Anaerobic Degradation of the Invasive Weed Solidago canadensis L. (goldenrod) and Copper Immobilization by a Community of Sulfate-Reducing and Methane-Producing Bacteria
Abstract
:1. Introduction
- 1.
- Hydrolysis: [C6H12O6]n (plant biomass) → C6H12O6 (Bacillus, Bacteroides, Clostridia)
- 2.
- Acidogenecis: 2 C6H12O6 = 3 CH3CH2COOH + 3 H2 + 3 CO2 (Actinomyces, Bacillus)
- 3.
- Acetogenesis: CH3CH2COOH + 2 H2O = CH3COOH + 3 H2 + CO2 + H2O(Acetoanaerobacterium, Acetobacterium, Clostridium, Desulfotomaculum)
- 4.
- Sulfate reduction [30]:2 CH3CHOHCOOH + SO42− = 2 CH3COOH + 2 HCO3− + H2S4 H2 + SO42− + 2H+ = H2S + 4 H2O
- 5.
2. Materials and Methods
2.1. Sampling and Plant Biomass Preparation for Anaerobic Degradation
2.2. Extraction of Solidago canadensis L. Bioactive Compounds
2.3. Determination of Antioxidant Activity, Concentration of Phenols, Flavonoids and Total Carbohydrates in the Whole Plant of Solidago canadensis L. Weed
2.4. Inoculum Preparation
2.5. The Measurement of the Main Metabolic Parameters of Anaerobic Degradation of Solidago canadensis L. Weed
2.6. The Measurement of the Dynamic of Copper Detoxification during the Degradation of Solidago canadensis L. Weed
3. Results
3.1. Biochemical Composition of the Extracts of Solidago canadensis L.
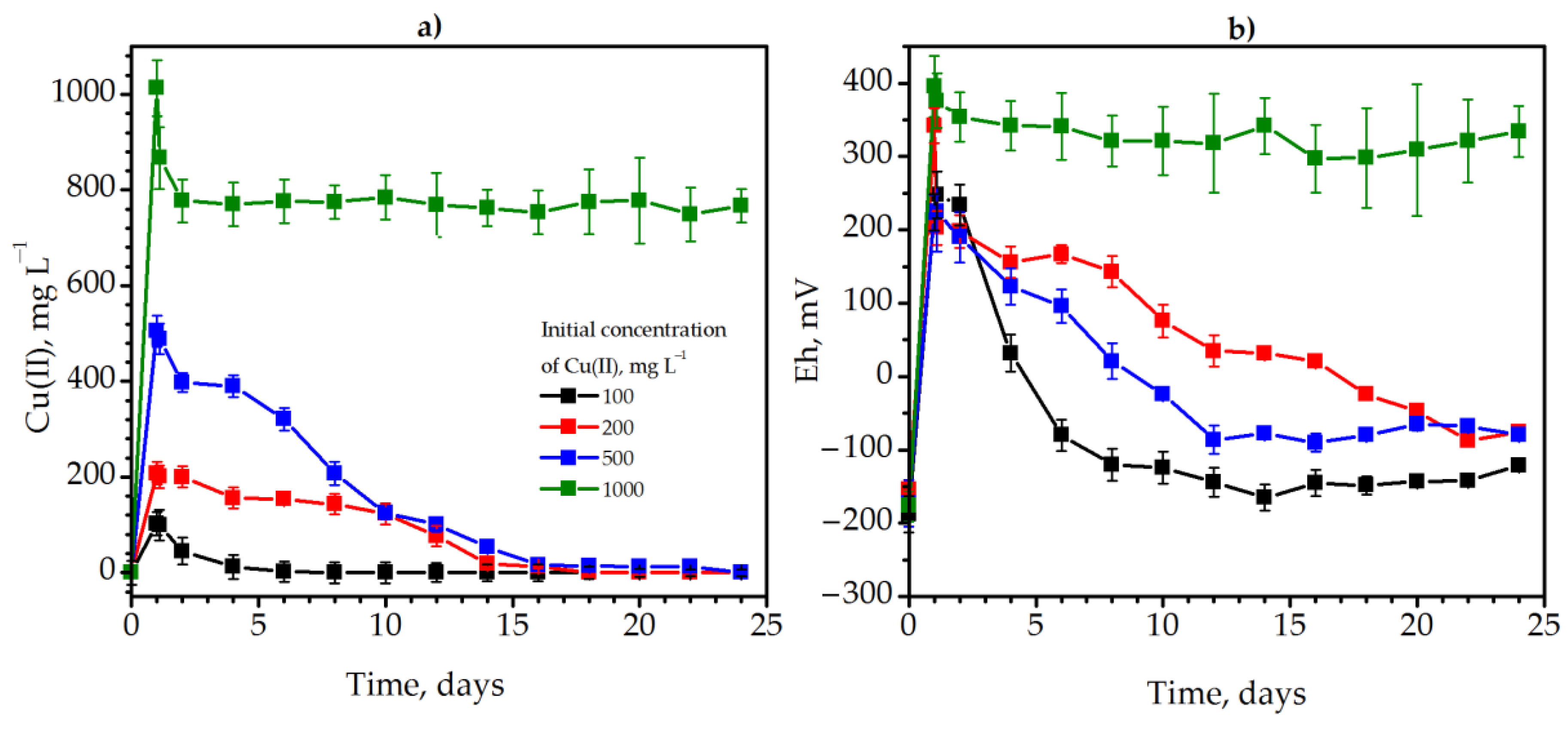
3.2. Detoxification of Copper via Methane Fermentation of Solidago canadensis L. Weed Biomass
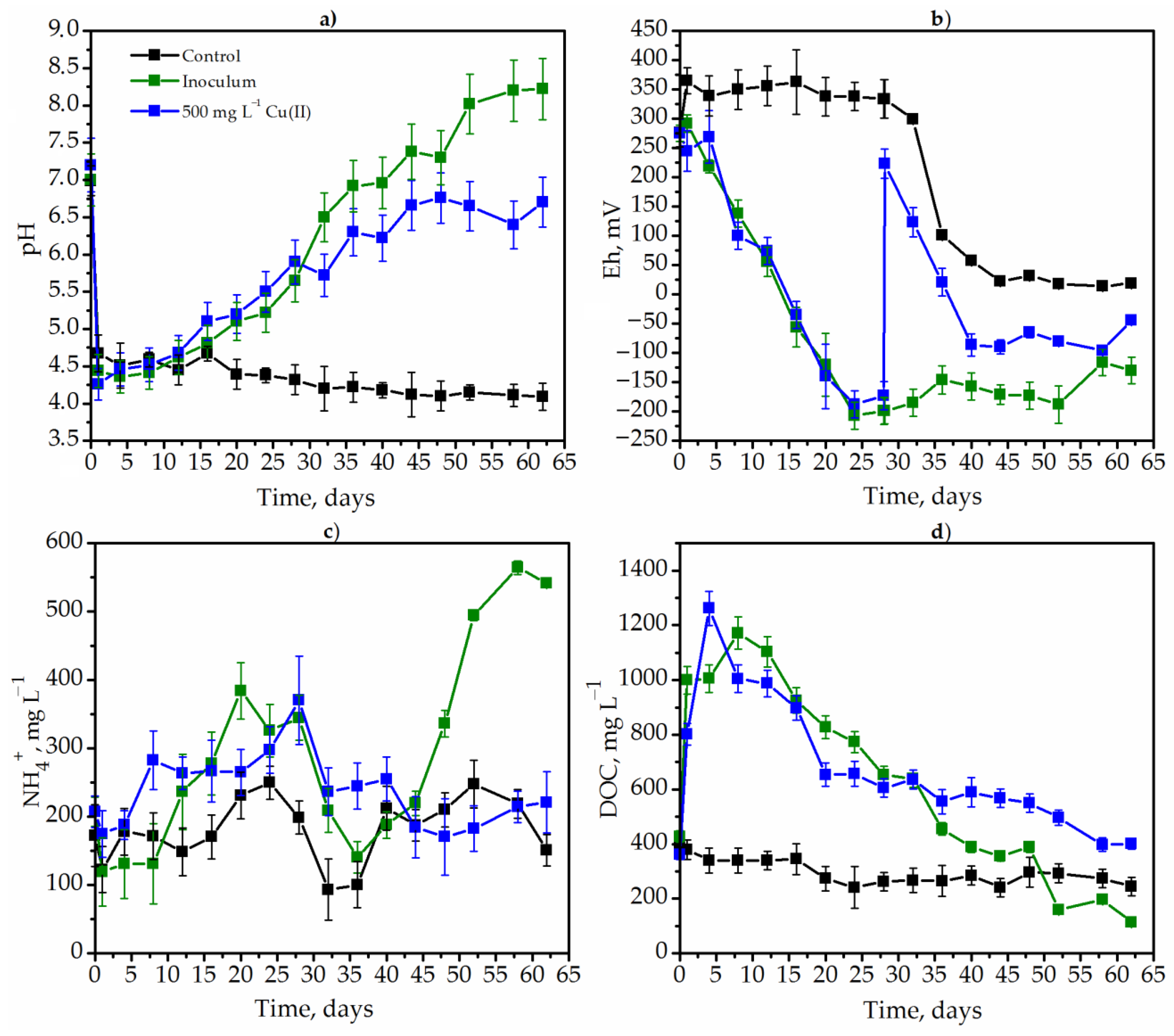
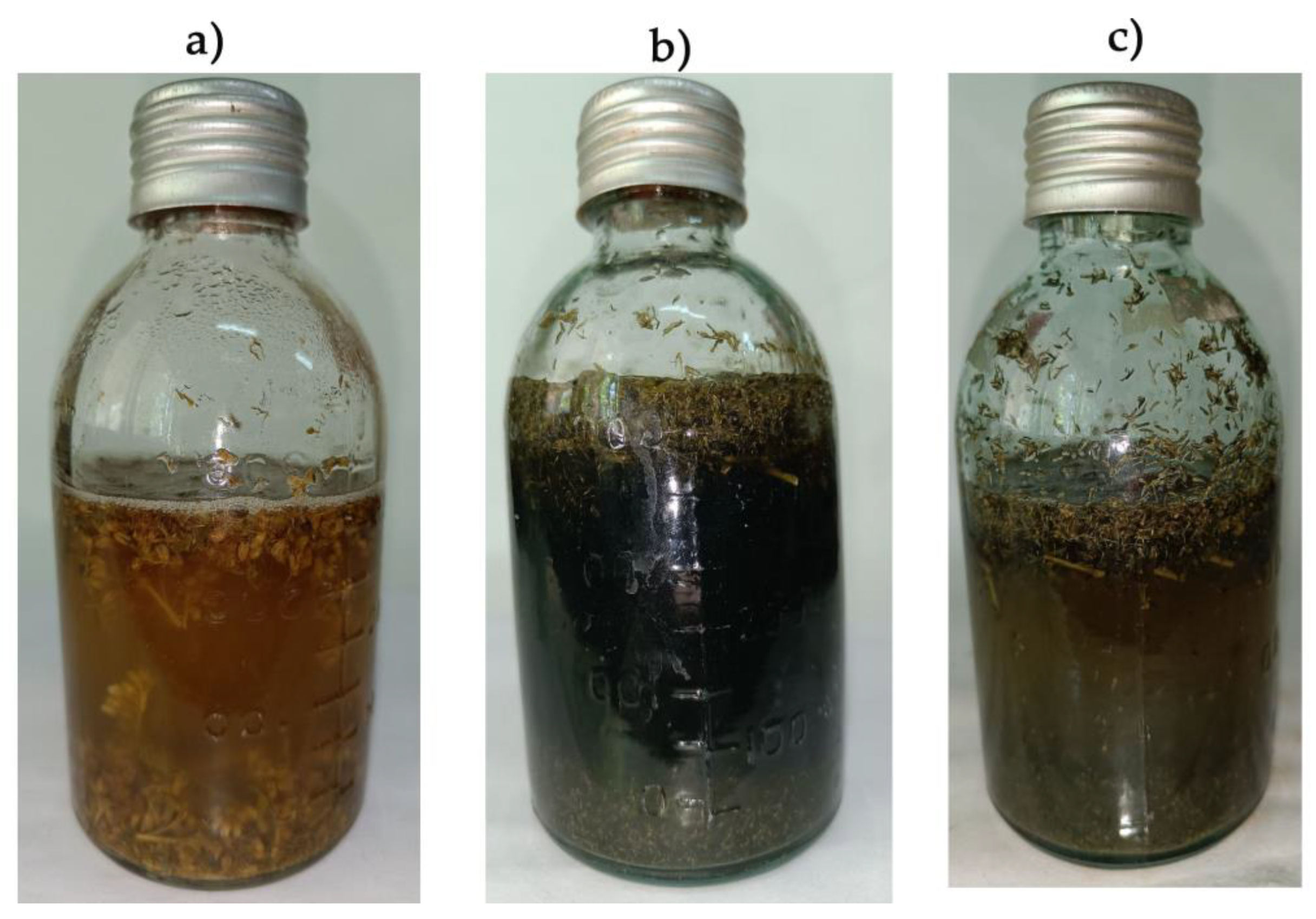
3.3. Anaeribic Degradation of Goldenrod by Methane-Producing and Sulfate-Reducing Bacteria
3.4. Biogas Synthesis and the Efficiency of the Goldenrod Fermentation Process
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, X.; Li, W.; Shao, H.; Tang, S. Selected Aspects of Invasive Solidago Canadensis with an Emphasis on Its Allelopathic Abilities: A Review. Chem. Biodivers. 2022, 19, e202200728. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liang, Q.; Wu, N.; Javed, Q.; Huang, P.; Du, D. Allelopathic Effects of Aqueous Extracts from Different Plant Parts of Canada Goldenrod (Solidago canadensis L.) On Seed Germination and Seedling Growth of Korean Lawngrass (Zoysia japonica Steud.). Appl. Ecol. Environ. Res. 2022, 20, 1009–1022. [Google Scholar] [CrossRef]
- Szymura, M.; Świerszcz, S.; Szymura, T.H. Restoration of Ecologically Valuable Grassland on Sites Degraded by Invasive Solidago: Lessons from a 6-year Experiment. Land Degrad. Dev. 2022, 33, 1985–1998. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Cianfaglione, K.; Nagy, D.U.; Canale, A.; Maggi, F. Evaluation of Two Invasive Plant Invaders in Europe (Solidago canadensis and Solidago gigantea) as Possible Sources of Botanical Insecticides. J. Pest Sci. 2019, 92, 805–821. [Google Scholar] [CrossRef]
- Likhanov, A.; Oliinyk, M.; Pashkevych, N.; Churilov, A.; Kozyr, M. The Role of Flavonoids in Invasion Strategy of Solidago canadensis L. Plants 2021, 10, 1748. [Google Scholar] [CrossRef]
- Shelepova, O.; Vinogradova, Y.; Vergun, O.; Grygorieva, O.; Brindza, J. Assessment of Flavonoids and Phenolic Compound Accumulation in Invasive Solidago canadensis L. in Slovakia. Potravinarstvo 2020, 14, 587–594. [Google Scholar] [CrossRef]
- Sili, A. The Antioxidant profile of solidago species. In Proceedings of the International Medical Congress for Students and Young Doctors, Chisinau, Moldova, 24–26 September 2020; pp. 377–378. Available online: https://ibn.idsi.md/vizualizare_articol/120972 (accessed on 31 January 2021).
- Souvannasouk, V.; Shen, M.; Trejo, M.; Bhuyar, P. Biogas Production from Napier Grass and Cattle Slurry Using a Green Energy Technology. Int. J. Innov. Res. Sci. Stud. 2021, 4, 174–180. [Google Scholar] [CrossRef]
- Shelepova, O.; Vinogradova, Y.; Vergun, O.; Grygorieva, O.; Brindza, J. Invasive Solidago canadensis L. As A Resource of Valuable Biological Compounds. Potravinarstvo 2019, 13, 280–286. [Google Scholar] [CrossRef] [Green Version]
- Luo, B.; Khan, A.A.; Ali, M.A.S.; Yu, J. An Evaluation of Influencing Factors and Public Attitudes for the Adoption of Biogas System in Rural Communities to Overcome Energy Crisis: A Case Study of Pakistan. Sci. Total Environ. 2021, 778, 146208. [Google Scholar] [CrossRef]
- Mthethwa, N.P.; Nasr, M.; Bux, F.; Kumari, S. Utilization of Pistia stratiotes (Aquatic Weed) for Fermentative Biohydrogen: Electron-Equivalent Balance, Stoichiometry, and Cost Estimation. Int. J. Hydrog. Energy 2018, 43, 8243–8255. [Google Scholar] [CrossRef]
- Saratale, R.G.; Cho, S.-K.; Ghodake, G.S.; Shin, H.-S.; Saratale, G.D.; Park, Y.; Lee, H.-S.; Bharagava, R.N.; Kim, D.-S. Utilization of Noxious Weed Water Hyacinth Biomass as a Potential Feedstock for Biopolymers Production: A Novel Approach. Polymers 2020, 12, 1704. [Google Scholar] [CrossRef] [PubMed]
- González, R.; Peña, D.C.; Gómez, X. Anaerobic Co-Digestion of Wastes: Reviewing Current Status and Approaches for Enhancing Biogas Production. Appl. Sci. 2022, 12, 8884. [Google Scholar] [CrossRef]
- Prakash, J.; Sharma, R.; Patel, S.K.; Kim, I.-W.; Kalia, V.C. Bio-Hydrogen Production by Co-Digestion of Domestic Wastewater and Biodiesel Industry Effluent. PloS ONE 2018, 13, e0199059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Chen, L.; Wang, J.; Yao, J.; Li, J. Biochar Preparation from Solidago canadensis and Its Alleviation of the Inhibition of Tomato Seed Germination by Allelochemicals. RSC Adv. 2018, 8, 22370–22375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marmann, A.; Aly, A.H.; Lin, W.; Wang, B.; Proksch, P. Co-Cultivation—A Powerful Emerging Tool for Enhancing the Chemical Diversity of Microorganisms. Mar. Drugs 2014, 12, 1043–1065. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Ai, W.; Dong, W. Lignocellulose Degradation, Biogas Production and Characteristics of the Microbial Community in Solid-State Anaerobic Digestion of Wheat Straw Waste. Life Sci. Space Res. 2022, 32, 1–7. [Google Scholar] [CrossRef]
- Patel, S.K.; Das, D.; Kim, S.C.; Cho, B.-K.; Kalia, V.C.; Lee, J.-K. Integrating Strategies for Sustainable Conversion of Waste Biomass into Dark-Fermentative Hydrogen and Value-Added Products. Renew. Sustain. Energy Rev. 2021, 150, 111491. [Google Scholar] [CrossRef]
- Nozhevnikova, A.; Russkova, Y.I.; Litti, Y.V.; Parshina, S.; Zhuravleva, E.; Nikitina, A. Syntrophy and Interspecies Electron Transfer in Methanogenic Microbial Communities. Microbiology 2020, 89, 129–147. [Google Scholar] [CrossRef]
- Paritosh, K.; Mathur, S.; Pareek, N.; Vivekanand, V. Enhancing Hydrolysis and Syntropy Simultaneously in Solid State Anaerobic Digestion: Digester Performance and Techno-Economic Evaluation. Bioresour. Technol. 2021, 338, 125538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wen, G.; Bu, D.; Sun, G.; Qiang, S. Long−Distance Wind Dispersal Drives Population Range Expansion of Solidago canadensis. Plants 2022, 11, 2734. [Google Scholar] [CrossRef] [PubMed]
- Wiatrowska, B.M.; Wawro, A.; Gieparda, W.; Waliszewska, B. Bioethanol Production Potential and Other Biomass Energy Properties of Invasive Reynoutria, Solidago, and Spiraea Plants. Forests 2022, 13, 1582. [Google Scholar] [CrossRef]
- Havryliuk, O.; Hovorukha, V.; Savitsky, O.; Trilis, V.; Kalinichenko, A.; Dołhańczuk-Śródka, A.; Janecki, D.; Tashyrev, O. Anaerobic Degradation of Environmentally Hazardous Aquatic Plant Pistia Stratiotes and Soluble Cu(II) Detoxification by Methanogenic Granular Microbial Preparation. Energies 2021, 14, 3849. [Google Scholar] [CrossRef]
- Liao, R.; Gao, B.; Fang, J. Invasive Plants as Feedstock for Biochar and Bioenergy Production. Bioresour. Technol. 2013, 140, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Kapun, T.; Zule, J.; Fabjan, E.; Hočevar, B.; Grilc, M.; Likozar, B. Engineered Invasive Plant Cellulose Fibers as Resources for Papermaking. Eur. J. Wood Wood Prod. 2022, 80, 501–514. [Google Scholar] [CrossRef]
- Lynd, L.R.; Weimer, P.J.; Van Zyl, W.H.; Pretorius, I.S. Microbial Cellulose Utilization: Fundamentals and Biotechnology. Microbiol. Mol. Biol. Rev. 2002, 66, 506–577. [Google Scholar] [CrossRef] [Green Version]
- Hattori, S. Syntrophic Acetate-Oxidizing Microbes in Methanogenic Environments. Microbes Environ. 2008, 23, 118–127. [Google Scholar] [CrossRef] [Green Version]
- Lyu, Z.; Shao, N.; Akinyemi, T.; Whitman, W.B. Methanogenesis. Curr. Biol. 2018, 28, R727–R732. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Dong, B. Anaerobic Co-Digestion of Sewage Sludge and Solidago canadensis L. with Different Volatile Solid Ratios in Feedstock. Environ. Sci. Technol. China 2014, 37, 126–131. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=FJKS201405025&DbName=CJFQ2014 (accessed on 22 December 2022).
- Kushkevych, I.; Cejnar, J.; Treml, J.; Dordević, D.; Kollar, P.; Vítězová, M. Recent Advances in Metabolic Pathways of Sulfate Reduction in Intestinal Bacteria. Cells 2020, 9, 698. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.V.S.R.; Germida, J.J. 18—Microbial transformations of sulfur in soil. In Principles and Applications of Soil Microbiology, 3rd ed.; Gentry, T.J., Fuhrmann, J.J., Zuberer, D.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 489–522. ISBN 978-0-12-820202-9. [Google Scholar] [CrossRef]
- Stams, A.J.M.; Teusink, B.; Sousa, D.Z.M. Ecophysiology of acetoclastic methanogens. In Biogenesis of Hydrocarbons. Handbook of Hydrocarbon and Lipid Microbiology; Stams, A., Sousa, D., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Khan, A.; Akbar, S.; Okonkwo, V.; Smith, C.; Khan, S.; Shah, A.A.; Adnan, F.; Ijaz, U.Z.; Ahmed, S.; Badshah, M. Enrichment of the Hydrogenotrophic Methanogens for, in-Situ Biogas up-Gradation by Recirculation of Gases and Supply of Hydrogen in Methanogenic Reactor. Bioresour. Technol. 2022, 345, 126219. [Google Scholar] [CrossRef]
- Shi, X.; Gao, G.; Tian, J.; Wang, X.C.; Jin, X.; Jin, P. Symbiosis of Sulfate-Reducing Bacteria and Methanogenic Archaea in Sewer Systems. Environ. Int. 2020, 143, 105923. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Wang, X.; Liu, H.; Liang, M.; Zhu, Y.; Li, Z. Chemical Speciation, Pollution and Ecological Risk of Toxic Metals in Readily Washed off Road Dust in a Megacity (Nanjing), China. Ecotoxicol. Environ. Saf. 2019, 173, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Chileshe, M.N.; Syampungani, S.; Festin, E.S.; Tigabu, M.; Daneshvar, A.; Odén, P.C. Physico-Chemical Characteristics and Heavy Metal Concentrations of Copper Mine Wastes in Zambia: Implications for Pollution Risk and Restoration. J. For. Res. 2020, 31, 1283–1293. [Google Scholar] [CrossRef] [Green Version]
- Mykolenko, S.; Liedienov, V.; Kharytonov, M.; Makieieva, N.; Kuliush, T.; Queralt, I.; Marguí, E.; Hidalgo, M.; Pardini, G.; Gispert, M. Presence, Mobility and Bioavailability of Toxic Metal (Oids) in Soil, Vegetation and Water around a Pb-Sb Recycling Factory (Barcelona, Spain). Environ. Pollut. 2018, 237, 569–580. [Google Scholar] [CrossRef]
- Li, L.; Zhang, K.; Gill, R.A.; Islam, F.; Farooq, M.A.; Wang, J.; Zhou, W. Ecotoxicological and Interactive Effects of Copper and Chromium on Physiochemical, Ultrastructural, and Molecular Profiling in Brassica napus L. BioMed Res. Int. 2018, 2018, 9248123. [Google Scholar] [CrossRef] [Green Version]
- Havryliuk, O.; Hovorukha, V.; Patrauchan, M.; Youssef, N.H.; Tashyrev, O. Draft Whole Genome Sequence for Four Highly Copper Resistant Soil Isolates Pseudomonas lactis Strain UKR1, Pseudomonas panacis Strain UKR2, and Pseudomonas veronii Strains UKR3 and UKR4. Curr. Res. Microb. Sci. 2020, 1, 44–52. [Google Scholar] [CrossRef]
- Werner, P.A.; Gross, R.S.; Bradbury, I.K. The Biology of Canadian Weeds.: 45. Solidago canadensis L. Can. J. Plant Sci. 1980, 60, 1393–1409. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, Y.; Zhang, S.; Hu, W. Simulated Warming Enhances Biological Invasion of Solidago Canadensis and Bidens Frondosa by Increasing Reproductive Investment and Altering Flowering Phenology Pattern. Sci. Rep. 2018, 8, 16073. [Google Scholar] [CrossRef] [Green Version]
- Fridh, L.; Volpé, S.; Eliasson, L. An Accurate and Fast Method for Moisture Content Determination. Int. J. For. Eng. 2014, 25, 222–228. [Google Scholar] [CrossRef]
- Semenov, V.; Iarosh, A. A Method of Determining the Antioxidative Activity of Biological Matter. Ukr. Biokhimicheskii Zhurnal 1978 1985, 57, 50–52. [Google Scholar] [CrossRef]
- Wrolstad, R.E.; Acree, T.E.; Decker, E.A.; Penner, M.H.; Reid, D.S.; Schwartz, S.J.; Shoemaker, C.F.; Smith, D.M.; Sporns, P. Handbook of Food Analytical Chemistry, Volume 1: Water, Proteins, Enzymes, Lipids, and Carbohydrates; John Wiley & Sons: New York, NY, USA, 2005; ISBN 0-471-70909-3. [Google Scholar]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Wong, P.; Dutka, B. Determination of Carbohydrate in Lake Sediment by a Modified Phenol-Sulfuric Acid Method. Water Res. 1973, 7, 741–746. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, Y.; Zhao, Y.; Zhu, X. New Sludge Pretreatment Method to Improve Methane Production in Waste Activated Sludge Digestion. Environ. Sci. Technol. 2010, 44, 4802–4808. [Google Scholar] [CrossRef]
- Harada, H.; Uemura, S.; Momonoi, K. Interaction between Sulfate-Reducing Bacteria and Methane-Producing Bacteria in UASB Reactors Fed with Low Strength Wastes Containing Different Levels of Sulfate. Water Res. 1994, 28, 355–367. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Li, Y.; Cheng, Y.; Zhu, W. The Enrichment of Anaerobic Fungi and Methanogens Showed Higher Lignocellulose Degrading and Methane Producing Ability than That of Bacteria and Methanogens. World J. Microbiol. Biotechnol. 2020, 36, 125. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ji, Q.; Fu, X.; Yu, X.; Ye, Z.; Zhang, M.; Sun, C.; Qiu, Y. Low-Cost Detection of Methane Gas in Rice Cultivation by Gas Chromatography-Flame Ionization Detector Based on Manual Injection and Split Pattern. Molecules 2022, 27, 3968. [Google Scholar] [CrossRef]
- Kushkevych, I.; Dordević, D.; Kollar, P.; Vítězová, M.; Drago, L. Hydrogen Sulfide as a Toxic Product in the Small–Large Intestine Axis and Its Role in IBD Development. J. Clin. Med. 2019, 8, 1054. [Google Scholar] [CrossRef] [Green Version]
- Moran, M.A.; Durham, B.P. Sulfur Metabolites in the Pelagic Ocean. Nat. Rev. Microbiol. 2019, 17, 665–678. [Google Scholar] [CrossRef]
- Govorukha, V.; Havrylyuk, O.; Tashyrev, O. Regularities of Quantitative Distribution for Fe (III)-Reducing Bacteria in Natural Ecosystems. Biotechnol. Acta 2015, 8, 123–128. [Google Scholar] [CrossRef]
- Acree, W.E. Basic Gas Chromatography (McNair, Harold M.; Miller, James M.). J. Chem. Educ. 1998, 75, 1094. [Google Scholar] [CrossRef] [Green Version]
- Tashyrev, O.; Prekrasna, I. Express Method for Redox Potential and PH Measuring in Microbial Cultures. Int. J. Bioautom. 2014, 18, 217–230. [Google Scholar]
- Zehnder, A.J.B.; Wuhrmann, K. Titanium (III) Citrate as a Nontoxic Oxidation-Reduction Buffering System for the Culture of Obligate Anaerobes Vertebrate Central Nervous System: Same Neurons Mediate Both Electrical and Chemical Inhibitions. Science 1975, 194, 1165–1166. [Google Scholar] [CrossRef] [PubMed]
- Suslova, O.; Govorukha, V.; Brovarskaya, O.; Matveeva, N.; Tashyreva, H.; Tashyrev, O. Method for Determining Organic Compound Concentration in Biological Systems by Permanganate Redox Titration. Int. J. Bioautom. 2014, 18, 45–52. [Google Scholar]
- Prekrasna, I.P.; Tashyrev, O.B. Copper Resistant Strain Candida Tropicalis RomCu5 Interaction with Soluble and Insoluble Copper Compounds. Biotechnol. Acta 2015, 8, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Selective Extraction of Bioactive Compounds from Plants Using Recent Extraction Techniques: A Review. J. Chromatogr. A 2021, 1635, 461770. [Google Scholar] [CrossRef]
- Sun, X.; Cheng, L.; Jonker, A.; Munidasa, S.; Pacheco, D. A Review: Plant Carbohydrate Types—The Potential Impact on Ruminant Methane Emissions. Front. Vet. Sci. 2022, 9, 880115. [Google Scholar] [CrossRef]
- Jomová, K.; Hudecova, L.; Lauro, P.; Simunkova, M.; Alwasel, S.H.; Alhazza, I.M.; Valko, M. A Switch between Antioxidant and Prooxidant Properties of the Phenolic Compounds Myricetin, Morin, 3′,4′-Dihydroxyflavone, Taxifolin and 4-Hydroxy-Coumarin in the Presence of Copper(II) Ions: A Spectroscopic, Absorption Titration and DNA Damage Study. Molecules 2019, 24, 4335. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Gao, X. Biogas: Potential, Challenges, and Perspectives in a Changing China. Biomass Bioenergy 2021, 150, 106127. [Google Scholar] [CrossRef]
- Li, W.; Wang, L.; Tian, B.; Ding, J.; Siemann, E. Introduced Populations of an Invasive Tree Have Higher Soluble Sugars but Lower Starch and Cellulose. Front. Plant Sci. 2020, 11, 587414. [Google Scholar] [CrossRef]
- Peter, A.; Žlabur, J.Š.; Šurić, J.; Voća, S.; Purgar, D.D.; Pezo, L.; Voća, N. Invasive Plant Species Biomass—Evaluation of Functional Value. Molecules 2021, 26, 3814. [Google Scholar] [CrossRef]
- Banunle, A.; Fei-Baffoe, B.; Miezah, K.; Ewusi-Mensah, N.; Jørgensen, U.; Aidoo, R.; Amoah, A.; Abaidoo, R.C.; Agbeshie, A.A. Utilisation Potentials of Invasive Plants in the Owabi Dam in the Ashanti Region of Ghana. BioResources 2021, 16, 3075–3095. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Wang, G. Two-stage Hydrolysis of Invasive Algal Feedstock for Ethanol Fermentation F. J. Integr. Plant Biol. 2011, 53, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Witt, A.B. Biofuels and Invasive Species from an African Perspective–a Review. Gcb Bioenergy 2010, 2, 321–329. [Google Scholar] [CrossRef]
- Zhang, J.; Bi, F.; Wang, Q.; Wang, W.; Liu, B.; Lutts, S.; Wei, W.; Zhao, Y.; Wang, G.; Han, R. Characteristics and Influencing Factors of Cadmium Biosorption by the Stem Powder of the Invasive Plant Species Solidago canadensis. Ecol. Eng. 2018, 121, 12–18. [Google Scholar] [CrossRef]
- Yao, Y.; Sheng, H.; Luo, Y.; He, M.; Li, X.; Zhang, H.; He, W.; An, L. Optimization of Anaerobic Co-Digestion of Solidago canadensis L. Biomass and Cattle Slurry. Energy 2014, 78, 122–127. [Google Scholar] [CrossRef]
- Dubrovskis, V.; Plume, I.; Straume, I. Suitability of Common Nettle (Urtica dioica) and Canadian Goldenrod (Solidago canadensis) for Methane Production. Agron. Res. 2018, 16, 16421648. [Google Scholar] [CrossRef]
- Tyagi, S.; Malik, W.; Annachhatre, A.P. Heavy Metal Precipitation from Sulfide Produced from Anaerobic Sulfidogenic Reactor. Mater. Today Proc. 2020, 32, 936–942. [Google Scholar] [CrossRef]
- Hnatush, S.O.; Moroz, O.M.; Yavorska, G.V.; Borsukevych, B.M. Sulfidogenic and Metal Reducing Activities of Desulfuromonas Genus Bacteria under the Influence of Copper Chloride. Biosyst. Divers. 2018, 26, 218–226. [Google Scholar] [CrossRef]
- Jarrell, K.F.; Saulnier, M.; Ley, A. Inhibition of Methanogenesis in Pure Cultures by Ammonia, Fatty Acids, and Heavy Metals, and Protection against Heavy Metal Toxicity by Sewage Sludge. Can. J. Microbiol. 1987, 33, 551–554. [Google Scholar] [CrossRef]


| Type of Analysis | Value mg mL−1 of Extract | Value mg g−1 of Extract | Value mg g−1 of Plant |
|---|---|---|---|
| Phenols (GAE) | 4.3 ± 0.3 | 485.6 ± 28.4 | 105.7 ± 6.2 |
| Flavonoids (RUE) | 3.4 ± 0.1 | 385.7 ± 16.4 | 84.0 ± 3.6 |
| Total carbohydrates | 4.5 ± 0.2 | 511.5 ± 23.1 | 111.4 ± 5.0 |
| DOC | 8.5 ± 0.5 | 956.8 ± 45.5 | 208.33 ± 17.7 |
| Antioxidant activity, % | 86.3 ± 4.2 | - | - |
| Treatments | CH4 Max (vol%) * | CH4 Yield (L kg−1 TSplant) | CO2 Yield (L kg−1 TSplant) | Kd (Times) |
|---|---|---|---|---|
| Control with native bacteria | 12.3 ± 2.6 | 5.2 ± 1.3 | 22.8 ± 3.5 | 1.5 ± 0.7 |
| Inoculum of MPB # and SRB ** | 72.4 ± 4.3 | 64.2 ± 9.1 | 68.2 ± 12.2 | 21.4 ± 3.2 |
| 500 mg L−1 Cu(II) | 58.6 ± 6.2 | 38.4 ± 4.6 | 44.4 ± 11.5 | 7.4 ± 5.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Havryliuk, O.; Hovorukha, V.; Bida, I.; Gladka, G.; Tymoshenko, A.; Kyrylov, S.; Mariychuk, R.; Tashyrev, O. Anaerobic Degradation of the Invasive Weed Solidago canadensis L. (goldenrod) and Copper Immobilization by a Community of Sulfate-Reducing and Methane-Producing Bacteria. Plants 2023, 12, 198. https://doi.org/10.3390/plants12010198
Havryliuk O, Hovorukha V, Bida I, Gladka G, Tymoshenko A, Kyrylov S, Mariychuk R, Tashyrev O. Anaerobic Degradation of the Invasive Weed Solidago canadensis L. (goldenrod) and Copper Immobilization by a Community of Sulfate-Reducing and Methane-Producing Bacteria. Plants. 2023; 12(1):198. https://doi.org/10.3390/plants12010198
Chicago/Turabian StyleHavryliuk, Olesia, Vira Hovorukha, Iryna Bida, Galyna Gladka, Artem Tymoshenko, Semen Kyrylov, Ruslan Mariychuk, and Oleksandr Tashyrev. 2023. "Anaerobic Degradation of the Invasive Weed Solidago canadensis L. (goldenrod) and Copper Immobilization by a Community of Sulfate-Reducing and Methane-Producing Bacteria" Plants 12, no. 1: 198. https://doi.org/10.3390/plants12010198










