Combined Effects of Different Alleles of FLO2, Wx and SSIIa on the Cooking and Eating Quality of Rice
Abstract
:1. Introduction
2. Results
2.1. Variation Analysis of Quality Traits in Parents and RIL
2.2. Genotyping of RIL
2.3. Effects of FLO2, Wx, and SSIIa Single Gene on the AAC, Viscosity Properties, and Textural Properties in the RIL Population
2.4. Differences in the AAC, Viscosity and Textural Properties among Eight Genetic Combinations
2.5. Effects of Interaction between FLO2, Wx, and SSIIa on AAC, Viscosity Properties, and Textural Properties of RIL
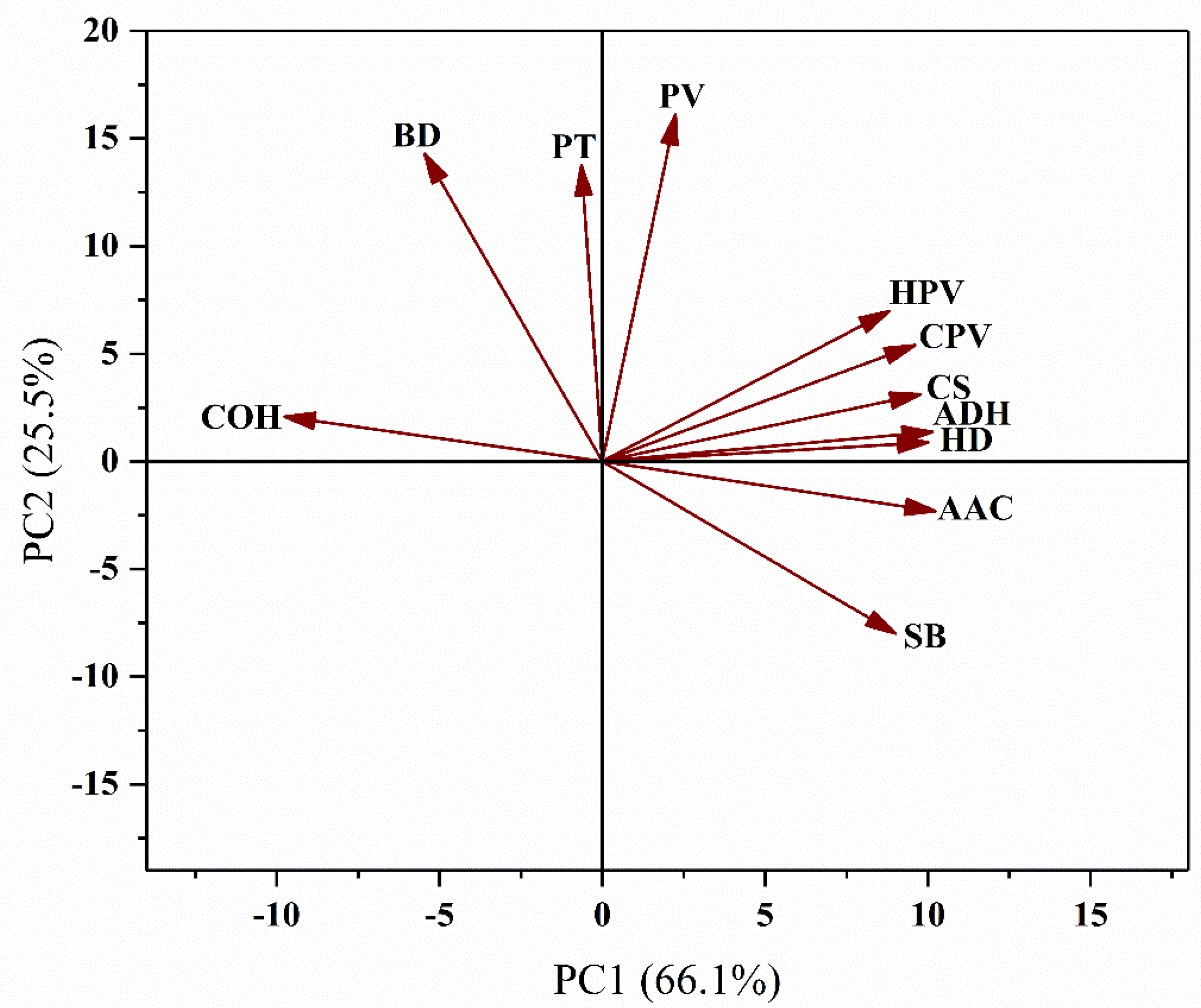
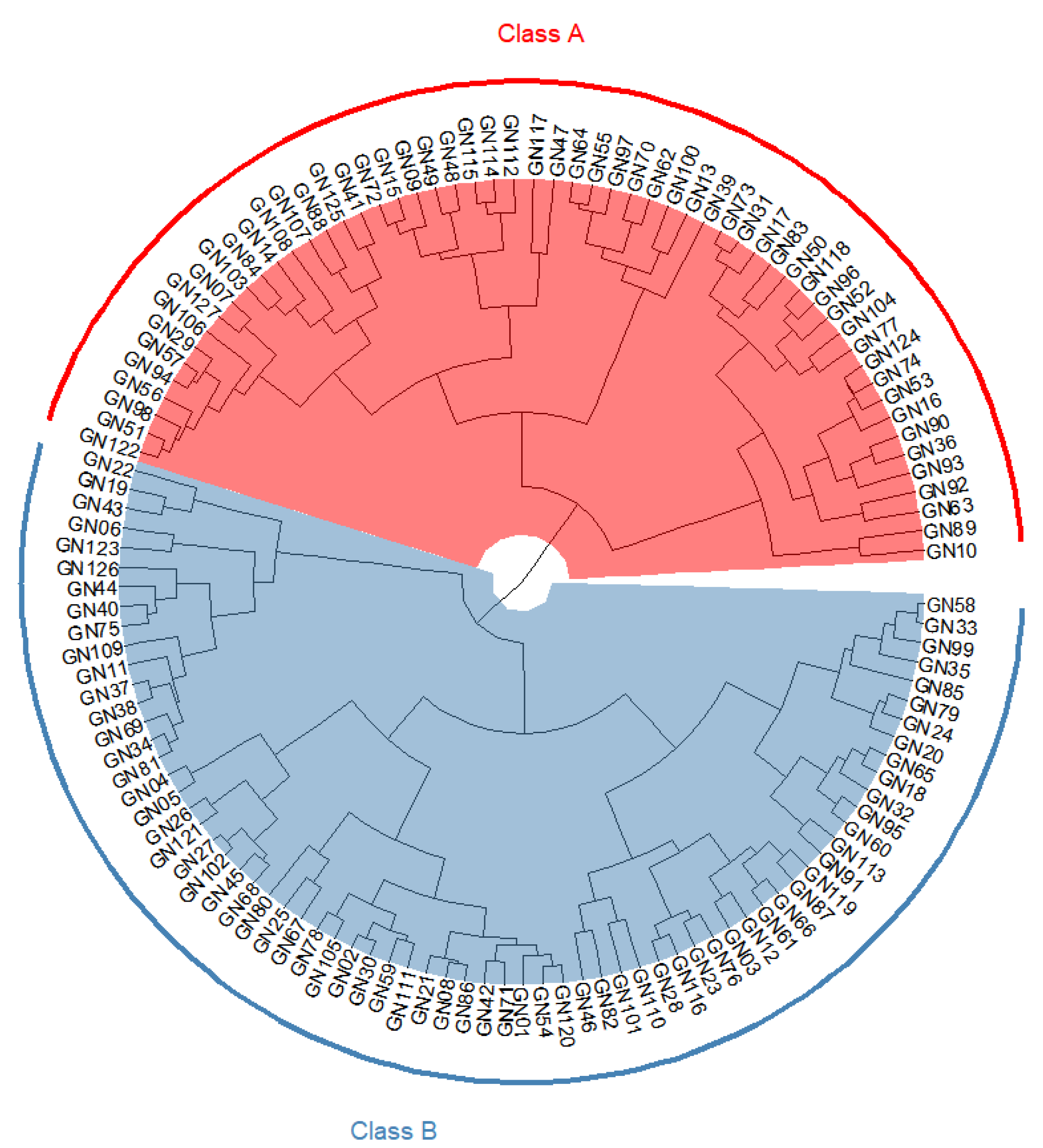
2.6. Cluster Analysis Based on the Quality Traits of RIL Population
3. Discussion
3.1. FLO2 Affects Rice Quality
3.2. Interaction Effect of FLO2, Wx and SSIIa
4. Materials and Methods
4.1. Plant Materials
4.2. Preparation of Rice Flour
4.3. DNA Extraction and Genotyping
4.4. Apparent Amylose Content (AAC)
4.5. Pasting Viscosity
4.6. Gel textural Properties
4.7. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAC | Apparent amylose content |
| ADH | Gel adhesiveness |
| BD | Breakdown |
| CPV | Cold paste viscosity |
| COH | Gel cohesiveness |
| CS | Consistency viscosity |
| CEQ | Cooking and eating quality |
| FLO | Floury |
| HD | Gel hardness |
| GT | Gelatinization temperature |
| HPV | Hot paste viscosity |
| PC | Principal component |
| PCA | Principal component analysis |
| PT | Pasting temperature |
| PV | Peak viscosity |
| RIL | Recombinant inbred line |
| RVA | Rapid Visco-Analyzer |
| RVU | Rapid Visco Unit |
| SB | Setback |
| SNP | Single nucleotide polymorphism |
| SS | Soluble starch synthase |
| Wx | Waxy |
References
- Wing, R.A.; Purugganan, M.D.; Zhang, Q. The rice genome revolution: From an ancient grain to Green Super Rice. Nat. Rev. Genet. 2018, 19, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Qian, Q.; Liu, Q.; Yan, M.; Liu, X.; Yan, C.; Liu, G.; Gao, Z.; Tang, S.; Zeng, D.; et al. Allelic diversities in rice starch biosynthesis lead to a diverse array of rice eating and cooking qualities. Proc. Natl. Acad. Sci. USA 2009, 106, 21760–21765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q. Strategies for developing Green Super Rice. Proc. Natl. Acad. Sci. USA 2007, 104, 16402–16409. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-H.; Zhuang, J.-Y.; Fan, Y.-Y.; Du, J.-H.; Cao, L.-Y. Progress in research and development on hybrid rice: A super-domesticate in China. Ann. Bot. 2007, 100, 959–966. [Google Scholar] [CrossRef]
- Bao, J. Toward understanding the genetic and molecular bases of the eating and cooking qualities of rice. Cereal Foods World 2012, 57, 148–156. [Google Scholar] [CrossRef]
- Bao, J.; Ying, Y.; Zhou, X.; Xu, Y.; Wu, P.; Xu, F.; Pang, Y. Relationships among starch biosynthesizing protein content, fine structure and functionality in rice. Carbohydr. Polym. 2020, 237, 116118. [Google Scholar] [CrossRef] [PubMed]
- Ayaad, M.; Han, Z.; Zheng, K.; Hu, G.; Abo-Yousef, M.; Sobeih, S.E.S.; Xing, Y. Bin-based genome-wide association studies reveal superior alleles for improvement of appearance quality using a 4-way MAGIC population in rice. J. Adv. Res. 2021, 28, 183–194. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, Y.; Zhao, J.; Zhang, Y.; Ying, Y.; Xu, F.; Bao, J. The role of different Wx and BEIIb allele combinations on fine structures and functional properties of indica rice starches. Carbohydr. Polym. 2022, 278, 118972. [Google Scholar] [CrossRef]
- Xu, Y.; Ying, Y.; Ouyang, S.; Duan, X.; Sun, H.; Jiang, S.; Sun, S.; Bao, J. Factors affecting sensory quality of cooked japonica rice. Rice Sci. 2018, 25, 330–339. [Google Scholar] [CrossRef]
- Huang, L.; Gu, Z.; Chen, Z.; Yu, J.; Chu, R.; Tan, H.; Zhao, D.; Fan, X.; Zhang, C.; Li, Q.; et al. Improving rice eating and cooking quality by coordinated expression of the major starch synthesis-related genes, SSII and Wx, in endosperm. Plant Mol. Biol. 2021, 106, 419–432. [Google Scholar] [CrossRef]
- Buléon, A.; Colonna, P.; Planchot, V.; Ball, S. Starch granules: Structure and biosynthesis. Int. J. Biol. Macromol. 1998, 23, 85–112. [Google Scholar] [CrossRef]
- Pérez, S.; Bertoft, E. The molecular structures of starch components and their contribution to the architecture of starch granules: A comprehensive review. Starch-Stärke 2010, 62, 389–420. [Google Scholar] [CrossRef]
- Nishi, A.; Nakamura, Y.; Tanaka, N.; Satoh, H. Biochemical and genetic analysis of the effects of amylose-extender mutation in rice endosperm. Plant Physiol. 2001, 127, 459–472. [Google Scholar] [CrossRef]
- Nakamura, Y. Towards a Better Understanding of the Metabolic System for Amylopectin Biosynthesis in Plants: Rice Endosperm as a Model Tissue. Plant Cell Physiol. 2002, 43, 718–725. [Google Scholar] [CrossRef]
- Jeon, J.-S.; Ryoo, N.; Hahn, T.-R.; Walia, H.; Nakamura, Y. Starch biosynthesis in cereal endosperm. Plant Physiol. Biochem. 2010, 48, 383–392. [Google Scholar] [CrossRef]
- Wanchana, S.; Toojinda, T.; Tragoonrung, S.; Vanavichit, A. Duplicated coding sequence in the waxy allele of tropical glutinous rice (Oryza sativa L.). Plant Sci. 2003, 165, 1193–1199. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, J.; Chen, S.; Fan, X.; Li, Q.; Lu, Y.; Wang, M.; Yu, H.; Yi, C.; Tang, S.; et al. Wxlv, the ancestral allele of rice Waxy gene. Mol. Plant 2019, 12, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Mikami, I.; Uwatoko, N.; Ikeda, Y.; Yamaguchi, J.; Hirano, H.Y.; Suzuki, Y.; Sano, Y. Allelic diversification at the wx locus in landraces of Asian rice. Theor. Appl. Genet. 2008, 116, 979–989. [Google Scholar] [CrossRef]
- Sato, H.; Suzuki, Y.; Sakai, M.; Imbe, T. Molecular Characterization of Wx-mq, a Novel Mutant Gene for Low-amylose Content in Endosperm of Rice (Oryza sativa L.). Breed. Sci. 2002, 52, 131–135. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Fan, F.-J.; Zhu, J.-Y.; Chen, T.; Wang, C.-L.; Zheng, T.-Q.; Zhang, J.; Zhong, W.-G.; Xu, J.-L. Development of AS-PCR marker based on a key mutation confirmed by resequencing of Wx-mp in Milky Princess and its application in japonica soft rice (Oryza sativa L.) breeding. Plant Breed. 2013, 132, 595–603. [Google Scholar] [CrossRef]
- Cai, X.L.; Wang, Z.Y.; Xing, Y.Y.; Zhang, J.L.; Hong, M.M. Aberrant splicing of intron 1 leads to the heterogeneous 5′ UTR and decreased expression of waxy gene in rice cultivars of intermediate amylose content. Plant J. 1998, 14, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Bligh, H.F.J.; Till, R.I.; Jones, C.A. A microsatellite sequence closely linked to the Waxy gene of Oryza sativa. Euphytica 1995, 86, 83–85. [Google Scholar] [CrossRef]
- Ayres, N.M.; McClung, A.M.; Larkin, P.D.; Bligh, H.F.J.; Jones, C.A.; Park, W.D. Microsatellites and a single-nucleotide polymorphism differentiate apparent amylose classes in an extended pedigree of US rice germ plasm. Theor. Appl. Genet. 1997, 94, 773–781. [Google Scholar] [CrossRef]
- Bao, J.S.; Corke, H.; Sun, M. Microsatellites, single nucleotide polymorphisms and a sequence tagged site in starch-synthesizing genes in relation to starch physicochemical properties in nonwaxy rice (Oryza sativa L.). Theor. Appl. Genet. 2006, 113, 1185–1196. [Google Scholar] [CrossRef]
- Gao, Z.; Zeng, D.; Cui, X.; Zhou, Y.; Yan, M.; Huang, D.; Li, J.; Qian, Q. Map-based cloning of the ALK gene, which controls the gelatinization temperature of rice. Sci. China Ser. C Life Sci. 2003, 46, 661–668. [Google Scholar] [CrossRef]
- Nakamura, Y.; Francisco, P.B.; Hosaka, Y.; Sato, A.; Sawada, T.; Kubo, A.; Fujita, N. Essential amino acids of starch synthase IIa differentiate amylopectin structure and starch quality between japonica and indica rice varieties. Plant Mol. Biol. 2005, 58, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.S.; Corke, H.; Sun, M. Nucleotide diversity in starch synthase IIa and validation of single nucleotide polymorphisms in relation to starch gelatinization temperature and other physicochemical properties in rice (Oryza sativa L.). Theor. Appl. Genet. 2006, 113, 1171–1183. [Google Scholar] [CrossRef]
- Jin, L.; Lu, Y.; Shao, Y.; Zhang, G.; Xiao, P.; Shen, S.; Corke, H.; Bao, J. Molecular marker assisted selection for improvement of the eating, cooking and sensory quality of rice (Oryza sativa L.). J. Cereal Sci. 2010, 51, 159–164. [Google Scholar] [CrossRef]
- Mo, Y.; Jeung, J.-U. The use of floury endosperm mutants to develop rice cultivars suitable for dry milling. Plant Biotechnol. Rep. 2020, 14, 185–191. [Google Scholar] [CrossRef]
- She, K.-C.; Kusano, H.; Koizumi, K.; Yamakawa, H.; Hakata, M.; Imamura, T.; Fukuda, M.; Naito, N.; Tsurumaki, Y.; Yaeshima, M.; et al. A novel factor FLOURY ENDOSPERM2 Is involved in regulation of rice grain size and starch quality. Plant Cell 2010, 22, 3280–3294. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.G.; Park, S.; Matsuoka, M.; An, G. White-core endosperm floury endosperm-4 in rice is generated by knockout mutations in the C-type pyruvate orthophosphate dikinase gene (OsPPDKB). Plant J. 2005, 42, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, N.; Yu, C.; Park, C.-S.; Baik, M.-Y.; Park, I.M.; Cho, M.-H.; Bhoo, S.H.; An, G.; Hahn, T.-R.; Jeon, J.-S. Knockout of a starch synthase gene OsSSIIIa/Flo5 causes white-core floury endosperm in rice (Oryza sativa L.). Plant Cell Rep. 2007, 26, 1083–1095. [Google Scholar] [CrossRef]
- Fujita, N.; Yoshida, M.; Kondo, T.; Saito, K.; Utsumi, Y.; Tokunaga, T.; Nishi, A.; Satoh, H.; Park, J.-H.; Jane, J.-L.; et al. Characterization of SSIIIa-deficient mutants of rice: The function of SSIIIa and pleiotropic effects by SSIIIa deficiency in the rice endosperm. Plant Physiol. 2007, 144, 2009–2023. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Wang, Y.; Liu, F.; Ren, Y.; Zhou, K.; Lv, J.; Zheng, M.; Zhao, S.; Zhang, L.; Wang, C.; et al. FLOURY ENDOSPERM6 encodes a CBM48 domain-containing protein involved in compound granule formation and starch synthesis in rice endosperm. Plant J. 2014, 77, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ren, Y.; Lu, B.; Yang, C.; Feng, Z.; Liu, Z.; Chen, J.; Ma, W.; Wang, Y.; Yu, X.; et al. FLOURY ENDOSPERM7 encodes a regulator of starch synthesis and amyloplast development essential for peripheral endosperm development in rice. J. Exp. Bot. 2016, 67, 633–647. [Google Scholar] [CrossRef]
- Long, W.; Dong, B.; Wang, Y.; Pan, P.; Wang, Y.; Liu, L.; Chen, X.; Liu, X.; Liu, S.; Tian, Y.; et al. FLOURY ENDOSPERM8, encoding the UDP-glucose pyrophosphorylase 1, affects the synthesis and structure of starch in rice endosperm. J. Plant Biol. 2017, 60, 513–522. [Google Scholar] [CrossRef]
- Wu, M.; Ren, Y.; Cai, M.; Wang, Y.; Zhu, S.; Zhu, J.; Hao, Y.; Teng, X.; Zhu, X.; Jing, R.; et al. Rice FLOURY ENDOSPERM10 encodes a pentatricopeptide repeat protein that is essential for the trans-splicing of mitochondrial nad1 intron 1 and endosperm development. New Phytol. 2019, 223, 736–750. [Google Scholar] [CrossRef]
- Zhu, X.; Teng, X.; Wang, Y.; Hao, Y.; Jing, R.; Wang, Y.; Liu, Y.; Zhu, J.; Wu, M.; Zhong, M.; et al. FLOURY ENDOSPERM11 encoding a plastid heat shock protein 70 is essential for amyloplast development in rice. Plant Sci. 2018, 277, 89–99. [Google Scholar] [CrossRef]
- Zhong, M.; Liu, X.; Liu, F.; Ren, Y.; Wang, Y.; Zhu, J.; Teng, X.; Duan, E.; Wang, F.; Zhang, H.; et al. FLOURY ENDOSPERM12 encoding alanine aminotransferase 1 regulates carbon and nitrogen metabolism in rice. J. Plant Biol. 2019, 62, 61–73. [Google Scholar] [CrossRef]
- Hu, T.; Tian, Y.; Zhu, J.; Wang, Y.; Jing, R.; Lei, J.; Sun, Y.; Yu, Y.; Li, J.; Chen, X.; et al. OsNDUFA9 encoding a mitochondrial complex I subunit is essential for embryo development and starch synthesis in rice. Plant Cell Rep. 2018, 37, 1667–1679. [Google Scholar] [CrossRef]
- Xue, M.; Liu, L.; Yu, Y.; Zhu, J.; Gao, H.; Wang, Y.; Wan, J. Lose-of-function of a rice nucleolus-localized pentatricopeptide repeat protein is responsible for the floury endosperm14 mutant phenotypes. Rice 2019, 12, 100. [Google Scholar] [CrossRef]
- You, X.; Zhang, W.; Hu, J.; Jing, R.; Cai, Y.; Feng, Z.; Kong, F.; Zhang, J.; Yan, H.; Chen, W.; et al. FLOURY ENDOSPERM15 encodes a glyoxalase I involved in compound granule formation and starch synthesis in rice endosperm. Plant Cell Rep. 2019, 38, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.; Zhong, M.; Zhu, X.; Wang, C.; Ren, Y.; Wang, Y.; Zhang, H.; Jiang, L.; Wang, D.; Hao, Y.; et al. FLOURY ENDOSPERM16 encoding a NAD-dependent cytosolic malate dehydrogenase plays an important role in starch synthesis and seed development in rice. Plant Biotechnol. J. 2019, 17, 1914–1927. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wu, M.; Ren, Y.; Wang, Y.; Li, J.; Lei, C.; Sun, Y.; Bao, X.; Wu, H.; Yang, H.; et al. Rice FLOURY ENDOSPERM 18 encodes a pentatricopeptide repeat protein required for 5′ processing of mitochondrial nad5 messenger RNA and endosperm development. J. Integr. Plant Biol. 2021, 63, 834–847. [Google Scholar] [CrossRef] [PubMed]
- Lou, G.; Chen, P.; Zhou, H.; Li, P.; Xiong, J.; Wan, S.; Zheng, Y.; Alam, M.; Liu, R.; Zhou, Y.; et al. FLOURY ENDOSPERM19 encoding a class I glutamine amidotransferase affects grain quality in rice. Mol. Breed. 2021, 41, 36. [Google Scholar] [CrossRef]
- Wu, Y.; Pu, C.; Lin, H.; Huang, H.; Huang, Y.; Hong, C.; Chang, M.; Lin, Y. Three novel alleles of FLOURY ENDOSPERM2 (FLO2) confer dull grains with low amylose content in rice. Plant Sci. 2015, 233, 44–52. [Google Scholar] [CrossRef]
- Qiao, Y.; Lee, S.I.; Piao, R.; Jiang, W.; Ham, T.H.; Chin, J.H.; Piao, Z.; Han, L.; Kang, S.Y.; Koh, H.J. Fine mapping and candidate gene analysis of the floury endosperm gene, FLO(a), in rice. Mol. Cells 2010, 29, 167–174. [Google Scholar] [CrossRef]
- Kong, X.; Sun, X.; Xu, F.; Umemoto, T.; Chen, H.; Bao, J. Morphological and physicochemical properties of two starch mutants induced from a high amylose indica rice by gamma irradiation. Starch-Stärke 2014, 66, 157–165. [Google Scholar] [CrossRef]
- Xiang, X.; Kang, C.; Xu, S.; Yang, B. Combined effects of Wx and SSIIa haplotypes on rice starch physicochemical properties. J. Sci. Food Agric. 2017, 97, 1229–1234. [Google Scholar] [CrossRef]
- Bao, J.S.; Jin, L.; Xiao, P.; Shen, S.; Sun, M.; Corke, H. Starch physicochemical properties and their associations with microsatellite alleles of starch-synthesizing genes in a rice RIL population. J. Agric. Food Chem. 2008, 56, 1589–1594. [Google Scholar] [CrossRef]
- Bao, J.S.; Zhang, Y.; Zhao, J.J.; Chen, Y.L.; Wu, W.X.; Cao, L.Y.; Xu, F.F. Identification of a new allele of FLOURY ENDOSPERM2 in a white-core endosperm mutant of rice. Rice Sci. 2022, 29, 407–411. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, H.; Jiang, Y.; Zhang, H.; Wang, S.; Wang, F.; Zhu, Y. Genetic control and high temperature effects on starch biosynthesis and grain quality in rice. Front. Plant Sci. 2021, 12, 2971. [Google Scholar] [CrossRef]
- Huang, L.; Tan, H.; Zhang, C.; Li, Q.; Liu, Q. Starch biosynthesis in cereal endosperms: An updated review over the last decade. Plant Commun. 2021, 2, 100237. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J. Effects of Flo2 with Different Mutation Sites on Synthesis of Storage Substances in Rice Endosperm. Master’s Thesis, Yangzhou University, Yangzhou, China, 2021. [Google Scholar]
- Das, A.K.; Cohen, P.T.W.; Barford, D. The structure of the tetratricopeptide repeats of protein phosphatase 5: Implications for TPR-mediated protein–protein interactions. EMBO J. 1998, 17, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Blatch, G.L.; Lässle, M. The tetratricopeptide repeat: A structural motif mediating protein-protein interactions. BioEssays 1999, 21, 932–939. [Google Scholar] [CrossRef]
- Sen, A.; Kalvakuri, S.; Bodmer, R.; Cox, R.T. Clueless, a protein required for mitochondrial function, interacts with the PINK1-Parkin complex in Drosophila. Dis. Models Mech. 2015, 8, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, L.; Lin, L.; Zhao, L.; Liu, Q.; Wei, C. A novel mutation of OsPPDKB, encoding pyruvate orthophosphate dikinase, affects metabolism and structure of starch in the rice endosperm. Int. J. Mol. Sci. 2018, 19, 2268. [Google Scholar] [CrossRef]
- Kowittaya, C.; Lumdubwong, N. Molecular weight, chain profile of rice amylopectin and starch pasting properties. Carbohydr. Polym. 2014, 108, 216–223. [Google Scholar] [CrossRef]
- Tao, K.; Li, C.; Yu, W.; Gilbert, R.G.; Li, E. How amylose molecular fine structure of rice starch affects functional properties. Carbohydr. Polym. 2019, 204, 24–31. [Google Scholar] [CrossRef]
- Wang, K.; Hasjim, J.; Wu, A.C.; Henry, R.J.; Gilbert, R.G. Variation in amylose fine structure of starches from different botanical sources. J. Agric. Food Chem. 2014, 62, 4443–4453. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pang, Y.; Bao, J. Expression profiles and protein complexes of starch biosynthetic enzymes from white-core and waxy mutants induced from high amylose indica rice. Rice Sci. 2020, 27, 152–161. [Google Scholar] [CrossRef]
- Huang, J.; Tang, X.; Pu, H. Research progress on the micro-structure, texture property and stability of starch gel. J. Food Sci. Biotechnol. 2017, 36, 673–679. [Google Scholar]
- Pfister, B.; Zeeman, S.C. Formation of starch in plant cells. Cell. Mol. Life Sci. 2016, 73, 2781–2807. [Google Scholar] [CrossRef] [PubMed]
- Ball, S.G.; Morell, M.K. From bacterial glycogen to starch: Understanding the biogenesis of the plant starch granule. Annu. Rev. Plant Biol. 2003, 54, 207–233. [Google Scholar] [CrossRef]
- Zeeman, S.C.; Kossmann, J.; Smith, A.M. Starch: Its metabolism, evolution, and biotechnological modification in plants. Annu. Rev. Plant Biol. 2010, 61, 209–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Parameter | Parents | RIL | |||||
|---|---|---|---|---|---|---|---|
| GM645 | TN67 | Mean ± SD | CV (%) | Range | Skewness | Kurtosis | |
| AAC (%) | 22.6 A | 16.3 B | 18 ± 6.8 | 37.8 | 6.7–29.1 | 0.2 | −1.5 |
| PV (RUV) | 215.3 B | 269.9 A | 246.5 ± 56.4 | 22.9 | 96.5–351.3 | −0.4 | −0.7 |
| HPV (RUV) | 176.7 A | 171.1 A | 163.7 ± 43.1 | 26.3 | 62.3–281.7 | 0.1 | −0.3 |
| CPV (RUV) | 279.1 A | 262.9 A | 271.7 ± 69.8 | 25.7 | 115.4–412.7 | −0.3 | −0.6 |
| BD (RUV) | 38.6 A | 98.8 A | 82.8 ± 44.0 | 53.1 | 11.3–198.2 | −0.3 | −0.5 |
| SB (RUV) | 63.8 A | −7.0 B | 25.2 ± 62.5 | 247.8 | −121.1 to 135.4 | −0.3 | −0.9 |
| PT (°C) | 72.8 B | 73.6 A | 75.5 ± 4.6 | 6.1 | 66.9–83.9 | 0.0 | −1.2 |
| CS (RUV) | 102.4 A | 91.8 A | 108.0 ± 34.9 | 32.4 | 34.7–182.6 | 0.1 | −0.6 |
| HD (g) | 11.5 A | 7.5 B | 11.6 ± 10.4 | 89.7 | 1.37–52.72 | 1.5 | 1.9 |
| ADH (g·s) | 24.8 A | 10.7 B | 23.2 ± 20.6 | 88.8 | 0.00–103.4 | 1.5 | 1.9 |
| COH | 0.58 B | 0.76 A | 0.61 ± 0.08 | 13.1 | 0.45–0.96 | 0.5 | 1.7 |
| Parameter | FLO2 | Wx | SSIIa | |||
|---|---|---|---|---|---|---|
| FLO2 (86) | flo2 (41) | Wxa (59) | Wxb (68) | TT (50) | GC (77) | |
| AAC (%) | 19.2 ± 7.1 A | 15.6 ± 5.2 B | 24.6 ± 3.1 A | 12.3 ± 2.6B | 18.7 ± 6.7 A | 17.6 ± 6.7 A |
| PV (RUV) | 274.2 ± 40.4 A | 188.4 ± 37.8 B | 239.0 ± 46.7 A | 253.8 ± 62.9 A | 248.8 ± 60.9 A | 245.1 ± 53.2 A |
| HPV (RUV) | 180.6 ± 37.7 A | 128.4 ± 30.4 B | 188.1 ± 37.5 A | 142.6 ± 35.9 B | 175.4 ± 50.5 A | 156.2 ± 35.6 B |
| CPV (RUV) | 93.6 ± 43.8 A | 60.0 ± 34.7 B | 50.9 ± 22.9 B | 110.5 ± 38.9 A | 73.4 ± 40.7 A | 88.9 ± 45.0 A |
| BD (RUV) | 300.2 ± 57.3 A | 212.0 ± 54.2 B | 321.8 ± 46.8 A | 228.3 ± 56.1 B | 284.0 ± 71.2 A | 263.8 ± 67.8 A |
| SB (RUV) | 26.0 ± 64.8 A | 23.6 ± 57.3 A | 82.8 ± 21.1 A | −24.8 ± 39.1 B | 35.2 ± 54.1 A | 18.7 ± 66.6 A |
| PT (°C) | 76.6 ± 4.7 A | 73.3 ± 3.7 B | 74.6 ± 4.1 B | 76.3 ± 4.9 A | 71.0 ± 2.3 B | 78.4 ± 3.2 A |
| CS (RUV) | 119.6 ± 31.2 A | 83.7 ± 29.4 B | 133.7 ± 27.6 A | 85.7 ± 23.5 B | 108.6 ± 30.9 A | 107.6 ± 37.3 A |
| HD (g) | 13.9 ± 11.5 A | 6.8 ± 4.2 B | 19.7 ± 10.3 A | 4.5 ± 1.0 B | 11.1 ± 9.3 A | 11.9 ± 10.9 A |
| ADH (g·s) | 27.9 ± 22.5 A | 13.3 ± 10.3 B | 38.8 ± 20.9 A | 9.7 ± 4.2 B | 22.7 ± 18.5 A | 23.5 ± 21.8 A |
| COH | 0.60 ± 0.08 A | 0.63 ± 0.08 A | 0.55 ± 0.06 B | 0.65 ± 0.06 A | 0.61 ± 0.07 A | 0.60 ± 0.08 A |
| Genetic Combination | AAC (%) | PV (RUV) | HPV (RUV) | BD (RUV) | CPV (RUV) | SB (RUV) |
|---|---|---|---|---|---|---|
| FLO2/Wxa/SSIIa(GC) | 25.70 ± 2.07 A | 264.5 ± 28.6 A | 193.3 ± 23.5 AB | 71.2 ± 17.8 D | 345.4 ± 25.1 A | 80.9 ± 22.7 A |
| FLO2/Wxa/SSIIa(TT) | 26.23 ± 2.67 A | 257.9 ± 38.0 A | 214.5 ± 38.7 A | 43.4 ± 13.1 EF | 343.5 ± 40.6 A | 85.6 ± 24.4 A |
| FLO2/Wxb/SSIIa(GC) | 11.30 ± 1.94 D | 283.3 ± 48.3 A | 147.3 ± 21.7 D | 136.0 ± 35.1 A | 239.0 ± 33.8 C | −44.4 ± 39.7 D |
| FLO2/Wxb/SSIIa(TT) | 14.41 ± 2.45 C | 289.7 ± 35.4 A | 174.8 ± 30.7 BC | 114.9 ± 27.6 B | 280.5 ± 38.7 B | −9.2 ± 31.9 BC |
| flo2/Wxa/SSIIa(GC) | 20.89 ± 1.62 B | 184.8 ± 22.2 BC | 154.2 ± 24.0 CD | 30.6 ± 10.5 F | 262.8 ± 24.1 BC | 78.1 ± 11.1 A |
| flo2/Wxa/SSIIa(TT) | 22.20 ± 1.59 B | 176.8 ± 13.0 BC | 148.1 ± 10.4 D | 28.6 ± 11.4 F | 274.1 ± 14.2 B | 97.4 ± 6.0 A |
| flo2/Wxb/SSIIa(GC) | 12.01 ± 2.39 D | 207.5 ± 37.0 B | 115.5 ± 15.9 E | 92.0 ± 27.4 C | 179.4 ± 30.3 D | −28.1 ± 36.0 BC |
| flo2/Wxb/SSIIa(TT) | 10.87 ± 2.08 D | 161.9 ± 46.8 C | 102.2 ± 30.8 E | 59.7 ± 21.8 DE | 163.7 ± 40.1 D | 1.7 ± 25.3 B |
| Genetic Combination | PT (°C) | CS (RUV) | HD (g) | ADH (g·s) | COH | |
| FLO2/Wxa/SSIIa(GC) | 79.0 ± 1.4 B | 152.1 ± 19.6 A | 25.14 ± 10.38 A | 48.68 ± 21.91 A | 0.52 ± 0.04 E | |
| FLO2/Wxa/SSIIa(TT) | 70.7 ± 1.8 E | 129.0 ± 29.0 B | 21.21 ± 7.78 A | 41.95 ± 16.23 A | 0.55 ± 0.05 DE | |
| FLO2/Wxb/SSIIa(GC) | 81.7 ± 1.2 A | 91.7 ± 16.0 D | 4.70 ± 0.91 CD | 10.25 ± 2.58 CD | 0.66 ± 0.04 AB | |
| FLO2/Wxb/SSIIa(TT) | 72.8 ± 1.6 D | 105.7 ± 13.6 CD | 4.72 ± 0.76 CD | 11.59 ± 3.60 CD | 0.67 ± 0.04 A | |
| flo2/Wxa/SSIIa(GC) | 73.8 ± 1.2 D | 108.7 ± 14.3 C | 9.64 ± 3.45 BC | 19.96 ± 9.77 BC | 0.61 ± 0.07 BC | |
| flo2/Wxa/SSIIa(TT) | 68.7 ± 0.9 F | 126.0 ± 15.5 B | 12.70 ± 5.46 B | 25.87 ± 10.99 B | 0.58 ± 0.05CD | |
| flo2/Wxb/SSIIa(GC) | 76.5 ± 2.0 C | 63.9 ± 18.0 E | 4.48 ± 1.34 CD | 8.49 ± 5.66 D | 0.64 ± 0.10AB | |
| flo2/Wxb/SSIIa(TT) | 68.2 ± 1.0 F | 61.4 ± 12.3 E | 3.71 ± 0.52 D | 5.65 ± 2.36 D | 0.65 ± 0.06AB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhao, J.; Hu, Y.; Zhang, Y.; Ying, Y.; Xu, F.; Bao, J. Combined Effects of Different Alleles of FLO2, Wx and SSIIa on the Cooking and Eating Quality of Rice. Plants 2022, 11, 2249. https://doi.org/10.3390/plants11172249
Zhang Y, Zhao J, Hu Y, Zhang Y, Ying Y, Xu F, Bao J. Combined Effects of Different Alleles of FLO2, Wx and SSIIa on the Cooking and Eating Quality of Rice. Plants. 2022; 11(17):2249. https://doi.org/10.3390/plants11172249
Chicago/Turabian StyleZhang, Yu, Jiajia Zhao, Yaqi Hu, Yanni Zhang, Yining Ying, Feifei Xu, and Jinsong Bao. 2022. "Combined Effects of Different Alleles of FLO2, Wx and SSIIa on the Cooking and Eating Quality of Rice" Plants 11, no. 17: 2249. https://doi.org/10.3390/plants11172249





