulkarni, N.N.; Stewart, D. Berry polyphenols inhibit pancreatic lipase activity in vitro. Food Chem. 2009, 115, 193–199. [Google Scholar] [CrossRef]
- Liu, S.; Li, D.; Huang, B.; Chen, Y.; Lu, X.; Wang, Y. Inhibition of pancreatic lipase, α-glucosidase, α-amylase, and hypolipidemic effects of the total flavonoids from Nelumbo nucifera leaves. J. Ethnopharmacol. 2013, 149, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Gulua, L.; Nikolaishvili, L.; Jgenti, M.; Turmanidze, T.; Dzneladze, G. Polyphenol content, anti-lipase and antioxidant activity of teas made in Georgia. Ann. Agrar. Sci. 2018, 16, 357–361. [Google Scholar] [CrossRef]
- Nadeem, M.; Mumtaz, M.W.; Danish, M.; Rashid, U.; Mukhtar, H.; Anwar, F.; Raza, S.A. Calotropis procera: UHPLC-QTOF-MS/MS based profiling of bioactives, antioxidant and anti-diabetic potential of leaf extracts and an insight into molecular docking. J. Food Meas. Charact. 2019, 13, 3206–3220. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: Causes and therapeutic strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higuchi, M.; Dusting, G.J.; Peshavariya, H.; Jiang, F.; Hsiao, S.T.-F.; Chan, E.C.; Liu, G.-S. Differentiation of human adipose-derived stem cells into fat involves reactive oxygen species and Forkhead box O1 mediated upregulation of antioxidant enzymes. Stem Cells Dev. 2013, 22, 878–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadeem, M.; Mumtaz, M.W.; Danish, M.; Rashid, U.; Mukhtar, H.; Irfan, A. Antidiabetic functionality of Vitex negundo L. leaves based on UHPLC-QTOF-MS/MS based bioactives profiling and molecular docking insights. Ind. Crop. Prod. 2020, 152, 112445. [Google Scholar] [CrossRef]
- Jaradat, N.A.; Zaid, A.N.; Hussein, F. Investigation of the anti-obesity and antioxidant properties of wild Plumbago europaea and Plumbago auriculata from North Palestine. Chem. Biol. Technol. Agric. 2016, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Riccardi, G.; Capaldo, B.; Vaccaro, O. Functional foods in the management of obesity and type 2 diabetes. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Ideraabdullah, F.Y.; Zeisel, S.H. Dietary modulation of the epigenome. Physiol. Rev. 2018, 98, 667–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrawal, K.; Ghildiyal, S.; Gautam, M.; Joshi, V.; Goel, R. Studies on laxative effect of extract of dried fruit pulp of Cassia fistula. J. Nat. Remedies 2012, 12, 28. [Google Scholar] [CrossRef]
- Mondal, M.; Hossain, M.M.; Rahman, M.A.; Mubarak, M.S.; PK, M.M.U.; Islam, M.S.; Pervin, R.; Rahman, M.; Morad, R.U.; Chowdhury, M.M.H. Cassia fistula Linn: A review of phytochemical and pharmacological studies. Int. J. Pharm. Sci. Res. 2014, 5, 2125–2130. [Google Scholar] [CrossRef]
- Rahmani, A.H. Cassia fistula Linn: Potential candidate in the health management. Pharmacogn. Res. 2015, 7, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chichioco-Hern, C.L.; Leonido, F.M.G. Weight-lowering effects of Caesalpinia pulcherrima, Cassia fistula and Senna alata leaf extracts. J. Med. Plant Res. 2011, 5, 452–455. [Google Scholar]
- Bahorun, T.; Neergheen, V.S.; Aruoma, O.I. Phytochemical constituents of Cassia fistula. Afr. J. Biotechnol. 2005, 4, 1530–1540. [Google Scholar] [CrossRef]
- Nadeem, M.; Mumtaz, M.W.; Danish, M. Ultrasonication-assisted extraction, antioxidant activity and α-amylase inhibition potential of vitex negundo leaves. Biologia 2019, 65, 1–9. [Google Scholar]
- Nadeem, M.; Mumtaz, M.W.; Danish, M.; Rashid, U.; Mukhtar, H.; Irfan, A.; Anwar, F.; Saari, N. UHPLC-QTOF-MS/MS metabolites profiling and antioxidant/antidiabetic attributes of Cuscuta reflexa grown on Casearia tomentosa: Exploring phytochemicals role via molecular docking. Int. J. Food Prop. 2020, 23, 918–940. [Google Scholar] [CrossRef]
- Khorasani Esmaeili, A.; Mat Taha, R.; Mohajer, S.; Banisalam, B. Antioxidant activity and total phenolic and flavonoid content of various solvent extracts from in vivo and in vitro grown Trifolium pratense L. (Red Clover). BioMed Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sembiring, E.N.; Elya, B.; Sauriasari, R. Phytochemical screening, total flavonoid and total phenolic content and antioxidant activity of different parts of Caesalpinia bonduc (L.) Roxb. Pharmacogn. J. 2018, 10, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Wanigasekera, W.M.A.P.; Joganathan, A.; Pethiyagoda, R.; Yatiwella, L.N.; Attanayake, H.M.D.A.B. Comparison of antioxidant activity, Phenolic and Flavonoid contents of selected medicinal plants in Sri Lanka. Ceylon J. Sci. 2019, 48, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.P.; Sulaiman Rahman, H. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2018, 9, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Deng, Z.; Ramdath, D.D.; Tang, Y.; Chen, P.X.; Liu, R.; Liu, Q.; Tsao, R. Phenolic profiles of 20 Canadian lentil cultivars and their contribution to antioxidant activity and inhibitory effects on α-glucosidase and pancreatic lipase. Food Chem. 2015, 172, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Boukhris, M.A.; Destandau, É.; El Hakmaoui, A.; El Rhaffari, L.; Elfakir, C. A dereplication strategy for the identification of new phenolic compounds from Anvillea radiata (Coss. & Durieu). Comptes Rendus Chim. 2016, 19, 1124–1132. [Google Scholar] [CrossRef] [Green Version]
- Al-Yousef, H.M.; Hassan, W.H.; Abdelaziz, S.; Amina, M.; Adel, R.; El-Sayed, M.A. UPLC-ESI-MS/MS profile and antioxidant, cytotoxic, antidiabetic, and anti-obesity activities of the aqueous extracts of three different Hibiscus Species. J. Chem. 2020, 2020, 1–17. [Google Scholar] [CrossRef]
- Akbari, S.; Abdurahman, N.H.; Yunus, R.M. Optimization of saponins, phenolics, and antioxidants extracted from fenugreek seeds using microwave-assisted extraction and response surface methodology as an optimizing tool. Comptes Rendus Chim. 2019, 22, 714–727. [Google Scholar] [CrossRef]
- Ambati, C.R.; Vantaku, V.; Donepudi, S.R.; Amara, C.S.; Ravi, S.S.; Mandalapu, A.; Perla, M.; Putluri, V.; Sreekumar, A.; Putluri, N. Measurement of methylated metabolites using liquid chromatography-mass spectrometry and its biological application. Anal. Methods 2019, 11, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.Y.; Fan, M.X.; Wu, X.; Wang, H.J.; Yang, J.; Si, N.; Bian, B.L. Chemical profiling of the Chinese herb formula Xiao-Cheng-Qi decoction using liquid chromatography coupled with electrospray ionization mass spectrometry. J. Chromatogr. Sci. 2013, 51, 273–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aabideen, Z.U.; Mumtaz, M.W.; Akhtar, M.T.; Mukhtar, H.; Raza, S.A.; Touqeer, T.; Saari, N. Anti-Obesity Attributes; UHPLC-QTOF-MS/MS-Based Metabolite Profiling and Molecular Docking Insights of Taraxacum officinale. Molecules 2020, 25, 4935. [Google Scholar] [CrossRef] [PubMed]
- Ammar, S.; del Mar Contreras, M.; Belguith-Hadrich, O.; Segura-Carretero, A.; Bouaziz, M. Assessment of the distribution of phenolic compounds and contribution to the antioxidant activity in Tunisian fig leaves, fruits, skins and pulps using mass spectrometry-based analysis. Food Funct. 2015, 6, 3663–3677. [Google Scholar] [CrossRef] [PubMed]
- Bujor, O.C. Extraction, Identification and Antioxidant Activity of the Phenolic Secondary Metabolites Isolated from the Leaves, Stems and Fruits of Two Shrubs of the Ericaceae Family. Ph.D. Thesis, Université d’Avignon, Avignon, France, 2016. [Google Scholar] [CrossRef]
- Yao, H.; Chen, B.; Zhang, Y.; Ou, H.; Li, Y.; Li, S.; Shi, P.; Lin, X. Analysis of the total biflavonoids extract from Selaginella doederleinii by HPLC-QTOF-MS and its in vitro and in vivo anticancer effects. Molecules 2017, 22, 325. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.X.; Li, Q.Y.; Yan, L.L.; Shi, Y. Structural characterization and identification of biflavones in Selaginella tamariscina by liquid chromatography-diode-array detection/electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 2173–2186. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Li, L.; Zhang, H.; Li, B.; Liu, C. Preparative separation of hydroxyl safflower yellow A and anhydrosafflor yellow B in plant extract of Carthamus tinctorius L. by reverse-phase medium-pressure liquid chromatography. J. Liq. Chrom. Rel. Technol. 2013, 36, 1947–1958. [Google Scholar] [CrossRef]
- Veitch, N.C.; Grayer, R.J. Flavonoids and their glycosides, including anthocyanins. Nat. Prod. Rep. 2008, 25, 555–611. [Google Scholar] [CrossRef]
- Calderon, A.I.; Wright, B.J.; Hurst, W.J.; Van Breemen, R.B. Screening antioxidants using LC-MS: Case study with cocoa. J. Agric. Food Chem. 2009, 57, 5693–5699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Liang, F.; Bin, Y.; Li, P.; Duan, C. Screening non-colored phenolics in red wines using liquid chromatography/ultraviolet and mass spectrometry/mass spectrometry libraries. Molecules 2007, 12, 679–693. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, A. Phytochemical Analysis of Rhododendron Species; IRC-Library, Information Resource Center der Jacobs University Bremen: Bremen, Germany, 2016. [Google Scholar]
- Spínola, V.; Llorent-Martínez, E.J.; Castilho, P.C. Antioxidant polyphenols of Madeira sorrel (Rumex maderensis): How do they survive to in vitro simulated gastrointestinal digestion? Food Chem. 2018, 259, 105–112. [Google Scholar] [CrossRef] [PubMed]
- De Marino, S.; Borbone, N.; Zollo, F.; Ianaro, A.; Di Meglio, P.; Iorizzi, M. Megastigmane and phenolic components from Laurus nobilis L. leaves and their inhibitory effects on nitric oxide production. J. Agric. Food Chem. 2004, 52, 7525–7531. [Google Scholar] [CrossRef]
- Karar, M.E.; Kuhnert, N. UPLC-ESI-Q-TOF-MS/MS characterization of phenolics from Crataegus monogyna and Crataegus laevigata (Hawthorn) leaves, fruits and their herbal derived drops (Crataegutt Tropfen). J. Chem. Biol. Ther. 2015, 1, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zhang, R.; Li, C.; Fan, P.; Zhang, Q.; Jia, Z. Development of a validated HPLC-PAD-APCI/MS method for the identification and determination of iridoid glycosides in Lamiophlomis rotata. Anal. Methods. 2010, 2, 714–721. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Jungfer, E.; Ritter, C.; Santiago-Schübel, B.; Thiele, B.; Fett, R.; Galensa, R. Characterization of flavan-3-ols in seeds of grape pomace by CE, HPLC-DAD-MSn and LC-ESI-FTICR-MS. Food Res. Int. 2012, 48, 848–855. [Google Scholar] [CrossRef] [Green Version]
- Michailidis, D.; Angelis, A.; Aligiannis, N.I.; Mitakou, S.; Skaltsounis, L.A. Recovery of sesamin, sesamolin and minor lignans from sesame oil using solid support free liquid-liquid extraction and chromatography techniques and evaluation of their enzymatic inhibition properties. Front. Pharmacol. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hoang, N.H.; Hong, S.-Y.; Huong, N.L.; Park, J.W. Biochemical characterization of recombinant UDP-glucose: Sterol 3-O-glycosyltransferase from Micromonospora rhodorangea ATCC 31603 and enzymatic biosynthesis of sterol-3-O-β-glucosides. J. Microbiol. Biotechnol. 2016, 26, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Kishi, A.; Matsuda, H.; Hattori, M.; Yoshikawa, M. Medicinal foodstuffs. XXIV. Chemical constituents of the processed leaves of Apocynum venetum L.: Absolute stereostructures of apocynosides I and II. Chem. Pharm. Bull. 2001, 49, 845–848. [Google Scholar] [CrossRef] [Green Version]
- Tao, H.; Wang, L.; Cui, Z.; Zhao, D.; Liu, Y. Dimeric proanthocyanidins from the roots of Ephedra sinica. Planta Med. 2008, 74, 1823–1825. [Google Scholar] [CrossRef] [PubMed]
- Parejo, I.; Jauregui, O.; Sánchez-Rabaneda, F.; Viladomat, F.; Bastida, J.; Codina, C. Separation and characterization of phenolic compounds in Fennel (Foeniculum vulgare) Using liquid chromatography−negative electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 2004, 52, 3679–3687. [Google Scholar] [CrossRef] [PubMed]
- Martucci, M.E.P.; De Vos, R.C.; Carollo, C.A.; Gobbo-Neto, L. Metabolomics as a potential chemotaxonomical tool: Application in the genus Vernonia Schreb. PLoS ONE 2014, 9, e93149. [Google Scholar] [CrossRef] [Green Version]
- Kicel, A.; Kolodziejczyk-Czepas, J.; Owczarek, A.; Marchelak, A.; Sopinska, M.; Ciszewski, P.; Nowak, P.; Olszewska, M.A. Polyphenol-rich extracts from Cotoneaster leaves inhibit pro-inflammatory enzymes and protect human plasma components against oxidative stress in vitro. Molecules 2018, 23, 2472. [Google Scholar] [CrossRef] [Green Version]
- Ramabulana, A.T.; Steenkamp, P.; Madala, N.; Dubery, I.A. Profiling of chlorogenic acids from Bidens pilosa and differentiation of closely related positional isomers with the aid of UHPLC-QTOF-MS/MS-based in-source collision-induced dissociation. Metabolites 2020, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.B.C. A Hexa-Herbal Chinese Formula for Treatment of Atopic Dermatitis: Phytochemical Analysis and Selected Anti-Inflammatory Activities. Ph.D. Thesis, UCL (University College London), London, UK, 2017. [Google Scholar]
- Llorent-Martínez, E.J.; Ortega-Barrales, P.; Zengin, G.; Mocan, A.; Simirgiotis, M.J.; Ceylan, R.; Uysal, S.; Aktumsek, A. Evaluation of antioxidant potential, enzyme inhibition activity and phenolic profile of Lathyrus cicera and Lathyrus digitatus: Potential sources of bioactive compounds for the food industry. Food Chem. Toxicol. 2017, 107, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Hong, S.M.; Lee, H.S.; Moon, B.C.; Kim, D.; Kwon, H. Identification and characterization of matrix components in spinach during QuEChERS sample preparation for pesticide residue analysis by LC-ESI-MS/MS, GC-MS and UPLC-DAD. J. Food Sci. Technol. 2018, 55, 3930–3938. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.R.; Chowdhury, K.H.; Hanif, N.B.; Sayeed, M.A.; Mouah, J.; Mahmud, I.; Kamal, A.M.; Chy, M.N.U.; Adnan, M. An integrated exploration of pharmacological potencies of Bischofia javanica (Blume) leaves through experimental and computational modeling. Heliyon 2020, 6, 1–13. [Google Scholar] [CrossRef]
- Zheng, Y.Z.; Deng, G.; Guo, R.; Chen, D.F.; Fu, Z.M. DFT studies on the antioxidant activity of Naringenin and its derivatives: Effects of the substituents at C3. Int. J. Mol. Sci. 2019, 20, 1450. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, M.; Asghar, B.H.; Zaafarany, I.; Fouda, A. The inhibition of carbon steel corrosion in hydrochloric acid solution using some phenolic compounds. Int. J. Electrochem. Sci. 2012, 7, 282–304. [Google Scholar]
- Rasool, N.; Akhtar, A.; Hussain, W. Insights into the inhibitory potential of selective phytochemicals against Mpro of 2019-nCoV: A computer-aided study. Struct. Chem. 2020, 31, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Morokuma, K.; Kitaura, K. Chemical Applications of Atomic and Molecular Electrostatic Potentials; Plenumnew: York, NY, USA, 1981; Volume 215. [Google Scholar]
- Murray, J.S.; Politzer, P. The electrostatic potential: An overview. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2011, 1, 153–163. [Google Scholar] [CrossRef]
- Wright, J.S.; Johnson, E.R.; DiLabio, G.A. Predicting the activity of phenolic antioxidants: Theoretical method, analysis of substituent effects, and application to major families of antioxidants. J. Am. Chem. Soc. 2001, 123, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; Santos, T.C.d.; Coube, C.S.; Leitão, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef]
- Galano, A.; Raúl Alvarez-Idaboy, J. Computational strategies for predicting free radical scavengers’ protection against oxidative stress: Where are we and what might follow? Int. J. Quantum Chem. 2019, 119, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Rajan, V.K.; Ragi, C.; Muraleedharan, K. A computational exploration into the structure, antioxidant capacity, toxicity and drug-like activity of the anthocyanidin “Petunidin”. Heliyon 2019, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Irfan, A.; Chaudhry, A.R.; Al-Sehemi, A.G.; Assiri, M.A.; Hussain, A. Charge carrier and optoelectronic properties of phenylimidazo [1, 5-a] pyridine-containing small molecules at molecular and solid-state bulk scales. Comput. Mater. Sci. 2019, 170, 109179. [Google Scholar] [CrossRef]
- Irfan, A.; Chaudhry, A.R.; Al-Sehemi, A.G.; Assiri, M.A.; Ullah, S. Exploration of optoelectronic and photosensitization properties of triphenylamine-based organic dye on TiO 2 surfaces. J. Comput. Electron. 2019, 18, 1119–1127. [Google Scholar] [CrossRef]
- Irfan, A.; Al-Sehemi, A.G.; Assiri, M.A.; Mumtaz, M.W. Exploring the electronic, optical and charge transfer properties of acene-based organic semiconductor materials. Bull. Mater. Sci. 2019, 42, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Irfan, A.; Mahmood, A. Designing of efficient acceptors for organic solar cells: Molecular modelling at dft level. J. Clust. Sci. 2018, 29, 359–365. [Google Scholar] [CrossRef]
- Preat, J.; Jacquemin, D.; Perpète, E.A. Design of new triphenylamine-sensitized solar cells: A theoretical approach. Environ. Sci. Technol. 2010, 44, 5666–5671. [Google Scholar] [CrossRef] [PubMed]
- Preat, J.; Michaux, C.; Jacquemin, D.; Perpete, E.A. Enhanced efficiency of organic dye-sensitized solar cells: Triphenylamine derivatives. J. Phys. Chem. C 2009, 113, 16821–16833. [Google Scholar] [CrossRef]


| Extract Type | TPCs (mg GAE/g Extract) | TFCs (mg RE/g Extract) |
|---|---|---|
| 20% hydroethanolic | 152.19 ± 1.4 b | 78.4 ± 2 b |
| 40% hydroethanolic | 181.2 ± 1.9 c | 99.8 ± 1.18 c |
| 60% hydroethanolic | 190.6 ± 30 d | 102.6 ± 1.0 c |
| 80% hydroethanolic | 201.3 ± 2.6 e | 116.3 ± 2.4 d |
| Pure ethanolic | 138.5± 2.3 a | 66 ± 1.7 a |
| Sr. # | Observed Mass (mDa) | Predicted Formula | Mass Error (mDa) | Matched Identity | MS/MS Fragments (m/z) |
|---|---|---|---|---|---|
| 1 | 332.05 | C16H12O8 | −0.7 | Patuletin | 317,300,164 |
| 2 | 558.14 | C27H26O13 | 2.7 | Erigoster A | 517,437,113 |
| 3 | 132.04 | C5H8O4 | −0.3 | Methyl succinic acid | 101, 85,71,59 |
| 4 | 313.11 | C14H19NO7 | −1.5 | Menisdaurin | 277,211,174,113 |
| 5 | 332.07 | C13H16O10 | −0.1 | 1-Galloyl-β-D-glucose | 168 |
| 6 | 538.09 | C30H18O10 | 1.9 | Robustaflavone | 501,485, 399, 335,250 |
| 7 | 612.16 | C27H32O16 | 0.7 | Hydroxysafflor yellow A | 611,431, 269, 163 |
| 8 | 354.09 | C16H18O9 | −0.1 | Undulatoside A | 233,205 |
| 9 | 756.21 | C34H44O19 | 1.1 | 3-O-[β-D-Glucopyranosyl-(1→2)]-β-D-glucopyranosyl-7-O-α-L-glucopyranosyl-kaempferol | 723, 635,617,419,295 |
| 10 | 660.23 | C33H40O14 | −2.3 | 2″-O-Rhamno-sylicariside II | 637,599,497,479,395 |
| 11 | 526.12 | C30H22O9 | 2.2 | Dendrocandin H | 495,479,467,185,141 |
| 12 | 578.14 | C30H26O12 | −0.1 | Procyanidin B2 | 451,425,407,311,289,245,161 |
| 13 | 402.15 | C18H26O10 | −0.6 | Benzyl alcohol xylopyranosyl(1→6)glucopyranoside | 343,269,161,101 |
| 14 | 448.15 | C19H28O12 | −0.4 | 8-O-Acetyl shanzhiside methyl ester | 437,401,269,161,101 |
| 15 | 798.22 | C35H42O21 | 0.7 | Rindoside | 635,577,487,446 |
| 16 | 290.07 | C15H14O6 | −0.6 | Epicatechin | 271,245,203,161,123 |
| 17 | 138.03 | C7H6O3 | −0.5 | Sesamol | 125,121 |
| 18 | 562.42 | C34H58O6 | 2.5 | Campesterol-β-D-glucoside | 543,435,407,381,311,289,245,203,164 |
| 19 | 386.19 | C19H30O8 | −0.6 | Apocynoside I | 367, 205,153 |
| 20 | 866.20 | C45H38O18 | 3.8 | Arecatannin A1 | 748,695,577,559,405,287 |
| 21 | 256.07 | C15H12O4 | −0.5 | Furoaloesone | 229,187,171,97 |
| 22 | 528.14 | C30H24O9 | 1.4 | Mahuannin E | 419,301,273,255,165 |
| 23 | 610.15 | C27H30O16 | 0.3 | Rutin | 545,489,301,243,109 |
| Sr. # | Compound Name | Scores (S-value) | Hydrogen Bonding | Other Nonbonding Interactions |
|---|---|---|---|---|
| 1 | Patuletin | −14.2007 | ARG 256 | PHE77, PHE215, ILE78, HIS151 |
| 2 | Erigoster A | −14.5517 | SER152, TYR114, THR255, ASP78, HIS151 | PHE77, ILE78, PHE215, HIS263 |
| 3 | Methyl succinic acid | −10.2645 | HIS263 | SER152 |
| 4 | Menisdaurin | −13.3161 | SER152, TYR114, HIS151 | PHE77, ILE78, ALA260, ARG26, LEU264 |
| 5 | 1-Galloyl-β-D-glucose | −14.3628 | TYR114, PHE77, HIS151 | GLY76 |
| 6 | Robustaflavone | −12.5894 | PHE77, ARG 256, ARG111 | TRP252, ILE78, ALA260, TYR114, PHE215, LEU264 |
| 7 | Hydroxysafflor yellow A | −14.6837 | ARG256, ASP79, ARG111 | ALA260 |
| 8 | Undulatoside A | −15.0210 | SER152, TYR114 | ILE78, ALA260, LEU264, ARG256, TRP252 |
| 9 | 3-O-[β-D-Glucopyranosyl-(1→2)]-β-D-glucopyranosyl-7-O-α-L-glucopyranosylkaempferol | −12.0339 | SER152, TYR114, THR255 | PHE215, ILE78, LEU265, ALA260, ALA259 |
| 10 | 2″-O-Rhamno-sylicariside II | −13.5572 | PHE215, LEU264, TYR267, ALA260, ALA259, ILE78, ARG256, TRP252 | |
| 11 | Dendrocandin H | −14.3445 | HIS151 | ILE78, LEU264, GLY76, TYR114, PHE215, PRO180, ILE209, ALA259, ALA260 |
| 12 | Procyanidin B2 | −12.3625 | THR255 | ALA260, LEU264, ARG11, ALA259, ILE78 |
| 13 | Benzyl alcohol xylopyranosyl (1→6) glucopyranoside | −13.9147 | SER152, PHE77, TYR114, HIS151 | PRO180 |
| 14 | 8-O-Acetyl shanzhiside methyl ester | −12.9175 | SER152 | TYR114, ILE78, PHE77, HIS151 |
| 15 | Rindoside | −10.0161 | PHE77 | LEU264, ILE78, ARG260, ARG256, ALA259, ILE245 |
| 16 | Epicatechin | −13.6194 | ASP79, TYR114, TYR267 | HIS263, LEU264, ARG256, ALA260, HIS151, ILE78 |
| 17 | Sesamol | −10.5688 | PHE77, HIS263 | PHE215, TYR114 |
| 18 | Campesterol-β-D-glucoside | −11.5302 | PHE77, TYR114, HIS263 | ALA259, ALA260, ARG256, ILE78, LEU264, TRP252 |
| 19 | Apocynoside I | −12.3624 | PHE77, HIS151 | ALA259, ILE78, PHE215 |
| 20 | Arecatannin A1 | −11.4091 | TRP252 | ILE78, ARG256, ALA259, ALA260 |
| 21 | Furoaloesone | −11.0088 | HIS263 | PHE77, ALA260, PHE215, TYR114, ILE78 |
| 22 | Mahuannin E | −13.4295 | SER152, TYR114, ASP79, TYR267 | ILE78, ALA259, PHE215, ALA260, LEU264, ARG356 |
| 23 | Rutin | −14.2859 | SER152, HIS151, ARG256, TYR114 | HIS263, ALA260, ILE78, ALA259, PRO180, PHE215 |
| 24 | Orlistat | −9.1309 | TYR114, PHE77 | ILE78, TYR114, SER152, PRO180, PHE215, ARG256, ALA259, ALA260, LEU264 |
| Compounds | EHOMO | ELUMO | Egap | EA | IP |
|---|---|---|---|---|---|
| 1 | −5.77 | −1.41 | 4.36 | 1.41 | 5.77 |
| 2 | −5.79 | −1.68 | 4.11 | 1.68 | 5.79 |
| 3 | −7.45 | −0.22 | 7.67 | 0.22 | 7.45 |
| 4 | −6.55 | −2.11 | 4.44 | 2.11 | 6.55 |
| 5 | −3.74 | −0.04 | 3.74 | 0.04 | 3.74 |
| 6 | −5.95 | −1.58 | 4.37 | 1.58 | 5.95 |
| 7 | −4.84 | −2.63 | 2.21 | 2.63 | 4.84 |
| 8 | −6.20 | −1.44 | 4.76 | 1.44 | 6.20 |
| 9 | −5.71 | −0.84 | 4.87 | 0.84 | 5.71 |
| 10 | −5.88 | −1.50 | 4.38 | 1.50 | 5.88 |
| 11 | −5.42 | −2.95 | 2.47 | 2.95 | 5.42 |
| 12 | −5.31 | −0.04 | 5.27 | 0.04 | 5.31 |
| 13 | −6.06 | −0.32 | 5.74 | 0.32 | 6.06 |
| 14 | −6.66 | −0.96 | 5.70 | 0.96 | 6.66 |
| 15 | −6.37 | −1.90 | 4.47 | 1.90 | 6.37 |
| 16 | −5.52 | −0.15 | 5.37 | 0.15 | 5.52 |
| 17 | −5.26 | 0.06 | 5.32 | −0.06 | 5.26 |
| 18 | −6.07 | 0.84 | 6.91 | −0.84 | 6.07 |
| 19 | −6.18 | −1.21 | 4.97 | 1.21 | 6.18 |
| 20 | −5.10 | −0.17 | 4.93 | 0.17 | 5.10 |
| 21 | −5.90 | −1.15 | 4.74 | 1.15 | 5.90 |
| 22 | −5.30 | −0.37 | 4.92 | 0.37 | 5.30 |
| 23 | −5.57 | −1.57 | 5.00 | 1.57 | 5.57 |
| Phenol | −5.96 | 0.036 | 5.99 | −0.036 | 8.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aabideen, Z.U.; Mumtaz, M.W.; Akhtar, M.T.; Raza, M.A.; Mukhtar, H.; Irfan, A.; Raza, S.A.; Touqeer, T.; Nadeem, M.; Saari, N. Cassia fistula Leaves; UHPLC-QTOF-MS/MS Based Metabolite Profiling and Molecular Docking Insights to Explore Bioactives Role towards Inhibition of Pancreatic Lipase. Plants 2021, 10, 1334. https://doi.org/10.3390/plants10071334
Aabideen ZU, Mumtaz MW, Akhtar MT, Raza MA, Mukhtar H, Irfan A, Raza SA, Touqeer T, Nadeem M, Saari N. Cassia fistula Leaves; UHPLC-QTOF-MS/MS Based Metabolite Profiling and Molecular Docking Insights to Explore Bioactives Role towards Inhibition of Pancreatic Lipase. Plants. 2021; 10(7):1334. https://doi.org/10.3390/plants10071334
Chicago/Turabian StyleAabideen, Zain Ul, Muhammad Waseem Mumtaz, Muhammad Tayyab Akhtar, Muhammad Asam Raza, Hamid Mukhtar, Ahmad Irfan, Syed Ali Raza, Tooba Touqeer, Muhammad Nadeem, and Nazamid Saari. 2021. "Cassia fistula Leaves; UHPLC-QTOF-MS/MS Based Metabolite Profiling and Molecular Docking Insights to Explore Bioactives Role towards Inhibition of Pancreatic Lipase" Plants 10, no. 7: 1334. https://doi.org/10.3390/plants10071334








