Phytochemicals Profiling, Antimicrobial Activity and Mechanism of Action of Essential Oil Extracted from Ginger (Zingiber officinale Roscoe cv. Bentong) against Burkholderia glumae Causative Agent of Bacterial Panicle Blight Disease of Rice
Abstract
:1. Introduction
2. Results
2.1. Analysis of Chemical Constituents of Ginger Essential Oils by Gas Chromatography-Mass Spectrometry
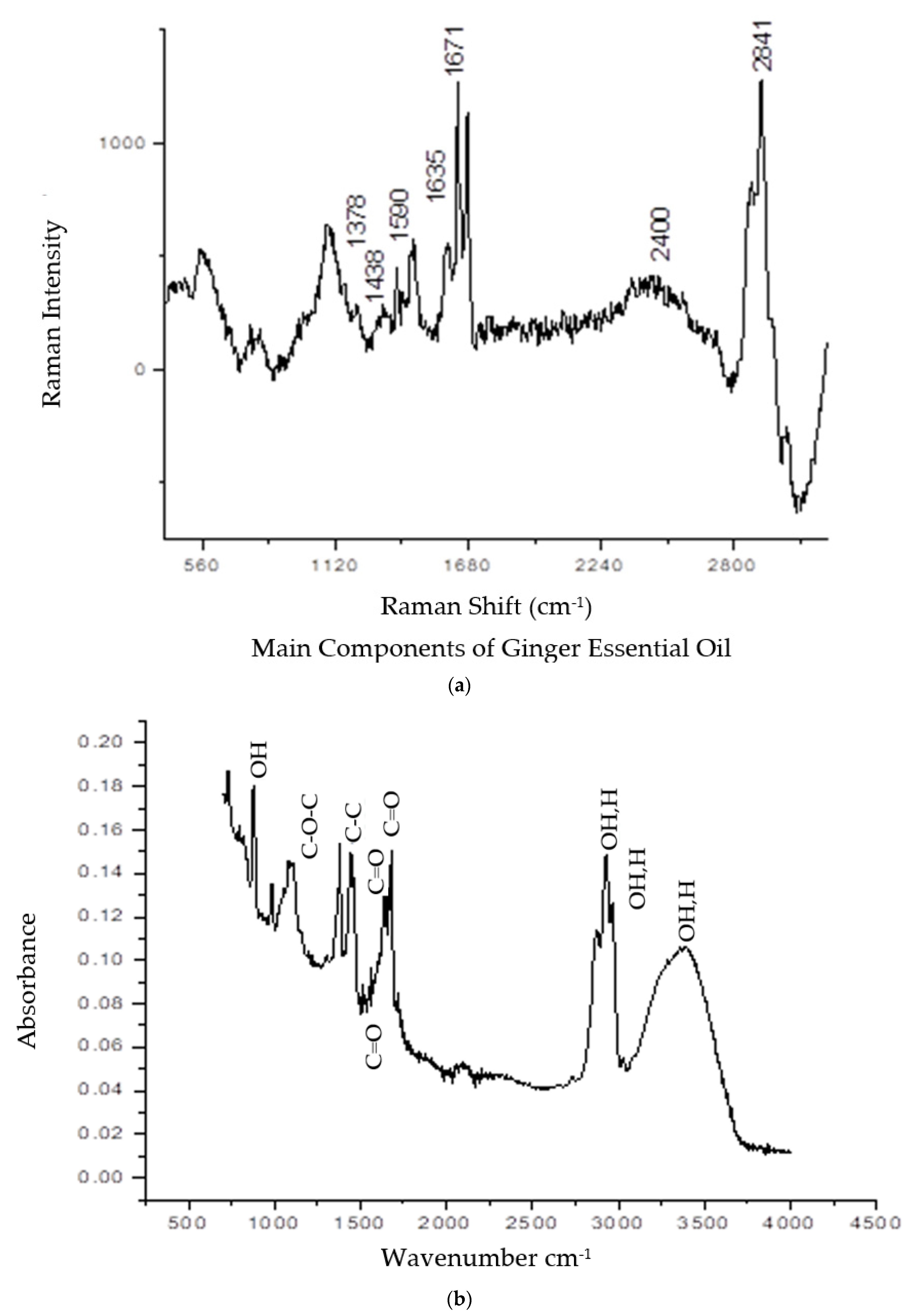
2.2. Vibrational Spectroscopy Analyses by Raman and FTIR
2.3. Antibacterial Activity, Minimum Inhibitory Concentration (MIC), and Minimum Bactericidal Concentration (MBC) of Ginger Essential Oil
2.4. Time Killing Analysis
2.5. Morphological Changes of B. glumae by Scanning Electron Microscope (SEM)
2.6. Morphological Changes of B. glumae by Transmission Electron Microscope (TEM)
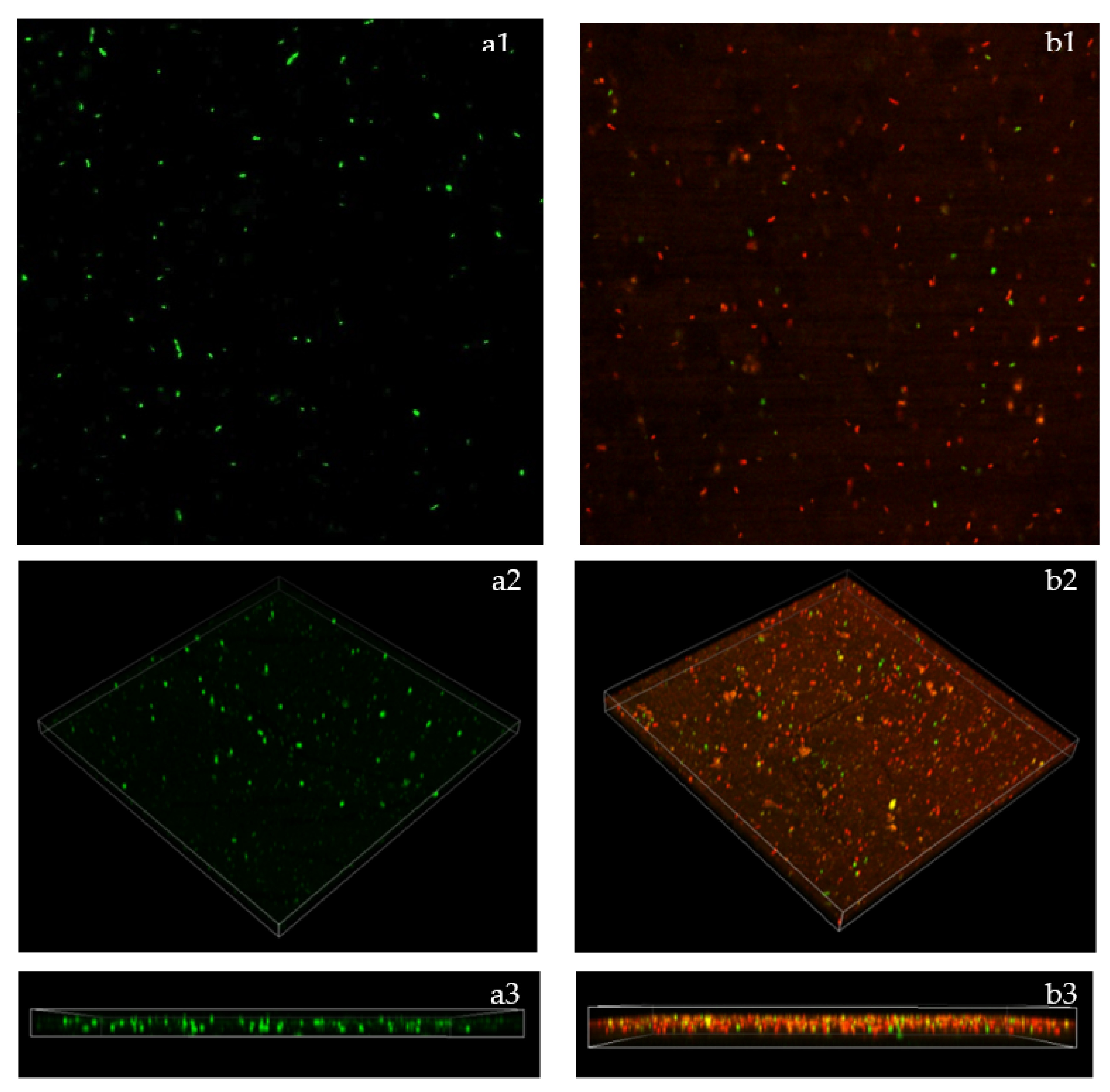
2.7. Biofilm Breakdown Observed by Confocal Laser Scanning Microscope (CLSM)
3. Discussion
4. Materials and Methods
4.1. Ginger Essential Oil Extraction
4.2. Analysis of the Chemical Compound in Ginger Essential Oil
4.3. Antibacterial Activity of Ginger Essential Oil
4.4. Assessment of MIC and MBC
4.5. Time Killing Analysis
4.6. Morphological Changes Observed by Scanning Electron Microscope
4.7. Morphological Damage Observed by Transmission Electron Microscope (TEM)
4.8. Biofilm Breakdown Observations under Confocal Laser Scanning Microscope
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Shew, A.M.; Durand-Morat, A.; Nalley, L.L.; Zhou, X.G.; Rojas, C.; Thoma, G. Warming increases Bacterial Panicle Blight (Burkholderia glumae) occurrences and impacts USA rice production. PLoS ONE 2019, 14, e021919. [Google Scholar] [CrossRef] [Green Version]
- Zhou-qi, C.; Bo, Z.; Guan-lin, X.; Bin, L.; Shi-wen, H. Research Status and Prospect of Burkholderia glumae, the Pathogen Causing Bacterial Panicle Blight. Rice Sci. 2016, 23, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Meshram, S.; Sinha, A. Bacterial Diseases in Rice and Their Eco-Friendly Management. Int. J. Agric. Sci. Res. 2017, 7, 31–42. Available online: http://www.tjprc.org/publishpapers/2-50-1486807962-5.IJASRAPR20175.pdf (accessed on 5 June 2021).
- CABI. Burkholderia glumae (Bacterial Grain Rot). Modified 20 November 2019. 2019. Available online: http://www.cabi.org/isc/datasheet/44964 (accessed on 2 February 2021).
- Lee, H.H.; Park, J.; Kim, J.; Park, I.; Seo, Y.S. Understanding the direction of evolution in Burkholderia glumae through comparative genomics. Curr. Genet. 2016, 62, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Syahri, S.; Somantri, R.U.; Sasmita, P. Detection and Control Bacteria Cause Grain Rot Burkholderia Glumae on Rice. J. Perlindungan Tanam. Indones. 2019, 23, 163–170. [Google Scholar] [CrossRef]
- Rao, J.; Chen, B.; McClements, D.J. Improving the Efficacy of Essential Oils as Antimicrobials in Foods: Mechanisms of Action. Annu. Rev. Food Sci. Technol. 2019, 10, 365–387. [Google Scholar] [CrossRef]
- Ashraf, S.A.; Al-Shammari, E.; Hussain, T.; Tajuddin, S.; Panda, B.P. In-vitro antimicrobial activity and identification of bioactive components using GC–MS of commercially available essential oils in Saudi Arabia. J. Food Sci. Technol. 2017, 54, 3948–3958. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.; Hu, F. Antibacterial Activity and Mechanism of Ginger Essential Oil against Escherichia coli and Staphylococcus aureus. Molecules 2020, 25, 3955. [Google Scholar] [CrossRef]
- Souza, J.E.T.; Siqueira, L.M.; Lucas, A.M.; Silva, C.G.F.D.; Cassel, E.; Vargas, R.M.F. Comparison of Different Extraction Techniques of Zingiber officinale Essential Oil. Braz. Arch. Biol. Technol. 2020, 63, e20190213. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, K.P.; Zhang, X.; Pan, D.D.; Sun, Y.Y.; Cao, J.X. Antibacterial activity and mechanism of action of black pepper essential oil on meat-borne Escherichia coli. Front. Microbiol. 2017, 7, 2094. [Google Scholar] [CrossRef] [Green Version]
- Chadwick, M.; Trewin, H.; Gawthrop, F.; Wagstaff, C. Sesquiterpenoids lactones: Benefits to plants and people. Int. J. Mol. Sci. 2013, 14, 12780–12805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi-Rad, M.; Varoni, E.M.; Salehi, B.; Sharifi-Rad, J.; Matthews, K.R.; Ayatollahi, S.A.; Kobarfard, F.; Ibrahim, S.A.; Mnayer, D.; Zakaria, Z.A.; et al. Plants of the Genus Zingiber as a Source of Bioactive Phytochemicals: From Tradition to Pharmacy. Molecules 2017, 22, 2145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogodo, A.C.; Abia, U. In-vitro antibacterial activity of garlic cloves and ginger rhizomes on food-borne pathogens. Int. J. Basic Appl. Sci. 2013, 2, 387–392. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.K.; Singh, V.; Ali, M. Chemical Composition and Antimicrobial Activity of Fresh Rhizome Essential Oil of Zingiber Officinale Roscoe. Pharmacogn. J. 2016, 8, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Rahamah, B.M.; Farhana, M.D.S.N.; Khairulmazmi, A.; Idris, A.S.; Susilawati, K.; Sariah, M. Assessment of plant secondary metabolites in oil palm seedlings after being treated with calcium, copper ions and salicylic acid. Arch. Phytopathol. Plant Prot. 2014, 47, 1120–1135. [Google Scholar] [CrossRef]
- Mao, Q.; Xu, X.; Shi-Yu Cao, S.; Gan, R.; Corke, H.; Trust, T.; Li, H. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef] [Green Version]
- Beristain-Bauza, S.D.C.; Hernandez-Carranza, P.; Cid-Perez, T.S.; Avila-Sosa, R.; Ruiz-Lopez, I.I.; Ochoa-Velasco, C.E. Antimicrobial Activity of Ginger (Zingiber Officinale) and Its Application in Food Products. Food Rev. Int. 2019, 35, 407–426. [Google Scholar] [CrossRef]
- O’Bryan, C.A.; Pendleton, S.J.; Crandall, P.G.; Ricke, S.C. Potential of plant essential oils and their components in animal agriculture—In vitro studies on the antibacterial mode of action. Front. Vet. Sci. 2015, 2, 35. [Google Scholar] [CrossRef] [Green Version]
- Ju, J.; Xie, Y.F.; Guo, Y.H.; Cheng, Y.L.; Qian, H.; Yao, W.R. Application of edible coating with essential oil in food preservation. Crit. Rev. Food Sci. Nutr. 2018, 59, 2467–2480. [Google Scholar] [CrossRef]
- Mesomo, M.C.; Corazza, M.L.; Ndiaye, P.M.; Dalla Santa, O.R.; Cardozo, L.; Scheer, A.D.P. Supercritical CO2 extracts and essential oil of ginger (Zingiber officinale R.): Chemical composition and antibacterial activity. J. Supercrit. Fluids 2013, 80, 44–49. [Google Scholar] [CrossRef]
- Babushok, V.I.; Zenkevich, I.G. Retention indices for most frequently reported essential oil compounds in GC. Chromatographia 2009, 69, 257–269. [Google Scholar] [CrossRef]
- Abdullahi, A.; Khairulmazmi, A.; Yasmeen, S.; Ismail, I.S.; Norhayu, A. Phytochemical profiling and antimicrobial activity of ginger (Zingiber officinale) essential oils against important phytopathogens. Arab. J. Chem. 2020, 13, 8012–8025. [Google Scholar] [CrossRef]
- Jentzsch, P.V.; Ramos, L.A.; Ciobota, V. Handheld Raman spectroscopy for the distinction of essential oils used in the cosmetics industry. Cosmetics 2015, 2, 162–176. [Google Scholar] [CrossRef] [Green Version]
- Farshbaf-Sadigh, A.; Jafarizadeh-Malmiri, H.; Anarjan, N.; Najian, Y. Preparation of ginger oil in water nanoemulsion using phase inversion composition technique: Effects of stirring and water addition rates on their Physico-chemical properties and stability. Z. Phys. Chem. 2021, 235, 295–314. [Google Scholar] [CrossRef]
- Li, Z.H.; Cai, M.; Liu, Y.S.; Sun, P.L.; Luo, S.L. Antibacterial Activity and Mechanisms of Essential Oil from Citrus medica L. var. sarcodactylis. Molecules 2019, 24, 1577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teerarak, M.; Laosinwattana, C. Essential oil from ginger as a novel agent in delaying senescence of cut fronds of the fern (Davallia solida (G. Forst.) Sw.). Postharvest Biol. Technol. 2019, 156, 110927. [Google Scholar] [CrossRef]
- Mahboubi, M. Zingiber officinale Rosc. essential oil, a review on its composition and bioactivity. Clin. Phytoscience 2019, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Nampoothiri, S.V.; Venugopalan, V.V.; Joy, B.; Sreekumar, M.M.; Menon, A.N. Comparison of Essential oil Composition of Three Ginger Cultivars from Sub Himalayan Region. Asian Pac. J. Trop. Biomed. 2012, 2 (Suppl. S3), S1347–S1350. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Rahmat, A. Changes in antioxidant and antibacterial activities as well as phytochemical constituents associated with ginger storage and polyphenol oxidase activity. BMC Complement. Altern. Med. 2016, 16, 382. [Google Scholar] [CrossRef] [Green Version]
- Guimaraes, A.C.; Meireles, L.M.; Lemos, M.F.; Guimaraes, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [Green Version]
- Hossain, S.; De Silva, B.C.J.; Wimalasena, S.H.M.P.; Pathirana, H.N.K.S.; Heo, G.J. In vitro antibacterial effect of ginger (Zingiber officinale) essential oil against fish pathogenic bacteria isolated from farmed olive flounder (Paralichthys olivaceus) in Korea. Iran. J. Fish. Sci. 2019, 18, 386–394. [Google Scholar]
- Brozyna, M.; Paleczny, J.; Kozłowska, W.; Chodaczek, G.; Dudek-Wicher, R.; Felinczak, A.; Gołębiewska, J.; Gorniak, A.; Junka, A. The antimicrobial and antibiofilm in vitro activity of liquid and vapour phases of selected essential oils against Staphylococcus aureus. Pathogens 2021, 10, 1207. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, M.; Vasic, S.; Djurdjevic, J.; Stefanovic, O.; Comic, L. Antibacterial and anti-biofilm activity of ginger (Zingiber officinale (Roscoe)) ethanolic extract. Kragujev. J. Sci. 2014, 36, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Dannenberg, G.d.S.; Funck, G.D.; da Silva, W.P.; Fiorentini, A.M. Essential oil from pink pepper (Schinus terebinthifolius Raddi): Chemical composition, antibacterial activity, and mechanism of action. Food Control 2018, 95, 115–120. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [Green Version]
- Oz, M.; Lozon, Y.; Sultan, A.; Yang, K.H.S.; Galadari, S. Effects of monoterpenes on ion channels of excitablecells. Pharmacol. Ther. 2015, 152, 83–97. [Google Scholar] [CrossRef]
- Ouattara, B.; Simard, R.E.; Holley, R.A.; Piette, G.J.P.; Begin, A. Antibacterial activity of selected fatty acids and essential oils against six meat spoilage organisms. Int. J. Food Microbiol. 1997, 37, 155–162. [Google Scholar] [CrossRef]
- Li, B.; Liu, B.; Shan, C.; Ibrahim, M.; Lou, Y.; Wang, Y.; Xie, G.; Li, H.Y.; Sun, G. Antibacterial activity of two chitosan solutions and their effect on rice bacterial leaf blight and leaf streak. Pest Manag. Sci. 2013, 69, 312–320. [Google Scholar] [CrossRef]
- Sahu, S.K.; Zheng, P.; Yao, N. Niclosamide Blocks Rice Leaf Blight by Inhibiting Biofilm Formation of Xanthomonas oryzae. Front. Plant Sci. 2018, 9, 408. [Google Scholar] [CrossRef] [Green Version]
- Kerekes, E.B.; Deak, E.; Tako, M.; Tserennadmid, R.; Petkovits, T.; Vagvolgyi, C.; Krisch, J. Anti-biofilm forming and an-ti-quorum sensing activity of selected essential oils and their main components on food-related micro-organisms. J. Appl. Microbiol. 2013, 115, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Lucero, M.; Estell, R.; Tellez, M.; Fredrickson, E. A retention index calculator simplifies the identification of plant volatile organic compounds. Phytochem. Anal. 2009, 20, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.F.; Hu, F.; Thakura, K.; Li, X.L.; Zhang, Y.S.; Wei, Z.J. Comparison of antibacterial effects and fumigant toxicity of essential oils extracted from different plants. Ind. Crops Prod. 2018, 124, 192–200. [Google Scholar] [CrossRef]
- Khalid, A.; Ullah, H.; Ul-Islam, M.; Khan, R.; Khan, S.; Ahmad, F.; Khan, T.; Wahid, F. Bacterial cellulose-TiO2 nanocomposites promote healing and tissue regeneration in burn mice model. RSC Adv. 2017, 7, 47662–47668. [Google Scholar] [CrossRef] [Green Version]
- Al-Shuneigat, J.; Al-Sarayreh, S.; Al-Saraireh, Y.; Al-Qudah, M. Effect of Achillea santolina essential oil on bacterial biofilm and its mode of action. Curr. Issues Pharm. Med. Sci. 2020, 33, 83–89. [Google Scholar] [CrossRef]
- Cai, L.; Chen, J.; Liu, Z.; Wang, H.; Yang, H.; Ding, W. Magnesium oxide nanoparticles: Effective agricultural antibacterial agent against Ralstonia solanacearum. Front. Microbiol. 2018, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.G.; Murate, L.S.; Spago, F.R.; Lopes, L.d.P.; Beranger, J.P.d.O.; Martin, J.A.B.S.; Nogueira, M.A.; de Mello, J.C.P.; Andrade, C.G.T.d.J.; Andrade, G. Evaluation of the antibiotic activity of extracellular compounds produced by the Pseudomonas strain against the Xanthomonas citri pv. citri 306 strain. Biol. Control 2011, 56, 125–131. [Google Scholar] [CrossRef]
- Adamu, A.; Ahmad, K.; Siddiqui, Y.; Ismail, I.S.; Asib, N.; Bashir Kutawa, A.; Adzmi, F.; Ismail, M.R.; Berahim, Z. Ginger Essential Oils-Loaded Nanoemulsions: Potential Strategy to Manage Bacterial Leaf Blight Disease and Enhanced Rice Yield. Molecules 2021, 26, 3902. [Google Scholar] [CrossRef]




| Chemical Compound | Rt (min) | RI (This Work) | RI [15] | RI [22] | Area (%) | Molecular Formula | MS | |
|---|---|---|---|---|---|---|---|---|
| 1 | Heptan-2-ol | 8.074 | 900 | - | - | 1.23 | C7H16O | 331 |
| 2 | α-Pinene | 9.386 | 933 | 937 | 922–955 | 2.76 | C10H16 | 412 |
| 3 | Camphene | 10.087 | 951 | 943 | 933–962 | 7.53 | C10H16 | 451 |
| 4 | β-Pinene | 11.173 | 978 | - | 959–986 | 0.52 | C10H16 | 349 |
| 5 | 6-Methyl-5-hepten-2-one | 11.344 | 983 | - | - | 0.95 | C8 H14 O | 347 |
| 6 | Myrcene | 11.588 | 989 | 975 | 973–993 | 2.31 | C10H16 | 359 |
| 7 | Sulcatol | 11.706 | 992 | - | - | 0.25 | C8H16O | 332 |
| 8 | α- Phellandrene | 12.370 | 1007 | 987 | 989–1013 | 0.30 | C10 H16 | 322 |
| 9 | 1,8-Cineole | 13.646 | 1034 | - | 1005–1039 | 14.96 | C10H18O | 363 |
| 10 | Terpinolene | 16.074 | 1086 | - | - | 0.47 | C10H16 | 408 |
| 11 | Methyl lavender ketone | 16.220 | 1089 | - | - | 0.44 | C10H20O2 | 290 |
| 12 | β-Linalool | 16.705 | 1100 | - | - | 2.19 | C10H18O | 396 |
| 13 | Camphor | 19.081 | 1149 | - | 1045 | 0.26 | C10 H16 O | 299 |
| 14 | Citronellal | 19.224 | 1152 | - | - | 0.95 | C10H18O | 290 |
| 15 | Isoneral | 19.667 | 1161 | - | - | 0.22 | C10H16O | 407 |
| 16 | Endo-Borneol | 20.349 | 1175 | - | - | 1.70 | C10H18O | 355 |
| 17 | Isogeranial | 20.581 | 1179 | - | - | 0.34 | C10H18O | 404 |
| 18 | 4-Terpineol | 20.719 | 1182 | - | - | 0.39 | C10H18O | 361 |
| 19 | Cryptone | 21.021 | 1188 | - | - | 0.19 | C9H14O | 361 |
| 20 | α -Terpineol | 21.442 | 1197 | - | 1159–1193 | 1.77 | C10H18O | 404 |
| 21 | Citronellol | 23.055 | 1231 | - | 1203–1229 | 3.27 | C10H20O | 358 |
| 22 | Neral | 23.666 | 1244 | - | - | 13.99 | C10H16O | 364 |
| 23 | Geraniol | 24.170 | 1254 | - | 1228–1258 | 2.35 | C10H18O | 364 |
| 24 | Geranial | 25.146 | 1274 | - | 1232–1267 | 17.88 | C10H16O | 342 |
| 25 | 2-Undecanone | 26.014 | 1293 | - | - | 0.90 | C11H22O | 369 |
| 26 | Methyl nonyl carbinol | 26.466 | 1302 | - | - | 0.20 | C11H24O | 334 |
| 27 | Citronellyl acetate | 28.595 | 1348 | - | - | 0.31 | C12H22O2 | 339 |
| 28 | Geranyl acetate | 29.911 | 1377 | 1383 | 1344–1385 | 0.63 | C12H20O2 | 357 |
| 29 | β-Elemene | 30.585 | 1392 | - | 1370–1404 | 0.51 | C15H24 | 385 |
| 30 | Caryophyllene | 32.007 | 1424 | - | - | 0.37 | C15H24 | 353 |
| 31 | Curcumene | 34.588 | 1483 | - | - | 1.58 | C15H22 | 423 |
| 32 | Germacrene | 34.698 | 1485 | - | - | 0.67 | C15 H24 | 371 |
| 33 | α-Zingiberene | 35.247 | 1498 | - | - | 5.19 | C15H24 | 312 |
| 34 | α-Farnesene | 35.551 | 1505 | 1433 | 1479–1518 | 3.51 | C15H24 | 429 |
| 35 | β-Bisabolene | 35.766 | 1510 | - | 1485–1513 | 1.16 | C15H24 | 396 |
| 36 | β-Sesquiphellandrene | 36.460 | 1527 | 1525 | - | 2.40 | C15H24 | 418 |
| 37 | Muurola-4,10(14)-dien-1.beta.-ol | 37.348 | 1548 | - | - | 0.23 | C15H24O | 386 |
| 38 | α-Elemol | 37.494 | 1552 | - | - | 0.89 | C15H26O | 401 |
| 39 | Nerolidiol | 37.882 | 1561 | 1558 | 1535–1565 | 0.65 | C15H26O | 309 |
| 40 | Sesquisabinene hydrate | 39.174 | 1592 | - | - | 0.34 | C15H26O | 315 |
| 41 | Zingiberenol | 40.152 | 1617 | 1601 | - | 0.68 | C15H26O | 370 |
| 42 | trans-Sesquisabinene hydrate | 40.830 | 1634 | - | - | 0.49 | C15H26O | 394 |
| 43 | Globulol | 40.956 | 1637 | - | - | 0.51 | C15H26O | 402 |
| 44 | Rosifoliol | 41.911 | 1661 | - | - | 0.89 | C15H26O | 372 |
| 45 | Shyobunol | 43.007 | 1689 | - | - | 0.66 | C15H26O | 358 |
| Peaks Number | Wavelength cm−1 | Chemical Bonds | Functional Compounds |
|---|---|---|---|
| 1 | 3271 | OH stretch;H-bonded | Carboxylic acid |
| 2 | 2920 | OH stretch;H-bonded | Carboxylic acid |
| 3 | 2871 | OH stretch;H-bonded | Carboxylic acid |
| 4 | 1739 | C=O stretch | Esters RCOOR |
| 5 | 1678 | C=O stretch | Carbonyl Compound |
| 6 | 1640 | C=O stretch | Carbonyl Compound |
| 7 | 1590 | C-C stretch | Aromatic stretch ring |
| 8 | 1260 | C-O-C | (R-O-R) Ether |
| 9 | 870 | OH bond | Phenol |
| GEO Concentration (µL/mL) | DMSO | Antibiotic Streptomycin | |||||
|---|---|---|---|---|---|---|---|
| 100 | 200 | 300 | 400 | 500 | |||
| Dimeter of zone of inhibition (mm) | 7.1 ± 0.16 f | 8.0 ± 0.28 e | 10.6 ± 0.44 d | 14.3 ± 0.16 c | 15.1 ± 0.16 b | 0.0 ± 0 g | 24.1 ± 0.44 a |
| Inhibition (%) | 29.46% | 33.19% | 43.98% | 59.33% | 62.65% | 0.0% | |
| Efficacy * | Not sensitive | Moderate sensitive | Moderate sensitive | Moderate Sensitive | Sensitive | Not sensitive | Extremely sensitive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunasena, M.T.; Rafi, A.; Mohd Zobir, S.A.; Hussein, M.Z.; Ali, A.; Kutawa, A.B.; Abdul Wahab, M.A.; Sulaiman, M.R.; Adzmi, F.; Ahmad, K. Phytochemicals Profiling, Antimicrobial Activity and Mechanism of Action of Essential Oil Extracted from Ginger (Zingiber officinale Roscoe cv. Bentong) against Burkholderia glumae Causative Agent of Bacterial Panicle Blight Disease of Rice. Plants 2022, 11, 1466. https://doi.org/10.3390/plants11111466
Gunasena MT, Rafi A, Mohd Zobir SA, Hussein MZ, Ali A, Kutawa AB, Abdul Wahab MA, Sulaiman MR, Adzmi F, Ahmad K. Phytochemicals Profiling, Antimicrobial Activity and Mechanism of Action of Essential Oil Extracted from Ginger (Zingiber officinale Roscoe cv. Bentong) against Burkholderia glumae Causative Agent of Bacterial Panicle Blight Disease of Rice. Plants. 2022; 11(11):1466. https://doi.org/10.3390/plants11111466
Chicago/Turabian StyleGunasena, Mahesh Tiran, Amara Rafi, Syazwan Afif Mohd Zobir, Mohd Zobir Hussein, Asgar Ali, Abdulaziz Bashir Kutawa, Mohd Aswad Abdul Wahab, Mohd Roslan Sulaiman, Fariz Adzmi, and Khairulmazmi Ahmad. 2022. "Phytochemicals Profiling, Antimicrobial Activity and Mechanism of Action of Essential Oil Extracted from Ginger (Zingiber officinale Roscoe cv. Bentong) against Burkholderia glumae Causative Agent of Bacterial Panicle Blight Disease of Rice" Plants 11, no. 11: 1466. https://doi.org/10.3390/plants11111466








