Metal- and Organ-Specific Response to Heavy Metal-Induced Stress Mediated by Antioxidant Enzymes’ Activities, Polyamines, and Plant Hormones Levels in Populus deltoides
Abstract
:1. Introduction
- ✓
- The metal content (calcium and magnesium), translocation (TF), and bioconcentration factors (BCF) in P. deltoides clone Pe19/66;
- ✓
- The activities of different ROS scavenging enzymes, such as guaiacol peroxidase, glutathione reductase, and superoxide dismutase;
- ✓
- Total antioxidant and reducing activities (estimated by biochemical assays DPPH and FRAP, respectively) and radical scavenger capacity (against NO and OH radicals), as well as total polyphenol compounds (TPC) accumulation; and
- ✓
- Endogenous hormone levels (ABA and IAA), as well as plant hormone, regulators-polyamines content (putrescine, spermine, and spermidine) that is both free and conjugated.
2. Results
2.1. Metal and Non-Metal Contents, Translocation (TF), and Bioconcentration Factors (BCF)
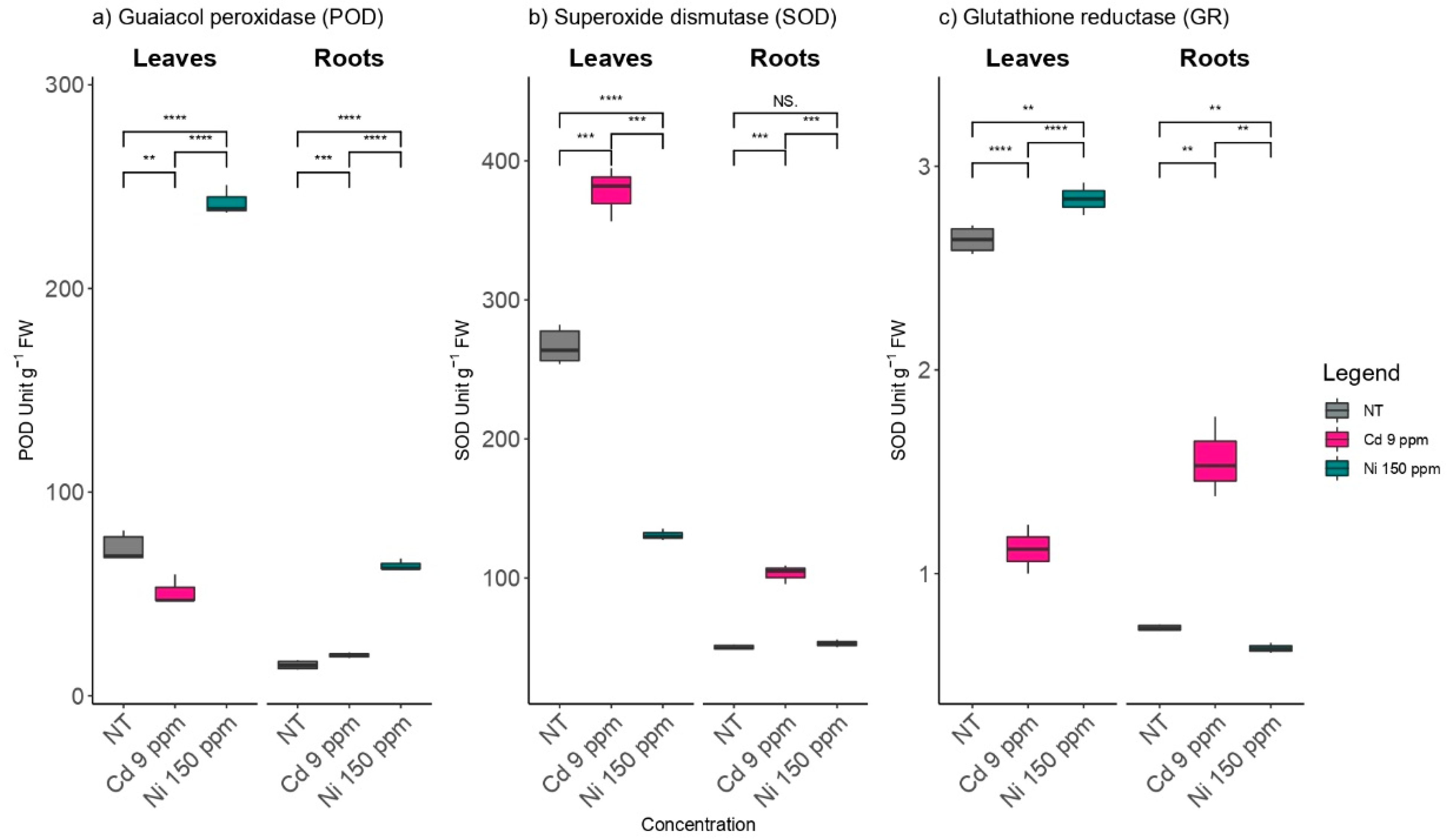
2.2. The Effects of Cd and Ni on Antioxidant Enzymes Activities in Poplar Leaves and Roots
2.3. The Effects of Cd and Ni on the Antioxidant Capacity of Poplar Leaves and Roots
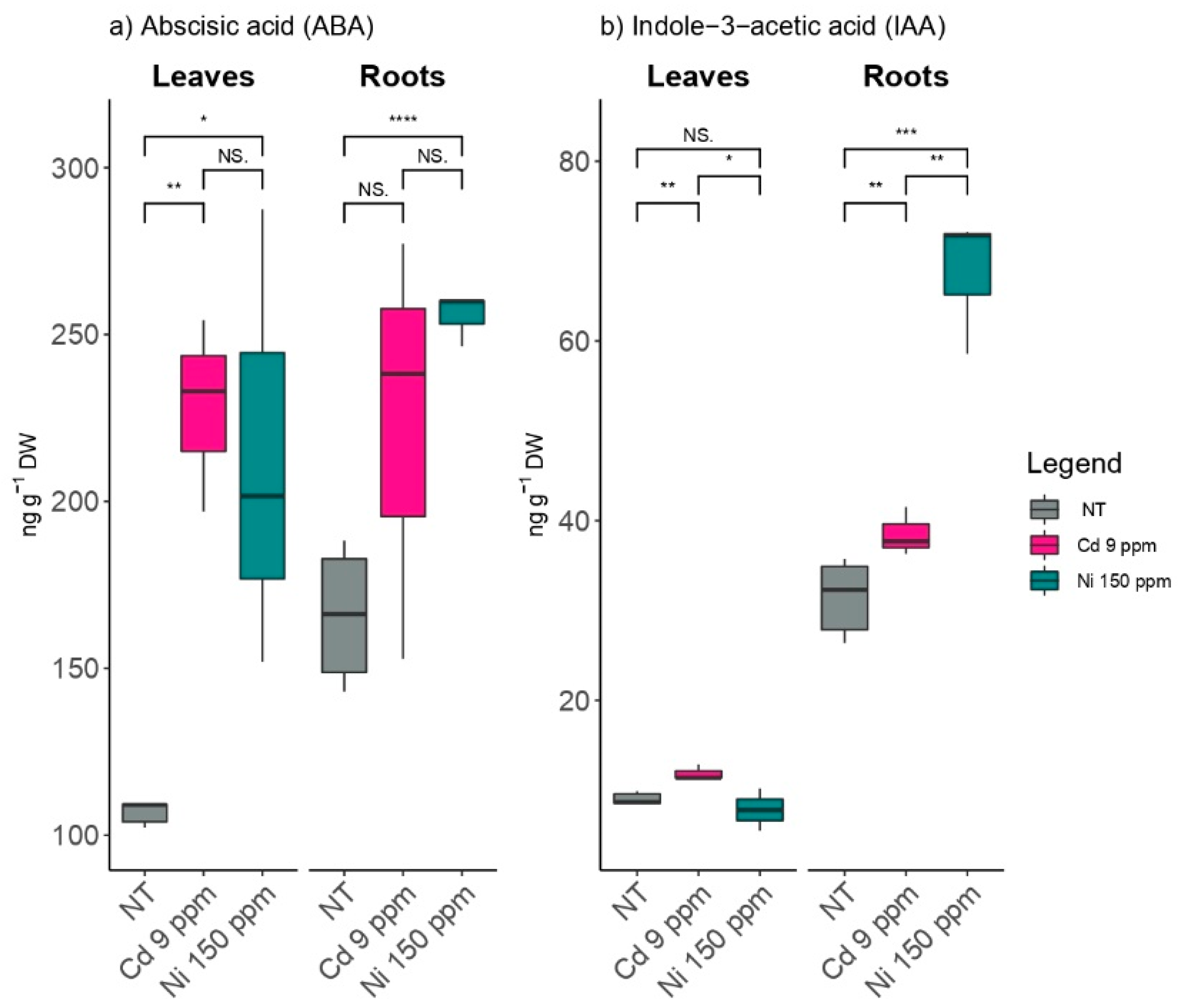
2.4. The Effects of Cd and Ni on Plant Hormones and Hormone Regulators Content
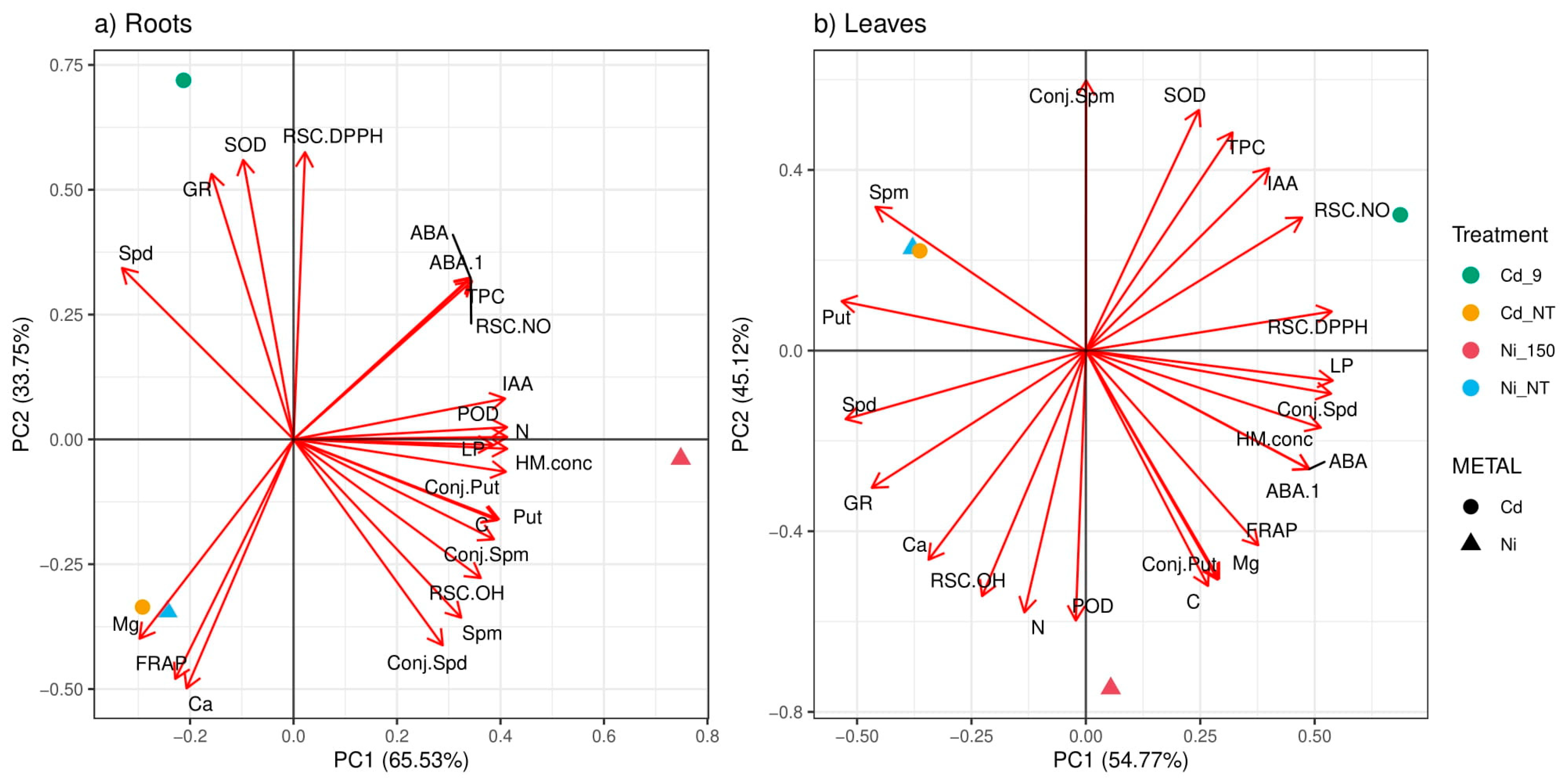
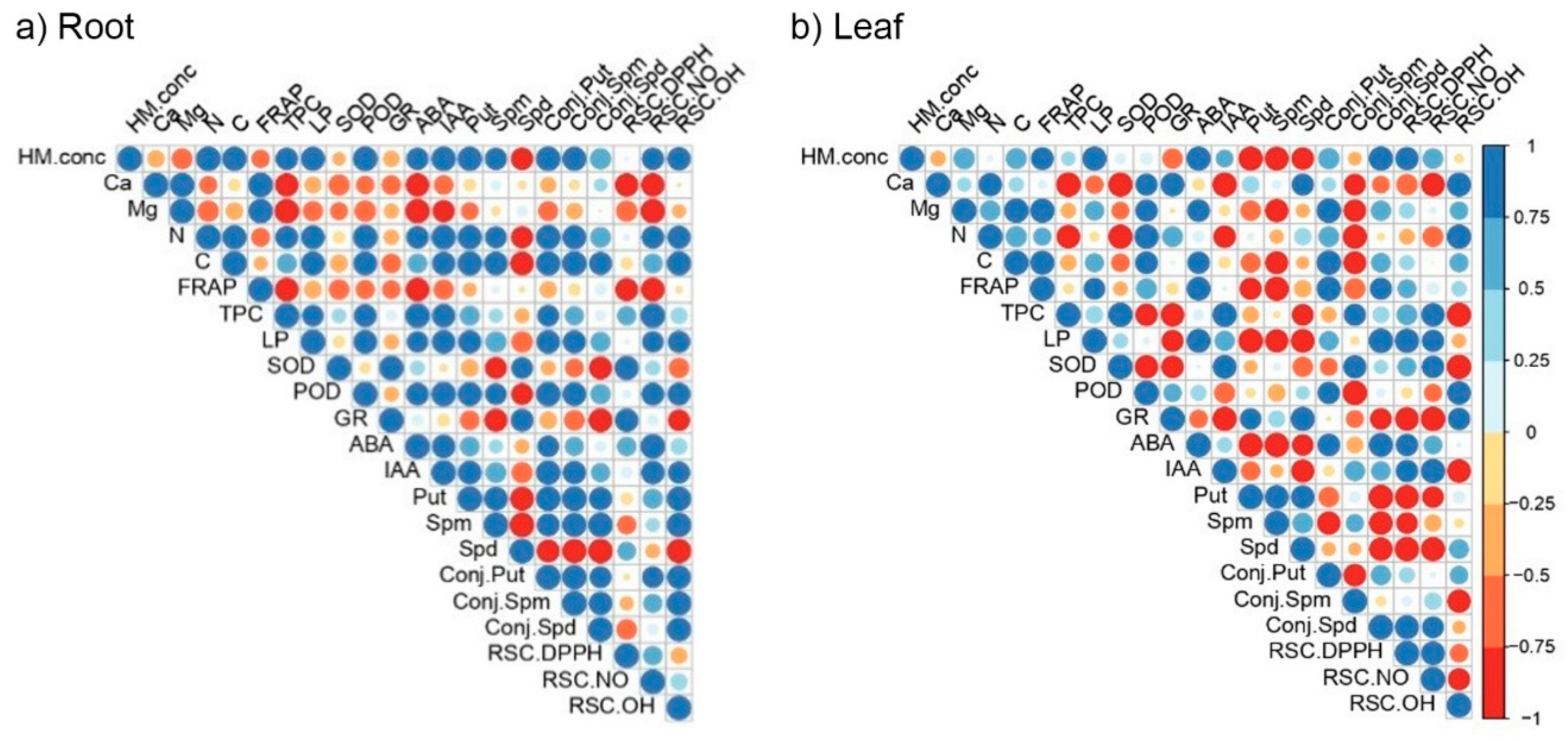
2.5. The Principal Component and Correlation Analysis
3. Discussion
3.1. The Effects of Cd and Ni on the Antioxidant Defense System
3.2. HM Induced Stress Affected Plant Developmental and Stress Hormones (IAA and ABA)
3.3. Polyamines Exhibited Metal and Organ Specific Responses to HM-Induced Stress
4. Materials and Methods
4.1. Experimental Design and Sampling
4.2. Metal Content, Translocation (TF), and Bioconcentration Factors (BCF)
4.3. Activities of Antioxidant Enzymes, Radical Scavenger Capacity, Lipid Peroxidation Intensity and Content of Total Polyphenol Compounds
- (A)
- Enzymes activities (POD, SOD, and GR)
- (B)
- Assays of Antioxidant Defense Systems
4.4. Plant Hormones and Hormone Regulators Content
- (A)
- Plant hormone analysis (ABA and IAA)
- (B)
- Polyamines determination
4.5. Elemental Analysis of Nitrogen and Carbon Content
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, W.; Zhao, F.; Wang, Y.; Ding, Z.; Yang, X.; Zhu, Z. Differences in Uptake and Accumulation of Copper and Zinc by Salix Clones under Flooded versus Non-Flooded Conditions. Chemosphere 2020, 241, 125059. [Google Scholar] [CrossRef]
- Oyewo, O.A.; Adeniyi, A.; Bopape, M.F.; Onyango, M.S. Chapter 4—Heavy Metal Mobility in Surface Water and Soil, Climate Change, and Soil Interactions. In Climate Change and Soil Interactions; Prasad, M.N.V., Pietrzykowski, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 51–88. [Google Scholar] [CrossRef]
- Zafar-ul-Hye, M.; Naeem, M.; Danish, S.; Fahad, S.; Datta, R.; Abbas, M.; Rahi, A.A.; Brtnicky, M.; Holátko, J.; Tarar, Z.H.; et al. Alleviation of Cadmium Adverse Effects by Improving Nutrients Uptake in Bitter Gourd through Cadmium Tolerant Rhizobacteria. Environments 2020, 7, 54. [Google Scholar] [CrossRef]
- Shah, K.; Nongkynrih, J.M. Metal Hyperaccumulation and Bioremediation. Biol. Plantarum. 2007, 51, 618–634. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, e6730305. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.J.; Kalamdhad, A. Effects of Heavy Metals on Soil, Plants, Human Health and Aquatic Life. Int. J. Res. Chem. Environ. 2011, 1, 15–21. [Google Scholar]
- Mahajan, P.; Kaushal, J. Role of Phytoremediation in Reducing Cadmium Toxicity in Soil and Water. J. Toxicol. 2018, 2018, e4864365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef] [Green Version]
- Dixit, P.; Mukherjee, P.K.; Ramachandran, V.; Eapen, S. Glutathione Transferase from Trichoderma Virens Enhances Cadmium Tolerance without Enhancing Its Accumulation in Transgenic Nicotiana Tabacum. PLoS ONE 2011, 6, e16360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, K.S.; Mohapatra, M.; Anand, S.; Venkateswarlu, P. Review on Cadmium Removal from Aqueous Solutions. Int. J. Eng. Sci. Technol. 2010, 2, 81–103. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Ogunseitan, O.A.; Wang, J.; Chen, H.; Wang, B.; Chen, S. Evolution of Electronic Waste Toxicity: Trends in Innovation and Regulation. Environ. Int. 2016, 89–90, 147–154. [Google Scholar] [CrossRef]
- Chen, M.; Qin, X.; Zeng, G.; Li, J. Impacts of Human Activity Modes and Climate on Heavy Metal “Spread” in Groundwater Are Biased. Chemosphere 2016, 152, 439–445. [Google Scholar] [CrossRef]
- Kumar, P.; Fulekar, M.H. Multivariate and Statistical Approaches for the Evaluation of Heavy Metals Pollution at E-Waste Dumping Sites. SN Appl. Sci. 2019, 1, 1506. [Google Scholar] [CrossRef]
- Moreira, T.F.M.; Santana, I.L.; Moura, M.N.; Ferreira, S.A.D.; Lelis, M.F.F.; Freitas, M.B.J.G. Recycling of Negative Electrodes from Spent Ni-Cd Batteries as CdO with Nanoparticle Sizes and Its Application in Remediation of Azo Dye. Mater. Chem. Phys. 2017, 195, 19–27. [Google Scholar] [CrossRef]
- Arya, S.; Kumar, S. E-Waste in India at a Glance: Current Trends, Regulations, Challenges and Management Strategies. J. Clean. Prod. 2020, 271, 122707. [Google Scholar] [CrossRef]
- Nedjimi, B. Phytoremediation: A Sustainable Environmental Technology for Heavy Metals Decontamination. SN Appl. Sci. 2021, 3, 286. [Google Scholar] [CrossRef]
- Habibul, N.; Chen, J.-J.; Hu, Y.-Y.; Hu, Y.; Yin, H.; Sheng, G.-P.; Yu, H.-Q. Uptake, Accumulation and Metabolization of 1-Butyl-3-Methylimidazolium Bromide by Ryegrass from Water: Prospects for Phytoremediation. Water Res. 2019, 156, 82–91. [Google Scholar] [CrossRef]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, Plant Selection and Enhancement by Natural and Synthetic Agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Q.; Wang, L.; Liu, W. The Influence of Different Growth Stages and Dosage of EDTA on Cd Uptake and Accumulation in Cd-Hyperaccumulator (Solanum Nigrum L.). Bull. Environ. Contam. Toxicol. 2009, 82, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.J.M.; McGrath, S.P.; Reeves, R.D.; Smith, J.A.C. Metal Hyperaccumulator Plants: A Review of the Ecology and Physiology of a Biological Resource for Phytoremediation of Metal-Polluted Soils. In Phytoremediation of Contaminated Soil; Terry, N., Vangronsveld, J., Banuelos, G., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 85–107. [Google Scholar]
- Suman, J.; Uhlik, O.; Viktorova, J.; Macek, T. Phytoextraction of Heavy Metals: A Promising Tool for Clean-Up of Polluted Environment? Front. Plant Sci. 2018, 9, 1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garbisu, C.; Alkorta, I. Phytoextraction: A Cost-Effective Plant-Based Technology for the Removal of Metals from the Environment. Bioresour. Technol. 2001, 77, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Sarma, H.; Islam, N.F.; Prasad, R.; Prasad, M.N.V.; Ma, L.Q.; Rinklebe, J. Enhancing Phytoremediation of Hazardous Metal(Loid)s Using Genome Engineering CRISPR–Cas9 Technology. J. Hazard. Mater. 2021, 414, 125493. [Google Scholar] [CrossRef]
- Matanzas, N.; Afif, E.; Díaz, T.E.; Gallego, J.R. Phytoremediation Potential of Native Herbaceous Plant Species Growing on a Paradigmatic Brownfield Site. Water Air Soil Pollut. 2021, 232, 290. [Google Scholar] [CrossRef]
- Yıldırım, K.; Kasım, G.Ç. Phytoremediation Potential of Poplar and Willow Species in Small Scale Constructed Wetland for Boron Removal. Chemosphere 2018, 194, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Malá, J.; Cvrčková, H.; Máchová, P.; Dostál, J.; Šíma, P. Heavy Metal Accumulation by Willow Clones in Short-Time Hydroponics. J. For. Sci. 2010, 56, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Greger, M.; Landberg, T. Use of Willow in Phytoextraction. Int. J. Phytoremed. 1999, 1, 115–123. [Google Scholar] [CrossRef]
- Zacchini, M.; Pietrini, F.; Mugnozza, G.S.; Iori, V.; Pietrosanti, L.; Massacci, A. Metal Tolerance, Accumulation and Translocation in Poplar and Willow Clones Treated with Cadmium in Hydroponics. Water Air Soil Pollut. 2008, 197, 23. [Google Scholar] [CrossRef]
- Pajević, S.; Borišev, M.; Nikolić, N.; Krstić, B.; Pilipović, A.; Orlović, S. Phytoremediation Capacity of Poplar (Populus spp.) and Willow (Salix spp.) Clonesin Relation to Photosynthesis. Arch. Biol. Sci. 2009, 61, 239–247. [Google Scholar] [CrossRef]
- Wullschleger, S.D.; Weston, D.J.; DiFazio, S.P.; Tuskan, G.A. Revisiting the Sequencing of the First Tree Genome: Populus Trichocarpa. Tree Physiol. 2013, 33, 357–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kebert, M.; Rapparini, F.; Neri, L.; Bertazza, G.; Orlović, S.; Biondi, S. Copper-Induced Responses in Poplar Clones Are Associated with Genotype- and Organ-Specific Changes in Peroxidase Activity and Proline, Polyamine, ABA, and IAA Levels. J. Plant Growth Regul. 2017, 36, 131–147. [Google Scholar] [CrossRef]
- Luo, J.-S.; Zhang, Z. Mechanisms of Cadmium Phytoremediation and Detoxification in Plants. Crop. J. 2021, 9, 521–529. [Google Scholar] [CrossRef]
- Ge, W.; Jiao, Y.Q.; Sun, B.L.; Qin, R.; Jiang, W.S.; Liu, D.H. Cadmium-Mediated Oxidative Stress and Ultrastructural Changes in Root Cells of Poplar Cultivars. S. Afr. J. Bot. 2012, 83, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Yang, D.; Tian, J.; Wang, S.; Yan, Y.; He, X.; Du, Z.; Zhong, F. Physiological and Transcriptional Response of Carbohydrate and Nitrogen Metabolism in Tomato Plant Leaves to Nickel Ion and Nitrogen Levels. Sci. Hortic. 2022, 292, 110620. [Google Scholar] [CrossRef]
- Chaoui, A.; El Ferjani, E. Effects of Cadmium and Copper on Antioxidant Capacities, Lignification and Auxin Degradation in Leaves of Pea (Pisum sativum L.) Seedlings. Comptes Rendus Biol. 2005, 328, 23–31. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium Toxicity in Plants: Impacts and Remediation Strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Rehman, A.; Cheema, S.A.; Fahad, S.; Rehman, S.; Sharma, A. Nickel; Whether Toxic or Essential for Plants and Environment—A Review. Plant Physiol. Biochem. 2018, 132, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Seregin, I.V.; Kozhevnikova, A.D.; Davydova, M.A.; Bystrova, E.I.; Schat, H.; Ivanov, V.B. Role of Root and Shoot Tissues of Excluders and Hyperaccumulators in Nickel Transport and Accumulation. Dokl. Biol. Sci. 2007, 415, 295–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobbett, C.; Goldsbrough, P. Phytochelatins and Metallothioneins: Roles in Heavy Metal Detoxification and Homeostasis. Annu. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimeri, A.M.; Dhankher, O.P.; McCaig, B.; Meagher, R.B. The Plant MT1 Metallothioneins Are Stabilized by Binding Cadmiums and Are Required for Cadmium Tolerance and Accumulation. Plant Mol. Biol. 2005, 58, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy Metals and Living Systems: An Overview. Indian J. Pharmacol. 2011, 43, 246–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valko, M.; Morris, H.; Cronin, M.T.D. Metals, Toxicity and Oxidative Stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive Oxygen Gene Network of Plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Danouche, M.; El Ghachtouli, N.; El Baouchi, A.; El Arroussi, H. Heavy Metals Phycoremediation Using Tolerant Green Microalgae: Enzymatic and Non-Enzymatic Antioxidant Systems for the Management of Oxidative Stress. J. Environ. Chem. Eng. 2020, 8, 104460. [Google Scholar] [CrossRef]
- Santovito, G.; Trentin, E.; Gobbi, I.; Bisaccia, P.; Tallandini, L.; Irato, P. Non-Enzymatic Antioxidant Responses of Mytilus Galloprovincialis: Insights into the Physiological Role against Metal-Induced Oxidative Stress. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 240, 108909. [Google Scholar] [CrossRef]
- Velikova, V.B.; Edreva, A.M.; Tsonev, T.D.; Jones, H.G. Singlet Oxygen Quenching by Phenylamides and Their Parent Compounds. Z. Naturforsch. C 2007, 62, 833–838. [Google Scholar] [CrossRef]
- Mandal, C.; Ghosh, N.; Maiti, S.; Das, K.; Gupta, S.; Dey, N.; Adak, M.K. Antioxidative Responses of Salvinia (Salvinia natans Linn.) to Aluminium Stress and It’s Modulation by Polyamine. Physiol. Mol. Biol. Plants 2013, 19, 91–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minocha, R.; Majumdar, R.; Minocha, S.C. Polyamines and Abiotic Stress in Plants: A Complex Relationship. Front. Plant Sci. 2014, 5, 175. [Google Scholar] [CrossRef] [Green Version]
- Franchin, C.; Fossati, T.; Pasquini, E.; Lingua, G.; Castiglione, S.; Torrigiani, P.; Biondi, S. High Concentrations of Zinc and Copper Induce Differential Polyamine Responses in Micropropagated White Poplar (Populus alba). Physiol. Plant. 2007, 130, 77–90. [Google Scholar] [CrossRef]
- Castiglione, S.; Todeschini, V.; Franchin, C.; Torrigiani, P.; Gastaldi, D.; Cicatelli, A.; Rinaudo, C.; Berta, G.; Biondi, S.; Lingua, G. Clonal Differences in Survival Capacity, Copper and Zinc Accumulation, and Correlation with Leaf Polyamine Levels in Poplar: A Large-Scale Field Trial on Heavily Polluted Soil. Environ. Pollut. 2009, 157, 2108. [Google Scholar] [CrossRef]
- Song, Y.; Ci, D.; Tian, M.; Zhang, D. Comparison of the Physiological Effects and Transcriptome Responses of Populus Simonii under Different Abiotic Stresses. Plant Mol. Biol. 2014, 86, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Gangwar, S.; Singh, V.P. Indole Acetic Acid Differently Changes Growth and Nitrogen Metabolism in Pisum sativum L. Seedlings under Chromium (VI) Phytotoxicity: Implication of Oxidative Stress. Sci. Hortic. 2011, 129, 321–328. [Google Scholar] [CrossRef]
- Elobeid, M.; Polle, A. Interference of Heavy Metal Toxicity with Auxin Physiology. In Metal Toxicity in Plants: Perception, Signaling and Remediation; Gupta, D., Sandalio, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 249–259. [Google Scholar]
- Asgher, M.; Khan, M.I.R.; Anjum, N.A.; Khan, N.A. Minimising Toxicity of Cadmium in Plants-Role of Plant Growth Regulators. Protoplasma 2015, 252, 399–413. [Google Scholar] [CrossRef]
- Schwitzguébel, J.-P.; van der Lelie, D.; Baker, A.; Glass, D.; Vangronsveld, J. Phytoremediation: European and American Trends. Successes, Obstacles and Needs. J. Soils Sediments 2002, 2, 91–99. [Google Scholar] [CrossRef]
- Sharma, R.K.; Agrawal, M. Biological Effects of Heavy Metals: An Overview. J. Environ. Biol. 2005, 26, 301–313. [Google Scholar]
- Drążkiewicz, M.; Skórzyńska-Polit, E.; Krupa, Z. Response of the Ascorbate–Glutathione Cycle to Excess Copper in Arabidopsis thaliana (L.). Plant Sci. 2003, 164, 195–202. [Google Scholar] [CrossRef]
- Wang, H.; Shan, X.; Wen, B.; Zhang, S.; Wang, Z. Responses of Antioxidative Enzymes to Accumulation of Copper in a Copper Hyperaccumulator of Commoelina Communis. Arch. Environ. Contam. Toxicol. 2004, 47, 185–192. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ma, C.; Ma, Y.; Li, H.; Kang, J.; Liu, T.-X.; Polle, A.; Peng, C.; Luo, Z.-B. Cadmium Tolerance in Six Poplar Species. Environ. Sci. Pollut. Res. Int. 2012, 20, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Wang, X.; Chen, J. Involvement of Plasma-Membrane NADPH Oxidase in Nickel-Induced Oxidative Stress in Roots of Wheat Seedlings. Plant Sci. 2006, 170, 151–158. [Google Scholar] [CrossRef]
- del Carmen, E.M.; Souza, V.; Bucio, L.; Hernández, E.; Damián-Matsumura, P.; Zaga, V.; Gutiérrez-Ruiz, M.C. Cadmium Induces Alpha(1)Collagen (I) and Metallothionein II Gene and Alters the Antioxidant System in Rat Hepatic Stellate Cells. Toxicology 2002, 170, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, E.; Skłodowska, M. Effect of Nickel on ROS Content and Antioxidative Enzyme Activities in Wheat Leaves. Biometals 2007, 20, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, E.; Skłodowska, M. Antioxidative Responses and Proline Level in Leaves and Roots of Pea Plants Subjected to Nickel Stress. Acta Physiol. Plant. 2005, 27, 329–340. [Google Scholar] [CrossRef]
- Kısa, D.; Elmastaş, M.; Öztürk, L.; Kayır, Ö. Responses of the Phenolic Compounds of Zea Mays under Heavy Metal Stress. Appl. Biol. Chem. 2016, 59, 813–820. [Google Scholar] [CrossRef]
- Torres, J.; Barrientos, E.; Wrobel, K.; Wrobel, K. Effect of Cadmium (Cd(II)), Selenium (Se(IV)) and Their Mixtures on Phenolic Compounds and Antioxidant Capacity in Lepidium Sativum. Acta Physiol. Plant. 2014, 35, 431–441. [Google Scholar] [CrossRef]
- Schützendübel, A.; Schwanz, P.; Teichmann, T.; Gross, K.; Langenfeld-Heyser, R.; Godbold, D.L.; Polle, A. Cadmium-Induced Changes in Antioxidative Systems, Hydrogen Peroxide Content, and Differentiation in Scots Pine Roots. Plant Physiol. 2001, 127, 887–898. [Google Scholar] [CrossRef]
- Díaz, J.; Bernal, A.; Pomar, F.; Merino, F. Induction of Shikimate Dehydrogenase and Peroxidase in Pepper (Capsicum annuum L.) Seedlings in Response to Copper Stress and Its Relation to Lignification. Plant Sci. 2001, 161, 179. [Google Scholar] [CrossRef]
- Hale, K.L.; Tufan, H.A.; Pickering, I.J.; George, G.N.; Terry, N.; Pilon, M.; Pilon-Smits, E.A.H. Anthocyanins Facilitate Tungsten Accumulation in Brassica. Physiol. Plant 2002, 116, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Vuksanović, V.; Kovačević, B.; Stojnić, S.; Kebert, M.; Kesić, L.; Galović, V.; Orlović, S. Variability of Tolerance of Wild Cherry Clones to PEG-Induced Osmotic Stress in Vitro. iForest 2022, 15, 265. [Google Scholar] [CrossRef]
- Król, A.; Amarowicz, R.; Weidner, S. Changes in the Composition of Phenolic Compounds and Antioxidant Properties of Grapevine Roots and Leaves (Vitis vinifera L.) under Continuous of Long-Term Drought Stress. Acta Physiol. Plant. 2014, 36, 1491–1499. [Google Scholar] [CrossRef] [Green Version]
- Solecka, D.; Kacperska, A. Phenylpropanoid Deficiency Affects the Course of Plant Acclimation to Cold. Physiol. Plant. 2003, 119, 253–262. [Google Scholar] [CrossRef]
- Kisa, D.; Kayir, O.; Saglam, N.; şahin, S.; Öztürk, L.; Elmastaş, M. Changes of Phenolic Compounds in Tomato Associated With The Heavy Metal Stress. Bartın Univ. Int. J. Nat. Appl. Sci. 2019, 2, 35–43. [Google Scholar]
- Vuksanović, V.; Kovaćević, B.; Kebert, M.; Katanić, M.; Pavlović, L.; Kesić, L.; Orlović, S. Clone Specificity of White Poplar (Populus alba L.) Acidity Tolerance in Vitro. Fresenius Environ. Bull. 2019, 28, 8307–8313. [Google Scholar]
- Puente-Garza, C.A.; Meza-Miranda, C.; Ochoa-Martínez, D.; García-Lara, S. Effect of in Vitro Drought Stress on Phenolic Acids, Flavonols, Saponins, and Antioxidant Activity in Agave Salmiana. Plant Physiol. Biochem. 2017, 115, 400–407. [Google Scholar] [CrossRef]
- Hamooh, B.T.; Sattar, F.A.; Wellman, G.; Mousa, M.A.A. Metabolomic and Biochemical Analysis of Two Potato (Solanum tuberosum L.) Cultivars Exposed to In Vitro Osmotic and Salt Stresses. Plants 2021, 10, 98. [Google Scholar] [CrossRef]
- Gould, K.S.; Neill, S.O.; Vogelmann, T.C. A Unified Explanation for Anthocyanins in Leaves? Adv. Bot. Res. 2002, 37, 167–192. [Google Scholar]
- Kebert, M.; Trudić, B.; Stojnić, S.; Orlović, S.; Štajner, D.; Popović, B.; Galić, Z. Estimation of Antioxidant Capacities of Poplar Clones Involved in Phytoremediation Processes. In Proceedings of the STREPOW International Workshop, Andrevlje-Novi Sad, Serbia, 23–24 February 2011. [Google Scholar]
- Kapoor, D.; Kaur, S.; Bhardwaj, R. Physiological and Biochemical Changes in Brassica juncea Plants under Cd-Induced Stress. BioMed Res. Int. 2014, 2014, e726070. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.M.; Abbasi, B.H.; Ahmad, N.; Ali, M.; Mahmood, T. Toxic Effects of Heavy Metals (Cd, Cr and Pb) on Seed Germination and Growth and DPPH-Scavenging Activity in Brassica Rapa Var. Turnip. Toxicol. Ind. Health 2014, 30, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Georgiadou, E.C.; Kowalska, E.; Patla, K.; Kulbat, K.; Smolińska, B.; Leszczyńska, J.; Fotopoulos, V. Influence of Heavy Metals (Ni, Cu, and Zn) on Nitro-Oxidative Stress Responses, Proteome Regulation and Allergen Production in Basil (Ocimum basilicum L.) Plants. Front. Plant Sci. 2018, 9, 862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metwally, A.; Safronova, V.I.; Belimov, A.A.; Dietz, K.-J. Genotypic Variation of the Response to Cadmium Toxicity in Pisum sativum L. J. Exp. Bot. 2005, 56, 167–178. [Google Scholar] [CrossRef]
- Wu, F.B.; Zhang, G.P.; Dominy, P. Four Barley Genotypes Respond Differently to Cadmium: Lipid Peroxidation and Activities of Antioxidant Capacity. Environ. Exp. Bot. 2003, 50, 67–78. [Google Scholar] [CrossRef]
- Groppa, M.D.; Tomaro, M.L.; Benavides, M.P. Polyamines as Protectors against Cadmium or Copper-Induced Oxidative Damage in Sunflower Leaf Discs. Plant Sci. 2001, 161, 481. [Google Scholar] [CrossRef]
- Cho, U.-H.; Seo, N.-H. Oxidative Stress in Arabidopsis Thaliana Exposed to Cadmium Is Due to Hydrogen Peroxide Accumulation. Plant Sci. 2005, 168, 113–120. [Google Scholar] [CrossRef]
- Balestrasse, K.B.; Gallego, S.M.; Tomaro, M.L. Cadmium-Induced Senescence in Nodules of Soybean (Glycine max L.) Plants. Plant Soil 2004, 262, 373–381. [Google Scholar] [CrossRef]
- Juknys, R.; Vitkauskaitė, G.; Račaitė, M.; Venclovienė, J. The Impacts of Heavy Metals on Oxidative Stress and Growth of Spring Barley. Open Life Sci. 2012, 7, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Giannakoula, A.; Therios, I.; Chatzissavvidis, C. Effect of Lead and Copper on Photosynthetic Apparatus in Citrus (Citrus aurantium L.) Plants. The Role of Antioxidants in Oxidative Damage as a Response to Heavy Metal Stress. Plants 2021, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Bartels, D.; Sunkar, R. Drought and Salt Tolerance in Plants. Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Tuteja, N. Abscisic Acid and Abiotic Stress Signaling. Plant Signal. Behav. 2007, 2, 135–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danquah, A.; de Zelicourt, A.; Colcombet, J.; Hirt, H. The Role of ABA and MAPK Signaling Pathways in Plant Abiotic Stress Responses. Biotechnol. Adv. 2014, 32, 40–52. [Google Scholar] [CrossRef]
- Choudhary, S.P.; Bhardwaj, R.; Gupta, B.D.; Dutt, P.; Gupta, R.K.; Biondi, S.; Kanwar, M. Epibrassinolide Induces Changes in Indole-3-Acetic Acid, Abscisic Acid and Polyamine Concentrations and Enhances Antioxidant Potential of Radish Seedlings under Copper Stress. Physiol. Plant. 2010, 140, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, H.; Cohen, M.F. NO Signal at the Crossroads: Polyamine-Induced Nitric Oxide Synthesis in Plants? Trends Plant Sci. 2006, 11, 522–524. [Google Scholar] [CrossRef]
- Shevyakova, N.I.; Cheremisina, A.I.; Kuznetsov, V.V. Phytoremediation Potential of Amaranthus Hybrids: Antagonism between Nickel and Iron and Chelating Role of Polyamines. Russ. J. Plant Physiol. 2011, 58, 634–642. [Google Scholar] [CrossRef]
- Kishor, P.K.; Sangam, S.; Amrutha, R.N.; Laxmi, P.S.; Naidu, K.R.; Rao, K.S.; Rao, S.; Reddy, K.J.; Theriappan, P.; Sreenivasulu, N. Regulation of Proline Biosynthesis, Degradation, Uptake and Transport in Higher Plants: Its Implications in Plant Growth and Abiotic Stress Tolerance. Curr. Sci. India 2005, 88, 424–438. [Google Scholar]
- Siripornadulsil, S.; Traina, S.; Verma, D.P.S.; Sayre, R.T. Molecular Mechanisms of Proline-Mediated Tolerance to Toxic Heavy Metals in Transgenic Microalgae. Plant Cell 2002, 14, 2837–2847. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, A.S.; Gonugunta, V.K.; Christmann, A.; Grill, E. ABA Perception and Signalling. Trends Plant Sci. 2010, 15, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Bishnoi, N.R.; Sheoran, I.S.; Singh, R. Influence of Cadmium and Nickel on Photosynthesis and Water Relations in Wheat Leaves of Different Insertion Level. Photosynthetica 1993, 28, 473–479. [Google Scholar]
- Li, S.-W.; Leng, Y.; Feng, L.; Zeng, X.-Y. Involvement of Abscisic Acid in Regulating Antioxidative Defense Systems and IAA-Oxidase Activity and Improving Adventitious Rooting in Mung Bean [Vigna radiata (L.) Wilczek] Seedlings under Cadmium Stress. Environ. Sci. Pollut. Res. Int. 2014, 21, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Haag-Kerwer, A.; Schäfer, H.J.; Heiss, S.; Walter, C.; Rausch, T. Cadmium Exposure in Brassica Juncea Causes a Decline in Transpiration Rate and Leaf Expansion without Effect on Photosynthesis. J. Exp. Bot. 1999, 50, 1827–1835. [Google Scholar] [CrossRef]
- Perfus-Barbeoch, L.; Leonhardt, N.; Vavasseur, A.; Forestier, C. Heavy Metal Toxicity: Cadmium Permeates through Calcium Channels and Disturbs the Plant Water Status. Plant J. 2002, 32, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Sanità di Toppi, L.; Lambardi, M.; Pazzagli, L.; Cappugi, G.; Durante, M.; Gabbrielli, R. Response to Cadmium in Carrot in Vitro Plants and Cell Suspension Cultures. Plant Sci. 1998, 137, 119–129. [Google Scholar] [CrossRef]
- Chen, S.L.; Kao, C.H. Glutathione Reduces the Inhibition of Rice Seedling Root Growth Caused by Cadmium. Plant Growth Regul. 1995, 16, 249–252. [Google Scholar] [CrossRef]
- Hollenbach, B.; Schreiber, L.; Hartung, W.; Dietz, K.J. Cadmium Leads to Stimulated Expression of the Lipid Transfer Protein Genes in Barley: Implications for the Involvement of Lipid Transfer Proteins in Wax Assembly. Planta 1997, 203, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Sanità Di Toppi, L.; Lambardi, M.; Pecchion, N.; Pazzagli, L.; Durante, M.; Gabbrielli, R. Effects of Cadmium Stress on Hairy Roots of Daucus Carota. J. Plant Physiol. 1999, 154, 385–391. [Google Scholar] [CrossRef]
- Chen, C.T.; Chen, L.-M.; Lin, C.C.; Kao, C.H. Regulation of Proline Accumulation in Detached Rice Leaves Exposed to Excess Copper. Plant Sci. 2001, 160, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.I.; Escrig, I.; Martinez-Cortina, C.; Lopez-Benet, F.J.; Sanz, A. Cadmium and Nickel Accumulation in Rice Plants. Effects on Mineral Nutrition and Possible Interactions of Abscisic and Gibberellic Acids. Plant Growth Regul. 1994, 14, 151–157. [Google Scholar] [CrossRef]
- Salt, D.E.; Rauser, W.E. MgATP-Dependent Transport of Phytochelatins Across the Tonoplast of Oat Roots. Plant Physiol. 1995, 107, 1293–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz, F.J.; Dopico, B.; Labrador, E. A CDNA Encoding a Proline-Rich Protein from Cicer Arietinum. Changes in Expression during Development and Abiotic Stresses. Physiol. Plant. 1998, 102, 582–590. [Google Scholar] [CrossRef]
- Das, P.; Samantaray, S.; Rout, G.R. Studies on Cadmium Toxicity in Plants: A Review. Environ. Pollut. 1997, 98, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Srivastava, A.K.; Suprasanna, P.; D’Souza, S.F. Identification and Profiling of Arsenic Stress-Induced MicroRNAs in Brassica juncea. J. Exp. Bot. 2013, 64, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Zelinová, V.; Alemayehu, A.; Bočová, B.; Huttová, J.; Tamás, L. Cadmium-Induced Reactive Oxygen Species Generation, Changes in Morphogenic Responses and Activity of Some Enzymes in Barley Root Tip Are Regulated by Auxin. Biologia 2015, 70, 356–364. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Sharma, I.; Handa, N.; Kapoor, D.; Kaur, H.; Gautam, V.; Kohli, S. Role of polyamines in stress management. In Plant Adaptation to Environmental Changes: Significance of Amino Acids and Their Derivatives; Anjum, N.A., Gill, S.S., Gill, R., Eds.; CABI Publishers: Wallingford, CT, USA, 2014; pp. 245–265. [Google Scholar]
- Saha, J.; Brauer, E.K.; Sengupta, A.; Popescu, S.C.; Gupta, K.; Gupta, B. Polyamines as Redox Homeostasis Regulators during Salt Stress in Plants. Front. Environ. Sci. 2015, 3, 21. [Google Scholar] [CrossRef] [Green Version]
- Benavides, M.P.; Groppa, M.D.; Recalde, L.; Verstraeten, S.V. Effects of Polyamines on Cadmium- and Copper-Mediated Alterations in Wheat (Triticum aestivum L) and Sunflower (Helianthus annuus L) Seedling Membrane Fluidity. Arch. Biochem. Biophys. 2018, 654, 27–39. [Google Scholar] [CrossRef]
- Shevyakova, N.I.; Il’ina, E.N.; Stetsenko, L.A.; Kuznetsov, V.V. Nickel Accumulation in Rape Shoots (Brassica napus L.) Increased by Putrescine. Int. J. Phytoremediation 2011, 13, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Pottosin, I.; Velarde-Buendía, A.M.; Bose, J.; Zepeda-Jazo, I.; Shabala, S.; Dobrovinskaya, O. Cross-Talk between Reactive Oxygen Species and Polyamines in Regulation of Ion Transport across the Plasma Membrane: Implications for Plant Adaptive Responses. J. Exp. Bot. 2014, 65, 1271–1283. [Google Scholar] [CrossRef] [Green Version]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative Stress and Some Antioxidant Systems in Acid Rain-Treated Bean Plants: Protective Role of Exogenous Polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Rahman, H.; Sabreen, S.; Alam, S.; Kawai, S. Effects of Nickel on Growth and Composition of Metal Micronutrients in Barley Plants Grown in Nutrient Solution. J. Plant Nutr. 2005, 28, 393–404. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Regulatory role of polyamines in growth, development and abiotic stress tolerance in plants. In Plant Adaptation to Environmental Changes: Significance of Amino Acids and Their Derivatives; Anjum, N.A., Gill, S.S., Gill, R., Eds.; CABI Publishers: Wallingford, CT, USA, 2014; pp. 157–193. [Google Scholar]
- Liu, J.-H.; Wang, W.; Wu, H.; Gong, X.; Moriguchi, T. Polyamines Function in Stress Tolerance: From Synthesis to Regulation. Front. Plant Sci. 2015, 6, 827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brüggemann, L.I.; Pottosin, I.I.; Schönknecht, G. Cytoplasmic Polyamines Block the Fast-Activating Vacuolar Cation Channel. Plant J. 1998, 16, 101–105. [Google Scholar] [CrossRef]
- Pál, M.; Horváth, E.; Janda, T.; Páldi, E.; Szalai, G. Physiological Changes and Defense Mechanisms Induced by Cadmium Stress in Maize. J. Plant Nutr. Soil Sci. 2006, 169, 239–246. [Google Scholar] [CrossRef]
- Chen, L.; Wang, L.; Chen, F.; Korpelainen, H.; Li, C. The Effects of Exogenous Putrescine on Sex-Specific Responses of Populus cathayana to Copper Stress. Ecotoxicol. Environ. Saf. 2013, 97, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Pietrini, F.; Iori, V.; Cheremisina, A.; Shevyakova, N.I.; Radyukina, N.; Kuznetsov, V.V.; Zacchini, M. Evaluation of Nickel Tolerance in Amaranthus paniculatus L. Plants by Measuring Photosynthesis, Oxidative Status, Antioxidative Response and Metal-Binding Molecule Content. Environ. Sci. Pollut. Res. Int. 2015, 22, 482–494. [Google Scholar] [CrossRef]
- Shevyakova, N.I.; Il’ina, E.N.; Kuznetsov, V.V. Polyamines Increase Plant Potential for Phytoremediation of Soils Polluted with Heavy Metals. Dokl. Biol. Sci. 2008, 423, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, S.S.; Pramanick, P.; Talukder, P.; Basak, A. Chapter 6-Polyamines, Metallothioneins, and Phytochelatins—Natural Defense of Plants to Mitigate Heavy Metals. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Bioactive Natural Products; Elsevier: Amsterdam, The Netherlands, 2021; Volume 69, pp. 227–261. [Google Scholar]
- Kuthanová, A.; Gemperlová, L.; Zelenková, S.; Eder, J.; Machácková, I.; Opatrný, Z.; Cvikrová, M. Cytological Changes and Alterations in Polyamine Contents Induced by Cadmium in Tobacco BY-2 Cells. Plant Physiol. Biochem. 2004, 42, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.; Singh, R.P. Cadmium-Induced Changes in Diamine Oxidase Activity and Polyamine Levels in Vigna Radiata Wilczek Seedlings. J. Plant Physiol. 2000, 156, 704–710. [Google Scholar] [CrossRef]
- Pál, M.; Csávás, G.; Szalai, G.; Oláh, T.; Khalil, R.; Yordanova, R.; Gell, G.; Birinyi, Z.; Németh, E.; Janda, T. Polyamines May Influence Phytochelatin Synthesis during Cd Stress in Rice. J. Hazard. Mater. 2017, 340, 272–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rady, M.; El-Yazal, M.; Taie, H.; Ahmed, S. Response of Wheat Growth and Productivity to Exogenous Polyamines under Lead Stress. J. Crop. Sci. Biotechnol. 2016, 19, 363–371. [Google Scholar] [CrossRef]
- Rady, M.M.; Hemida, K.A. Modulation of Cadmium Toxicity and Enhancing Cadmium-Tolerance in Wheat Seedlings by Exogenous Application of Polyamines. Ecotoxicol. Environ. Saf. 2015, 119, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Bagni, N.; Tassoni, A. Biosynthesis, Oxidation and Conjugation of Aliphatic Polyamines in Higher Plants. Amino Acids 2001, 20, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-J.; Harding, S.A.; Tschaplinski, T.J.; Lindroth, R.L.; Yuan, Y. Genome-Wide Analysis of the Structural Genes Regulating Defense Phenylpropanoid Metabolism in Populus. New Phytol 2006, 172, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Shi, G.X.; Li, W.L.; Wu, W.L. Exogenous Spermidine Enhances Hydrocharis Dubia Cadmium Tolerance. Russ J. Plant Physiol. 2013, 60, 770–775. [Google Scholar] [CrossRef]
- Zimmerlin, A.; Wojtaszek, P.; Bolwell, G.P. Synthesis of Dehydrogenation Polymers of Ferulic Acid with High Specificity by a Purified Cell-Wall Peroxidase from French Bean (Phaseolus vulgaris L.). Biochem. J. 1994, 299 Pt 3, 747–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fridovich, I. Superoxide Radical and Superoxide Dismutases. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, I.; Mannervik, B. Glutathione Reductase. Methods Enzymol. 1985, 113, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B. Some Methodological Problems in the Determination of Antioxidant Activity Using Chromogen Radicals: A Practical Case. Trends Food Sci. Technol. 2000, 11, 419–421. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.; Aruoma, O.I. The Deoxyribose Method: A Simple “Test-Tube” Assay for Determination of Rate Constants for Reactions of Hydroxyl Radicals. Anal. Biochem. 1987, 165, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Hensley, K.; Mou, S.; Pye, Q.N. Nitrite determination by colorimetric and fluorometric Griess diazotization assays. In Methods in Pharmacology and Toxicology: Methods in Biological Oxidative Stress; Hensley, K., Floyd, R.A., Eds.; Humana Press Inc.: Totowa, NJ, USA, 2009; pp. 185–193. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devasagayam, T.; Boloor, K.; Ramasarma, T. Methods for Estimating Lipid Peroxidation: An Analysis of Merits and Demerits. Indian J. Biochem. Biophys. 2003, 40, 300–308. [Google Scholar] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Chen, K.H.; Miller, A.N.; Patterson, G.W.; Cohen, J.D. A Rapid and Simple Procedure for Purification of Indole-3-Acetic Acid Prior to GC-SIM-MS Analysis. Plant Physiol. 1988, 86, 822–825. [Google Scholar] [CrossRef] [PubMed]
- Rapparini, F.; Tam, Y.Y.; Cohen, J.D.; Slovin, J.P. Indole-3-Acetic Acid Metabolism in Lemna Gibba Undergoes Dynamic Changes in Response to Growth Temperature. Plant Physiol. 2002, 128, 1410–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baraldi, R.; Bertazza, G.; Bogino, J.; Luna, V.; Bottini, R. Effect of Light Quality on Prunus Cerasus II. Changes in Hormone Levels in Plants Grown under Different Light Conditions. Photochem. Photobiol. 1995, 62, 800–803. [Google Scholar] [CrossRef]
- Scaramagli, S.; Biondi, S.; Capitani, F.; Gerola, P.; Altamura, M.M.; Torrigiani, P. Polyamine Conjugate Levels and Ethylene Biosynthesis: Inverse Relationship with Vegetative Bud Formation in Tobacco Thin Layers. Physiol. Plant. 1999, 105, 366–375. [Google Scholar] [CrossRef]
- Kassambara, A. Pipe-Friendly Framework for Basic Statistical Tests [R Package Rstatix Version 0.7.0]; Free Software Foundation Inc.: Boston, MA, USA, 2021. [Google Scholar]
- Wickham, H. Ggplot2. WIREs Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]


| Ni | Cd | |
|---|---|---|
| Root metal accumulation (mg kg−1) | 152.77 ± 8.01 | 17.46 ± 2.46 |
| Leaf metal accumulation (mg kg−1) | 23.36 ± 0.75 | 31.98 ± 2.52 |
| Leaf calcium accumulation (mg kg−1) | 11.86 ± 0.60 | 10.70 ± 0.29 |
| Root calcium accumulation (mg kg−1) | 8.24 ± 1.16 | 7.22 ± 0.97 |
| Leaf magnesium accumulation (mg kg−1) | 9.07 ± 0.29 | 8.35 ± 0.23 |
| Root magnesium accumulation (mg kg−1) | 5.55 ± 0.55 | 6.49 ± 0.99 |
| Root bioconcentration factor (rBCF) | 0.75 | 1.97 |
| Aboveground bioconcentration factor (aBCF) | 0.18 | 5.02 |
| Translocation factor (TF) | 24.62 | 261.8 |
| Leaf nitrogen content (mg g−1) | 19.9 ± 3.3 | 15.3 ± 1.4 |
| Root nitrogen content (mg g−1) | 9.75 ± 3.2 | 6.77 ± 1.2 |
| Leaf carbon content (mg g−1) | 420.5 ± 13.3 | 417.3 ± 12.5 |
| Root carbon content (mg g−1) | 391.3 ± 27.8 | 344.4 ± 10.8 |
| Horizon | Depth (cm) | pH (in H2O) | Humus (%) | CaCO2 (%) | Particle Size Composition | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coarse Sand (˃0.2) | Fine Sand (0.2–0.02) | Silt (0.02–0.002) | Clay (<0.002) | Total Sand (˃0.02) | Total Clay (<0.02) | |||||
| Ap | 0–30 | 7.55 | 2.64 | 17.08 | 0.5 | 37.4 | 40.4 | 21.7 | 37.9 | 62.1 |
| I | 30–58 | 7.91 | 1.58 | 19.56 | 0.3 | 45.9 | 34.8 | 19.0 | 46.2 | 53.8 |
| II | 58–72 | 8.08 | 1.00 | 16.06 | 0.3 | 71.0 | 15.9 | 12.8 | 71.3 | 28.7 |
| III Geo | 72–110 | 8.22 | 1.09 | 19.10 | 1.9 | 40.5 | 37.7 | 19.9 | 42.4 | 57.6 |
| IV Geo | 110–175 | 8.53 | 0.46 | 15.93 | 2.5 | 88.5 | 1.5 | 7.5 | 91.0 | 9.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kebert, M.; Kostić, S.; Vuksanović, V.; Gavranović Markić, A.; Kiprovski, B.; Zorić, M.; Orlović, S. Metal- and Organ-Specific Response to Heavy Metal-Induced Stress Mediated by Antioxidant Enzymes’ Activities, Polyamines, and Plant Hormones Levels in Populus deltoides. Plants 2022, 11, 3246. https://doi.org/10.3390/plants11233246
Kebert M, Kostić S, Vuksanović V, Gavranović Markić A, Kiprovski B, Zorić M, Orlović S. Metal- and Organ-Specific Response to Heavy Metal-Induced Stress Mediated by Antioxidant Enzymes’ Activities, Polyamines, and Plant Hormones Levels in Populus deltoides. Plants. 2022; 11(23):3246. https://doi.org/10.3390/plants11233246
Chicago/Turabian StyleKebert, Marko, Saša Kostić, Vanja Vuksanović, Anđelina Gavranović Markić, Biljana Kiprovski, Martina Zorić, and Saša Orlović. 2022. "Metal- and Organ-Specific Response to Heavy Metal-Induced Stress Mediated by Antioxidant Enzymes’ Activities, Polyamines, and Plant Hormones Levels in Populus deltoides" Plants 11, no. 23: 3246. https://doi.org/10.3390/plants11233246










