Effects of LED Light Spectra on the Development, Phytochemical Profile, and Antioxidant Activity of Curcuma longa from Easter Island
Abstract
:1. Introduction
2. Results
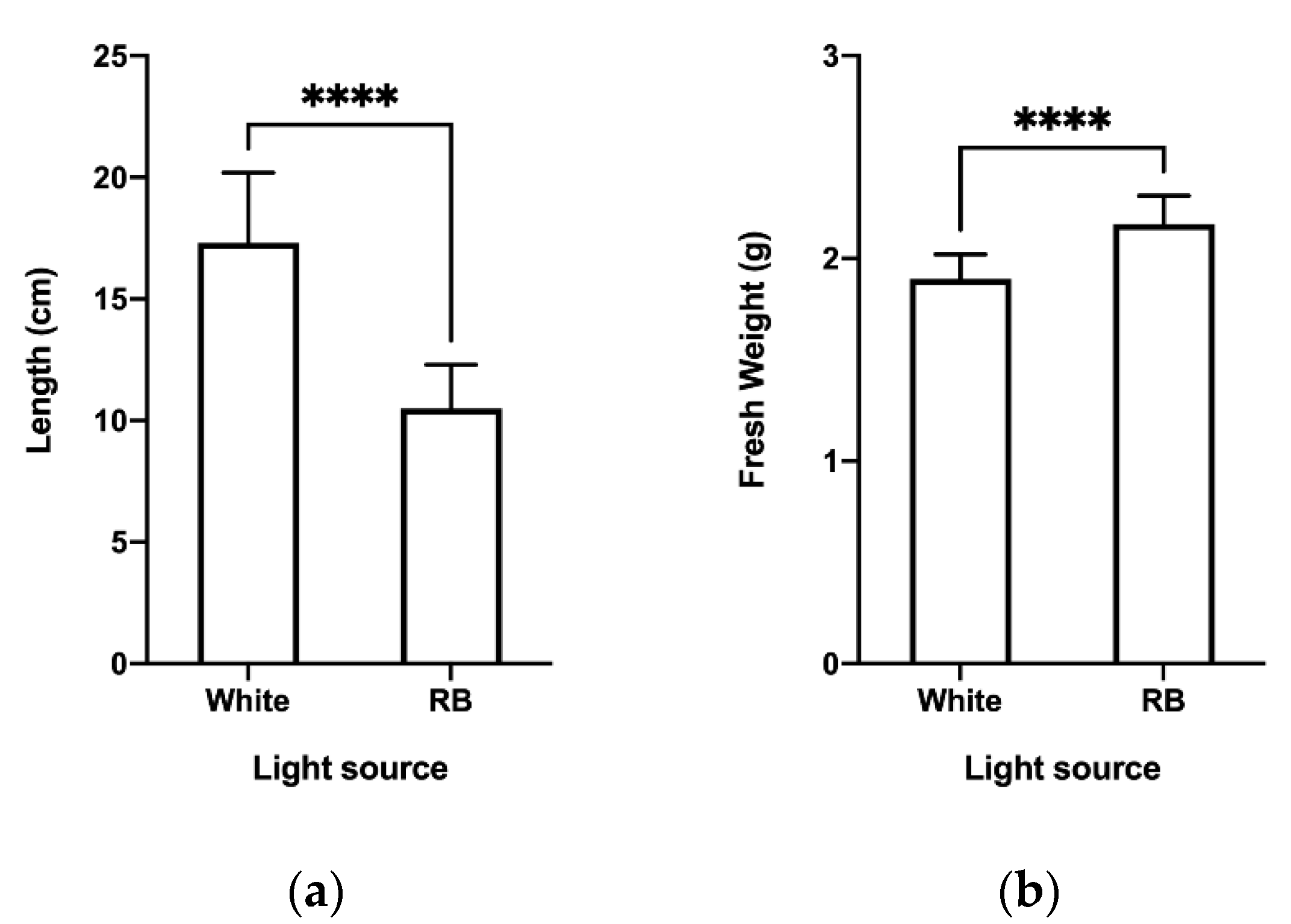
2.1. Effect of Light Quality on In Vitro Growth and Development of C. longa
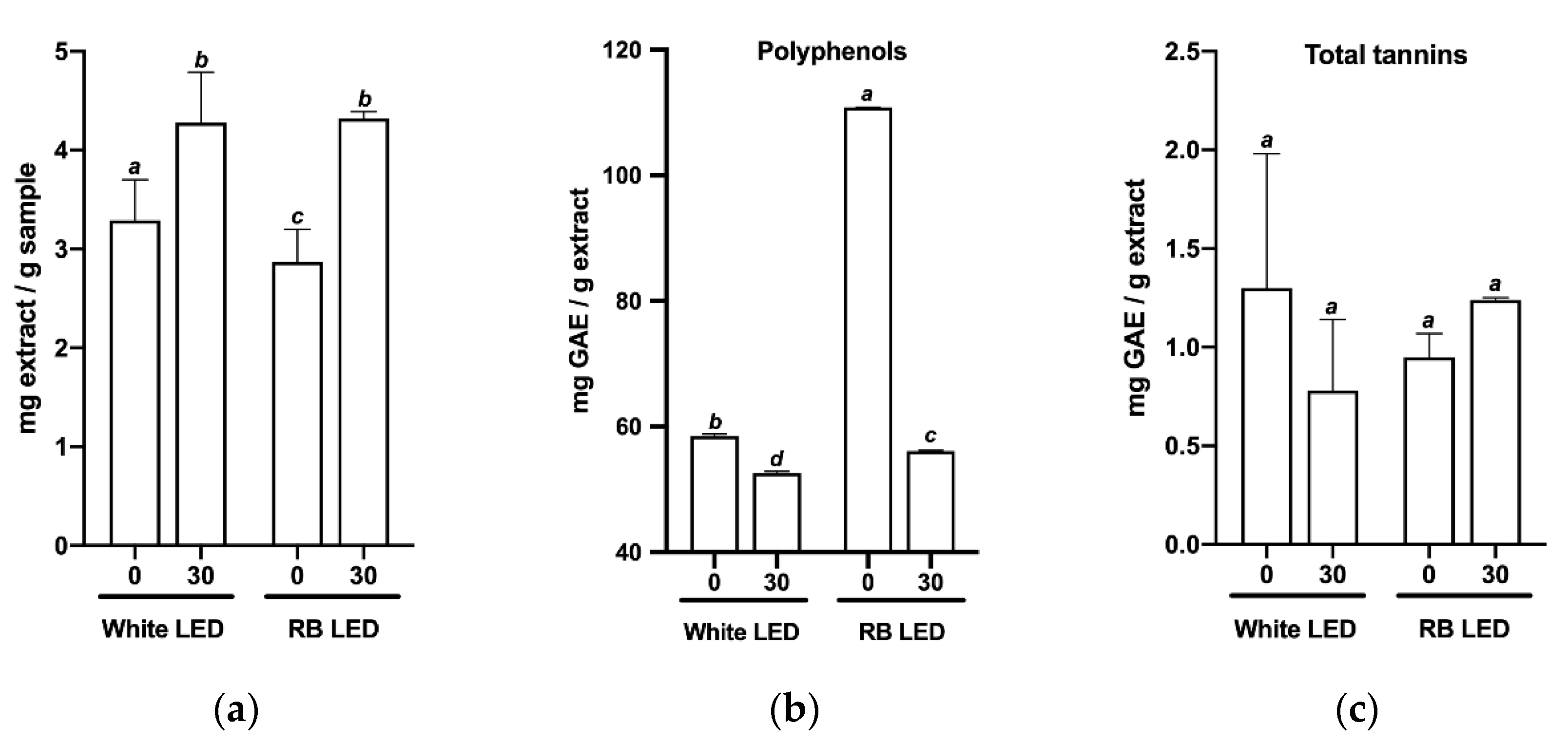
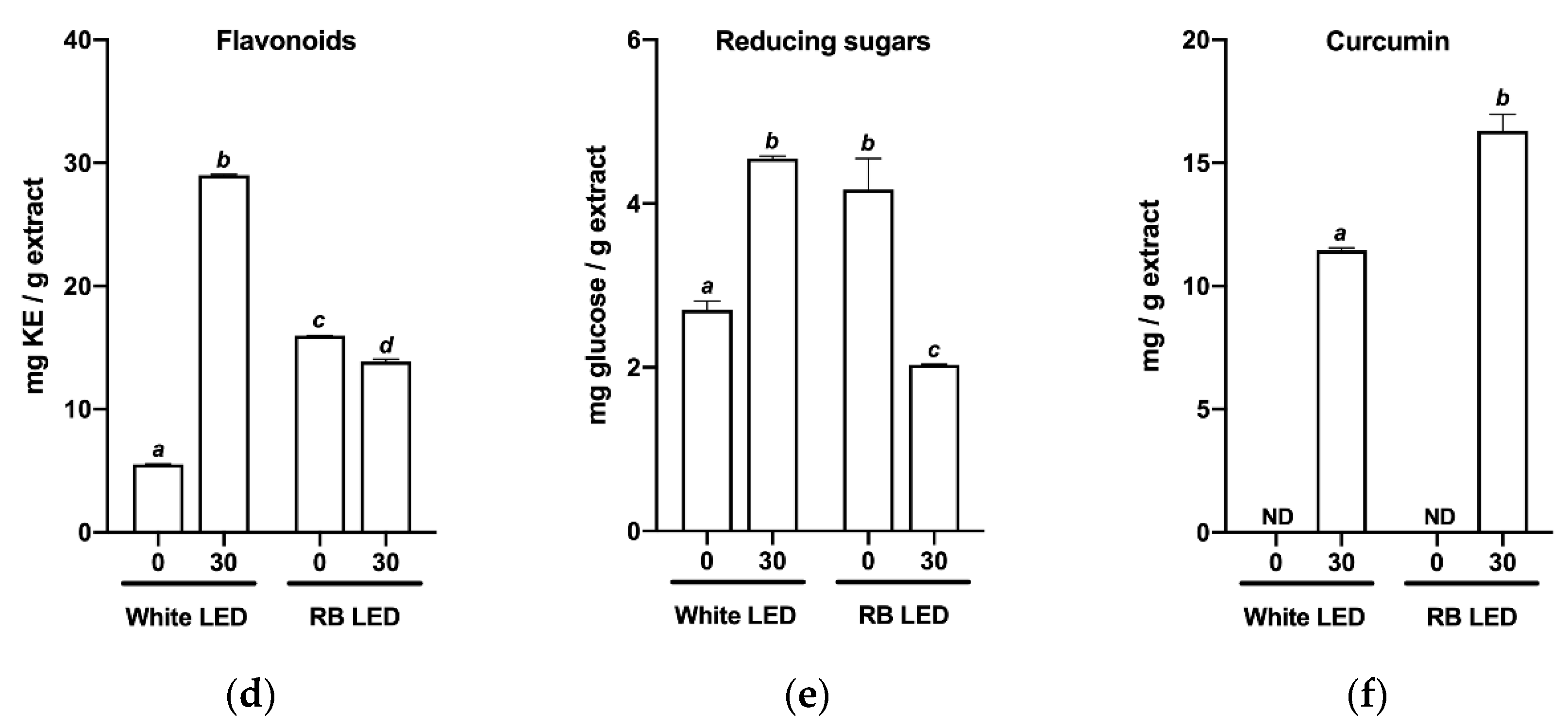
2.2. Effects of Light Quality on the Phytochemical Profile of C. longa
2.3. Effect of Light Spectra on C. longa Radical Scavenging Activity
3. Discussion
4. Materials and Methods
4.1. Explant Collection, Disinfection, and Establishment of Rhizome Cultures
4.2. In Vitro Growth of Explants
4.3. In Vitro Proliferation of Curcuma longa by TIS
4.4. Spectral Light Conditions
4.5. Phytochemical Composition
4.5.1. Polyphenols
4.5.2. Total Tannins
4.5.3. Flavonoids
4.5.4. Reducing Sugars
4.5.5. Determination of Curcumin
4.6. Radical Scavenging Capacity
4.6.1. DPPH Assay
4.6.2. ORAC-FL Assay
4.6.3. FRAP Assay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sharifi-Rad, J.; El Rayess, Y.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef]
- Balada, C.; Castro, M.; Fassio, C.; Zamora, A.; Marchant, M.J.; Acevedo, W.; Guzmán, L. Genetic diversity and biological activity of Curcuma longa ecotypes from Rapa Nui using molecular markers. Saudi J. Biol. Sci. 2021, 28, 707–716. [Google Scholar] [CrossRef]
- Marchant, M.J.; Molina, P.; Montecinos, M.; Guzmán, L.; Balada, C.; Fassio, C.; Castro, M. In Vitro Propagation of Easter Island Curcuma longa from Rhizome Explants Using Temporary Immersion System. Agronomy 2021, 11, 2121. [Google Scholar] [CrossRef]
- Al Murad, M.; Razi, K.; Jeong, B.; Samy, P.; Muneer, S. Light Emitting Diodes (LEDs) as Agricultural Lighting: Impact and Its Potential on Improving Physiology, Flowering, and Secondary Metabolites of Crops. Sustainability 2021, 13, 1985. [Google Scholar] [CrossRef]
- Le, A.T.; Yu, J.-K.; Han, G.-D.; Do, T.K.; Chung, Y.-S. Potential Use of Colored LED Lights to Increase the Production of Bioactive Metabolites Hedyotis corymbosa (L.) Lam. Plants 2022, 11, 225. [Google Scholar] [CrossRef]
- Cioć, M.; Szewczyk, A.; Żupnik, M.; Kalisz, A.; Pawłowska, B. LED lighting affects plant growth, morphogenesis and phytochemical contents of Myrtus communis L. in vitro. Plant Cell Tissue Organ Cult. PCTOC 2018, 132, 433–447. [Google Scholar] [CrossRef] [Green Version]
- Azad, M.O.K.; Kjaer, K.H.; Adnan, M.; Naznin, M.T.; Lim, J.D.; Sung, I.J.; Park, C.H.; Lim, Y.S. The Evaluation of Growth Performance, Photosynthetic Capacity, and Primary and Secondary Metabolite Content of Leaf Lettuce Grown under Limited Irradiation of Blue and Red LED Light in an Urban Plant Factory. Agriculture 2020, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Samuolienė, G.; Brazaitytė, A.; Sirtautas, R.; Novičkovas, A.; Duchovskis, P. The Effect of Supplementary LED Lighting on the Antioxidant and Nutritional Properties of Lettuce. Acta Hortic. 2012, 952, 835–841. [Google Scholar] [CrossRef]
- Dong, C.; Fu, Y.; Liu, G.; Liu, H. Growth, Photosynthetic Characteristics, Antioxidant Capacity and Biomass Yield and Quality of Wheat (Triticum aestivum L.) Exposed to LED Light Sources with Different Spectra Combinations. J. Agron. Crop Sci. 2014, 200, 219–230. [Google Scholar] [CrossRef]
- Alvarenga, I.C.A.; Pacheco, F.V.; Silva, S.T.; Bertolucci, S.K.V.; Pinto, J.E.B.P. In vitro culture of Achillea millefolium L.: Quality and intensity of light on growth and production of volatiles. Plant Cell Tissue Organ Cult. PCTOC 2015, 122, 299–308. [Google Scholar] [CrossRef]
- Li, H.; Tang, C.; Xu, Z. The effects of different light qualities on rapeseed (Brassica napus L.) plantlet growth and morphogenesis in vitro. Sci. Hortic. 2013, 150, 117–124. [Google Scholar] [CrossRef]
- Darko, É.; Heydarizadeh, P.; Schoefs, B.; Sabzalian, M.R. Photosynthesis under artificial light: The shift in primary and secondary metabolism. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, W.-S.; Chung, I.-M.; Hwang, M.; Kim, S.-H.; Yu, C.; Ghimire, B. Application of Light-Emitting Diodes for Improving the Nutritional Quality and Bioactive Compound Levels of Some Crops and Medicinal Plants. Molecules 2021, 26, 1477. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Bashir, T.; Ghosh, R.; Lee, S.K.; Bae, H. An Overview of LEDs’ Effects on the Production of Bioactive Compounds and Crop Quality. Molecules 2017, 22, 1420. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Chory, J.; Fankhauser, C. Light Signal Transduction in Higher Plants. Annu. Rev. Genet. 2004, 38, 87–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, J.; Liu, B.; Liao, J.; Yang, Z.; Lin, C.; Oka, Y. Coordination of Cryptochrome and Phytochrome Signals in the Regulation of Plant Light Responses. Agronomy 2017, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Agrios, G. Enviromental Factors That Cause Plant Diseases. In Plant Pathology; Breibelbis, D., Sonnack, K.D., Beattie, L., DeCicco, E., Eds.; Elsevier: Berkeley, CA, USA, 2005; pp. 357–384. ISBN 0-12-473276-3. [Google Scholar]
- Wang, Y.; Folta, K.M. Contributions of green light to plant growth and development. Am. J. Bot. 2013, 100, 70–78. [Google Scholar] [CrossRef]
- Pham, M.D.; Hwang, H.; Park, S.W.; Cui, M.; Lee, H.; Chun, C. Leaf chlorosis, epinasty, carbohydrate contents and growth of tomato show different responses to the red/blue wavelength ratio under continuous light. Plant Physiol. Biochem. 2019, 141, 477–486. [Google Scholar] [CrossRef]
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review. J. Sci. Food Agric. 2015, 95, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Dutta Gupta, S.; Pradhan, S. Regulation of Gene Expression by LED Lighting. In Light Emitting Diodes for Agriculture: Smart Lighting; Dutta Gupta, S., Ed.; Springer: Singapore, 2017; pp. 237–258. ISBN 9789811058073. [Google Scholar]
- Meng, X.; Xing, T.; Wang, X. The role of light in the regulation of anthocyanin accumulation in Gerbera hybrida. Plant Growth Regul. 2004, 44, 243. [Google Scholar] [CrossRef]
- Ahmadi, T.; Shabani, L.; Sabzalian, M.R. LED light mediates phenolic accumulation and enhances antioxidant activity in Melissa officinalis L. under drought stress condition. Protoplasma 2020, 257, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Klimek-Szczykutowicz, M.; Prokopiuk, B.; Dziurka, K.; Pawłowska, B.; Ekiert, H.; Szopa, A. The influence of different wavelengths of LED light on the production of glucosinolates and phenolic compounds and the antioxidant potential in in vitro cultures of Nasturtium officinale (watercress). Plant Cell Tissue Organ Cult. 2022, 149, 113–122. [Google Scholar] [CrossRef]
- Lü, J.-M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef]
- Kim, K.; Kook, H.-S.; Jang, Y.-J.; Lee, W.-H.; Kamala-Kannan, S.; Chae, J.-C.; Lee, K.-J. The Effect of Blue-light-emitting Diodes on Antioxidant Properties and Resistance to Botrytis cinerea in Tomato. J. Plant Pathol. Microbiol. 2013, 4, 203. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-C.; Kim, J.-H.; Jeong, S.-M.; Kim, D.-R.; Ha, J.-U.; Nam, K.C.; Ahn, D.U. Effect of Far-Infrared Radiation on the Antioxidant Activity of Rice Hulls. J. Agric. Food Chem. 2003, 51, 4400–4403. [Google Scholar] [CrossRef]
- Alrifai, O.; Hao, X.; Marcone, M.F.; Tsao, R. Current Review of the Modulatory Effects of LED Lights on Photosynthesis of Secondary Metabolites and Future Perspectives of Microgreen Vegetables. J. Agric. Food Chem. 2019, 67, 6075–6090. [Google Scholar] [CrossRef]
- Borra, S.K.; Gurumurthy, P.; Mahendra, J.; Jayamathi, K.M.; Cherian, C.N.; Chand, R. Antioxidant and free radical scavenging activity of curcumin determined by using different in vitro and ex vivo models. J. Med. Plant Res. 2013, 7, 2680–2690. [Google Scholar] [CrossRef]
- Kim, G.-H.; Duan, Y.; Kim, H.-S. Effects of Various Extracts from Turmeric (Curcuma longa L.) on Antioxidant Activity. J. Korean Oil Chem. Soc. 2016, 33, 521–528. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Zha, J.; Guleria, S.; Koffas, M.A.G. Recent Advances in the Recombinant Biosynthesis of Polyphenols. Front. Microbiol. 2017, 8, 2259. [Google Scholar] [CrossRef]
- Sharangi, A.; Gowda, M.P.; Das, S. Responses of turmeric to light intensities and nutrients in a forest ecosystem: Retrospective insight. Trees For. People 2022, 7, 100208. [Google Scholar] [CrossRef]
- Ricco, R.A.; Agudelo, I.J.; Wagner, M.L. Métodos Empleados En El Análisis de Los Polifenoles En Un Laboratorio de Baja Complejidad. Lilloa 2015, 52, 161–174. [Google Scholar]
- Liu, H.; Song, Y.; Zhang, X. Determination of total flavonoids in leek by alcl3 colorimetric assay. Chem. Eng. Trans. 2017, 59, 775–780. [Google Scholar] [CrossRef]
- Gusakov, A.V.; Kondratyeva, E.G.; Sinitsyn, A.P. Comparison of Two Methods for Assaying Reducing Sugars in the Determination of Carbohydrase Activities. Int. J. Anal. Chem. 2011, 2011, 283658. [Google Scholar] [CrossRef] [Green Version]
- Hazra, K.; Kumar, R.; Kumar Sarkar, B.; Chowdary, Y.A.; Devgan, M.; Ramaiah, M.; Educational, R.P. Uv-Visible Spectrophotometric Estimation of Curcumin in Nano-Formulation. Int. J. Pharma. Cognosy 2015, 2, 127–130. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovés, A.C.; Bartolomé, B. Extending Applicability of the Oxygen Radical Absorbance Capacity (ORAC−Fluorescein) Assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]



| Parameter | Spectral Fraction of Light Source (%) | |
|---|---|---|
| White | RB | |
| Photon flux (380–780 nm) | 100 a | 100 a |
| Ultraviolet light (380–399 nm) | 0.1 | 0.1 |
| Blue light (400–499 nm) | 32.3 | 30.9 |
| Green light (500–599 nm) | 40.7 | 0.5 |
| Red light (600–700 nm) | 26.2 | 68.4 |
| Far-red light (701–780 nm) | 0.7 | 0.1 |
| DLI (mol m−2) | 0.86 | 3.80 |
| R:B ratio b | 0.63 | 1.74 |
| R:FR ratio c | 8.41 | 113.20 |
| Light source | White LED light | LED with red and blue chips |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchant, M.J.; Molina, P.; Montecinos, M.; Guzmán, L.; Balada, C.; Castro, M. Effects of LED Light Spectra on the Development, Phytochemical Profile, and Antioxidant Activity of Curcuma longa from Easter Island. Plants 2022, 11, 2701. https://doi.org/10.3390/plants11202701
Marchant MJ, Molina P, Montecinos M, Guzmán L, Balada C, Castro M. Effects of LED Light Spectra on the Development, Phytochemical Profile, and Antioxidant Activity of Curcuma longa from Easter Island. Plants. 2022; 11(20):2701. https://doi.org/10.3390/plants11202701
Chicago/Turabian StyleMarchant, María José, Paula Molina, Miriam Montecinos, Leda Guzmán, Cristóbal Balada, and Mónica Castro. 2022. "Effects of LED Light Spectra on the Development, Phytochemical Profile, and Antioxidant Activity of Curcuma longa from Easter Island" Plants 11, no. 20: 2701. https://doi.org/10.3390/plants11202701





