Emulsification of Rosemary and Oregano Aqueous Extracts and Their In Vitro Bioavailability
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Average Feret Diameters of Emulsified Rosemary/Oregano Extracts
2.2. Physical and Chemical Properties of Oil-in-Water Emulsions Containing Rosemary/Oregano Extract
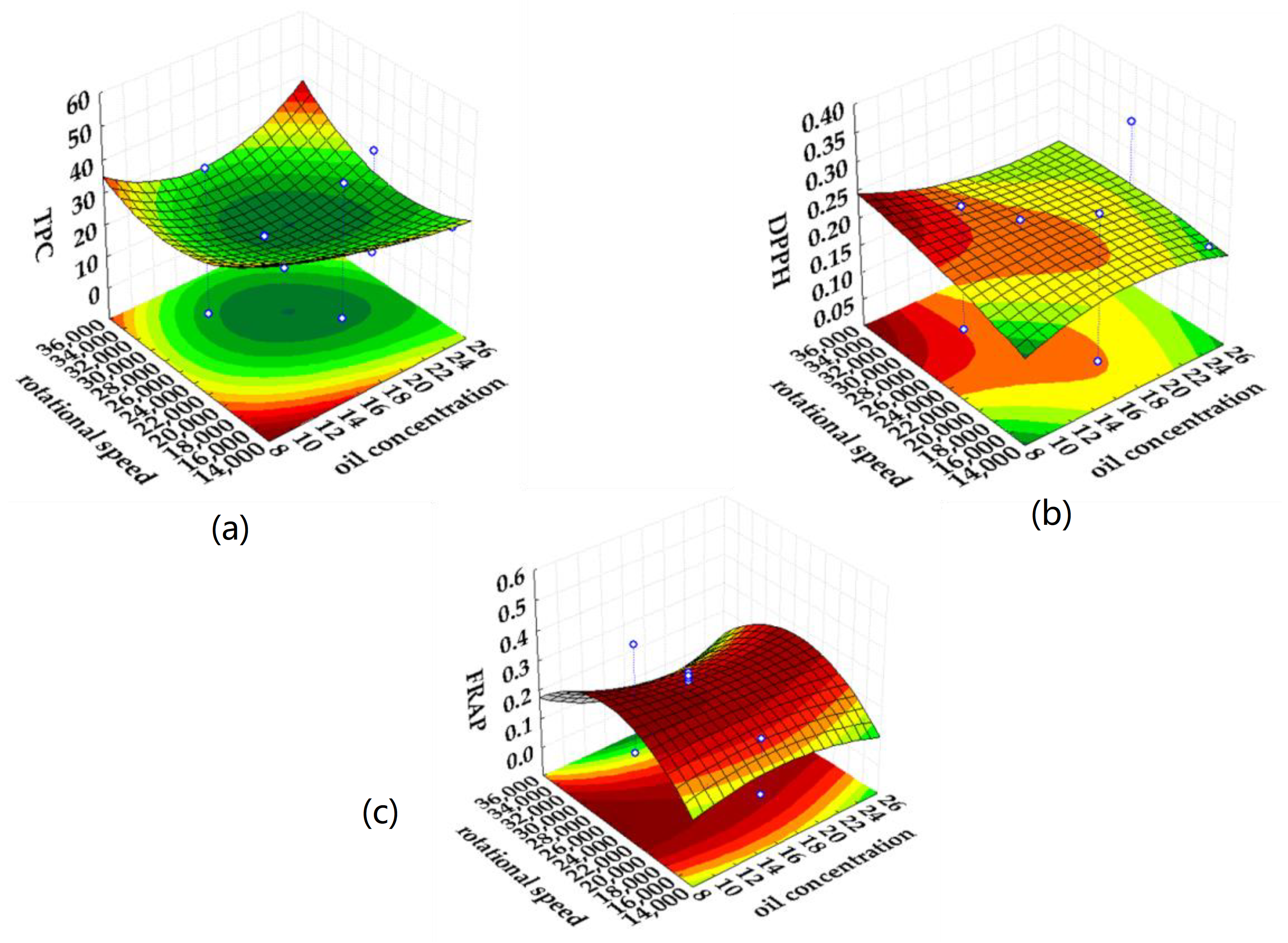
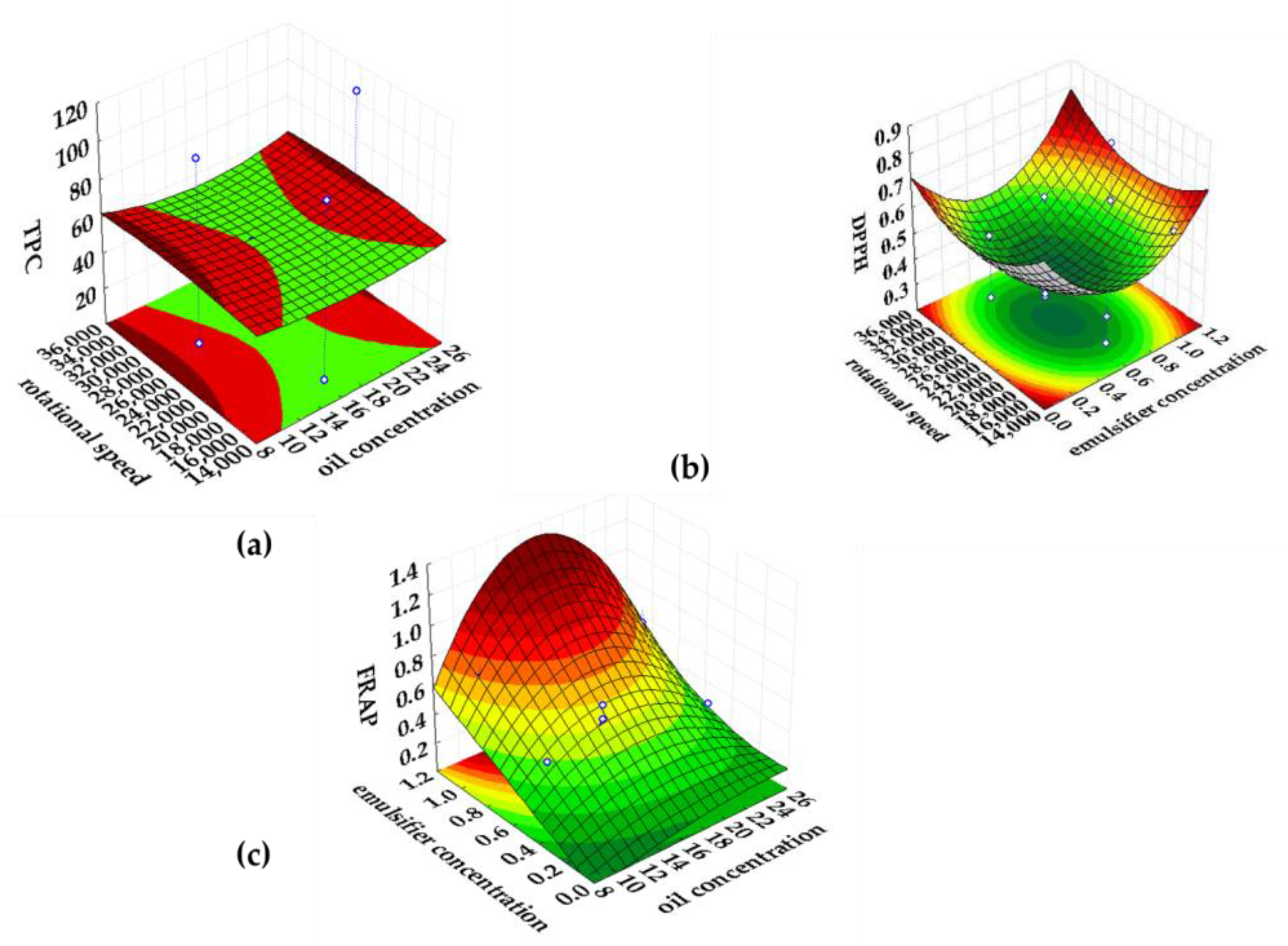
2.3. Response Surface Modeling of the Emulsification Process
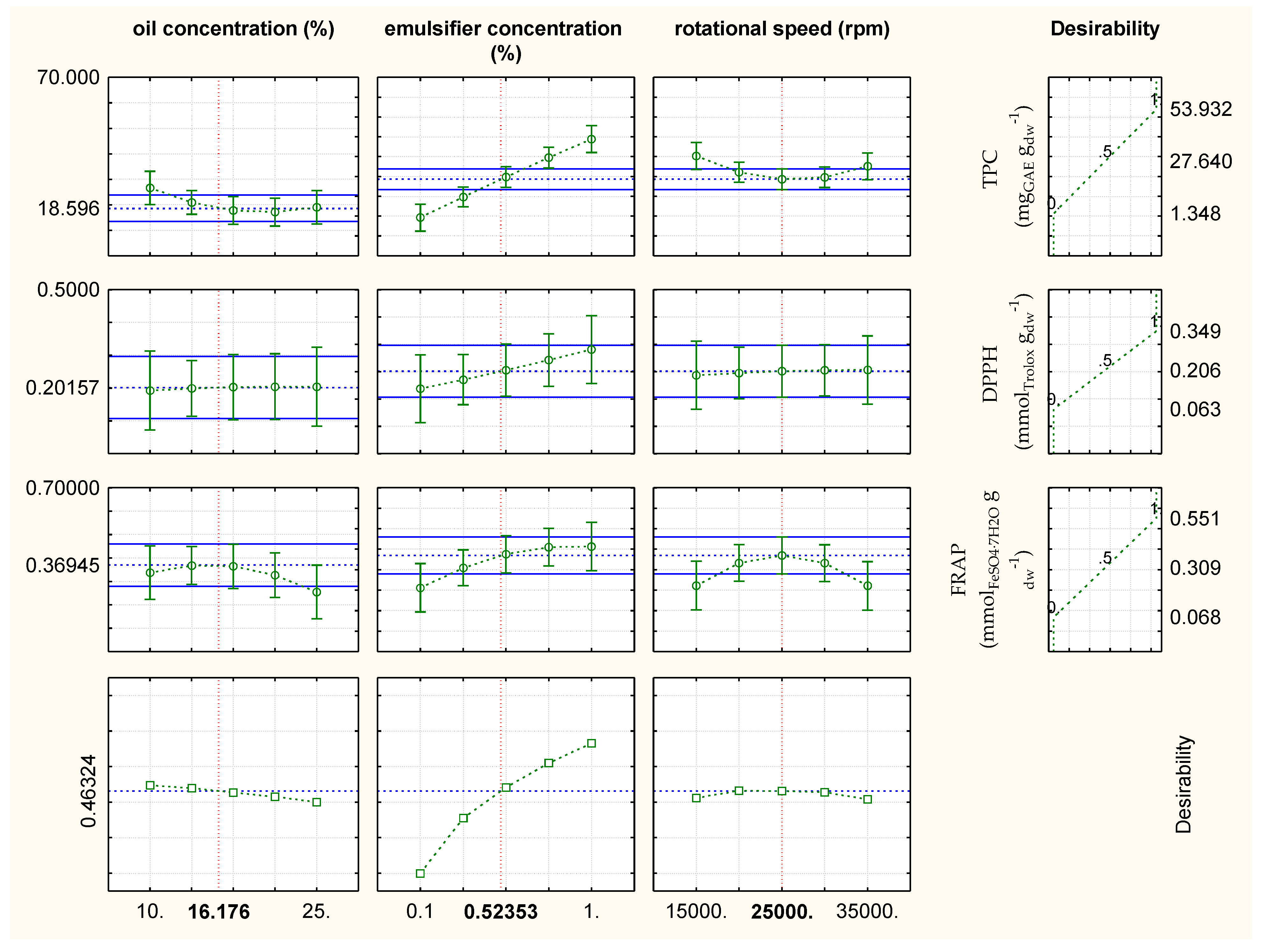
2.4. Optimization of the Emulsification Process with Respect to Chemical Properties of Oil-in-Water Emulsions Containing Rosemary/Oregano Extract with the Addition of Emulsifier
2.5. The In Vitro Digestion of Oil-in-Water Emulsions Containing Rosemary/Oregano Extract
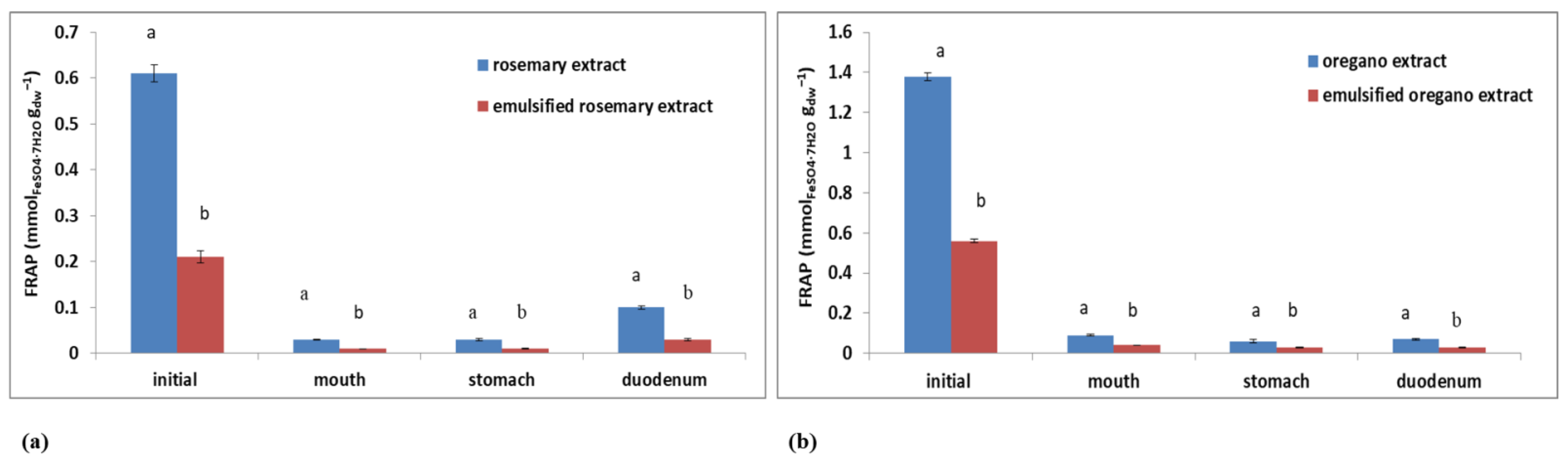
2.6. Bioavailability of Polyphenolic Compounds from Rosemary/Oregano Extracts and Oil-in-Water Emulsions Containing Rosemary/Oregano Extract
3. Materials and Methods
3.1. Materials
3.1.1. Plant Materials, Sunflower Oil and Emulsifier
3.1.2. Chemicals
3.2. Methods
3.2.1. Preparation of Aqueous Plant Extracts
3.2.2. Preparation of Pea Protein Suspensions in Aqueous Plant Extracts
3.2.3. Determination of the Concentration of Commercial Pea Protein According to the Bradford Method
3.2.4. Oil-in-Water Emulsions Containing Plant Extract Preparation
3.2.5. Extraction Yield
3.2.6. Determination of Physical Characteristics of Oil-in-Water Emulsions Containing Plant Extract
Determination of Zeta Potential, Electrical Conductivity and Total Dissolved Solids
Determination of the Average Feret’s Droplet Diameter of Oil-in-Water Emulsions Containing Rosemary/Oregano Extract
In Vitro Digestion
3.2.7. Determination of Chemical Characteristics of Aqueous Plant Extracts and Oil-in-Water Emulsions Containing Plant Extract
Determination of Total Polyphenolic Content (TPC)
Determination of Antioxidant Activity by DPPH Method
Determination of Antioxidant Activity by FRAP Method
3.2.8. Response Surface Methodology (RSM)
3.2.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chakrabartty, I.; Mohanta, Y.K.; Nongbet, A.; Mohanta, T.K.; Mahanta, S.; Das, N.; Saravanan, N.; Sharma, N. Exploration of Lamiaceae in cardio vascular diseases and functional foods: Medicine as food and food as medicine. Front. Pharmacol. 2022, 13, 894814. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, J.; Brusač Kovačević, D.; Putnik, P.; Gabrić, D.; Bilušić, T.; Krešić, G.; Stulić, V.; Barba, F.J.; Chemat, F.; Barbosa-Cánovas, G.; et al. Extraction of bioactive compounds and essential oils from Mediterra nean herbs by conventional and green innovative techniques: A review. Food Res. Int. 2018, 133, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Rattray, R.D.; Van Wyk, B.E. The botanical, chemical and ethnobotanical diversity of Southern African Lamiaceae. Molecules 2021, 26, 3712. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, E.; Kubaa Tovaa, A.; Senorans, F.J.; Cavero, S.; Reglero, G.; Hawthorne, S.B. Subcritical water extraction of antioxidant compounds from rosemary plants. J. Agric. Food Chem. 2003, 51, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Gutrièrrez-Grijalva, E.P.; Picos-Salas, M.A.; Leyva-Lòpez, N.; Criollo-Mendoza, M.S.; Vazquez-Olivo, G.; Heredia, B.J. Flavonoids and phenolic acids from oregano: Occurrence, biological activity and health benefits. Plants 2017, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Fernandez-Ochoa, A.; Borras-Linares, I.; Perez-Sanchez, A.; Barrajon-Catalan, E.; Gonzalez-Alvarez, I.; Arraez-Román, D.; Micol, V.; Segura-Carretero, A. Phenolic compounds in rosemary as potential source of bioactive compounds against colorectal cancer: In situ absorption and metabolism study. J. Funct. Food 2017, 33, 202–210. [Google Scholar] [CrossRef]
- Piementel-Moral, S.; Teixiera, M.C.; Fernandes, A.R.; Arrae-Roman, D.; Martínez-Férez, A.; Segura-Carretero, A.; Souto, E.B. Lipid nanocarriers for the loading of polyphenols—A comprehensive review. Adv. Colloid Interface Sci. 2018, 260, 85–94. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lèvy, F. Encapsulation of natural polyphenolic compounds; A review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Kelly, A.L.; Miao, S. Emulsion-based encapsulation and delivery systems for polyphenols. Trends Food Sci. Technol. 2016, 47, 1–9. [Google Scholar] [CrossRef]
- Tian, H.; Xiang, D.; Li, C. Tea polyphenols encapsulated in W/O/W emulsions with xanthan gum–locust bean gum mixture: Evaluation of their stability and protection. Int. J. Biol. Macromol. 2021, 175, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Li, T.; Li, J.; Liu, L.; Dou, X.; Zhang, X. Development and evaluation of tea polyphenols loaded water in oil emulsion with zein as stabilizer. J. Drug Deliv. Sci. Technol. 2020, 56, 101528. [Google Scholar] [CrossRef]
- Bamba, B.S.B.; Shi, J.; Tranchant, C.C.; Xue, S.J.; Forney, C.F.; Lim, L.-T.; Xu, W.; Xu, G. Coencapsulation of polyphenols and anthocyanins from blueberry pomace by double emulsion stabilized by whey proteins: Effect of homogenization parameters. Molecules 2018, 23, 2525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Östbring, K.; Nilsson, K.; Ahlström, C.; Fridolfsson, A.; Rayner, M. Emulsifying and anti-oxidative properties of proteins extracted from industrially cold-pressed rapeseed press-cake. Foods 2020, 9, 678. [Google Scholar] [CrossRef]
- Burger, T.; Zhang, Y. Recent progress in the utilization of pea protein as an emulsifier for food applications. Trends Food Sci. Technol. 2019, 86, 25–33. [Google Scholar] [CrossRef]
- Kutzli, I.; Griener, D.; Gibis, M.; Grossmann, L.; Baier, S.K.; Weiss, J. Improvement of emulsifying behavior of pea proteins as plant-based emulsifiers via Maillard-induced glycation in electrospun pea protein–maltodextrin fibers. Food Funct. 2020, 11, 4049–4056. [Google Scholar] [CrossRef]
- Loper Francisco, C.R.; de Oliviera Junior, F.D.; Marin, G.; Dutra Alvim, I.; Dupas Hubinger, M. Plant proteins at low concentrations as natural emulsifiers for an effective orange essential oil microencapsulation by spray drying. Colloids Surf. A 2020, 607, 125470. [Google Scholar] [CrossRef]
- Sridharan, S.; Meinders, M.B.J.; Bitter, J.H.; Nikiforidis, C.V. On the emulsifying properties of self-assembled pea protein particles. Langmuir 2020, 36, 12221–12229. [Google Scholar] [CrossRef]
- Singh, H.; Ye, A.; Horne, D. Structuring food emulsions in the gastrointestinal tract to modify lipid digestion. Prog. Lipid Res. 2009, 48, 92–100. [Google Scholar] [CrossRef]
- Zalewska, A.; Zwierz, K.; Zólkowski, K.; Gindzienski, A. Structure and biosynthesis of human salivary mucins. Acta Biochim. Pol. 2000, 47, 1067–1079. [Google Scholar] [CrossRef]
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Browning, A.M.; Steed, L.L.; Reed, S.G.; Walle, U.K. Flavonoid glucosides are hydrolyzed and thus activated in the oral cavity in humans. J. Nutr. 2005, 135, 48–52. [Google Scholar] [CrossRef] [Green Version]
- Németh, K.; Plumb, G.W.; Berrin, J.G.; Juge, N.; Jacob, R.; Naim, H.Y.; Williamson, G. Deglycosylation by small intestinal epitherial cell beta-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur. J. Clin. Nutr. 2003, 42, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itzel Peña-Vázquez, G.; Dominguez-Fernández, M.T.; Dianey Camacho-Zamora, B.; Hernandez-Salazar, M.; Urías-Orona, V.; De Peña, M.-P.; de la Garza, A.L. In vitro simulated gastrointestinal digestion impacts bioaccessibility and bioactivity of Sweet orange (Citrus sinensis) phenolic compounds. J. Funct. Foods 2022, 88, 104891. [Google Scholar] [CrossRef]
- Jurinjak Tušek, A.; Marić, L.; Benković, M.; Valinger, D.; Jurina, T.; Gajdoš Kljusurić, J. In-vitro digestion of the bioactives originating from the Lamiaceae family herbal teas: A kinetic and PLS modeling study. J. Food Biochem. 2020, 44, e13233. [Google Scholar] [CrossRef]
- Katsouli, M.; Polychniatou, V.; Tzia, C. Optimization of water in olive oil nano-emulsions composition with bioactive compounds by response surface methodology. LWT Food Sci. Technol. 2018, 89, 740–748. [Google Scholar] [CrossRef]
- McClements, D.J. Theoretical analysis of factors affecting the formation and stability of multilayered colloidal dispersions. Langmuir 2005, 21, 9777–9785. [Google Scholar] [CrossRef]
- van de Linde, S. Single-molecule localization microscopy analysis with ImageJ. J. Phys. D 2019, 52, 203002. [Google Scholar] [CrossRef]
- Karadag, A.; Yang, X.; Ozcelik, B.; Huang, Q. Optimization of preparation conditions for quercetion nanoemulsions using Response Surface Methodology. J. Agric. Food Chem. 2013, 61, 2130–2139. [Google Scholar] [CrossRef]
- Sohn, B.; Olenskyj, A.; Feng, Y. Correlation between emulsifier concentration and emulsion droplet size in oil-in-water emulsion stabilized by zein nanoparticles. i-ACES 2017, 3, 1. [Google Scholar]
- Larsson, M.; Hill, A.; Duffy, J. Suspension stability; Why particle size. zeta potential and rheology are important. Ann. T Nord. Rheol. Soc. 2012, 20, 6. [Google Scholar] [CrossRef] [Green Version]
- Tomac, V. Electrokinetic Potential. Bachelor’s Thesis, University of Josip Juraj Strossmayer Osijek, Osijek, Poland, 30 September 2021. [Google Scholar]
- Ivanković, M. Optimizing the Conditions of Emulsification of Biologically Active Compounds Originating from the Lamiaceae Family. Bachelor’s Thesis, University of Zagreb, Zagreb, Croatia, 30 September 2020. [Google Scholar]
- Rezvani, E.; Schleining, G.; Taherian, A.R. Assessment of physical and mechanical properties of orange oil-in-water beverage emulsions using response surface methodology. LWT 2012, 48, 82–88. [Google Scholar] [CrossRef]
- Hinderink, E.B.A.; Münch, K.; Sagis, L.; Scroënb, K.; Berton-Carabin, C.C. Synergistic stabilisation of emulsions by blends of dairy and soluble pea proteins: Contribution of the interfacial composition. Food Hydrocoll. 2019, 97, 105206. [Google Scholar] [CrossRef]
- Vallverdù-Queralt, A.; Regueiro, J.; Marìnez-Huélamo, M.; Alvarenga, J.F.; Leal, L.N.; Lamuela-Raventos, R.M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014, 154, 299–307. [Google Scholar] [CrossRef]
- USDA Ag Data Commons. U.S. Department of Agriculture. Available online: https://data.nal.usda.gov (accessed on 19 November 2022).
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, W.G.; Walker, R.B. Thorough study of various compound classes toward the Folin-Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastola, K.P.; Guragain, Y.N.; Bhadriraju, V.; Vadlani, P.V. Evaluation of standards and interfering compounds in the determination of phenolics by Folin-Ciocalteu assay method for effective bioprocessing of biomass. J. Anal. Chem. 2017, 8, 416–431. [Google Scholar] [CrossRef] [Green Version]
- Bhale, S.D.; Xu, Z.; Prinyawiwatkul, W.; King, J.M.; Godber, J.S. Oregano and rosemary extracts inhibit oxidation of long-chain n-3 fatty acids in menhaden oil. Food Chem. Toxicol. 2007, 72, C504–C508. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.; Kang, S.N. Antioxidant activities of hot water extracts from various spices. Int. J. Mol. Sci. 2012, 12, 4120–4131. [Google Scholar] [CrossRef]
- El-Guendouz, S.; Aazza, S.; Anahi Dandlen, S.; Majdoub, N.; Lyoussi, B.; Raposo, S.; Dulce Antunes, M.; Gomes, V.; Graça Miguel, M. Antioxidant activity of thyme waste extract in O/W emulsions. Antioxidants 2019, 8, 243. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Shin, J.-A.; Hong, S.T.; Lee, K.-T. Stability and antioxidant effect of rapeseed extract in oil-in-water emulsion. Korean J. Agric. Sci. 2016, 43, 249–257. [Google Scholar] [CrossRef]
- Zeb, A. A comprehensive review on different classes of polyphenolic compounds present in edible oils. Food Res. Int. 2021, 143, 110312. [Google Scholar] [CrossRef]
- Thanonkaew, A.; Wongyai, S.; Decker, E.A.; McClements, D.J. Formation, antioxidant property and oxidative stability of cold pressed rice bran oil emulsion. J. Food Sci. Technol. 2015, 52, 6520–6528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matešić, N.; Jurina, T.; Benković, M.; Panić, M.; Valinger, D.; Gajdoš Kljusurić, J.; Jurinjak Tušek, A. Microwave-assisted extraction of phenolic compounds from Cannabis sativa L.: Optimization and kinetics study. Sep. Sci. Technol. 2021, 56, 2047–2060. [Google Scholar] [CrossRef]
- Mehmood, T.; Ahmad, A.; Ahmed, A.; Ahmed, Z. Optimization of olive oil based O/W nanoemulsions prepared through ultrasonic homogenization: A response surfave methodology approach. Food Chem. 2017, 229, 790–793. [Google Scholar] [CrossRef]
- Chong, W.T.; Tan, C.P.; Cheah, Y.K.; Lajis, A.F.; Habi Mat Dian, N.L.; Kanagaratnam, S.; Lai, O.-M. Optimization of process parameters in preparation of tocotrienol-rich red palm oil-based nanoemulsion stabilized by Tween80-Span 80 using response surface methodology. PLoS ONE 2018, 13, e0202771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurinjak Tušek, A.; Benković, M.; Belščak-Cvitanović, A.; Valinger, D.; Jurina, T.; Gajdoš Kljusurić, J. Kinetics and thermodynamics of the solid-liquid extraction process of total polyphenols, antioxidants and extraction yield from Asteraceae plants. Ind. Crop. Prod. 2016, 91, 205–214. [Google Scholar] [CrossRef]
- Saad, R.; Asmni, F.; Saad, M.; Hussain, M.; Khan, J.; Kaleemullah, M. A new approach for predicting antioxidant propriety of herbal extracts. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 166–174. [Google Scholar]
- Tarko, T.; Duda-Chodak, A. Influence of food matrix on the bioaccessibility of fruit polyphenolic compound. J. Agric. Food Chem. 2020, 68, 1315–1325. [Google Scholar] [CrossRef]
- Đurđević, M. Bioavailability of Phenolic Compounds from Oil-in-Water Emulsions from Plants Belonging to Lamiaceae Family during in vitro digestion. Bachelor’s Thesis, University of Zagreb, Zagreb, Croatia, 30 September 2020. [Google Scholar]
- Gutiérrez-Grijalva, E.P.; Anqulo-Escalante, M.A.; León-Félix, J. Effect of in vitro digestion on the total antioxidant capacity and phenolic content of 3 species of oregano (Hedeoma patens, Lippia graveolens, Lippia palmeri). J. Food Sci. 2017, 82, 2832–2839. [Google Scholar] [CrossRef]
- Sridharan, S.; Meinders, M.B.J.; Bitter, J.H.; Nikiforidis, C.V. Pea flour as stabilizer of oil-in-water emulsions: Protein purification unnecessary. Food Hydrocoll. 2020, 101, 105533. [Google Scholar] [CrossRef]
- Ernst, O.; Zor, T. Linearization of the Bradford Protein Assay. J. Vis. Exp. 2010, 38, e1918. [Google Scholar] [CrossRef] [PubMed]
- Yolmeh, M.; Mahdi Jafari, S. Applications of Response Surface Methodology in the food industry processes. Food Bioprocess Techol. 2017, 10, 413–433. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis (Method 930.15); Association of Official Analytical Chemists: Arlington, VA, USA, 1995. [Google Scholar]
- Grgić, F.; Benković, M.; Valinger, D.; Jurina, T.; Gajdoš Kljusurić, J.; Jurinjak Tušek, A. Macro-batch and continuously operated microfluidic emulsification—Differences, Similarities and Optimization. Processes 2022, 10, 449. [Google Scholar] [CrossRef]
- Ortega, N.; Reguant, J.; Romero, M.P.; Macià, A.; Motilva, M.J. Effect of fat content on the digestibility and bioaccessibility of cocoa polyphenol by an in vitro digestion model. J. Agric. Food Chem. 2009, 57, 5743–5749. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power“: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]





| Sample | Zeta Potential (mV) | Conductivity (mS cm−1) | ||
|---|---|---|---|---|
| Rosemary | Oregano | Rosemary | Oregano | |
| 1 | −43.22 ± 1.76 A,a | −42.47 ± 2.73 A,a | 0.0063 ± 0.00 A,a | 0.0051 ± 0.00 B,a |
| 2 | −49.91 ± 3.37 A,a,c | −45.20 ± 0.95 A,a | 0.0067 ± 0.00 A,a,f | 0.0075 ± 0.00 A,b |
| 3 | −51.14 ± 2.87 A,a,c | −52.28 ± 1.35 A,b | 0.0099± 0.00 A,a,f | 0.0164 ± 0.00 B,c |
| 4 | −47.07 ± 1.98 A,a | −48.65 ± 2.78 A,a | 0.0104 ± 0.00 A,a,f | 0.0112 ± 0.00 A,b |
| 5 | −48.32 ± 1.41 A,a | −43.09 ± 2.45 A,a | 0.0117 ± 0.00 A,b,f | 0.0093 ± 0.00 A,b |
| 6 | −41.37 ± 4.50 A,a | −46.97 ± 3.76 A,a | 0.0103 ± 0.00 A,a,f | 0.0499 ± 0.00 B,a |
| 7 | −51.77 ± 0.67 A,a,c | −37.71 ± 2.31 B,a | 0.0301 ± 0.00 A,c | 0.0099 ± 0.00 B,b |
| 8 | −67.22 ± 2.24 A,b,c | −57.18 ± 4.47 B,c | 0.0453 ± 0.00 A,d | 0.0286 ± 0.00 B,d |
| 9 | −39.53 ± 0.26 A,a,c | −51.39 ± 3.53 B,a,c | 0.0234 ± 0.00 A,e,i | 0.0064 ± 0.00 B,b |
| 10 | −46.29 ± 5.87 A,a,b | −43.94 ± 1.73 A,a,d | 0.0114 ± 0.00 A,f | 0.0177 ± 0.00 B,c |
| 11 | −40.50 ± 1.84 A,a | −50.41 ± 1.20 B,a,c | 0.0043 ± 0.00 A,g,d | 0.0052 ± 0.00 B,b |
| 12 | −38.52 ± 3.28 A,a | −45.60 ± 3.98 A,a | 0.0092 ± 0.00 A,a,f | 0.0168 ± 0.00 B,c |
| 13 | −49.67 ± 2.88 A,a | −52.21 ± 1.92 A,d,c | 0.0223 ± 0.00 A,h,e,i | 0.0098 ± 0.00 B,b |
| 14 | −48.02 ± 2.95 A,a | −56.92 ± 1.22 A,e,c | 0.0251 ± 0.00 A,i,b | 0.0179 ± 0.00 B,c |
| 15 | −45.20 ± 2.16 A,a | −51.54 ± 3.50 A,f,c | 0.0072 ± 0.00 A,a,f | 0.0099 ± 0.00 A,b |
| 16 | −46.11 ± 0.60 A,a | −56.24 ± 2.48 B,g,c | 0.0148 ± 0.00 A,j,f | 0.0128 ± 0.00 A,c,b |
| 17 | −42.95 ± 3.73 A,a | −53.32 ± 0.30 B,h,c | 0.0098 ± 0.00 A,a,b,f | 0.0094 ± 0.00 A,b |
| Sample | TPC (mgGAE gdw−1) | DPPH (mmolTrolox gdw−1) | FRAP (mmolFeSO4·7H2O gdw−1) | |||
|---|---|---|---|---|---|---|
| Rosemary | Oregano | Rosemary | Oregano | Rosemary | Oregano | |
| 1 | 7.01 ± 0.00 A,a | 15.02 ±2.36 B,a | 0.08 ± 0.01 A,a | 0.64 ± 0.03 B,a | 0.13 ± 0.01 A,a | 0.06 ± 0.01 A,a |
| 2 | 1.35 ± 1.14 A,a | 9.18 ± 1.18 B,a,b | 0.32 ± 0.03 A,a | 0.41 ± 0.02 A,a | 0.12 ± 0.00 A,a | 0.15 ± 0.02 A,a |
| 3 | 50.97 ± 0.38 A,b | 113.79 ± 1.90 B,c | 0.30 ± 0.03 A,a | 0.78 ± 0.01 B,a,b | 0.49 ± 0.03 A,c | 0.71 ± 0.11 B,b,c |
| 4 | 33.71 ± 1.14 A,c | 111.28 ± 2.40 B,c,d | 0.13 ± 0.00 A,a | 0.56 ± 0.05 B,a,b | 0.22 ± 0.03 A,a | 0.62 ± 0.00 B,b,c |
| 5 | 37.48 ± 0.38 A,c | 54.25 ± 1.18 B,e | 0.17 ± 0.01 A,a | 0.40 ± 0.04 A,a,b | 0.16 ± 0.01 A,a,g | 0.43 ± 0.02 B,d,e |
| 6 | 25.35 ± 3.05 A,d | 53.97 ± 0.79 B,e | 0.18 ± 0.02 A,a | 0.30 ± 0.03 A,b,c | 0.13 ± 0.03 A,a | 0.14 ± 0.15 A,a |
| 7 | 28.04 ± 1.53 A,c,d | 55.92 ± 0.39 B,e | 0.22 ± 0.05 A,a | 0.80 ± 0.01 B,a | 0.15 ± 0.02 A,a | 0.18 ± 0.01 A,a |
| 8 | 32.63 ± 3.43 A,c,d | 56.20 ± 1.57 B,e,f | 0.19 ± 0.03 A,a | 0.83 ± 0.06 B,a | 0.13 ± 0.04 A,a | 0.33 ± 0.02 B,d,e |
| 9 | 13.75 ± 7.25 A,a,e | 9.74 ± 0.39 A,b,g,h | 0.07 ± 0.04 A,a,b | 0.66 ± 0.01 B,a,b | 0.07 ± 0.01 A,a,d | 0.16 ± 0.01 A,a |
| 10 | 53.93 ± 0.76 A,b | 105.16 ± 1.60 B,d,h | 0.33 ± 0.01 A,a | 0.60 ± 0.00 A,a,b | 0.26 ± 0.01 A,a,b | 1.01 ± 0.08 B,f |
| 11 | 3.51 ± 1.14 A,a | 6.68 ± 0.00 A,b,f | 0.06 ± 0.01 A,a,c | 0.66 ± 0.03 B,a,b | 0.09 ± 0.00 A,a,e | 0.07 ± 0.00 A,a |
| 12 | 45.57 ± 2.67 A,b | 107.39 ± 6.30 B,c,e,g | 0.35 ± 0.04 A,a,d | 0.67 ± 0.04 B,a,b | 0.24 ± 0.01 A,a | 0.99 ± 0.04 B,f |
| 13 | 22.92 ± 3.43 A,d | 54.81 ± 0.39 B,e | 0.18 ± 0.00 A,a | 0.31 ± 0.06 A,b,c | 0.32 ± 0.00 A,f | 0.51 ± 0.00 B,c,d |
| 14 | 22.65 ± 0.76 A,d | 57.03 ± 1.18 B,e | 0.18 ± 0.06 A,a | 0.34 ± 0.03 A,b,c | 0.29 ± 0.00 A,g | 0.48 ± 0.08 B,b,d |
| 15 | 17.53 ± 2.67 A,e | 52.58 ± 2.75 B,e,i | 0.20 ± 0.01 A,a | 0.32 ± 0.00 A,b,c | 0.30 ± 0.01 A,h | 0.65 ± 0.05 B,b,c |
| 16 | 15.64 ± 1.53 A,e | 56.48 ± 1.97 B,e | 0.20 ± 0.04 A,a | 0.35 ± 0.04 A,b,c | 0.31 ± 0.01 A,i | 0.55 ± 0.07 B,c,d |
| 17 | 14.02 ± 0.76 A,a,e | 45.90 ± 1.18 B,i | 0.23 ± 0.02 A,a | 0.69 ± 0.46 B,a | 0.13 ± 0.01 A,a,j | 0.36 ± 0.00 B,d,e |
| Analyzed Property | Input Variable | Intercept ± Standard Error | Regression Coefficients (L-Linear, Q-Quadratic) ± Standard Error | p | R2 | R2adj. |
|---|---|---|---|---|---|---|
| TPC (mgGAE gdw−1) | Oil concentration (%) | 27.647 ± 2.355 | −3.809 ± 1.725 (L) −2.232 ± 1.223 (Q) | 0.052 (L) 0.098 (Q) | 0.94 | 0.90 |
| Emulsifier concentration (%) | 19.197 ± 2.644 (L) 0.623 ± 2.147 (Q) | 0.000 (L) 0.778 (Q) | ||||
| Rotational speed (rpm) | −2.595 ± 1.725(L) −4.564 ± 1.189(Q) | 0.163(L) 0.003(Q) | ||||
| DPPH (mmolTrolox gdw−1) | Oil concentration (%) | 0.205 ± 0.043 | 0.006± 0.032 (L) 0.002 ± 0.022 (Q) | 0.857 (L) 0.932 (Q) | 0.35 | 0.00 |
| Emulsifier concentration (%) | 0.075 ± 0.048 (L) −0.003 ± 0.039 (Q) | 0.155 (L) 0.940 (Q) | ||||
| Rotational speed (rpm) | 0.01 ± 0.032 (L) 0.002 ± 0.022(Q) | 0.766 (L) 0.908 (Q) | ||||
| FRAP (mmolFeSO4·7H2O gdw−1) | Oil concentration (%) | 0.142 ± 0.041 | −0.041 ± 0.030 (L) 0.030 ± 0.021(Q) | 0.199 (L) 0.187 (Q) | 0.76 | 0.62 |
| Emulsifier concentration (%) | 0.045 ± 0.046 (L) 0.056 ± 0.037 (Q) | 0.354 (L) 0.165 (Q) | ||||
| Rotational speed (rpm) | −0.0008 ± 0.030 (L) 0.074 ± 0.021 (Q) | 0.979 (L) 0.005(Q) |
| Analyzed Property | Input Variable | Intercept ± Standard Error | Regression Coefficients (L-Linear, Q-Quadratic) ± Standard Error | p | R2 | R2adj. |
|---|---|---|---|---|---|---|
| TPC (mgGAE gdw−1) | Oil concentration (%) | 59.375 ± 0.978 | −1.043 ± 0.708 (L) −1.408 ± 0.515(Q) | 0.175 (L) 0.023(Q) | 0.99 | 0.99 |
| Emulsifier concentration (%) | 48.826 ± 1.104 (L) 0.800 ± 0.905 (Q) | 0.000 (L) 0.399 (Q) | ||||
| Rotational speed (rpm) | 0.38254 ± 0.708542 (L) 1.304 ± 0.501 (Q) | 0.60 (L) 0.029 (Q) | ||||
| DPPH (mmolTrolox gdw−1) | Oil concentration (%) | 0.706 ± 0.059 | −0.065 ± 0.043 (L) −0.062 ± 0.031 (Q) | 0.164 (L) 0.079 (Q) | 0.76 | 0.60 |
| Emulsifier concentration (%) | 0.176 ± 0.067 (L) −0.145 ± 0.055 (Q) | 0.028 (L) 0.026 (Q) | ||||
| Rotational speed (rpm) | 0.126 ± 0.043 (L) −0.076 ± 0.030 (Q) | 0.016 (L) 0.033 (Q) | ||||
| FRAP (mmolFeSO4·7H2O gdw−1) | Oil concentration (%) | 0.430 ± 0.059 | −0.018 ± 0.043 (L) 0.109 ± 0.031 (Q) | 0.687 (L) 0.006 (Q) | 0.91 | 0.84 |
| Emulsifier concentration (%) | 0.380 ± 0.066 (L) −0.019 ± 0.054 (Q) | 0.0003 (L) 0.731 (Q) | ||||
| Rotational speed (rpm) | −0.020 ± 0.043 (L) 0.027 ± 0.030 (Q) | 0.644 (L) 0.400 (Q) |
| Sample | Total Dissolved Solids (g L−1) | Conductivity (mS cm−1) | ||
|---|---|---|---|---|
| Rosemary | Oregano | Rosemary | Oregano | |
| Plant extract (initial) | 0.09 ± 0.00 A,a | 0.31 ± 0.01 B,a | 0.18 ± 0.00 A,a | 0.63 ± 0.00 B,a |
| Plant extract (mouth) | 2.46 ± 0.02 A,b | 2.42 ± 0.06 A,b | 4.93 ± 0.03 A,b | 4.90 ± 0.08 A,b |
| Plant extract (stomach) | 1.25 ± 0.04 A,c | 1.35 ± 0.02 A,c | 2.50 ± 0.01 A,c | 2.61 ± 0.15 A,c |
| Plant extract (duodenum) | 4.28 ± 0.04 A,d | 4.88 ± 0.05 A,d | 8.39 ± 0.21 A,d | 9.80 ± 0.06 B,d |
| Emulsion (initial) | 0.02 ±0.00 A,a | 0.04 ±0.00 A,a | 0.04 ± 0.00 A,a | 0.01 ± 0.00 B,a |
| Emulsion (mouth) | 1.18 ± 0.03 A,b | 2.36 ± 0.15 B,b | 2.39 ± 0.02 A,b | 4.92 ± 0.08 B,b |
| Emulsion (stomach) | 1.61 ± 0.04 A,c | 1.23 ± 0.01 B,c | 3.24 ± 0.06 A,c | 2.46 ± 0.01 B,c |
| Emulsion (duodenum) | 4.69 ± 0.08 A,d | 4.93 ± 0.00 B,d | 9.48 ± 0.06 A,d | 9.85 ± 0.04 B,d |
| Rosemary | Oregano | ||
|---|---|---|---|
| TPC (mgGAE gdw−1) | Plant extract | 5.09% | 5.99% |
| Emulsified plant extract | 67.78% | 3.39% | |
| DPPH (mmolTrolox gdw−1) | Plant extract | 42.11% | 17.07% |
| Emulsified plant extract | 69.23% | 76.47% | |
| FRAP (mmolFeSO4·7H2O gdw−1) | Plant extract | 16.39% | 5.07% |
| Emulsified plant extract | 14.29% | 5.36% |
| Experiment No. | Oil Concentration in Emulsified Plant Extracts (% w/w) | Emulsifier Concentration (% w/w) | Rotational Speed (rpm) |
|---|---|---|---|
| 1 | 10 (−1) | 0.10 (−1) | 25,000 (0) |
| 2 | 25 (+1) | 0.10 (−1) | 25,000 (0) |
| 3 | 10 (−1) | 1.00 (+1) | 25,000 (0) |
| 4 | 25 (+1) | 1.00 (+1) | 25,000 (0) |
| 5 | 10 (−1) | 0.50 (0) | 15,000 (−1) |
| 6 | 25 (+1) | 0.50 (0) | 15,000 (−1) |
| 7 | 10 (−1) | 0.50 (0) | 35,000 (+1) |
| 8 | 25 (+1) | 0.50 (0) | 35,000 (+1) |
| 9 | 15 (0) | 0.10 (−1) | 15,000 (−1) |
| 10 | 15 (0) | 1.00 (+1) | 15,000 (−1) |
| 11 | 15 (0) | 0.10 (−1) | 35,000 (+1) |
| 12 | 15 (0) | 1.00 (+1) | 35,000 (+1) |
| 13 | 15 (0) | 0.50 (0) | 25,000 (0) |
| 14 | 15 (0) | 0.50 (0) | 25,000 (0) |
| 15 | 15 (0) | 0.50 (0) | 25,000 (0) |
| 16 | 15 (0) | 0.50 (0) | 25,000 (0) |
| 17 | 15 (0) | 0.50 (0) | 25,000 (0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirovec, S.; Tušek, A.J.; Benković, M.; Valinger, D.; Cvetnić, T.S.; Kljusurić, J.G.; Jurina, T. Emulsification of Rosemary and Oregano Aqueous Extracts and Their In Vitro Bioavailability. Plants 2022, 11, 3372. https://doi.org/10.3390/plants11233372
Sirovec S, Tušek AJ, Benković M, Valinger D, Cvetnić TS, Kljusurić JG, Jurina T. Emulsification of Rosemary and Oregano Aqueous Extracts and Their In Vitro Bioavailability. Plants. 2022; 11(23):3372. https://doi.org/10.3390/plants11233372
Chicago/Turabian StyleSirovec, Sara, Ana Jurinjak Tušek, Maja Benković, Davor Valinger, Tea Sokač Cvetnić, Jasenka Gajdoš Kljusurić, and Tamara Jurina. 2022. "Emulsification of Rosemary and Oregano Aqueous Extracts and Their In Vitro Bioavailability" Plants 11, no. 23: 3372. https://doi.org/10.3390/plants11233372





