Biotization of Endophytes Trichoderma asperellum and Bacillus subtilis in Mentha spicata Microplants to Promote Growth, Pathogen Tolerance and Specialized Plant Metabolites
Abstract
:1. Introduction
2. Results
2.1. Antagonism of T. asperellum and B. subtilis against Rhizoctonia sp.
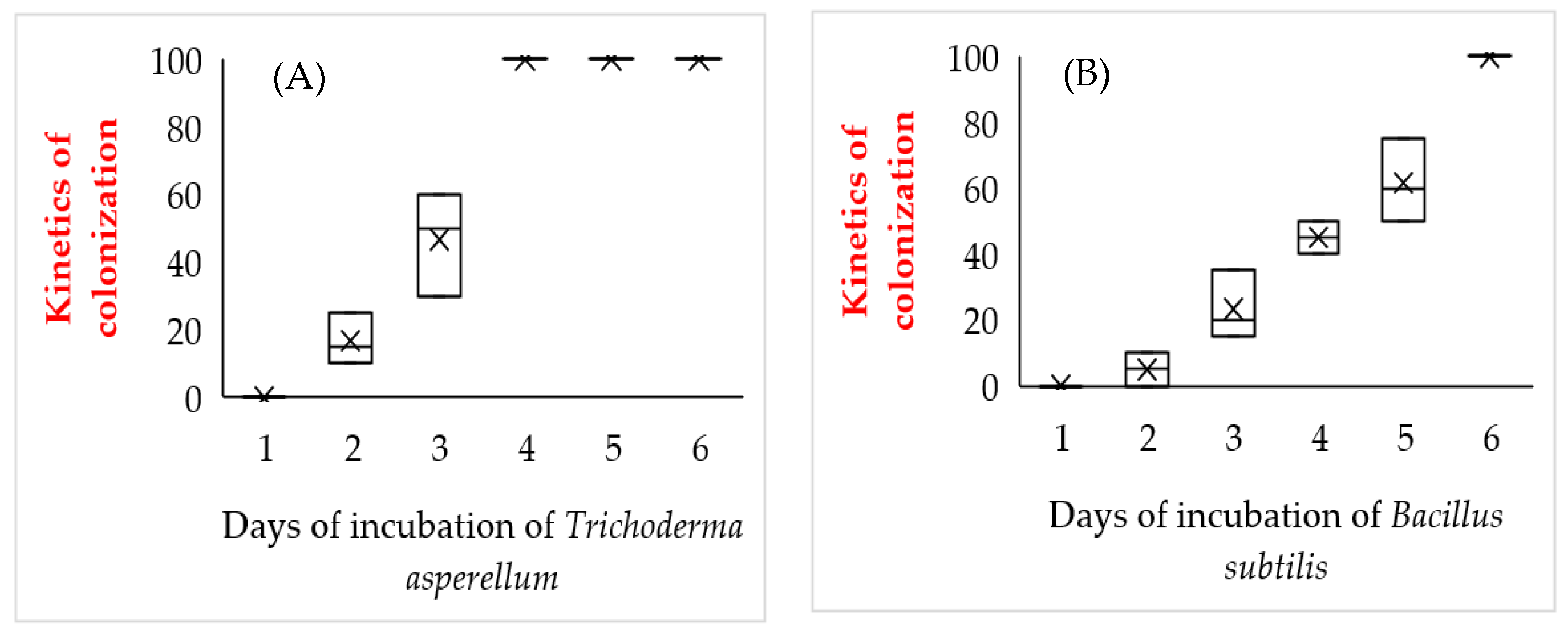
2.2. Determination of the Incubation Period of Endophytes on Roots of M. spicata Microplants
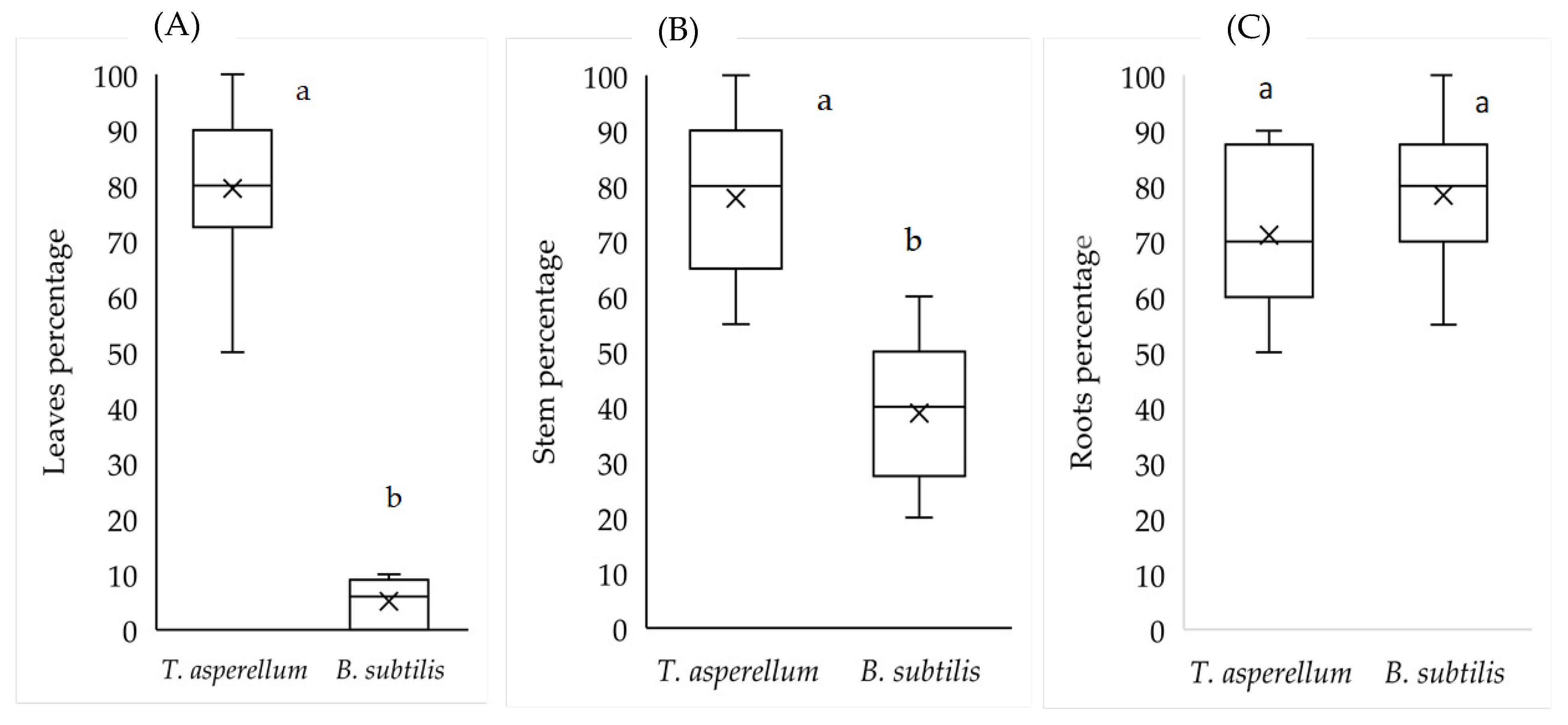
2.3. Evaluation of the Effect of Microplants Biotization on Survival during the Acclimatization Phase
2.4. Isolation of Endophytes in Tissues and Morphological and Molecular Identification of the Microorganisms under Greenhose Conditions
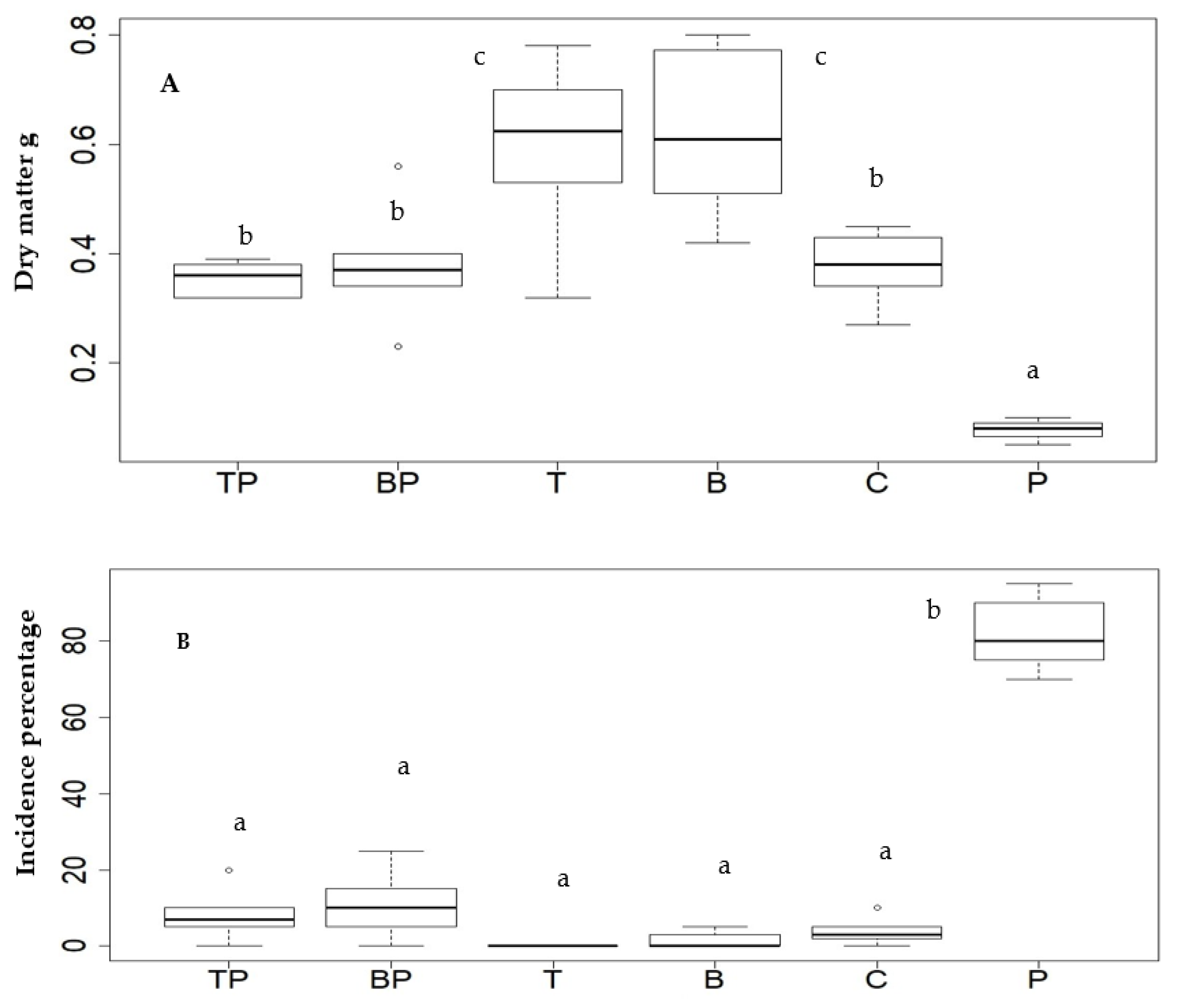
2.5. Effect of Biotizated M. spicata Plantlets on Dry Biomass and Incidence of Rhizoctonia sp. under Greenhouse Conditions
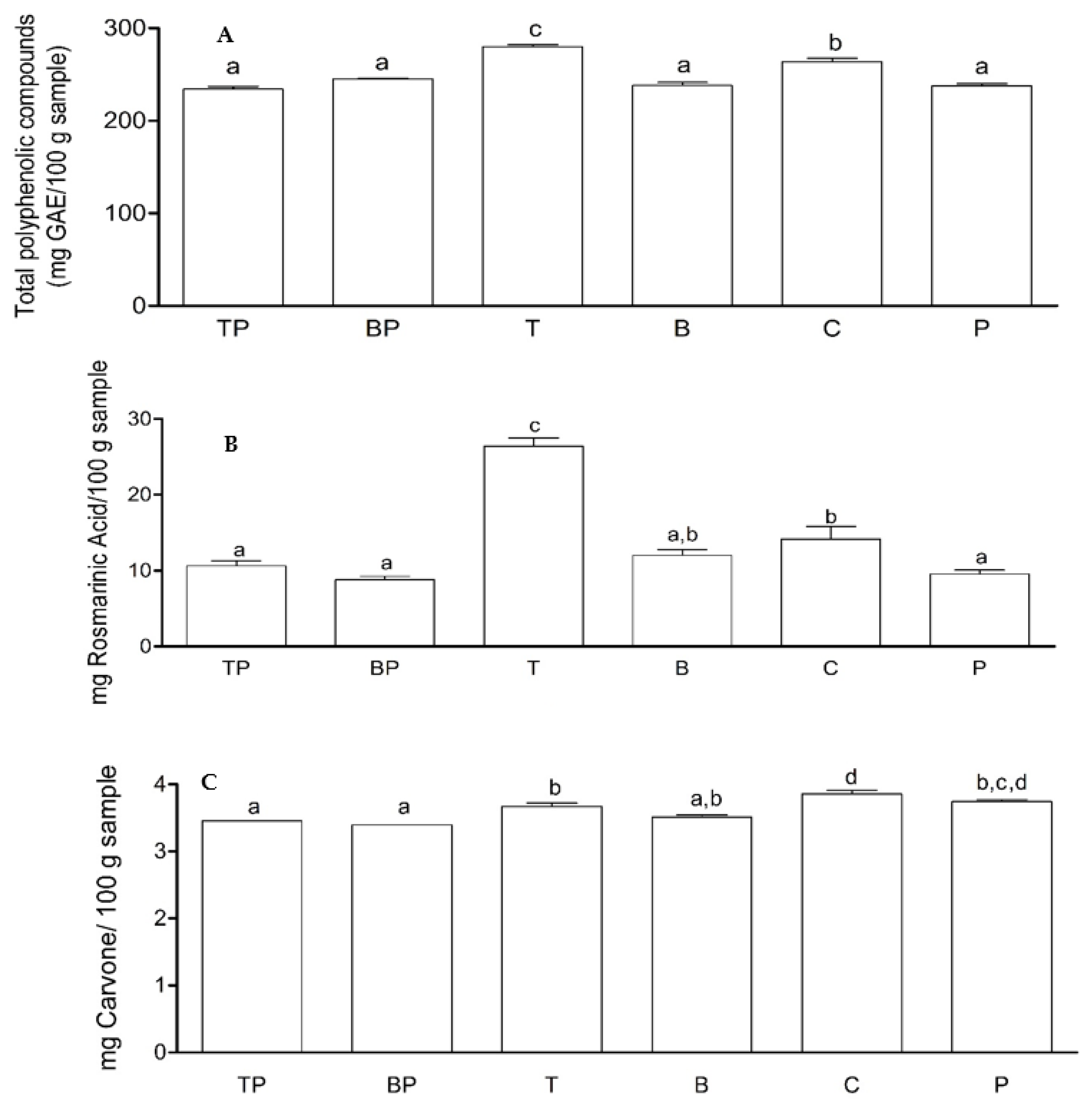
2.6. Effect of Biotizated M. spicata Plantlets on the Content of Some Specialized Metabolites under Nursery Conditions
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material
4.3. Endophytic Microorganism
4.4. Isolation of Rhizoctonia sp.
4.5. Antagonism Tests T. asperellum and B. subtilis vs Rhizoctonia sp.
4.6. Determination of the Incubation Period of the Endophytes in M. spicata Microplants
4.7. Evaluation of the Effect of Microplants Biotization on Survival during the Acclimatization Phase
4.8. Isolation of Endophytes in Tissues and Morphological and Molecular Identification of the Microorganisms under Greenhose Conditions
- ▪
- Backt_341 F (5′-CCTACGGGNGGCWGCAG-3′)
- ▪
- Backt_805 R (5′-GACTACHVGGGTATCTAATCC-3′)
4.9. Evaluation of the Effect of Inoculation of Biotized Plants with Rhizoctonia sp. under Greenhouse Conditions
4.10. Determination of the Disease Incidence
4.11. Determination of the Biomass of the Plants
4.12. Determination of Total Phenolic Content (TPC)
4.13. Phenolic Compounds Identification by HPLC-DAD
4.14. Gas Chromatography/Mass Spectrometry (GC-MS) Analysis
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kee, L.; Bakd, A.; Salihin, B. Bioactivity and health effects of Mentha spicata. Integr. Food Nutr. Metab. 2017, 5, 1–2. [Google Scholar]
- Bekut, M.; Brkić, S.; Kladar, N.; Dragović, G.; Gavarić, N.; Božin, B. Potential of selected Lamiaceae plants in anti(retro)viral therapy. Pharm. Res. 2018, 133, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Pina, L.; Serafini, M.; Oliveira, M.; Sampaio, L.; Guimaraes, J.; Guimaraes, A. Carvone and its pharmacological activities: A systematic review. Phytochemistry 2022, 196, 113080. [Google Scholar] [CrossRef] [PubMed]
- Sik, B.; Kapcsándi, V.; Székelyhidi, R.; Hanczné, E.; Ajtony, Z. Recent Advances in the analysis of rosmarinic acid from herbs in the Lamiaceae family. Nat. Prod. Commun. 2019, 14, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Akter, K.T.; Hoque, M.A. In vitro shoot regeneration of mint (Mentha sp. L.) using different types of explants and levels of benzylaminopurine. J. Agril. Res. 2018, 43, 703–716. [Google Scholar] [CrossRef]
- Nitzan, N.; Chaimovitsh, D.; Davidovitch-Rekanati, R.; Sharon, M.; Dudai, N. Rhizoctonia web blight—A new disease on mint in Israel. Plant Dis. 2012, 96, 370–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lokesh, R.; Yallanagouda, M. Role of Tissue Culture for Production of Disease-Free Planting Material. Agric. Food E-Newsletter. 2021, 3, 34–36. [Google Scholar]
- Soumare, A.; Diédhiou, A.G.; Arora, N.K.; Al-Ani, L.K.; Ngom, M.; Fall, S.; Hafidi, M.; Ouhdouch, Y.; Kouisni, L.; Sy, M.O. Potential Role and Utilization of Plant Growth Promoting Microbes in Plant Tissue Culture. Front. Microbiol. 2021, 12, 649878. [Google Scholar] [CrossRef]
- Orlikowska, T.; Nowak, K.; Reed, B. Bacteria in the plant tissue culture environment. Plant Cell Tissue Organ Cult. 2017, 128, 487–508. [Google Scholar] [CrossRef]
- Mirjani, L.; Salimi, A.; Matinizadeh, M.; Razavi, K.; Shahbazi, K. Biotization with Glomus fasciculatum to enhance the acclimatization and absorption of nutrients by micropropagated savory (Satureja khuzistanica Jamzad) plantlets. J. Elem. 2018, 24, 785–802. [Google Scholar] [CrossRef]
- Anjum, N.; Chandra, R. Endophytic bacteria: Optimization of isolation procedure from various medicinal plants and their preliminary characterization. Asian J. Pharm Clin. Res. 2015, 8, 233–238. [Google Scholar]
- Rahman, M.; Sabir, A.; Akter, J.; Khan, M.; Mohi-Ud-Din, M.; Miah, G.; Rahman, M.; Tofazzal, M. Plant probiotic bacteria Bacillus and Paraburkholderia improve growth, yield and content of antioxidants in strawberry fruit. Sci. Rep. 2018, 8, 2504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzmán–Guzmán, P.; Porras–Troncoso, M.D.; Olmedo–Monfil, V.; Herrera–Estrella, A. Trichoderma species: Versatile plant symbionts. Phytopathology 2019, 109, 6–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, A.; Ullah, S.K.; Ullah, W.K.; Saleh, T.; Ullah, M.K.; Ullah, S.; Ali, A.; Ikram, M. Antagonist effects of strains of Bacillus spp. against Rhizoctonia solani for their protection against several plant diseases: Alternatives to chemical pesticides. Comptes Rendus Biol. 2019, 342, 124–135. [Google Scholar] [CrossRef]
- Kanani, P.; Modi, A.; Kumar, A. Biotization of endophytes in micropropagation: A helpful enemy. In Microbial Endophytes: Prospects for Sustainable Agriculture, Food Science, Technology and Nutrition; Kumar, A., Singh, V.K., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2020; pp. 357–379. [Google Scholar]
- Indravathi1, G.; Babu, P. Enhancing acclimatization of tissue cultured plants of Albizia amara by biotization. Int. J. Sci. Res. Biol. Sci. 2019, 6, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Pushpakanth, P.; Khrisnamoorthy, R.; Anandham, R.; Senthilkumar, M. Biotization of tissue culture banana plantlets with Miethylobacterium salsuginis to enhance the survival and growth under greenhouse and open environment condition. J. Environ. Biol. 2021, 42, 1452–1460. [Google Scholar] [CrossRef]
- Rios, C.V.; Caro, M.C.; Berlanga, D.R.; Ruiz, M.C.; Ornelas, J.P.; Salas, M.; Villalobos, E.P.; Guerrero, V.P. Identification and antagonistic activity in vitro of Bacillus spp. and Trichoderma spp. isolates against common phytopathogenic fungi. Rev. Mex. Fitopatol. 2016, 34, 84–99. [Google Scholar]
- Schulz-Bohm, K.; Martín-Sánchez, L.; Garbeva, P. Microbial Volatiles: Small Molecules with an Important Role in Intra- and Inter-Kingdom Interactions. Front. Microbiol. 2017, 8, 2504. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Liu, Y.; Ai, G.M.; Mia, L.L.; Zhen, H.Y.; Liu, Z.P. The characteristics of a novel heterotrophic nitrification-aerobic denitrification, Bacillus methylotrophicus strain L7. Bioresour. Technol. 2012, 108, 35–44. [Google Scholar] [CrossRef]
- Kamalakannan, A.; Mohan, L.; Kavitha, K.; Harish, S.; Radjacommare, R.; Nakkeeran, S.; Parthiban, V.; Karuppiah, R.; Angayarkanni, T. Enhancing resistance to stem and stolon rot of peppermint (Mentha piperita Lin.) using biocontrol Agents. Acta Phytopathol. Entomol. Hung. 2003, 38, 293–305. [Google Scholar] [CrossRef]
- Abdallah, D.B.; Frikha-Gargouri, O.; Tounsi, S. Rizhospheric competence, plant growth promotion and biocontrol efficacy of Bacillus amyloliquefaciens subsp. plantarum strain 32a. Biol. Control 2018, 124, 61–67. [Google Scholar] [CrossRef]
- Andreolli, M.; Zapparoli, G.; Lampis, S.; Santi, C.; Angelini, E.; Bertazzon, N. In Vivo Endophytic, Rhizospheric and Epiphytic Colonization of Vitis vinifera by the Plant-Growth Promoting and Antifungal Strain Pseudomonas protegens MP12. Microorganisms 2021, 9, 234. [Google Scholar] [CrossRef] [PubMed]
- Nsofor, G.C.; Isaiah, T. Plant growth promoting microbes in plant tissue culture. Niger. Agric. J. 2021, 52, 229–235. [Google Scholar]
- Villamarín-Gallegos, D.; Oviedo-Pereira, D.G.; Evangelista-Lozano, D.G.; Sepúlveda-Jiménez, G.; Molina-Torres, J.; Rodríguez-Monroy, M. Trichoderma asperellum, an inoculant for the production of steviol glycosides in Stevia rebaudiana Bertoni plants micropropagated in a temporary immersion bioreactor. Rev. Mex. Ing. Química 2020, 19, 1153–1161. [Google Scholar] [CrossRef]
- Fontana, D.C.; de Paula, S.; Torres, A.G.; de Souza, V.H.M.; Pascholati, S.F.; Schmidt, D.; Dourado Neto, D. Endophytic Fungi Biological Control and Induced Resistance to Phytopathogens and Abiotic Stresses. Pathogens 2021, 10, 570. [Google Scholar] [CrossRef]
- Lim, S.; Subramaniam, S.; Zamzuri, I.; Amir, H. Biotization of in vitro calli and embryogenic calli of oil palm (Elaeis guineensis Jacq.) with diazotrophic bacteria Herbaspirillum seropedicae (Z78). Plant Cell Tiss Organ Cult. 2016, 127, 251–262. [Google Scholar] [CrossRef]
- Brotman, Y.; Landau, U.; Cuadros-Inostroza, A.; Takayuki, T.; Fernie, A.R.; Chet, I.; Viterbo, A.; Willmitzer, L. Trichoderma-Plant Root Colonization: Escaping Early Plant Defense Responses and Activation of the Antioxidant Machinery for Saline Stress Tolerance. PLoS Pathog. 2013, 9, e1003221. [Google Scholar] [CrossRef]
- Sperotto, P.A.; de Oliveira, V.M.; Romano, P.; Selari, G.; Martins, P.; Guimarães, F.S.; de Fátima, S. Multifunctional potential of endophytic bacteria from Anacardium othonianum Rizzini in promoting in vitro and ex vitro plant growth. Microbiol. Res. 2021, 242, 126600. [Google Scholar]
- Nataraja, K.N.; Suryanarayanan, T.S.; Shaanker, R.U.; Senthil-Kumar, M.; Oelmüller, R. Plant–Microbe Interaction: Prospects for Crop Improvement and Management. Plant Physiol. Rep. 2019, 24, 461–462. [Google Scholar] [CrossRef]
- Wu, W.; Chen, W.; Liu, S.; Wu, J.; Zhu, Y.; Qin, L.; Zhu, B. Beneficial Relationships Between Endophytic Bacteria and Medicinal Plants. Front. Plant Sci. 2021, 12, 646146. [Google Scholar] [CrossRef]
- Quambusch, M.; Winkelmann, T. Bacterial endophytes in plant tissue culture: Mode of action, detection, and control. Methods Mol. Biol. 2018, 1815, 69–88. [Google Scholar] [PubMed]
- Pereira, E.; Pimenta, A.I.; Calhelha, R.C.; Antonio, A.L.; Verde, S.C.; Barros, L.; Santos-buelga, C.; Ferreira, I.C. Effects of gamma irradiation on cytotoxicity and phenolic compounds of Thymus vulgaris L. and Mentha piperita L. LWT-Food Sci. Technol. 2016, 71, 370–377. [Google Scholar] [CrossRef] [Green Version]
- Franco-Bañuelos, F.; Contreras-Martínez, C.; Carranza-Tellez, J.; Carranza-Concha, J. Total phenolic content and antioxidant capacity of non-native wine grapes grown in Zacatecas, México. Agrociencia 2017, 51, 661–671. [Google Scholar]
- Song, X.; Wu, H.; Piao, X.; Yin, Z.; Yin, C. Microbial transformation of ginsenosides extracted from Panax ginseng adventitious roots in an airlift bioreactor. Electron. J. Biotechnol. 2017, 26, 20–26. [Google Scholar] [CrossRef]
- Fu, Y.; Yin, Z.; Yin, C. Biotransformation of ginsenoside Rb1 to Gyp- XVII and minor ginsenoside Rg3 by endophytic bacterium Flavobacterium sp. GE 32 isolated from Panax ginseng. Lett. Appl. Microbiol. 2018, 68, 134–141. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F.A. revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Łyczko, J.; Piotrowski, K.; Kolasa, K.; Galek, R.; Szumny, A. Mentha piperita L. Micropropagation and the potential Influence of Plant Growth Regulators on volatile organic compound composition. Molecules 2020, 25, 2652. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, Y.; Li, Y.; Dong, J.; Liu, X.; Li, C. Biocontrol of Rhizoctonia solani via induction of the defense mechanism and antimicrobial compounds produced by Bacillus subtilis SL-44 on Pepper (Capsicum annuum L.). Front. Microbiol. 2019, 10, 2676. [Google Scholar] [CrossRef] [Green Version]
- Ezziyyani, M.; Sánchez, C.P.; Ahmed, A.S.; Requena, M.E.; Candela, M.E.C. Trichoderma harzianum como biofungicida para el biocontrol de Phytophthora capsici en plantas de pimiento (Capsicum annuum L.). In An. Biol.; 2004; pp. 35–45. [Google Scholar]
- Madika, A.; Ameh, J.B.; Machido, D.A. Isolation and Screening of Bacillus subtilis from Soil for Amylase Production. UJMR 2017, 2, 82–86. [Google Scholar]
- Chandra, Y.S.; Khayum, S.A.; Prasad, T.N.; Sarada, R.J. Identification of Trichoderma species based on morphological characters isolated from rhizosphere of groundnut (Arachis hypogaea L). Int. J. Sci. Environ. Technol. 2017, 6, 2056–2063. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. A Rapid DNA Isolation Procedure for Small Quantities of Fresh Leaf Tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- White, T.J.; Bruns, T.D.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols, a Guide to Methods and Applications; Innis, M.A., Gelfand, D.R., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Cheng, T.; Xu, C.; Lei, L.; Li, C.; Zhang, Y.; Zhou, S. Barcoding the kingdom Plantae: New PCR primers for ITS regions of plants with improved universality and specificity. Mol. Ecol. Resour. 2016, 16, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.C.; Tamanini, R.; Martinez, A.L.; Ribeiro, J.; Beloti, V. Efficiency of boiling and four other methods for genomic DNA extraction of deteriorating spore-forming bacteria from milk. Semin. Cienc. Agrar. 2016, 37, 3069–3078. [Google Scholar] [CrossRef]
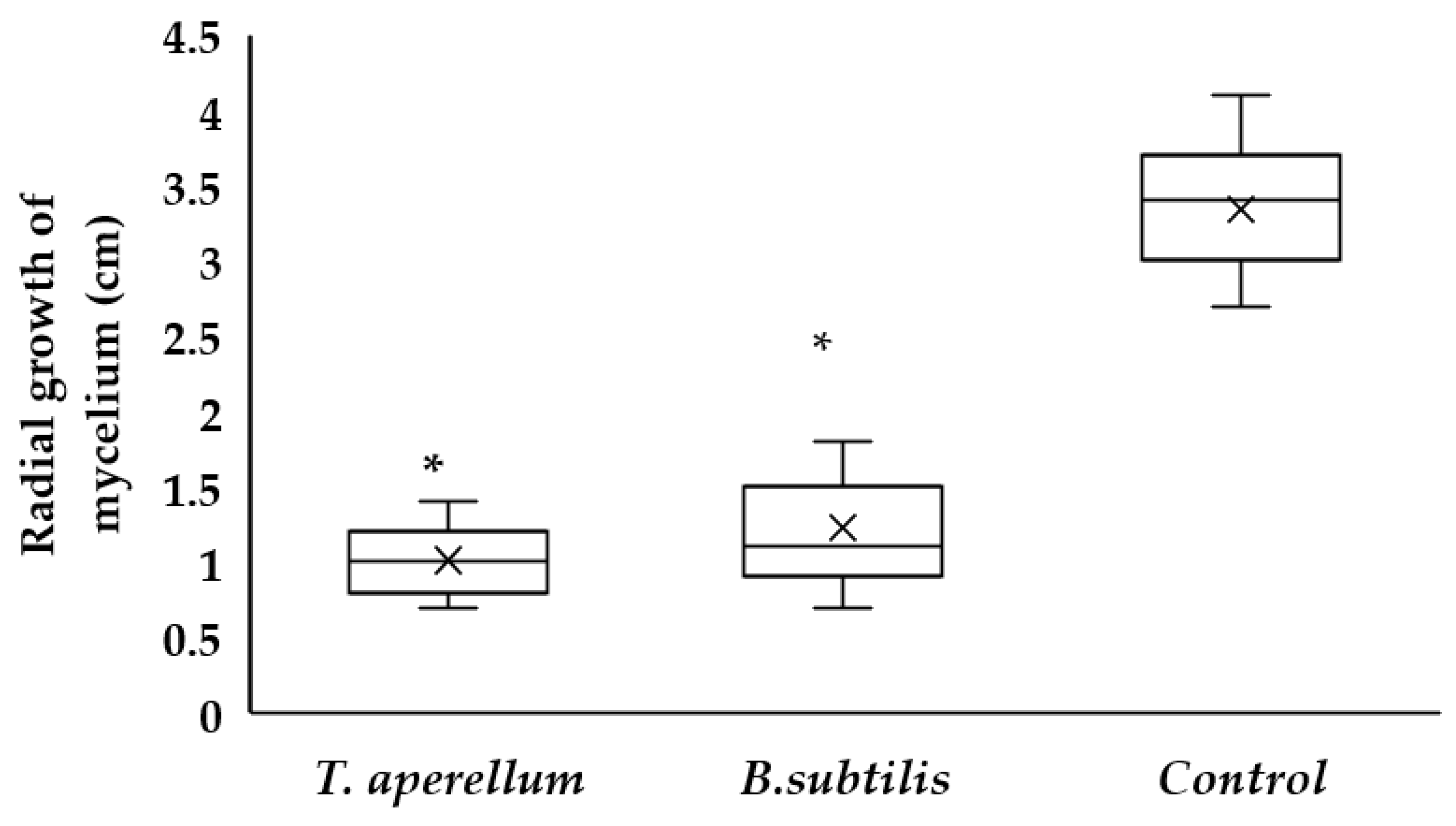
| Microorganism | Growth Inhibition (%) |
|---|---|
| T. asperellum | 69.5 |
| B. subtilis | 63.8 |
| Treatments | Survival Percentage | Plant Height (cm) |
|---|---|---|
| T. asperellum | 95a | 12a |
| B. subtilis | 93a | 15a |
| Control | 75b | 8b |
| Code | Treatment | Code | Treatment |
|---|---|---|---|
| (TP) | Spearmint plants with endophyte Trichoderma asperellum + Rhizoctonia sp. | (B) | Spearmint plants with endophyte Bacillus subtilis Rhizoctonia free |
| (BP) | Spearmint plants with endophyte Bacillus subtilis + Rhizoctonia sp. | (C) | Spearmint plants without endophyte (test) |
| (T) | Spearmint plants with endophyte T. asperellum Rhizoctonia free | (P) | Spearmint plants infected with Rhizoctonia without endophyte |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Restrepo, D.; Dominguez, M.I.; Gaviria-Gutiérrez, B.; Osorio, E.; Sierra, K. Biotization of Endophytes Trichoderma asperellum and Bacillus subtilis in Mentha spicata Microplants to Promote Growth, Pathogen Tolerance and Specialized Plant Metabolites. Plants 2022, 11, 1474. https://doi.org/10.3390/plants11111474
Castro-Restrepo D, Dominguez MI, Gaviria-Gutiérrez B, Osorio E, Sierra K. Biotization of Endophytes Trichoderma asperellum and Bacillus subtilis in Mentha spicata Microplants to Promote Growth, Pathogen Tolerance and Specialized Plant Metabolites. Plants. 2022; 11(11):1474. https://doi.org/10.3390/plants11111474
Chicago/Turabian StyleCastro-Restrepo, Dagoberto, Maria Isabel Dominguez, Bertha Gaviria-Gutiérrez, Edison Osorio, and Karina Sierra. 2022. "Biotization of Endophytes Trichoderma asperellum and Bacillus subtilis in Mentha spicata Microplants to Promote Growth, Pathogen Tolerance and Specialized Plant Metabolites" Plants 11, no. 11: 1474. https://doi.org/10.3390/plants11111474






