Cachrys spp. from Southern Italy: Phytochemical Characterization and JAK/STAT Signaling Pathway Inhibition
Abstract
:1. Introduction
2. Results and Discussion
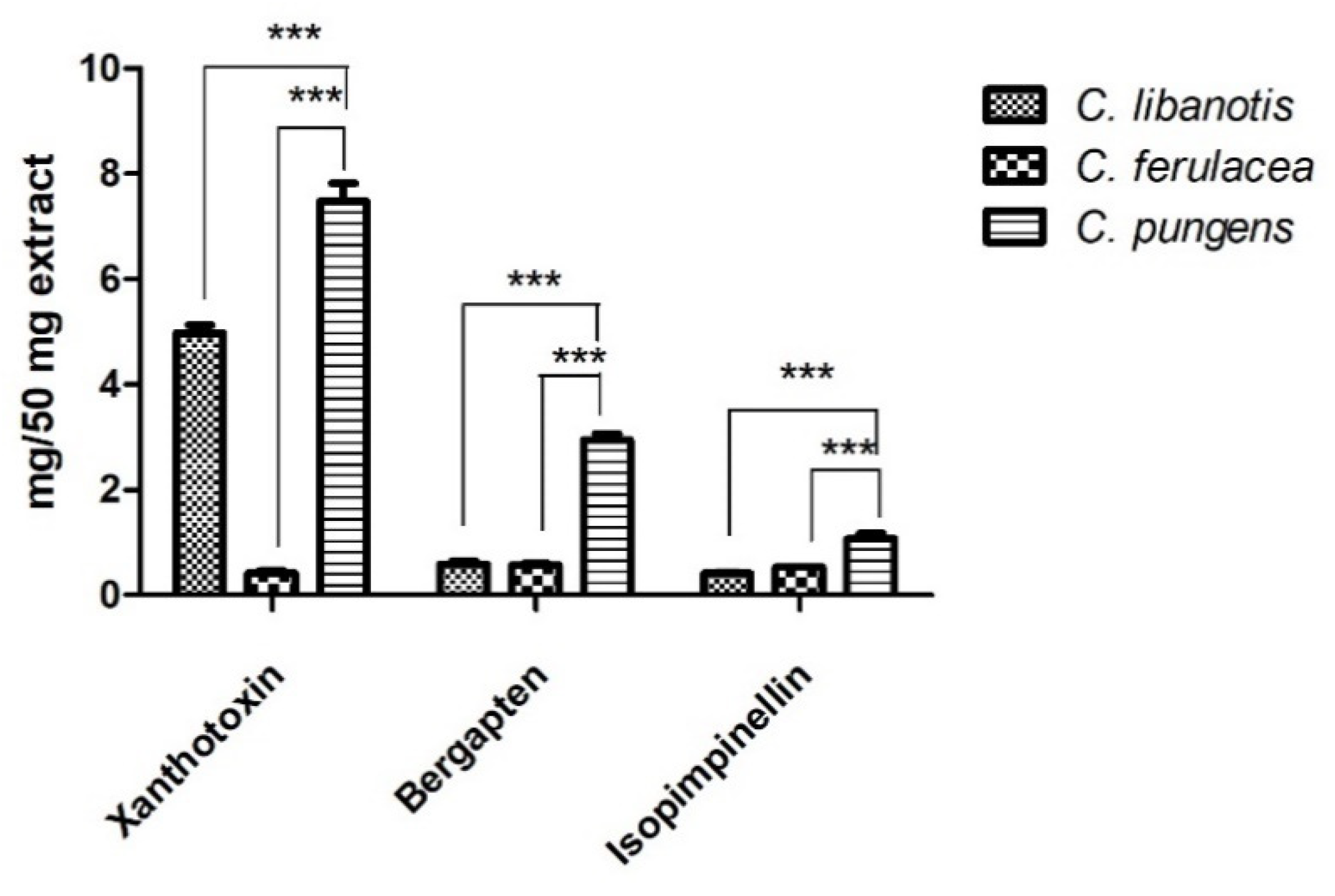
2.1. Phytochemical Profile
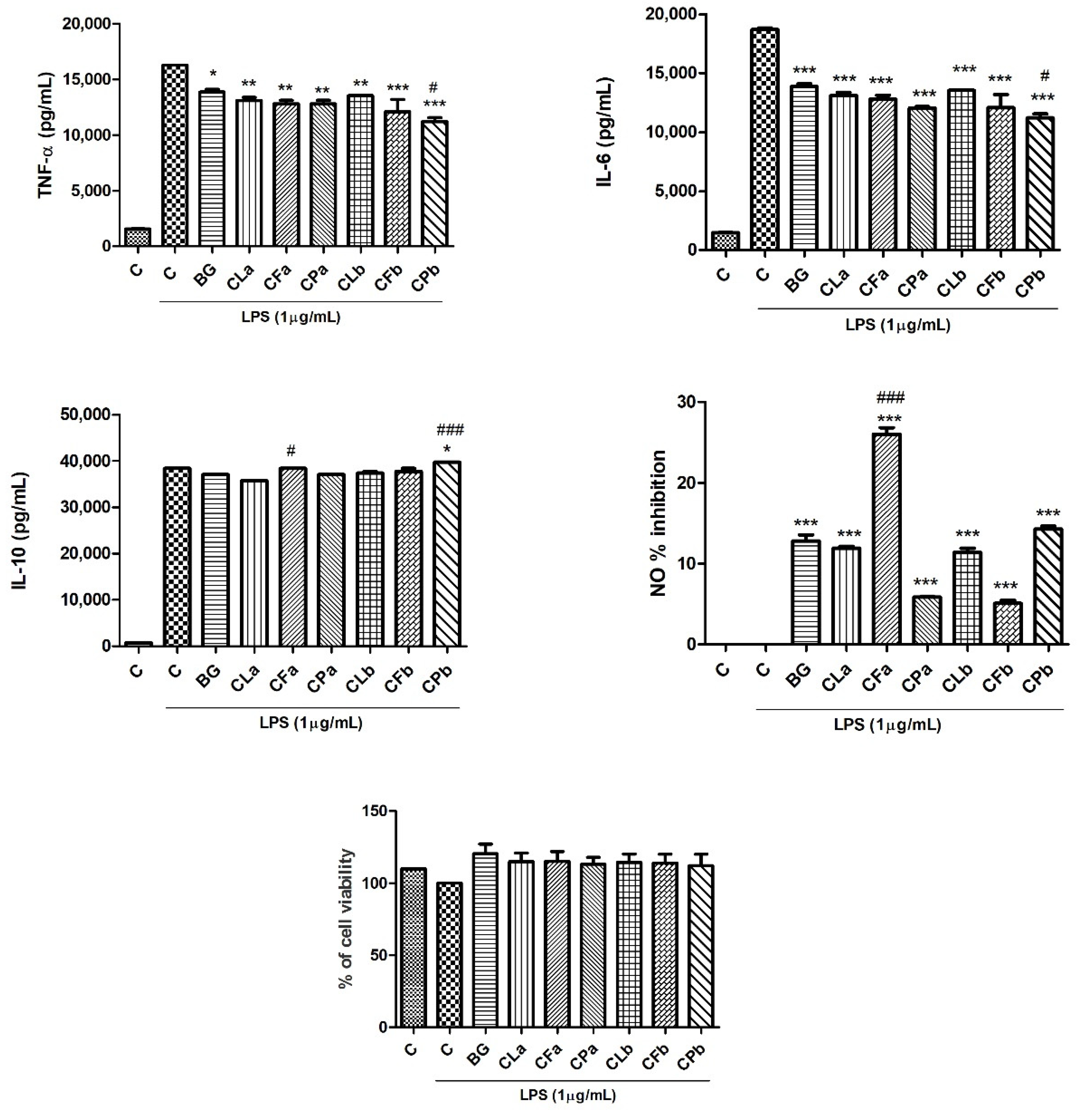
2.2. Phytochemical Profile Effects of Bergapten and Cachrys Extracts on LPS-Induced Production of Pro-Inflammatory, Anti-Inflammatory Cytokines and Mediators (NO) in RAW 264.7 Cells
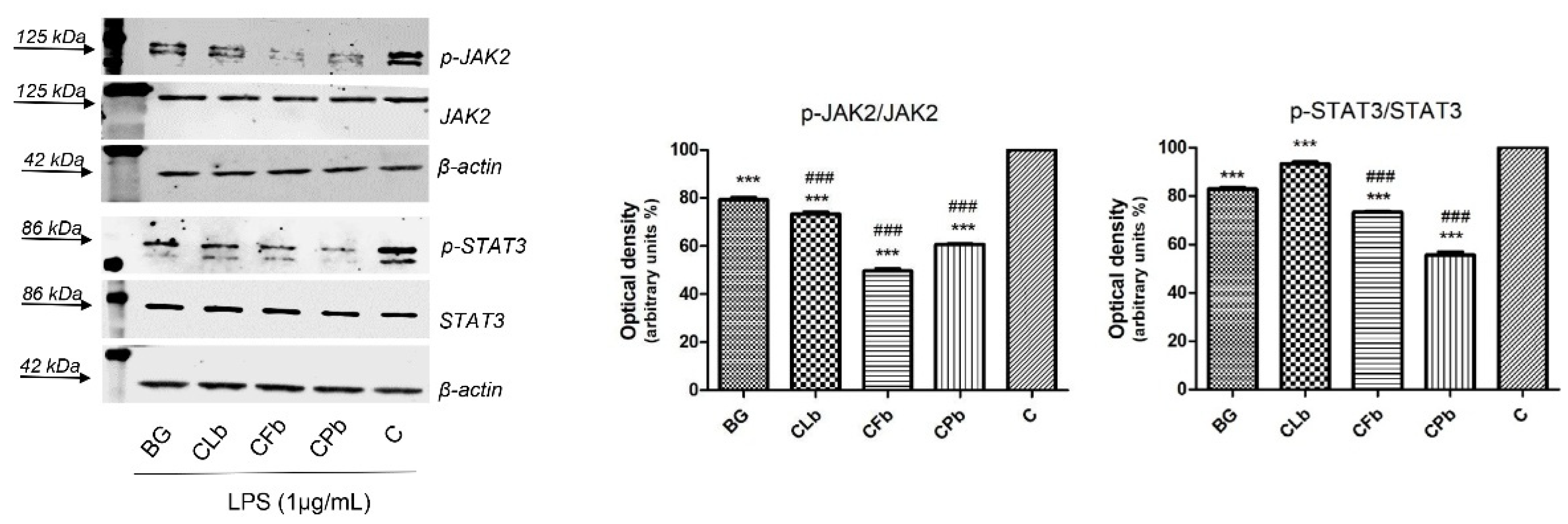
2.3. Effects of Bergapten and Cachrys Extracts on LPS-Induced JAK/STAT Signaling Pathway Activation
3. Materials and Methods
3.1. Reagents
3.2. Plant Material and Extraction Procedure
3.3. Determination of Total Phenolic and Flavonoid Content
3.4. Qualitative and Quantitative Analyses Using Gas Chromatography—Mass Spectrometry (GC-MS)
3.5. Cell Cultures
3.6. Cell Viability Assay (SRB)
3.7. Western Blot Analysis
3.8. Cytokine Measurements
3.9. Nitrite Analysis
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Les, F.; Cásedas, G.; López, V. Bioactivity of medicinal plants and extracts. Biology 2021, 10, 634. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.; Rana, C.S. Plant secondary metabolites: A review. IJERGS 2015, 3, 661–670. [Google Scholar]
- Ryabushkina, N.A. Synergism of metabolite action in plant responses to stresses. Russ. J. Plant Physiol. 2005, 52, 547–552. [Google Scholar] [CrossRef]
- Morris, R.; Kershaw, N.J.; Babon, J.J. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018, 27, 1984–2009. [Google Scholar] [CrossRef] [Green Version]
- Zobel, A.M.; Wang, J.; March, R.E.; Brown, S.A. Identification of eight coumarins occurring with psoralen, xanthotoxin, and bergapten on leaf surfaces. J. Chem. Ecol. 1991, 17, 1859–1870. [Google Scholar] [CrossRef]
- Quetglas-Llabrés, M.M.; Quispe, C.; Herrera-Bravo, J.; Catarino, M.D.; Pereira, O.R.; Cardoso, S.M.; Dua, K.; Chellappan, D.K.; Pabreja, K.; Satija, S.; et al. Pharmacological properties of bergapten: Mechanistic and therapeutic aspects. Oxid. Med. Cell Longev. 2022, 2022, 8615242. [Google Scholar] [CrossRef]
- Liang, Y.; Xie, L.; Liu, K.; Cao, Y.; Dai, X.; Wang, X.; Lu, J.; Zhang, X.; Li, X. Bergapten: A review of its pharmacology, pharmacokinetics, and toxicity. Phytother. Res. 2021, 35, 6131–6147. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Yang, W.; Qi, X.; Lan, L.; Luo, L.; Yin, Z. Bergapten prevents lipopolysaccharide-induced inflammation in RAW264. 7 cells through suppressing JAK/STAT activation and ROS production and increases the survival rate of mice after LPS challenge. Int. Immunopharmacol. 2017, 48, 159–168. [Google Scholar] [CrossRef]
- Denaro, M.; Smeriglio, A.; Xiao, J.; Cornara, L.; Burlando, B.; Trombetta, D. New insights into Citrus genus: From ancient fruits to new hybrids. Food Front. 2020, 1, 305–328. [Google Scholar] [CrossRef]
- Bruno, M.; Ilardi, V.; Lupidi, G.; Quassinti, L.; Bramucci, M.; Fiorini, D.; Venditti, A.; Maggi, F. The nonvolatile and volatile metabolites of Prangos ferulacea and their biological properties. Planta Med. 2019, 85, 815–824. [Google Scholar] [CrossRef] [Green Version]
- Marrelli, M.; Perri, M.R.; Amodeo, V.; Giordano, F.; Statti, G.A.; Panno, M.L.; Conforti, F. Assessment of photo-Induced cytotoxic activity of Cachrys sicula and Cachrys libanotis enriched-Coumarin extracts against human melanoma cells. Plants 2021, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Khan, H.; Aschner, M.; Mirzae, H.; Küpeli, A.E.; Capasso, R. Anticancer potential of furanocoumarins: Mechanistic and therapeutic aspects. Int. J. Mol. Sci. 2020, 21, 5622. [Google Scholar] [CrossRef] [PubMed]
- Martin, Y.C.; Kofron, J.L.; Traphagen, L.M. Do structurally similar molecules have similar biological activity? J. Med. Chem. 2002, 45, 4350–4358. [Google Scholar] [CrossRef] [PubMed]
- Willett, P. Similarity-Based virtual screening using 2D fingerprints. Drug Discov. Today 2006, 11, 1046–1053. [Google Scholar] [CrossRef] [Green Version]
- Naviglio, D. Naviglio’s principle and presentation of an innovative solid–Liquid extraction technology: Extractor Naviglio®. Anal. Lett. 2003, 36, 1647–1659. [Google Scholar] [CrossRef]
- Naviglio, D.; Pizzolongo, F.; Romano, R.; Ferrara, L.; Naviglio, B.; Santini, A. An innovative solid-Liquid extraction technology: Use of the Naviglio extractor® for the production of lemon liquor. Afr. J. Food Sci. 2007, 1, 42–50. [Google Scholar]
- Klepacka, J.; Gujska, E.; Michalak, J. Phenolic compounds as cultivar-and variety-Distinguishing factors in some plant products. Plant Foods Hum. Nutr. 2011, 66, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Eseberri, I.; Trepiana, J.; Léniz, A.; Gómez-García, I.; Carr-Ugarte, H.; González, M.; Portillo, M.P. Variability in the Beneficial Effects of Phenolic Compounds: A Review. Nutrients 2022, 14, 1925. [Google Scholar] [CrossRef]
- Boneza, M.M.; Niemeyer, E.D. Cultivar affects the phenolic composition and antioxidant properties of commercially available lemon balm (Melissa officinalis L.) varieties. Ind. Crops Prod. 2018, 112, 783–789. [Google Scholar] [CrossRef]
- Amin, I.; Norazaidah, Y.; Hainida, K.E. Antioxidant activity and phenolic content of raw and blanched Amaranthus species. Food Chem. 2006, 94, 47–52. [Google Scholar] [CrossRef]
- Jeshvaghani, Z.A.; Rahimmalek, M.; Talebi, M.; Goli, S.A.H. Comparison of total phenolic content and antioxidant activity in different Salvia species using three model systems. Ind. Crops Prod. 2015, 77, 409–414. [Google Scholar] [CrossRef]
- Nickavar, B.; Esbati, N. Evaluation of the antioxidant capacity and phenolic content of three Thymus species. J. Acupunct. Meridian Stud. 2012, 5, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Bazdar, M.; Sadeghi, H.; Hosseini, S. Evaluation of oil profiles, total phenols and phenolic compounds in Prangos ferulacea leaves and flowers and their effects on antioxidant activities. Biocatal. Agric. Biotechnol. 2018, 14, 418–423. [Google Scholar] [CrossRef]
- Marrelli, M.; Perri, M.R.; Amodeo, V.; Conforti, F.; Giordano, F.; Panno, M.L.; Statti, G. Cachrys ferulacea (L.) Calest. Extracts as Natural Photosensitizers: An In Vitro Photobiological Study. Biol. Life Sci. Forum 2021, 11, 17. [Google Scholar]
- Lee, S.B.; Lee, W.S.; Shin, J.S.; Jang, D.S.; Lee, K.T. Xanthotoxin suppresses LPS-induced expression of iNOS, COX-2, TNF-α, and IL-6 via AP-1, NF-κB, and JAK-STAT inactivation in RAW 264.7 macrophages. Int. Immunopharmacol. 2017, 49, 21–29. [Google Scholar] [CrossRef]
- Bose, S.K.; Dewanjee, S.; Sahu, R.; Dey, S.P. Effect of bergapten from Heracleum nepalense root on production of proinflammatory cytokines. Nat. Prod. Res. 2011, 25, 1444–1449. [Google Scholar] [CrossRef]
- Singh, G.; Kaur, A.; Kaur, J.; Bhatti, M.S.; Singh, P.; Bhatti, R. Bergapten inhibits chemically induced nociceptive behavior and inflammation in mice by decreasing the expression of spinal PARP, iNOS, COX-2 and inflammatory cytokines. Inflammopharmacology 2019, 27, 749–760. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, K.; Mei, W.; Wang, Y.; Yu, C.; Yu, B.; Deng, S.; Hu, J. Anti-Inflammatory and proresolution activities of bergapten isolated from the roots of Ficus hirta in an in vivo zebrafish model. Biochem. Biophys. Res. Commun. 2018, 496, 763–769. [Google Scholar] [CrossRef]
- Wang, C.C.; Lai, J.E.; Chen, L.G.; Yen, K.Y.; Yang, L.L. Inducible nitric oxide synthase inhibitors of Chinese herbs. Part 2: Naturally occurring furanocoumarins. Bioorg. Med. Chem. 2000, 8, 2701–2707. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, J.H.; Sethi, G.; Kim, C.; Baek, S.H.; Nam, D.; Chung, W.S.; Kim, S.H.; Shim, B.S.; Ahn, K.S. Bergamottin, a natural furanocoumarin obtained from grapefruit juice induces chemosensitization and apoptosis through the inhibition of STAT3 signaling pathway in tumor cells. Cancer Lett. 2014, 354, 153–163. [Google Scholar] [CrossRef]
- Sansone, P.; Bromberg, J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J. Clin. Oncol. 2012, 30, 1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.Z.; Chen, J.H.; Tsai, C.F.; Yeh, W.L. Anti-Inflammatory property of imperatorin on alveolar macrophages and inflammatory lung injury. J. Nat. Prod. 2019, 82, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-Phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Rababah, T.M.; Hettiarachchy, N.S.; Horax, R. Total phenolics and antioxidant activities of fenugreek, green tea, black tea, grape seed, ginger, rosemary, gotu kola, and ginkgo extracts, vitamin E, and tert-butylhydroquinone. J. Agric. Food Chem. 2004, 52, 5183–5186. [Google Scholar] [CrossRef] [PubMed]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Mazzei, R.; Piacentini, E.; Nardi, M.; Poerio, T.; Bazzarelli, F.; Procopio, A.; Di Gioia, M.L.; Rizza, P.; Ceraldi, R.; Morelli, C.; et al. Production of plant-derived oleuropein aglycone by a combined membrane process and evaluation of its breast anticancer properties. Front. Bioeng. Biotechnol. 2020, 8, 908. [Google Scholar] [CrossRef]
- Rizza, P.; Barone, I.; Zito, D.; Giordano, F.; Lanzino, M.; De Amicis, F.; Mauro, L.; Sisci, D.; Catalano, S.; Wright, K.D.; et al. Estrogen receptor beta as a novel target of androgen receptor action in breast cancer cell lines. Breast Cancer Res. 2014, 16, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Marrelli, M.; De Marco, C.T.; Statti, G.; Neag, T.A.; Toma, C.C.; Conforti, F. Ranunculus species suppress nitric oxide production in LPS-Stimulated RAW 264.7 macrophages. Nat. Prod. Res. 2022, 36, 2859–2863. [Google Scholar] [CrossRef]
- Bose, S.; Banerjee, S.; Mondal, A.; Chakraborty, U.; Pumarol, J.; Croley, C.R.; Bishayee, A. Targeting the JAK/STAT signaling pathway using phytocompounds for cancer prevention and therapy. Cells 2020, 9, 1451. [Google Scholar] [CrossRef]
- Abad, M.J.; De las Heras, B.; Silvan, A.M.; Pascual, R.; Bermejo, P.; Rodriguez, B.; Villar, A.M. Effects of furocoumarins from Cachrys trifida on some macrophage functions. J. Pharm. Pharmacol. 2001, 53, 1163–1168. [Google Scholar] [CrossRef]
- Grande, M.; Aguado, M.T.; Mancheño, B.; Piera, F. Coumarins and ferulol esters from Cachrys sicula. Phytochemistry 1986, 25, 505–507. [Google Scholar] [CrossRef]
- Pistelli, L.; Catalano, S.; Manunta, A.; Marsili, A. Coumarins from Cachrys ferulacea collected in Sardinia. Planta Med. 1989, 55, 203. [Google Scholar] [CrossRef]
- Tahar, S.; Hamdi, B.; Flamini, G.; Mehmet, Ö.; Duru, M.E.; Bruno, M.; Maggi, F. Chemical composition, antioxidant and anticholinesterase activity of the essential oil of algerian Cachrys sicula L. Nat. Prod. Res. 2022, 36, 4094–4102. [Google Scholar] [CrossRef] [PubMed]


| Species | TP 1 | TF 2 | Yield (%) |
|---|---|---|---|
| C. libanotis L. 3 | 12.80 ± 0.10 a | 0.09 ± 0.01 c | 12.6 |
| C. ferulacea (L.) Calest. | 4.14 ± 0.24 c | 0.18 ± 0.02 b | 3.6 |
| C. pungens Jan | 8.64 ± 0.42 b | 0.32 ± 0.01 a | 7.4 |
| Species | Xanthotoxin | Bergapten | Isopimpinellin |
|---|---|---|---|
| C. libanotis L. 1 | 4.98 ± 0.21 | 0.59 ± 0.08 | 0.42 ± 0.03 |
| C. ferulacea (L.) Calest. | 0.42 ± 0.05 | 0.57 ± 0.04 | 0.050 ± 0.001 |
| C. pungens Jan | 7.48 ± 0.48 | 2.94 ± 0.16 | 1.07 ± 0.14 |
| Sample | Extract Formulation | Abbreviation |
|---|---|---|
| C. libanotis L. | a | CLa |
| b | CLb | |
| C. ferulacea (L.) Calest. | a | CFa |
| b | CFb | |
| C. pungens Jan | a | CPa |
| b | CPb |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perri, M.R.; Pellegrino, M.; Aquaro, S.; Cavaliere, F.; Lupia, C.; Uzunov, D.; Marrelli, M.; Conforti, F.; Statti, G. Cachrys spp. from Southern Italy: Phytochemical Characterization and JAK/STAT Signaling Pathway Inhibition. Plants 2022, 11, 2913. https://doi.org/10.3390/plants11212913
Perri MR, Pellegrino M, Aquaro S, Cavaliere F, Lupia C, Uzunov D, Marrelli M, Conforti F, Statti G. Cachrys spp. from Southern Italy: Phytochemical Characterization and JAK/STAT Signaling Pathway Inhibition. Plants. 2022; 11(21):2913. https://doi.org/10.3390/plants11212913
Chicago/Turabian StylePerri, Maria Rosaria, Michele Pellegrino, Stefano Aquaro, Fabiola Cavaliere, Carmine Lupia, Dimitar Uzunov, Mariangela Marrelli, Filomena Conforti, and Giancarlo Statti. 2022. "Cachrys spp. from Southern Italy: Phytochemical Characterization and JAK/STAT Signaling Pathway Inhibition" Plants 11, no. 21: 2913. https://doi.org/10.3390/plants11212913






