The Recruitment of the Recalcitrant-Seeded Cryptocarya alba (Mol.) Looser, Established via Direct Seeding Is Mainly Affected by the Seed Source and Forest Cover
Abstract
1. Introduction
2. Results
2.1. Seed Assessment in the Laboratory
2.2. Field Experiment
3. Discussion
4. Materials and Methods
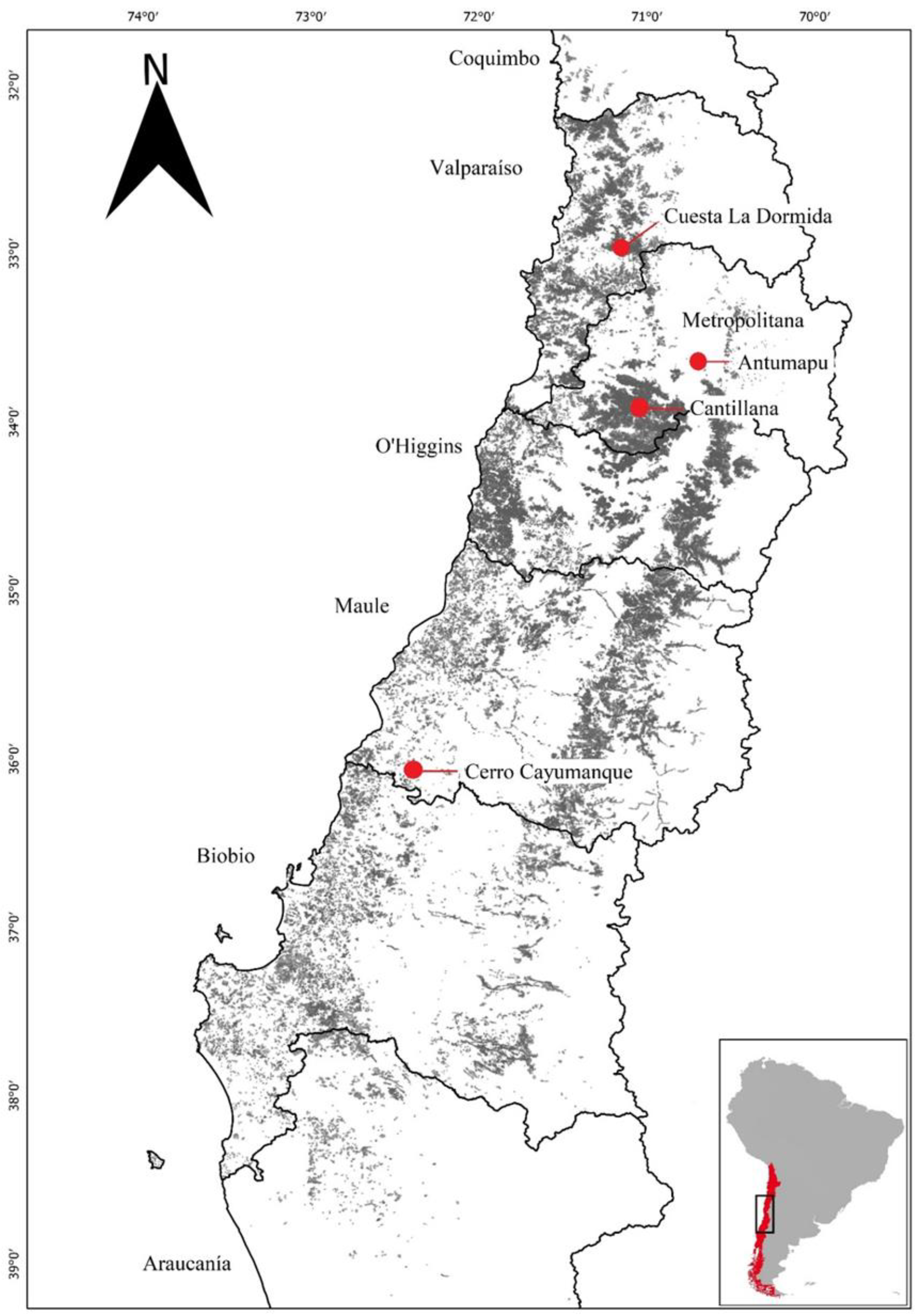
4.1. Seed Collection
4.2. Field Experiment
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peñuelas, J.; Sardans, J.; Filella, I.; Estiarte, M.; Llusià, J.; Ogaya, R.; Carnicer, J.; Bartrons, M.; Rivas-Ubach, A.; Grau, O.; et al. Impacts of Global Change on Mediterranean Forests and Their Services. Forests 2017, 8, 463. [Google Scholar] [CrossRef]
- Berjak, P.; Pammenter, N.W. Recalcitrant seeds. In Handbook of Seed Physiology: Applications to Agriculture; Haworth Press: New York, NY, USA, 2004; pp. 305–345. [Google Scholar]
- Pammenter, N.W.; Berjak, P. A review of recalcitrant seed physiology in relation to desiccation-tolerance mechanisms. Seed Sci. Res. 1999, 9, 13–37. [Google Scholar] [CrossRef]
- Berjak, P.; Pammenter, N.W. From Avicennia to Zizania: Seed recalcitrance in perspective. Ann. Bot. 2008, 101, 213–228. [Google Scholar] [CrossRef]
- Bannister, J.R.; Vargas-Gaete, R.; Ovalle, J.F.; Acevedo, M.; Fuentes-Ramirez, A.; Donoso, P.J.; Promis, A.; Smith-Ramírez, C. Major bottlenecks for the restoration of natural forests in Chile. Restor. Ecol. 2018, 26, 1039–1044. [Google Scholar] [CrossRef]
- Doust, S.J.; Erskine, P.D.; Lamb, D. Direct seeding to restore rainforest species: Microsite effects on the early establishment and growth of rainforest tree seedlings on degraded land in the wet tropics of Australia. For. Ecol. Manag. 2006, 234, 333–343. [Google Scholar] [CrossRef]
- Cole, R.J.; Holl, K.D.; Keene, C.L.; Zahawi, R.A. Direct seeding of late-successional trees to restore tropical montane forest. For. Ecol. Manag. 2011, 261, 1590–1597. [Google Scholar] [CrossRef]
- Sánchez-Gómez, D.; Valladares, F.; Zavala, M.A. Performance of seedlings of Mediterranean woody species under experimental gradients of irradiance and water availability: Trade-offs and evidence for niche differentiation. New Phytol. 2006, 170, 795–806. [Google Scholar] [CrossRef]
- Zavala, M.A.; Espelta, J.M.; Caspersen, J.; Retana, J. Interspecific differences in sapling performance with respect to light and aridity gradients in Mediterranean pine–oak forests: Implications for species coexistence. Can. J. For. Res. 2011, 41, 1432–1444. [Google Scholar] [CrossRef]
- Bischoff, A.; Vonlanthen, B.; Steinger, T.; Müller-Schärer, H. Seed provenance matters—Effects on germination of four plant species used for ecological restoration. Basic Appl. Ecol. 2006, 7, 347–359. [Google Scholar] [CrossRef]
- Legras, E.C. The role of germination microsite in the establishment of sugar pine and Jeffrey pine seedlings. For. Ecol. Manag. 2010, 260, 806–813. [Google Scholar] [CrossRef]
- Marañon, T.; Zamora, R.; Villar, R.; Zavala, M.; Quero, J.L.; Pérez-Ramos, I.M.; Mendoza, I.; Castro, J. Regeneration of tree species and restoration under constrasted Mediterranean habitats: Field and glasshouse experiments. Int. J. Ecol. Environ. Sci. 2004, 30, 187–196. [Google Scholar]
- Eriksson, O.; Ehrlén, J. Seedling recruitment and population ecology. In Seedling Ecology and Evolution; Cambridge University Press: New York, NY, USA, 2008; pp. 239–254. [Google Scholar]
- Jennings, S.; Brown, N.; Sheil, D. Assessing forest canopies and understorey illumination: Canopy closure, canopy cover and other measures. Forestry 1999, 72, 59–74. [Google Scholar] [CrossRef]
- Gavinet, J.; Prévosto, B.; Fernandez, C. Do shrubs facilitate oak seedling establishment in Mediterranean pine forest understory? For. Ecol. Manag. 2016, 381, 289–296. [Google Scholar] [CrossRef]
- Gómez-Aparicio, L.; Pérez-Ramos, I.M.; Mendoza, I.; Matías, L.; Quero, J.L.; Castro, J.; Zamora, R.; Marañón, T. Oak seedling survival and growth along resource gradients in Mediterranean forests: Implications for regeneration in current and future environmental scenarios. Oikos 2008, 117, 1683–1699. [Google Scholar] [CrossRef]
- Holmgren, M.; Scheffer, M. Strong facilitation in mild environments: The stress gradient hypothesis revisited. J. Ecol. 2010, 98, 1269–1275. [Google Scholar] [CrossRef]
- Mucina, L.; Bustamante-Sánchez, M.A.; Pedra, B.D.; Holmes, P.; Keeler-Wolf, T.; Armesto, J.; Vilagrosa, A. Ecological restoration in mediterranean-type shrublands and woodlands. In Routledge Handbook of Ecological and Environmental Restoration; Routledge: London, UK, 2017; pp. 173–196. [Google Scholar]
- Mendoza, I.; Gómez-Aparicio, L.; Zamora, R.; Matías, L. Recruitment limitation of forest communities in a degraded Mediterranean landscape. J. Veg. Sci. 2009, 20, 367–376. [Google Scholar] [CrossRef]
- Rodríguez-García, E.; Bravo, F.; Spies, T.A. Effects of overstorey canopy, plant–plant interactions and soil properties on Mediterranean maritime pine seedling dynamics. For. Ecol. Manag. 2011, 262, 244–251. [Google Scholar] [CrossRef]
- Castro, J.; Zamora, R.; Hódar, J.A.; Gómez, J.M. Use of Shrubs as Nurse Plants: A New Technique for Reforestation in Mediterranean Mountains. Restor. Ecol. 2002, 10, 297–305. [Google Scholar] [CrossRef]
- Becerra, P.I.; Montenegro, G. The widely invasive tree Pinus radiata facilitates regeneration of native woody species in a semi-arid ecosystem. Appl. Veg. Sci. 2013, 16, 173–183. [Google Scholar] [CrossRef]
- Fenner, M.; Thompson, K. The Ecology of Seeds; Cambridge University Press: Cambridge, UK, 2005; 250 p. [Google Scholar]
- Pérez-Ramos, I.M.; Marañón, T. Factors affecting post-dispersal seed predation in two coexisting oak species: Microhabitat, burial and exclusion of large herbivores. For. Ecol. Manag. 2008, 255, 3506–3514. [Google Scholar] [CrossRef]
- Kremer, K.N.; Promis, Á.A.; Mancilla, G.; Magni, C.R. Leaf litter and irrigation can increase seed germination and early seedling survival of the recalcitrant-seeded tree Beilschmiedia miersii. Austral. Ecol. 2019, 44, 86–94. [Google Scholar] [CrossRef]
- Vander Mijnsbrugge, K.; Bischoff, A.; Smith, B. A question of origin: Where and how to collect seed for ecological restoration. Basic Appl. Ecol. 2010, 11, 300–311. [Google Scholar] [CrossRef]
- Bischoff, A.; Steinger, T.; Müller-Schärer, H. The Importance of Plant Provenance and Genotypic Diversity of Seed Material Used for Ecological Restoration. Restor. Ecol. 2010, 18, 338–348. [Google Scholar] [CrossRef]
- Lucas-Borja, M.E.; Candel-Pérez, D.; Tíscar, P.A.; Prévosto, B.; Hedo, J. Pinus nigra Arn. ssp salzmannii early recruitment and initial seedling growth in warmer and drier locations: The role of seed and soil provenance. Plant Ecol. 2017, 218, 761–772. [Google Scholar] [CrossRef]
- McKay, J.K.; Christian, C.E.; Harrison, S.; Rice, K.J. “How Local Is Local?”—A Review of Practical and Conceptual Issues in the Genetics of Restoration. Restor. Ecol. 2005, 13, 432–440. [Google Scholar] [CrossRef]
- Mao, P.; Han, G.; Wang, G.; Yu, J.; Shao, H. Effects of age and stand density of mother trees on early Pinus thunbergii seedling establishment in the coastal zone, China. Sci. World J. 2014, 2014, 468036. [Google Scholar] [CrossRef]
- Fort, T.; Pauvert, C. Maternal effects shape the seed mycobiome in Quercus petraea. New Phytol. 2021, 230, 1594–1608. [Google Scholar] [CrossRef]
- Chacón, P.; Bustamante, R.O. The effects of seed size and pericarp on seedling recruitment and biomass in Cryptocarya alba (Lauraceae) under two contrasting moisture regimes. Plant Ecol. 2004, 152, 137–144. [Google Scholar] [CrossRef]
- Slot, M.; Palow, D.T.; Kitajima, K. Seed reserve dependency of Leucaena leucocephala seedling growth for nitrogen and phosphorus. Funct. Plant Biol. 2013, 40, 244–250. [Google Scholar] [CrossRef]
- Rodríguez, R.; Matthei, O.; Quezada, M. Flora arbórea de Chile; Editorial de la Universidad de Concepción: Concepción, Chile, 1983. [Google Scholar]
- Cabello, A.; Donoso, C. Cryptocarya alba (Mol.) Looser. Peumo Familia: Lauraceae. In Las especies arbóreas de los bosques templados de Chile y Argentina; Autoecología, Marisa Cuneo Ediciones: Valdivia, Chile, 2013; pp. 206–212. [Google Scholar]
- Espinoza, S.; Yáñez, M.; Magni, R.; Martínez, E.; Ovalle, J.; Vaswani, S. Growth of provenances of Criptocarya alba during water stress and re-watering in the nursery. Sci. Agric. 2021, 78, e20200292. [Google Scholar]
- Espinoza, S.E.; Yañez, M.A.; Martínez, E.E.; Carrasco-Benavides, M.; Vaswani, S.; Gajardo, J.; Magni, C. Influence of provenance origin on the early performance of two sclerophyllous Mediterranean species established in burned drylands. Sci. Rep. 2021, 11, 6212. [Google Scholar] [CrossRef] [PubMed]
- Joët, T.; Ourcival, J.M.; Capelli, M.; Dussert, S.; Morin, X. Explanatory ecological factors for the persistence of desiccation-sensitive seeds in transient soil seed banks: Quercus ilex as a case study. Ann. Bot. 2016, 117, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.; Razmilic, I.; San Martín, J.; Doll, U.; González, B. Plantas medicinales chilenas: Experiencia de domesticación y cultivo de boldo, matico, bailahuén, canelo, peumo y maqui; Editorial Universidad de Talca: Talca, Chile, 2005; 178 p. [Google Scholar]
- Vita, A. Reforestación por siembra directa con quillay (Quillaja saponaria Mol.) y peumo (Cryptocarya alba (Mol.) Looser); Universidad de Chile: Santiago, Chile, 1966; 86 p. [Google Scholar]
- Jiménez, H.E.; Armesto, J.J. Importance of the Soil Seed Bank of Disturbed Sites in Chilean Matorral in Early Secondary Succession. J. Veg. Sci. 1992, 3, 579–586. [Google Scholar] [CrossRef]
- Baskin, C.; Baskin, J. Seeds: Ecology, Biogeography, and, Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 2014; 1586 p. [Google Scholar]
- Garreaud, R.D.; Alvarez-Garreton, C.; Barichivich, J.; Boisier, J.P.; Christie, D.; Galleguillos, M.; LeQuesne, C.; McPhee, J.; Zambrano-Bigiarini, M. The 2010–2015 megadrought in central Chile: Impacts on regional hydroclimate and vegetation. Hydrol. Earth Syst. Sci. 2017, 21, 6307–6327. [Google Scholar] [CrossRef]
- Rodger, J.; Bennett, J.; Razanajatovo, M.; Knight, T.; van Kleunen, M.; Ashman, T.; Steets, J.; Hui, C.; Arceo-Gómez, G.; Burd, M.; et al. Widespread vulnerability of flowering plants seed production to pollinator declines. Sci. Adv. 2021, 7, eabd3524. [Google Scholar] [CrossRef]
- Vieli, L.; Murúa, M.; Flores-Prado, L.; Carvallo, G.; Valdivia, C.; Muschett, G.; López-Aliste, M.; Andía, C.; Jofré-Pérez, C.; Fortúrbel, F. Local actions to tackle a global problem: A multidimientional assessment of the pollination crisis in Chile. Diversity 2021, 13, 571. [Google Scholar] [CrossRef]
- Baskin, J.; Baskin, C. Pollen limitation and its effect on seed germination. Seed Sci. Res. 2018, 28, 253–260. [Google Scholar] [CrossRef]
- Alvarez, C.; Acevedo, M.; González, M.; Cartes, E.; Bannister, J. Detección de albinismo en la regeneración de Persea lingue y Cryptocarya alba procedentes del cerro Cayumanque, región del Biobio. Gayana Botánica 2017, 74, 296–298. [Google Scholar] [CrossRef][Green Version]
- Figueroa, P. Germinación de semillas de Cryptocarya alba (Mol.)Looser y Persea lingue Ness bajo distintas condiciones de temperatura; Universidad de Concepción: Concepción, Chile, 1999; 53 p. [Google Scholar]
- Urbieta, I.R.; Pére-Ramos, I.M.; Zavala, M.A.; Marañón, T.; Kobe, R.K. Soil water content and emergence time control seedling establishment in three co-occurring Mediterranean oak species. Can. J. For. Res. 2008, 38, 2382–2393. [Google Scholar] [CrossRef]
- Elliott, S.; Navakitbumrung, P.; Kuarak, C.; Zangkum, S.; Anusarnsunthorn, V.; Blakesley, D. Selecting framework tree species for restoring seasonally dry tropical forests in northern Thailand based on field performance. For. Ecol. Manag. 2003, 184, 177–191. [Google Scholar] [CrossRef]
- Caldeira, M.C.; Ibáñez, I.; Nogueira, C.; Bugalho, M.N.; Lecomte, X.; Moreira, A.; Pereira, J.S. Direct and indirect effects of tree canopy facilitation in the recruitment of Mediterranean oaks. J. Appl. Ecol. 2014, 51, 349–358. [Google Scholar] [CrossRef]
- Batlla, D.; Nicoletta, M.; Benech-Arnold, R. Sensitivity of Polygonum aviculare seeds to light as affected by soil moisture conditions. Ann. Bot. 2007, 99, 915–924. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bassett, I.E.; Simcock, R.C.; Mitchell, N.D. Consequences of soil compaction for seedling establishment: Implications for natural regeneration and restoration. Austral Ecol. 2005, 30, 827–833. [Google Scholar] [CrossRef]
- Busse, M.D.; Fiddler, G.O.; Shestak, C.J. Conifer Root Proliferation after 20 Years of Soil Compaction. For. Sci. 2016, 63, 147–150. [Google Scholar] [CrossRef]
- Skinner, A.K.; Lunt, I.D.; Spooner, P.; McIntyre, S.U.E. The effect of soil compaction on germination and early growth of Eucalyptus albensand an exotic annual grass. Austral Ecol. 2009, 34, 698–704. [Google Scholar] [CrossRef]
- Fuentes-Castillo, T.; Miranda, A.; Rivera-Hutinel, A.; Smith-Ramírez, C.; Holmgren, M. Nucleated regeneration of semiarid sclerophyllous forests close to remnant vegetation. For. Ecol. Manag. 2012, 274, 38–47. [Google Scholar] [CrossRef]
- Pardos, M.; Calama, R. Adaptive Strategies of Seedlings of Four Mediterranean Co-Occurring Tree Species in Response to Light and Moderate Drought: A Nursery Approach. Forests 2022, 13, 154. [Google Scholar] [CrossRef]
- Humara, J.M.; López, M.; Casares, A.; Majada, J. Temperature and provenance as two factors affecting Eucalyptus nitens seed germination. Forestry 2000, 73, 87–90. [Google Scholar] [CrossRef]
- Rix, K.D.; Gracie, A.J.; Potts, B.M.; Brown, P.H.; Gore, P.L. Genetic control of Eucalyptus globulus seed germination. Ann. For. Sci. 2015, 72, 457–467. [Google Scholar] [CrossRef]
- Aigbe, H.; Fredrick, C.; Omukhua, G. Effect of seed source on germination and early seedling growth of Heinsia crinita (Afzel.) G. Taylor. Appl. Trop. Agric. 2016, 21, 180–185. [Google Scholar]
- Santelices Moya, R.; Espinoza Meza, S.; Magni Díaz, C.; Cabrera Ariza, A.; Donoso Calderón, S.; Peña-Rojas, K. Variability in seed germination and seedling growth at the intra- and inter-provenance levels of Nothofagus glauca (Lophozonia glauca), an endemic species of Central Chile. New Zealand J. For. Sci. 2017, 47, 10. [Google Scholar] [CrossRef]
- Donohue, K.; Dorn, L.; Griffith, C.; Kim, E.; Aguilera, A.; Polisetty, C.R.; Schmitt, J. Environmental and genetic influences on the germination of Arabidopsis thaliana in the field. Evolution 2005, 59, 740–757. [Google Scholar] [CrossRef] [PubMed]
- Veblen, T.; Read, J.; Hill, R. The Ecology and Biogeography of Nothofagus Forests; Yale University Press: New Haven, CT, USA, 1996; 414p. [Google Scholar]
- Santelices-Moya, R.E.; González Ortega, M.; Acevedo Tapia, M.; Cartes Rodríguez, E.; Cabrera-Ariza, A.M. Effect of Temperature on the germination of five coastal Provenances of Nothofagus glauca (Phil.) Krasser, the Most Representative Species of the Mediterranean Forests of South America. Plants 2022, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Magni, C.; Espinoza, S.; Poch, P.; Abarca, B.; Grez, I.; Martínez, E.; Yáñez, M.; Santelices, R.; Cabrera, A. Growth and biomass partitioning of nine provenances of Quillaja saponaria seedlings to water stress. South. For. 2018, 81, 103–109. [Google Scholar] [CrossRef]
- Yáñez, M.A.; Gonzalez, B.; Espinoza, S.E.; Vogel, H.; Doll, U. Phenotypic variation of fruit and ecophysiological traits among maqui (Aristotelia chilensis [Molina] Stuntz) provenances established in a common garden. Sci. Rep. 2022, 12, 185. [Google Scholar] [CrossRef]
- Thomas, E.; Jalonen, R.; Loo, J.; Boshier, D.; Gallo, L.; Cavers, S.; Bordács, S.; Smith, P.; Bozzano, M. Genetic considerations in ecosystem restoration using native tree species. For. Ecol. Manag. 2014, 333, 66–75. [Google Scholar] [CrossRef]
- Broadhurst, L.; Boshier, D. Seed provenance for restoration and management: Conserving evolutionary potential and utility. In Genetic Considerartions in Ecosystem Restoration Using Native Tree Species; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014; pp. 27–38. [Google Scholar]
- Gutterman, Y. Maternal Effects on seeds during development. In Seeds: The Ecology of Regeneration in Plant Comunnities, 2nd ed.; CABI Publishing: Wallingford, UK, 2000; pp. 59–84. [Google Scholar]
- Chacón, P.; Bustamante, R.; Henriquez, C. The effect of seed size on germination and seedling growth of Cryptocarya alba (Lauraceae) in Chile. Rev. Chil. De Hist. Nat. 1998, 71, 189–197. [Google Scholar]
- Hallett, L.M.; Standish, R.J.; Hobbs, R.J. Seed mass and summer drought survival in a Mediterranean-climate ecosystem. Plant Ecol. 2011, 212, 1479. [Google Scholar] [CrossRef]
- Giménez-Benavides, L.; Escudero, A.; Pérez-García, F. Seed germination of high mountain Mediterranean species: Altitudinal, interpopulation and interannual variability. Ecol. Res. 2005, 20, 433–444. [Google Scholar] [CrossRef]
- Moles, A.T.; Westoby, M. Seed size and plant strategy across the whole life cycle. Oikos 2006, 113, 91–105. [Google Scholar] [CrossRef]
- Harper, J. Population Biology of Plants; Academic Press: Cambridge, MA, USA, 1977; 892 p. [Google Scholar]
- ISTA. International Rules for Seed Testing; ISTA (International Seed Testing Association): Zurich, Switzerland, 2006. [Google Scholar]
- Tichý, L. Field test of canopy cover estimation by hemispherical photographs taken with a smartphone. J. Veg. Sci. 2016, 27, 427–435. [Google Scholar] [CrossRef]
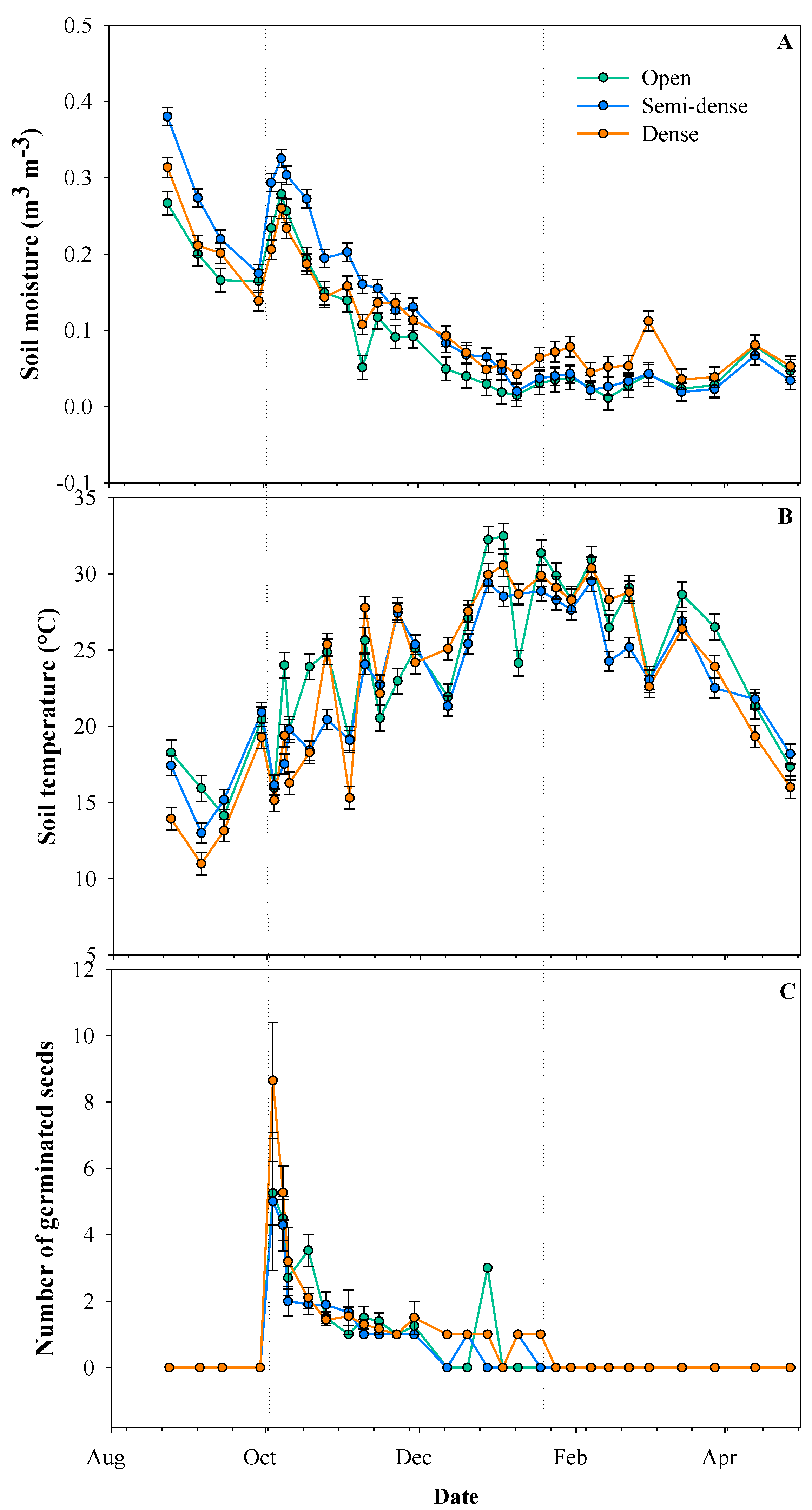
| Germin | Surv | H | SM | |
|---|---|---|---|---|
| p-values | ||||
| Seed source | <0.0001 | 0.0209 | 0.8291 | <0.0001 |
| Means | (%) | (%) | (cm) | (g) |
| CD | 10.4 ± 4.2 b | 3.3 ± 2.9 b | 5.9 ± 0.6 a | 0.73 ± 0.07 b |
| AN | 11.7 ± 5.2 b | 10.3 ± 4.1 ab | 6.6 ± 0.8 a | 1.37 ± 0.09 a |
| CA | 39.4 ± 3.8 a | 14.1 ± 1.9 a | 5.9 ± 0.4 a | 1.01 ± 0.07 b |
| CY | 21.3 ± 6.9 ab | 4.3 ± 3.5 b | 6.5 ± 0.7 a | 0.80 ± 0.12 b |
| Germin | Surv | |
|---|---|---|
| p-values | ||
| Forest Cover (FC) | 0.0045 | <0.0001 |
| Seed source (SSo) | 0.0498 | 0.0821 |
| FC×Sso | 0.8702 | 0.9734 |
| Mother (Sso) | 0.0032 | 0.1336 |
| Means per forest cover | (%) | (%) |
| Open | 19.0 (3.0) a | 0.0 (0.0) |
| Semi-dense | 7.0 (2.1) b | 0.0 (0.0) |
| Dense | 22.1 (4.2) a | 55.2 (5.2) |
| Means per seed sources | ||
| CD | 7.8 (2.1) c | 0.0 (0.0) |
| AN | 6.5 (2.3) c | 0.0 (0.0) |
| CA | 37.3 (5.4) a | 20.0 (3.4) |
| CY | 19.1 (6.4) b | 0.0 (0.0) |
| Seed Sources (Number of Mother Trees) | Climatic Classification | Latitude/Longitude | Elevation (m.a.s.l.) | M.A.R. 1 (mm) | M.A.T. 2 (°C) | DM |
|---|---|---|---|---|---|---|
| Cuesta la dormida CD (11) | Suprathermal warm temperate, semi-arid humid regime (Csb2Sa) | 33°04′17″/70°58′00″ | 743 | 429 | 14.4 | 17.5 |
| Antumapu AN (7) | Temperate Mediterranean with dry hot summer (Csb) | 33°34′19″/70°37′53″ | 629 | 371 | 14.5 | 15.1 |
| Cantillana CA (16) | Suprathermal warm temperate, semi-arid humid regime (Csb2Sa) | 33°52′07″/70°55′20″ | 373 | 543 | 12.0 | 24.7 |
| Cayumanque CY (4) | Temperate, warm summers and cold winters (Cfb) | 36°42′31″/72°31′52″ | 733 | 1292 | 10.0 | 64.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magni, C.R.; Saavedra, N.; Espinoza, S.E.; Yáñez, M.A.; Quiroz, I.; Faúndez, Á.; Grez, I.; Martinez-Herrera, E. The Recruitment of the Recalcitrant-Seeded Cryptocarya alba (Mol.) Looser, Established via Direct Seeding Is Mainly Affected by the Seed Source and Forest Cover. Plants 2022, 11, 2918. https://doi.org/10.3390/plants11212918
Magni CR, Saavedra N, Espinoza SE, Yáñez MA, Quiroz I, Faúndez Á, Grez I, Martinez-Herrera E. The Recruitment of the Recalcitrant-Seeded Cryptocarya alba (Mol.) Looser, Established via Direct Seeding Is Mainly Affected by the Seed Source and Forest Cover. Plants. 2022; 11(21):2918. https://doi.org/10.3390/plants11212918
Chicago/Turabian StyleMagni, Carlos R., Nicole Saavedra, Sergio E. Espinoza, Marco A. Yáñez, Iván Quiroz, Ángela Faúndez, Iván Grez, and Eduardo Martinez-Herrera. 2022. "The Recruitment of the Recalcitrant-Seeded Cryptocarya alba (Mol.) Looser, Established via Direct Seeding Is Mainly Affected by the Seed Source and Forest Cover" Plants 11, no. 21: 2918. https://doi.org/10.3390/plants11212918
APA StyleMagni, C. R., Saavedra, N., Espinoza, S. E., Yáñez, M. A., Quiroz, I., Faúndez, Á., Grez, I., & Martinez-Herrera, E. (2022). The Recruitment of the Recalcitrant-Seeded Cryptocarya alba (Mol.) Looser, Established via Direct Seeding Is Mainly Affected by the Seed Source and Forest Cover. Plants, 11(21), 2918. https://doi.org/10.3390/plants11212918








