The Contribution of PGPR in Salt Stress Tolerance in Crops: Unravelling the Molecular Mechanisms of Cross-Talk between Plant and Bacteria
Abstract
:1. Introduction
2. Rhizosphere Microbial Community
3. Plant-Growth-Promoting Rhizobacteria (PGPR)
4. Salt Stress Affects Plant Growth
4.1. Soil Salinization
4.2. Plant Response to Salt Stress
4.3. PGPR Growth and Activity in Salt Stress Conditions
4.4. Contribution of PGPR to Plant Salt Tolerance
4.4.1. Osmotic Adjustment
4.4.2. Antioxidant Activity
4.4.3. Ion Homeostasis
4.4.4. Hormonal Modulation
4.4.5. Nutrient Uptake
4.4.6. Biofilm Formation
4.4.7. Volatile Organic Compounds (VOCs)
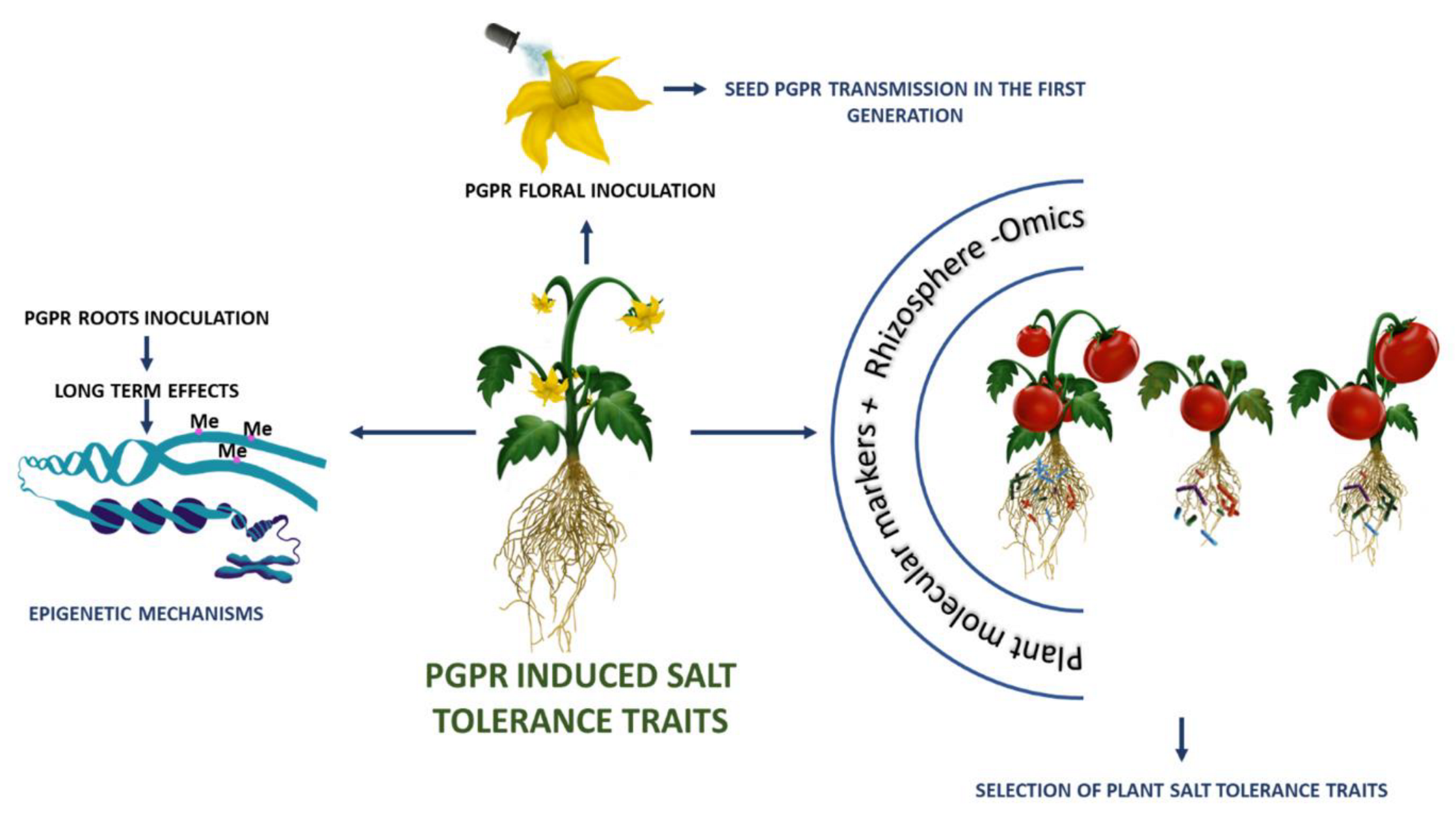
5. Future Perspective for Dissecting Plant–Microbe Molecular Interactions under Salt Stress
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. High Level Expert Forum—How to Feed the World in 2050; FAO: Rome, Italy, 2009; Available online: https://www.fao.org/fileadmin/templates/wsfs/docs/expert_paper/How_to_Feed_the_World_in_2050.pdf (accessed on 11 June 2020).
- Pingali, P.L. Green revolution: Impacts, limits, and the path ahead. Proc. Natl. Acad. Sci. USA 2012, 109, 12302–12308. [Google Scholar] [CrossRef]
- Yang, X.; Fang, S. Practices, perceptions, and implications of fertilizer use in East-Central China. Ambio 2015, 44, 647–652. [Google Scholar] [CrossRef]
- FAO. The Future of Food and Agriculture—Alternative Pathways to 2050; Licence: CC BY-NC-SA 3.0 IGO.; FAO: Rome, Italy, 2018; 224p, Available online: https://www.fao.org/3/I8429EN/i8429en.pdf (accessed on 11 June 2020).
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef] [PubMed]
- FAO. Global Map of Salt Affected Soils. 2021. Available online: https://www.fao.org/soils-portal/data-hub/soil-maps-and-databases/global-map-of-salt-affected-soils/en/ (accessed on 17 January 2022).
- Abdelraheem, A.; Esmaeili, N.; O’Connell, M.; Zhang, J. Progress and perspective on drought and salt stress tolerance in cotton. Ind. Crops Prod. 2019, 130, 118–129. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Ghassemi-Golezani, K. Improving amino acid composition of soybean under salt stress by salicylic acid and jasmonic acid. J. Appl. Bot. Food Qual. 2016, 89, 243–248. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, P.K. Soil salinity and food security in India. Front. Sustain. Food Syst. 2020, 4, 533781. [Google Scholar] [CrossRef]
- Liu, B.; Soundararajan, P.; Manivannan, A. Mechanisms of silicon-mediated amelioration of salt stress in plants. Plants 2017, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Smith, D.L.; Praslickova, D.; Ilangumaran, G. Inter-organismal signaling and management of the phytomicrobiome. Front. Plant Sci. 2015, 6, 722. [Google Scholar] [CrossRef]
- Bringhurst, R.M.; Cardon, Z.G.; Gage, D.J. Galactosides in the rhizosphere: Utilization by Sinorhizobium meliloti and development of a biosensor. Proc. Natl. Acad. Sci. USA 2001, 98, 4540–4545. [Google Scholar] [CrossRef] [PubMed]
- Hiltner, L. Über neuere erfahrungen und probleme auf dem debiete der bo denbakteriologie und unter besonderer berucksichtigung der grundund und brache. Zbl. Bakteriol. 1904, 2, 14–25. [Google Scholar]
- Gray, E.J.; Smith, D.L. Intracellular and extracellular PGPR: Commonalities and distinctions in the plant-bacterium signaling processes. Soil Biol. Biochem. 2005, 37, 395–412. [Google Scholar] [CrossRef]
- Zhang, R.; Vivanco, J.M.; Shen, Q. The unseen rhizosphere root-soil-microbe interactions for crop production. Curr. Opin. Microbiol. 2017, 37, 8–14. [Google Scholar] [CrossRef]
- Clarkson, D.T.; Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Ver Loren van Themaat, E.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant. Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef]
- Kumar, N.; Iyer-Pascuzzi, A.N. Shedding the last layer: Mechanisms of root cap cell release. Plants 2020, 9, 308. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Srivastava, A.K.; Rajput, V.D.; Chauhan, P.K.; Bhojiya, A.A.; Jain, D.; Chaubey, G.; Dwivedi, P.; Sharma, B.; Minkina, T. Root exudates: Mechanistic insight of plant growth promoting rhizobacteria for sustainable crop production. Front. Microbiol. 2022, 13, 916488. [Google Scholar] [CrossRef]
- Kawa, D.; Brady, S.M. Root cell types as an interface for biotic interactions. Trends Plant Sci. 2022, 27, 1173–1186. [Google Scholar] [CrossRef]
- Ganesh, A.; Shukla, V.; Mohapatra, A.; George, A.P.; Bhukya, D.P.N.; Das, K.K.; Kola, V.S.R.; Suresh, A.; Ramireddy, E. Root cap to soil interface: A driving force toward plant adaptation and development. Plant Cell Physiol. 2022, 63, 1038–1051. [Google Scholar] [CrossRef]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root exudation of primary metabolites: Mechanisms and their roles in plant responses to environmental stimuli. Front. Plant Sci. 2019, 10, 157. [Google Scholar] [CrossRef]
- Jones, P.; Garcia, B.J.; Furches, A.; Tuskan, G.A.; Jacobson, D. Plant host-associated mechanisms for microbial selection. Front. Plant Sci. 2019, 10, 862. [Google Scholar] [CrossRef] [PubMed]
- Burdman, S.; Jurkevitch, E.; Okon, Y. Recent advances in the use of plant growth promoting rhizobacteria (PGPR) in agriculture. In Microbial Interactions in Agriculture and Forestry; Subba Rao, N.S., Dommergues, Y.R., Eds.; Science Publishers: Enfield, NH, USA, 2000; pp. 229–250. [Google Scholar]
- Kaymak, D.C. Potential of PGPR in agricultural innovations. In Plant Growth and Health Promoting Bacteria; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Weller, D.M.; Thomashow, L.S. Current challenges in introducing beneficial microorganisms into the rhizosphere. In Molecular Ecology of Rhizosphere Microorganisms: Biotechnology and Release of GMOs; O’Gara, F., Dowling, D.N., Boesten, B., Eds.; VCH: New York, NY, USA, 1994; pp. 1–18. [Google Scholar]
- Soto, M.; Nogales, J.; Perez-Mendoza, D.; Gallegos, M.T.; Olivares, J.; Sanjuan, J. Pathogenic and mutualistic plant-bacteria interactions: Ever increasing similarities. Open Life Sci. 2011, 6, 911917. [Google Scholar] [CrossRef]
- Santoyo, G.; Urtis-Flores, C.A.; Loeza-Lara, P.D.; Orozco-Mosqueda, M.D.C.; Glick, B.R. Rhizosphere colonization determinants by plant growth-promoting rhizobacteria (PGPR). Biology 2021, 10, 475. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J.W.; Schroth, M.N. Plant growth-promoting rhizobacteria on radishes. In Proceedings of the 4th International Conference on Plant Pathogenic Bacteria, Angers, France, 27 August–2 September 1978; Gilbert-Clarey: Tours, France, 1978; pp. 879–882. [Google Scholar]
- Kloepper, J.W.; Schroth, M.N. Relationship of in vitro antibiosis of plant growth promoting rhizobacteria to plant growth and the displacement of root microflora. Phytopathology 1981, 71, 1020–1024. [Google Scholar] [CrossRef]
- Antoun, H.; Kloepper, J.W. Plant growth promoting rhizobacteria. In Encyclopedia of Genetics; Brenner, S., Miller, J.H., Eds.; Academic: New York, NY, USA, 2001; pp. 1477–1480. [Google Scholar]
- Nakkeeran, S.; Fernando, W.G.D.; Siddiqui, Z.A. Plant Growth Promoting Rhizobacteria formulations and its scope in commercialization for the management of pests and diseases. In PGPR: Biocontrol and Biofertilization; Siddiqui, Z.A., Ed.; Springer: Dordrecht, The Netherlands, 2005; pp. 257–296. [Google Scholar] [CrossRef]
- Martinez-Viveros, O.; Jorquera, M.A.; Crowley, D.E.; Gajardo, G.; Mora, M.L. Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. J. Soil Sci. Plant. Nutr. 2010, 10, 293–319. [Google Scholar] [CrossRef]
- Wang, E.T.; Martinez-Romero, E. Sesbania herbacea-Rhizobium huautlense nodulation in flooded soils and comparative characterization of S. herbacea-nodulating rhizobia in different environments. Microb. Ecol. 2000, 40, 25–32. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Sharma, K.P.; Gaur, R.K. Biotechnological perspectives of microbes in agro-ecosystems. Biotechnol. Lett. 2011, 33, 1905–1910. [Google Scholar] [CrossRef]
- Bertola, M.; Ferrarini, A.; Visioli, G. Improvement of soil microbial diversity through sustainable agricultural practices and its evaluation by -Omics approaches: A perspective for the environment, food quality and human safety. Microorganisms 2021, 9, 1400. [Google Scholar] [CrossRef]
- Giannelli, G.; Bisceglie, F.; Pelosi, G.; Bonati, B.; Cardarelli, M.; Antenozio, M.L.; Degola, F.; Visioli, G. Phyto-beneficial traits of rhizosphere bacteria: In vitro exploration of plant growth promoting and phytopathogen biocontrol ability of selected strains isolated from harsh environments. Plants 2022, 11, 230. [Google Scholar] [CrossRef]
- Omuto, C.T.; Vargas, R.R.; El Mobarak, A.M.; Mohamed, N.; Viatkin, K.; Yigini, Y. Mapping of Salt-Affected Soils: Technical Manual; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Maathuis, F.J.M. Sodium in plants: Perception, signalling, and regulation of sodium fluxes. J. Exp. Bot. 2014, 65, 849–858. [Google Scholar] [CrossRef]
- Carillo, P.; Annunziata, M.G.; Pontecorvo, G.; Fuggi, A.; Woodrow, P. Salinity stress and salt tolerance. In Abiotic Stress in Plants—Mechanisms and Adaptations; Shanker, A., Venkateswarlu, B., Eds.; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Soltabayeva, A.; Ongaltay, A.; Omondi, J.O.; Srivastava, S. Morphological, physiological and molecular markers for salt-stressed plants. Plants 2021, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- İbrahimova, U.; Kumari, P.; Yadav, S.; Rastogi, A.; Antala, M.; Suleymanova, Z.; Zivcak, M.; Tahjib-Ul-Arif, M.; Hussain, S.; Abdelhamid, M.; et al. Progress in understanding salt stress response in plants using biotechnological tools. J. Biotechnol. 2021, 329, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genomics 2014, 2014, 701596. [Google Scholar] [CrossRef] [PubMed]
- Ondrasek, G.; Rathod, S.; Manohara, K.K.; Gireesh, C.; Anantha, M.S.; Sakhare, A.S.; Parmar, B.; Yadav, B.K.; Bandumula, N.; Raihan, F.; et al. Salt stress in plants and mitigation approaches. Plants 2022, 11, 717. [Google Scholar] [CrossRef]
- Chele, K.H.; Tinte, M.M.; Piater, L.A.; Dubery, I.A.; Tugizimana, F. Soil salinity, a serious environmental issue and plant responses: A metabolomics perspective. Metabolites 2021, 11, 724. [Google Scholar] [CrossRef]
- Zhang, J.L.; Shi, H. Physiological and molecular mechanisms of plant salt tolerance. Photosynth. Res. 2013, 115, 1–22. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Mukherjee, A.; Rastogi, R.P.; Verma, J.P. Salt-tolerant plant growth-promoting Bacillus pumilus strain JPVS11 to enhance plant growth attributes of rice and improve soil health under salinity stress. Microbiol. Res. 2021, 242, 126616. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Ahmad, M.; Naveed, M.; Imran, M.; Zahir, Z.A.; Crowley, D.E. Relationship between in vitro characterization and comparative efficacy of plant growth-promoting rhizobacteria for improving cucumber salt tolerance. Arch. Microbiol. 2016, 198, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Kusale, S.P.; Attar, Y.C.; Sayyed, R.Z.; Malek, R.A.; Ilyas, N.; Suriani, N.L.; Khan, N.; El Enshasy, H.A. Production of plant beneficial and antioxidants metabolites by Klebsiella variicola under salinity stress. Molecules 2021, 26, 1894. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Y.; Nadeem, S.M.; Sohaib, M.; Waqas, M.R.; Alotaibi, F.; Ali, L.; Zahir, Z.A.; Al-Barakah, F.N.I. Potential of plant growth promoting bacterial consortium for improving the growth and yield of wheat under saline conditions. Front. Microbiol. 2022, 13, 958522. [Google Scholar] [CrossRef] [PubMed]
- Komaresofla, B.R.; Alikhani, H.A.; Etesami, H.; Khoshkholgh-Sima, N.A. Improved growth and salinity tolerance of the halophyte Salicornia sp. By co–inoculation with endophytic and rhizosphere bacteria. Appl. Soil Ecol. 2019, 138, 160–170. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, K.; Fan, Y.; Fang, H.; Nie, Y.; Wu, X.L. The bacterial MtrAB Two-Component System regulates the cell wall homeostasis responding to environmental alkaline stress. Microbiol. Spectr. 2022, 10, e0231122. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.; Eid, A.; Ewais, E. The interaction between plants and bacterial endophytes under salinity stress. In Endophytes and Secondary Metabolites; Jha, S., Ed.; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2019; pp. 1–17. [Google Scholar]
- Bhat, M.A.; Kumar, V.; Bhat, M.A.; Wani, I.A.; Dar, F.L.; Farooq, I.; Bhatti, F.; Koser, R.; Rahman, S.; Jan, A.T. Mechanistic insights of the interaction of plant growth-promoting rhizobacteria (PGPR) with plant roots toward enhancing plant productivity by alleviating salinity stress. Front. Microbiol. 2020, 11, 1952. [Google Scholar] [CrossRef]
- Ali, B.; Wang, X.; Saleem, M.H.; Sumaira; Hafeez, A.; Afridi, M.S.; Khan, S.; Zaib-Un-Nisa; Ullah, I.; Amaral Júnior, A.T.D.; et al. PGPR-mediated salt tolerance in maize by modulating plant physiology, antioxidant defense, compatible solutes accumulation and bio-surfactant producing genes. Plants 2022, 11, 345. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Alaraidh, I.A.; Alsahli, A.A.; Alzahrani, S.M.; Ali, H.M.; Alayafi, A.A.; Ahmad, M. Serratia liquefaciens KM4 improves salt stress tolerance in maize by regulating redox potential, ion homeostasis, leaf gas exchange and stress-related gene expression. Int. J. Mol. Sci. 2018, 19, 3310. [Google Scholar] [CrossRef]
- Niu, S.; Gao, Y.; Zi, H.; Liu, Y.; Liu, X.; Xiong, X.; Yao, Q.; Qin, Z.; Chen, N.; Guo, L.; et al. The osmolyte producing endophyte Streptomyces albidoflavus OsiLf-2 induces drought and salt tolerance in rice via a multi-level mechanism. Crop J. 2022, 10, 375–386. [Google Scholar] [CrossRef]
- Pérez-Rodriguez, M.M.; Pontin, M.; Piccoli, P.; Lobato Ureche, M.A.; Gordillo, M.G.; Funes-Pinter, I.; Cohen, A.C. Halotolerant native bacteria Enterobacter 64S1 and Pseudomonas 42P4 alleviate saline stress in tomato plants. Physiol. Plant. 2022, 174, e13742. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, F.; Mickan, B.; Wang, D.; Wang, W. Physiological, proteomic, and metabolomic analysis provide insights into Bacillus sp.-mediated salt tolerance in wheat. Plant Cell Rep. 2022, 41, 95–118. [Google Scholar] [CrossRef] [PubMed]
- El-Esawi, M.A.; Alaraidh, I.A.; Alsahli, A.A.; Alamri, S.A.; Ali, H.M.; Alayafi, A.A. Bacillus firmus (SW5) augments salt tolerance in soybean (Glycine max L.) by modulating root system architecture, antioxidant defense systems and stress-responsive genes expression. Plant Physiol. Biochem. 2018, 132, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Sapre, S.; Gontia-Mishra, I.; Tiwari, S. Klebsiella sp. Confers enhanced tolerance to salinity and plant growth promotion in oat seedlings (Avena sativa). Microbiol. Res. 2018, 206, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Chauhan, P.S. ACC deaminase-producing rhizosphere competent Bacillus spp. Mitigate salt stress and promote Zea mays growth by modulating ethylene metabolism. 3 Biotech 2020, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.N.; Lu, L.X.; Ye, J.R.; Shi, H.M. The endophytic strain ZS-3 enhances salt tolerance in Arabidopsis thaliana by regulating photosynthesis, osmotic stress, and ion homeostasis and inducing systemic tolerance. Front Plant Sci. 2022, 13, 820837. [Google Scholar] [CrossRef] [PubMed]
- Shabaan, M.; Asghar, H.N.; Zahir, Z.A.; Zhang, X.; Sardar, M.F.; Li, H. Salt-tolerant PGPR confer salt tolerance to maize through enhanced soil biological health, enzymatic activities, nutrient uptake and antioxidant defense. Front. Microbiol. 2022, 13, 901865. [Google Scholar] [CrossRef]
- Mellidou, I.; Ainalidou, A.; Papadopoulou, A.; Leontidou, K.; Genitsaris, S.; Karagiannis, E.; Van de Poel, B.; Karamanoli, K. Comparative transcriptomics and metabolomics reveal an intricate priming mechanism involved in PGPR-mediated salt tolerance in tomato. Front Plant Sci. 2021, 12, 713984. [Google Scholar] [CrossRef]
- Rabhi, N.E.H.; Silini, A.; Cherif-Silini, H.; Yahiaoui, B.; Lekired, A.; Robineau, M.; Esmaeel, Q.; Jacquard, C.; Vaillant-Gaveau, N.; Clément, C.; et al. Pseudomonas knackmussii MLR6, a rhizospheric strain isolated from halophyte, enhances salt tolerance in Arabidopsis thaliana. J. Appl. Microbiol. 2018, 125, 1836–1851. [Google Scholar] [CrossRef]
- Hou, Y.; Zeng, W.; Ao, C.; Luo, Y.; Wang, Z.; Hou, M.; Huang, J. Bacillus atrophaeus WZYH01 and Planococcus soli WZYH02 improve salt tolerance of maize (Zea mays L.) in saline soil. Front. Plant Sci. 2022, 13, 891372. [Google Scholar] [CrossRef]
- Barnawal, D.; Bharti, N.; Pandey, S.S.; Pandey, A.; Chanotiya, C.S.; Kalra, A. Plant growth-promoting rhizobacteria enhance wheat salt and drought stress tolerance by altering endogenous phytohormone levels and TaCTR1/TaDREB2 expression. Physiol. Plantarum 2017, 161, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Vives-Peris, V.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Salt stress alleviation in citrus plants by plant growth-promoting rhizobacteria Pseudomonas putida and Novosphingobium sp. Plant Cell Rep. 2018, 37, 1557–1569. [Google Scholar] [CrossRef] [PubMed]
- Arkhipova, T.; Martynenko, E.; Sharipova, G.; Kuzmina, L.; Ivanov, I.; Garipova, M.; Kudoyarova, G. Effects of plant growth promoting rhizobacteria on the content of abscisic acid and salt resistance of wheat plants. Plants 2020, 9, 1429. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Gupta, S.C.; Chauhan, P.S.; Lata, C. An OsNAM gene plays important role in root rhizobacteria interaction in transgenic Arabidopsis through abiotic stress and phytohormone crosstalk. Plant Cell Rep. 2021, 40, 143–155. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Evseeva, N.V.; Tkachenko, O.V.; Burygin, G.L.; Vysotskaya, L.B.; Akhtyamova, Z.A.; Kudoyarova, G.R. Rhizobacteria inoculation effects on phytohormone status of potato microclones cultivated in Vitro under osmotic stress. Biomolecules 2020, 10, 1231. [Google Scholar] [CrossRef]
- Gomez, M.Y.; Schroeder, M.M.; Chieb, M.; McLain, N.K.; Gachomo, E.W. Bradyrhizobium japonicum IRAT FA3 promotes salt tolerance through jasmonic acid priming in Arabidopsis thaliana. BMC Plant Biol. 2023, 23, 60. [Google Scholar] [CrossRef]
- Shahid, M.; Al-Khattaf, F.S.; Danish, M.; Zeyad, M.T.; Atef Hatamleh, A.; Mohamed, A.; Ali, S. PGPR Kosakonia Radicincitans KR-17 increases the salt tolerance of radish by regulating ion-homeostasis, photosynthetic molecules, redox potential, and stressor metabolites. Front. Plant Sci. 2022, 13, 919696. [Google Scholar] [CrossRef]
- Cai, D.; Xu, Y.; Zhao, F.; Zhang, Y.; Duan, H.; Guo, X. Improved salt tolerance of Chenopodium quinoa Willd. Contributed by Pseudomonas sp. Strain M30-35. PeerJ 2021, 9, e10702. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, S.; Vaishnav, A.; Jain, R.; Singh, D.; Singh, H.B.; Goel, A.; Singh, S. Salt-tolerant PGPR strain Priestia endophytica SK1 promotes fenugreek growth under salt stress by inducing nitrogen assimilation and secondary metabolites. J. Appl. Microbiol. 2022, 133, 2802–2813. [Google Scholar] [CrossRef]
- Hidri, R.; Mahmoud, O.M.; Zorrig, W.; Mahmoudi, H.; Smaoui, A.; Abdelly, C.; Azcon, R.; Debez, A. Plant growth-promoting rhizobacteria alleviate high salinity impact on the halophyte Suaeda fruticose by modulating antioxidant defense and soil biological activity. Front. Plant Sci. 2022, 13, 821475. [Google Scholar] [CrossRef]
- Safdarian, M.; Askari, H.; Shariati, J.V.; Nematzadeh, G. Transcriptional responses of wheat roots inoculated with Arthrobacter nitroguajacolicus to salt stress. Sci. Rep. 2019, 9, 1792. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Chen, Y.; Wang, Y.; Zheng, L.; Zhang, Q.; Liu, Y.; Hu, C.; Chen, C.; Ma, K.; Sun, Z. Halotolerant Bacillus altitudinis WR10 improves salt tolerance in wheat via a multi-level mechanism. Front. Plant Sci. 2022, 13, 941388. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Cheng, L.; Ma, Y.; Lei, P.; Wang, R.; Gu, Y.; Li, S.; Zhang, F.; Xu, H. Exopolysaccharides from Pantoea alhagi NX-11 specifically improve its root colonization and rice salt resistance. Int. J. Biol. Macromol. 2022, 209, 396–404. [Google Scholar] [CrossRef]
- Haroon, U.; Munis, M.F.H.; Liaquat, F.; Khizar, M.; Elahi, M.; Chaudhary, H.J. Biofilm formation and flocculation potential analysis of halotolerant Bacillus tequilensis and its inoculation in soil to mitigate salinity stress of chickpea. Physiol. Mol. Biol. Plants 2023, 29, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.W.; Ju, X.Y.; Li, X.W.; Gong, Y.; Xu, M.J.; Zhang, C.M.; Yuan, B.; Lv, Z.P.; Qin, S. Fermentation conditions optimization, purification, and antioxidant activity of exopolysaccharides obtained from the plant growth-promoting endophytic actinobacterium Glutamicibacter halophytocola KLBMP 5180. Int. J. Biol. Macromol. 2020, 153, 1176–1185. [Google Scholar] [CrossRef]
- Li, P.S.; Kong, W.L.; Wu, X.Q.; Zhang, Y. Volatile organic compounds of the plant growth-promoting rhizobacteria JZ-GX1 enhanced the tolerance of Robinia pseudoacacia to salt stress. Front. Plant Sci. 2021, 12, 753332. [Google Scholar] [CrossRef]
- Liu, S.; Tian, Y.; Jia, M.; Lu, X.; Yue, L.; Zhao, X.; Jin, W.; Wang, Y.; Zhang, Y.; Xie, Z.; et al. Induction of salt tolerance in Arabidopsis thaliana by volatiles from Bacillus amyloliquefaciens FZB42 via the jasmonic acid signaling pathway. Front. Microbiol. 2020, 11, 562934. [Google Scholar] [CrossRef]
- Luo, H.; Riu, M.; Ryu, C.M.; Yu, J.M. Volatile organic compounds emitted by Burkholderia pyrrocinia CNUC9 trigger induced systemic salt tolerance in Arabidopsis thaliana. Front. Microbiol. 2022, 13, 1050901. [Google Scholar] [CrossRef]
- Ledger, T.; Rojas, S.; Timmermann, T.; Pinedo, I.; Poupin, M.J.; Garrido, T.; Richter, P.; Tamayo, J.; Donoso, R. Volatile-mediated effects predominate in Paraburkholderia phytofirmans growth promotion and salt stress tolerance of Arabidopsis thaliana. Front. Microbiol. 2016, 7, 1838. [Google Scholar] [CrossRef]
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Ilangumaran, G.; Smith, D.L. Plant growth promoting rhizobacteria in amelioration of salinity stress: A systems biology perspective. Front. Plant Sci. 2017, 8, 1768. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, K.C.; Sharma, B.; Nagpal, S.; Kumar, A.; Tiwari, S.; Nair, R.M. Plant growth-promoting rhizobacteria: Salt stress alleviators to improve crop productivity for sustainable agriculture development. Front. Plant Sci. 2023, 13, 1101862. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Gilliham, M. Salinity tolerance of crops—What is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A. Regulation of Ph and generation of osmolarity in vascular plants—A cost-benefit analysis in relation to efficiency of use of energy, nitrogen and water. New Phytol. 1985, 101, 25–77. [Google Scholar] [CrossRef] [PubMed]
- Asensi-Fabado, M.A.; Amtmann, A.; Perrella, G. Plant responses to abiotic stress: The chromatin context of transcriptional regulation. Biochim. Biophys. Acta—Gene Regul. Mech. 2017, 1860, 106–122. [Google Scholar] [CrossRef]
- Cui, J.; Sun, T.; Li, S.; Xie, Y.; Song, X.; Wang, F.; Chen, L. Improved salt tolerance and metabolomics analysis of Synechococcus elongatus UTEX 2973 by overexpressing Mrp Antiporters. Front. Bioeng. Biotechnol. 2020, 8, 500. [Google Scholar] [CrossRef]
- Reza Amirjani, M. Effect of salinity stress on growth, sugar content, pigments and enzyme activity of rice. Int. J. Bot. 2011, 7, 73–81. Available online: https://scialert.net/abstract/?doi=ijb.2011.73.81 (accessed on 11 June 2020). [CrossRef]
- Jogawat, A. Osmolytes and their role in abiotic stress tolerance in plants. In Molecular Plant Abiotic Stress; Roychoudhury, A., Tripathi, D., Eds.; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- De Zelicourt, A.; Colcombet, J.; Hirt, H. The role of MAPK modules and ABA during abiotic stress signaling. Trends Plant Sci. 2016, 21, 677–685. [Google Scholar] [CrossRef]
- Kim, S.H.; Woo, D.H.; Kim, J.M.; Lee, S.Y.; Chung, W.S.; Moon, Y.H. Arabidopsis MKK4 mediates osmotic-stress response via its regulation of MPK3 activity. Biochem. Biophys. Res. Commun. 2011, 412, 150–154. [Google Scholar] [CrossRef]
- Fujii, H.; Zhu, J.K. Arabidopsis mutant deficient in 3 abscisic acid-activated protein kinases reveals critical roles in growth, reproduction, and stress. Proc. Natl. Acad. Sci. USA 2009, 106, 8380–8385. [Google Scholar] [CrossRef]
- Fujita, Y.; Nakashima, K.; Yoshida, T.; Katagiri, T.; Kidokoro, S.; Kanamori, N.; Umezawa, T.; Fujita, M.; Maruyama, K.; Ishiyama, K.; et al. Three SnRK2 protein kinases are the main positive regulators of abscisic acid signaling in response to water stress in Arabidopsis. Plant Cell Physiol. 2009, 50, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, T.; Sugiyama, N.; Mizoguchi, M.; Hayashi, S.; Myouga, F. Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 17588–17593. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Vinocur, B.; Altman, A. Recent advances in engineering plant tolerance to abiotic stress: Achievements and limitations. Curr. Opin. Biotech. 2005, 16, 123–132. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef]
- Damodaran, T.; Rai, R.B.; Jha, S.K.; Kannan, R.; Pandey, B.K.; Sah, V.; Mishra, V.K.; Sharma, D.K. Rhizosphere and endophytic bacteria for induction of salt tolerance in gladiolus grown in sodic soils. J. Plant Interact. 2014, 9, 577–584. [Google Scholar] [CrossRef]
- Etesami, H.; Beattie, G.A. Plant-Microbe interactions in adaptation of agricultural crops to abiotic stress conditions. In Probiotics and Plant Health; Kumar, V., Kumar, M., Sharma, S., Prasad, R., Eds.; Springer: Singapore, 2017; pp. 163–200. [Google Scholar] [CrossRef]
- Kim, K.; Jang, Y.J.; Lee, S.M.; Oh, B.T.; Chae, J.C.; Lee, K.J. Alleviation of salt stress by Enterobacter sp. EJ01 in tomato and Arabidopsis is accompanied by upregulation of conserved salinity responsive factors in plants. Mol. Cells 2014, 37, 109–117. [Google Scholar] [CrossRef]
- Armada, E.; Roldán, A.; Azcon, R. Differential activity of autochthonous bacteria in controlling drought stress in native Lavandula and Salvia plants species under drought conditions in natural arid soil. Microb. Ecol. 2014, 67, 410–420. [Google Scholar] [CrossRef]
- Kang, S.M.; Khan, A.L.; Waqas, M.; You, Y.H.; Kim, J.H.; Kim, J.G.; Hamayun, M.; Lee, I.J. Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J. Plant Interact. 2014, 9, 673–682. [Google Scholar] [CrossRef]
- Vardharajula, S.; Zulfikar Ali, S.; Grover, M.; Reddy, G.; Bandi, V. Drought-tolerant plant growth promoting Bacillus spp.: Effect on growth, osmolytes, and antioxidant status of maize under drought stress. J. Plant Interact. 2011, 6, 1–14. [Google Scholar] [CrossRef]
- Almeida, D.M.; Oliveira, M.M.; Saibo, N.J.M. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 2017, 40, 326–345. [Google Scholar] [CrossRef] [PubMed]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Roychoudhury, A. Gene regulation at transcriptional and post-transcriptional levels to combat salt stress in plants. Physiol. Plant. 2021, 173, 1556–1572. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Qiu, Q.S.; Quintero, F.J.; Pardo, J.M.; Ohta, M.; Zhang, C.; Schumaker, K.S.; Zhu, J.K. Transgenic evaluation of activated mutant alleles of SOS2 reveals a critical requirement for its kinase activity and C-terminal regulatory domain for salt tolerance in Arabidopsis thaliana. Plant Cell 2004, 16, 435–449. [Google Scholar] [CrossRef]
- Shi, H.; Ishitani, M.; Kim, C.; Zhu, J.K. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc. Natl. Acad. Sci. USA 2000, 97, 6896–6901. [Google Scholar] [CrossRef]
- Shi, H.; Quintero, F.J.; Pardo, J.M.; Zhu, J.K. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell 2002, 14, 465–477. [Google Scholar] [CrossRef]
- Apse, M.P.; Aharon, G.S.; Snedden, W.A.; Blumwald, E. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 1999, 285, 1256–1258. [Google Scholar] [CrossRef]
- Sottosanto, J.B.; Saranga, Y.; Blumwald, E. Impact of AtNHX1, a vacuolar Na+/H+ antiporter, upon gene expression during short- and long-term salt stress in Arabidopsis thaliana. BMC Plant Biol. 2007, 7, 18. [Google Scholar] [CrossRef]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How plant hormones mediate salt stress responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef]
- Finkelstein, R.R.; Rock, C.D. Abscisic acid biosynthesis and response. In The Arabidopsis Book; Somerville, C.R., Meyerowitz, E.M., Eds.; American Society of Plant Biologists: Rockville, MD, USA, 2002. [Google Scholar] [CrossRef]
- Sreenivasulu, N.; Harshavardhan, V.T.; Govind, G.; Seiler, C.; Kohli, A. Contrapuntal role of ABA: Does it mediate stress tolerance or plant growth retardation under long-term drought stress? Gene 2012, 506, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Ito, Y.; Yamaguchi-Shinozaki, K. Transcriptional regulatory networks in response to abiotic stresses in Arabidopsis and grasses. Plant Physiol. 2009, 149, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Yamaguchi-Shinozaki, K.; Seki, M. Regulatory network of gene expression in the drought and cold stress responses. Curr. Opin. Plant Biol. 2003, 6, 410–417. [Google Scholar] [CrossRef]
- Nemhauser, J.L.; Hong, F.; Chory, J. Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell 2006, 126, 467–475. [Google Scholar] [CrossRef]
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef] [PubMed]
- Cutler, A.J.; Krochko, J.E. Formation and breakdown of ABA. Trends Plant Sci. 1999, 4, 472–478. [Google Scholar] [CrossRef]
- Jaschke, W.D.; Peuke, A.D.; Pate, J.S.; Hartung, W. Transport, synthesis and catabolism of abscisic acid (ABA) in intact plants of castor bean (Ricinus communis L.) under phosphate deficiency and moderate salinity. J. Exp. Bot. 1997, 48, 1737–1747. [Google Scholar] [CrossRef]
- Li, J.; Han, G.; Sun, C.; Sui, N. Research advances of MYB transcription factors in plant stress resistance and breeding. Plant Signal. Behav. 2019, 14, 1613131. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef]
- Li, Q.; Saleh-Lakha, S.; Glick, B.R. The effect of native and ACC deaminase containing Azospirillum brasilense Cdl843 on the rooting of carnation cuttings. Can. J. Microbiol. 2005, 51, 511–514. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Ansari, M.W.; Sahoo, R.K.; Tuteja, N. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Fact. 2014, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 2007, 31, 425–448. [Google Scholar] [CrossRef] [PubMed]
- Castro-Camba, R.; Sánchez, C.; Vidal, N.; Vielba, J.M. Plant development and crop yield: The role of gibberellins. Plants 2022, 11, 2650. [Google Scholar] [CrossRef] [PubMed]
- Aloni, R.; Aloni, E.; Langhans, M. Role of cytokinin and auxin in shaping root architecture: Regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann. Bot. 2006, 97, 883–893. [Google Scholar] [CrossRef]
- Etesami, H.; Beattie, G.A. Mining halophytes for plant growth-promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Front. Microbiol. 2018, 9, 148. [Google Scholar] [CrossRef]
- Etesami, H.; Alikhani, H.A.; Mirseyed Hosseini, H. Indole-3-acetic Acid and 1-Aminocyclopropane-1-Carboxylate Deaminase: Bacterial traits required in rhizosphere, rhizoplane and/or endophytic competence by beneficial bacteria. In Bacterial Metabolites in Sustainable Agroecosystem; Maheshwari, D., Ed.; Sustainable Development and Biodiversity; Springer: Cham, Switzerland, 2015; Volume 12. [Google Scholar] [CrossRef]
- Glick, B.R. Bacterial ACC deaminase and the alleviation of plant stress. Adv. Appl. Microbiol. 2004, 56, 291–312. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. Bacterial modulation of plant ethylene levels. Plant Physiol. 2015, 169, 13–22. [Google Scholar] [CrossRef]
- Glick, B.R.; Cheng, Z.; Czarny, J.; Duan, J. Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur. J. Plant Pathol. 2007, 119, 329–339. [Google Scholar] [CrossRef]
- Martynenko, E.; Arkhipova, T.; Safronova, V.; Seldimirova, O.; Galin, I.; Akhtyamova, Z.; Veselov, D.; Ivanov, R.; Kudoyarova, G. Effects of phytohormone-producing rhizobacteria on casparian band formation, ion homeostasis and salt tolerance of durum wheat. Biomolecules 2022, 12, 230. [Google Scholar] [CrossRef]
- Akhtyamova, Z.; Arkhipova, T.; Martynenko, E.; Nuzhnaya, T.; Kuzmina, L.; Kudoyarova, G.; Veselov, D. Growth-promoting effect of rhizobacterium (Bacillus subtilis IB22) in salt-stressed barley depends on abscisic acid accumulation in the roots. Int. J. Mol. Sci. 2021, 22, 10680. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Park, S.W. 12-oxo-Phytodienoic acid: A fuse and/or switch of plant growth and defense responses? Front. Plant Sci. 2021, 12, 724079. [Google Scholar] [CrossRef] [PubMed]
- Taki, N.; Sasaki-Sekimoto, Y.; Obayashi, T.; Kikuta, A.; Kobayashi, K.; Ainai, T.; Yagi, K.; Sakurai, N.; Suzuki, H.; Masuda, T.; et al. 12-oxo-phytodienoic acid triggers expression of a distinct set of genes and plays a role in wound-induced gene expression in Arabidopsis. Plant Physiol. 2005, 139, 1268–1283. [Google Scholar] [CrossRef] [PubMed]
- Reginato, M.; Sgroy, V.; Llanes, A.; Cassán, F.; Luna, V. The american halophyte Prosopis strombulifera, a new potential source to confer salt tolerance to crops. In Crop Production for Agricultural Improvement; Ashraf, M., Öztürk, M., Ahmad, M., Aksoy, A., Eds.; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Hazman, M.; Hause, B.; Eiche, E.; Nick, P.; Riemann, M. Increased tolerance to salt stress in OPDA-deficient rice ALLENE OXIDE CYCLASE mutants is linked to an increased ROS-scavenging activity. J. Exp. Bot. 2015, 66, 3339–3352. [Google Scholar] [CrossRef] [PubMed]
- Forchetti, G.; Masciarelli, O.; Alemano, S.; Alvarez, D.; Abdala, G. Endophytic bacteria in sunflower (Helianthus annuus L.): Isolation, characterization, and production of jasmonates and abscisic acid in culture medium. Appl. Microbiol. Biotechnol. 2007, 76, 1145–1152. [Google Scholar] [CrossRef]
- Erice, G.; Ruíz-Lozano, J.M.; Zamarreño, A.M.; García-Mina, J.M.; Aroca, R. Transcriptomic analysis reveals the importance of JA-Ile turnover in the response of Arabidopsis plants to plant growth promoting rhizobacteria and salinity. Environ. Exp. Bot. 2017, 143, 10–19. [Google Scholar] [CrossRef]
- Burdiak, P.; Mielecki, J.; Gawro’ nski, P.; Karpi´ nski, S. The CRK5 and WRKY53 are conditional regulators of senescence and stomatal conductance in Arabidopsis. Cells 2022, 11, 3558. [Google Scholar] [CrossRef]
- Mittler, R.; Kim, Y.; Song, L.; Coutu, J.; Coutu, A.; Ciftci-Yilmaz, S.; Lee, H.; Stevenson, B.; Zhu, J.K. Gain- and loss-of-function mutations in Zat10 enhance the tolerance of plants to abiotic stress. FEBS Lett. 2006, 580, 6537–6542. [Google Scholar] [CrossRef] [PubMed]
- Pedranzani, H.; Rodríguez-Rivera, M.; Gutiérrez, M.; Porcel, R.; Hause, B.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis regulates physiology and performance of Digitaria eriantha plants subjected to abiotic stresses by modulating antioxidant and jasmonate levels. Mycorrhiza 2016, 26, 141–152. [Google Scholar] [CrossRef]
- Ben Abdallah, H.; Mai, H.J.; Alvarez-Fernandez, A.; Abadia, J.; Bauer, P. Natural variation reveals contrasting abilities to cope with alkaline and saline soil among different Medicago truncatula genotypes. Plant Soil 2017, 418, 45–60. [Google Scholar] [CrossRef]
- Läuchli, A.; Grattan, S. Plant growth and development under salinity stress. In Advances in Molecular Breeding toward Drought and Salt Tolerant Crops; Jenks, M.A., Hasegawa, P.M., Jain, S.M., Eds.; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar] [CrossRef]
- Hu, Y.; Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Singh, M.; Singh, D.; Gupta, A.; Pandey, K.D.; Singh, P.K.; Kumar, A. Plant Growth Promoting Rhizobacteria: Application in Biofertilizers and Biocontrol of Phytopathogens. In PGPR Amelioration in Sustainable Agriculture; Singh, A.K., Kumar, A., Singh, P.K., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 41–66. [Google Scholar] [CrossRef]
- Tilak, K.V.B.R.; Ranganayaki, N.; Pal, K.K.; De, R.; Saxena, A.K.; Nautiyal, C.S.; Mittal, S.; Tripathi, A.K.; Johri, B.N. Diversity of plant growth and soil health supporting bacteria. Curr. Sci. 2005, 89, 136–150. Available online: http://www.jstor.org/stable/24110439 (accessed on 11 June 2020).
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Damam, M.; Kaloori, K.; Gaddam, B.; Kausar, R. Plant growth promoting substances (phytohormones) produced by rhizobacterial strains isolated from the rhizosphere of medicinal plants. Int. J. Pharm. Sci. Rev. Res. 2016, 37, 130–136. [Google Scholar]
- Khan, M.S.; Zaidi, A.; Ahemad, M.; Oves, M.; Wani, P.A. Plant growth promotion by phosphate solubilizing fungi—Current perspective. Arch. Agron. Soil Sci. 2010, 56, 73–98. [Google Scholar] [CrossRef]
- Oteino, N.; Lally, R.D.; Kiwanuka, S.; Lloyd, A.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015, 6, 745. [Google Scholar] [CrossRef] [PubMed]
- Barragan, V.; Leidi, E.O.; Andrés, Z.; Rubio, L.; De Luca, A.; Fernandez, J.A.; Cubero, B.; Pardo, J.M. Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell 2012, 24, 1127–1142. [Google Scholar] [CrossRef]
- Parmar, P.; Sindhu, S.S. Potassium solubilization by rhizosphere bacteria: Influence of nutritional and environmental conditions. J. Microbiol. Res. 2013, 3, 25–31. [Google Scholar]
- Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. Bacterial siderophores: Classification, biosynthesis, perspectives of use in agriculture. Plants 2022, 11, 3065. [Google Scholar] [CrossRef]
- Ammari, T.; Mengel, K. Total soluble Fe in soil solutions of chemically different soils. Geoderma 2006, 136, 876–885. [Google Scholar] [CrossRef]
- Dertz, E.A.; Xu, J.; Stintzi, A.; Raymond, K.N. Bacillibactin-mediated iron transport in Bacillus subtilis. J. Am. Chem. Soc. 2006, 11, 22–23. [Google Scholar] [CrossRef]
- Scavino, A.F.; Pedraza, R.O. The role of siderophores in plant growth-promoting bacteria. In Bacteria in Agrobiology: Crop Productivity; Maheshwari, D., Saraf, M., Aeron, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Parisi, B.; Vallee, B.L. Metal enzyme complexes activated by zinc. J. Biol. Chem. 1969, 179, 803–807. [Google Scholar]
- Singh, B.P.; Natesan, S.; Singh, B.; Usha, K. Improving zinc efficiency of cereals under zinc deficiency. Curr. Sci. 2005, 88, 36–44. [Google Scholar]
- Saravanan, V.S.; Kumar, M.R.; Sa, T.M. Microbial zinc solubilization and their role on plants. In Bacteria in Agrobiology: Plant Nutrient Management; Maheshwari, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Danhorn, T.; Fuqua, C. Biofilm formation by plant-associated bacteria. Annu. Rev. Microbiol. 2007, 61, 401–422. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Solis, D.; Vences-Guzmán, M.Á.; Sohlenkamp, C.; Santoyo, G. Antifungal and plant growth–promoting Bacillus under saline stress modify their membrane composition. J. Soil Sci. Plant Nutr. 2020, 20, 1549–1559. [Google Scholar] [CrossRef]
- Morcillo, R.J.L.; Manzanera, M. The effects of plant-associated bacterial exopolysaccharides on plant abiotic stress tolerance. Metabolites 2021, 11, 337. [Google Scholar] [CrossRef]
- Kanchiswamy, C.N.; Malnoy, M.; Maffei, M.E. Chemical diversity of microbial volatiles and their potential for plant growth and productivity. Front. Plant Sci. 2015, 6, 151. [Google Scholar] [CrossRef]
- Maffei, M.E.; Gertsch, J.; Appendino, G. Plant volatiles production, function and pharmacology. Nat. Prod. Rep. 2011, 28, 1359–1380. [Google Scholar] [CrossRef]
- Korpi, A.; Järnberg, J.; Pasanen, A.L. Microbial volatile organic compounds. Crit. Rev. Toxicol. 2009, 39, 139–193. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Lee, Y.H. A cocktail of volatile compounds emitted from Alcaligenes faecalis JBCS1294 induces salt tolerance in Arabidopsis thaliana by modulating hormonal pathways and ion transporters. J. Plant Physiol. 2017, 214, 64–73. [Google Scholar] [CrossRef]
- De Palma, M.; Salzano, M.; Villano, C.; Aversano, R.; Lorito, M.; Ruocco, M.; Docimo, T.; Piccinelli, A.L.; D’Agostino, N.; Tucci, M. Transcriptome reprogramming, epigenetic modifications and alternative splicing orchestrate the tomato root response to the beneficial fungus Trichoderma harzianum. Hortic. Res. 2019, 6, 5. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J.K. Epigenetic regulation of stress responses in plants. Curr. Opin. Plant Biol. 2009, 12, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Seki, M. Epigenetic memory for stress response and adaptation in plants. Plant Cell Physiol. 2014, 55, 1859–6183. [Google Scholar] [CrossRef] [PubMed]
- Thiebaut, F.; Hemerly, A.S.; Ferreira, P.C.G. A role for epigenetic regulation in the adaptation and stress responses of non-model plants. Front. Plant Sci. 2019, 10, 246. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, M.; Zhu, J.; Tang, Y.; Zhang, H.; Zhao, Q.; Jing, M.; Chen, Y.; Xu, X.; Jiang, J.; et al. Long-term effect of epigenetic modification in plant–microbe interactions: Modification of DNA methylation induced by plant growth-promoting bacteria mediates promotion process. Microbiome 2022, 10, 36. [Google Scholar] [CrossRef]
- Neiverth, A.; Delai, S.; Garcia, D.M.; Saatkamp, K.; de Souza, E.M.; Pedrosa, F.d.O.; da Costa, A.C.T. Performance of different wheat genotypes inoculated with the plant growth promoting bacterium Herbaspirillum seropedicae. Eur. J. Soil Biol. 2014, 64, 1–5. [Google Scholar] [CrossRef]
- Vacheron, J.; Combes-Meynet, E.; Walker, V.; Gouesnard, B.; Muller, D.; Moënne-Loccoz, Y.; Prigent-Combaret, C. Expression on roots and contribution to maize phytostimulation of 1-aminocyclopropane- 1-decarboxylate deaminase gene acdS in Pseudomonas fluorescens F113. Plant Soil 2016, 407, 187–202. [Google Scholar] [CrossRef]
- Valente, J.; Gerin, F.; Le Gouis, J.; Moënne-Loccoz, Y.; Prigent–Combaret, C. Ancient wheat varieties have a higher ability to interact with plant growth-promoting rhizobacteria. Plant Cell Environ. 2020, 43, 246–260. [Google Scholar] [CrossRef]
- Corbin, K.E.; Bolt, B.; Lòpez, C.M.R. Breeding for beneficial microbial communities using epigenomics. Front. Microbiol. 2020, 11, 937. [Google Scholar] [CrossRef]
- Wei, Z.; Jousset, A. Plant breeding goes microbial. Trends Plant Sci. 2017, 22, 555–558. [Google Scholar] [CrossRef]
- Amoah, S.; Kurup, S.; Rodriguez Lopez, C.M.; Welham, S.J.; Powers, S.J.; Hopkins, C.J.; Wilkinson, M.J.; King, G.J. A hypoethylated population of Brassica rapa for forward and reverse epi-genetics. BMC Plant Biol. 2012, 12, 193. [Google Scholar] [CrossRef] [PubMed]
- Escudero-Martinez, C.; Coulter, M.; Alegria Terrazas, R.; Foito, A.; Kapadia, R.; Pietrangelo, L.; Maver, M.; Sharma, R.; Aprile, A.; Morris, J.; et al. Identifying plant genes shaping microbiota composition in the barley rhizosphere. Nat. Commun. 2022, 13, 3443. [Google Scholar] [CrossRef]
- Wallace, J.G.; Kremling, K.A.; Kovar, L.L.; Buckler, E.S. Quantitative genetics of the maize leaf microbiome. Phytobiomes J. 2018, 2, 208–224. [Google Scholar] [CrossRef]
- Deng, S.; Caddell, D.F.; Xu, G.; Dahlen, L.; Washington, L.; Yang, J.; Coleman-Derr, D. Genome wide association study reveals plant loci controlling heritability of the rhizosphere microbiome. ISME J. 2021, 15, 3181–3194. [Google Scholar] [CrossRef]
- Mitter, B.; Pfaffenbichler, N.; Flavell, R.; Company, S.; Antonielli, L.; Petric, A.; Berninger, T.; Naveed, M.; Sheibani-Tezerji, R.; von Maltzahn, G.; et al. A new approach to modify plant microbiomes and traits by introducing beneficial bacteria at flowering into progeny seeds. Front. Microbiol. 2017, 8, 11. [Google Scholar] [CrossRef] [PubMed]

| PGPR | Plant Species | PGPR-Induced Plant Salt Stress Responses | References |
|---|---|---|---|
| Osmotic adjustment | |||
| Enterobacter cloacae PM23 Serratia liquefaciens KM4 | Zea mays | ↑ Proline, glycine betaine, free amino acids, and soluble sugars | [61,62] |
| Streptomyces albidoflavus OsiLf-2 | Oryza sativa | ↑ Proline, total soluble sugars | [63] |
| Enterobacter 64S1 and Pseudomonas 42P4 | Solanum lycopersicum | ↑ Proline | [64] |
| Bacillus sp. Wp-6 | Triticum aestivum | ↑Proline, soluble sugars, and soluble proteins | [65] |
| Bacillus firmus SWS | Glycine max | ↑ Proline | [66] |
| Klebsiella sp. IG 3 | Avena sativa | ↑ Total soluble sugars; ↓ Proline | [67] |
| Bacillus spp. | Solanum lycopersicum | ↑ Total soluble sugars; ↓ Proline | [68] |
| Bacillus megaterium ZS-3 | Arabidopsis thaliana | ↑ Total soluble sugars; ↓ Proline | [69] |
| Antioxidant activity | |||
| Acinetobacter Johnsonii SUA-14 | Zea mays | ↓ Superoxide dismutase and catalase activity, MDA content | [70] |
| Enterobacter cloacae PM23 | Zea mays | ↑ SOD, POD, APX, ascorbic acid activity, flavonoid and phenolic content ↓ H2O2 and MDA content | [61] |
| Pseudomonas oryzihabitans AXSa06 | Solanum lycopersicum | ↑ Ascorbate and MDA content, POD, and CAT activity | [71] |
| Bacillus firmus SW5 | Glycine max | ↓ H2O2 and MDA content; ↑ APX, CAT, Fe-SOD, and POD | [66] |
| Ion homeostasis | |||
| Bacillus megaterium ZS-3 | Arabidopsis thaliana | ↑ K+/Na+ ratio, NHX1 and AVP1; ↓ HKT1 | [69] |
| Pseudomonas knackmussii MLR6 | Arabidopsis thaliana | ↑ SOS1 | [72] |
| Bacillus atrophaeus ZYH01 Planococcus soli WZYH02 | Zea mays | ↑ K+/Na+ ratio, NHX, and HKT | [73] |
| Hormonal modulation | |||
| Bacillus safenis NBRI 12M Bacillus subtilis NBRI 28B Bacillus subtilis NBRI 33N | Zea mays | ↓ Ethylene production, ACC-oxidase activity | [68] |
| Bacillus atrophaeus WZYH01 Planococcus soli WZYH02 | Zea mays | ↓ ABA content, NCED gene; ↑ DREB2A and WRKY58 genes | [73] |
| Arthrobacter protophormiae SA3 Dietzia natronolimnaea STR1 | Triticum aestivum | ↓ ABA content; ↑ DREB2 | [74] |
| Pseudomonas putida KT2440 Novosphingobium sp. HR1a | Citrus macrophylla | ↓ ABA, SA and IAA content | [75] |
| Bacillus subtilis IB-22 | Triticum durum | ↓ ABA content in the roots, HvNCED2; ↑ ABA content in the leaves, HvCYP707A1 | [76] |
| Bacillus amyloliquefaciens SN13 | Arabidopsis | ↑ OsNAM; ↑/↓ ABA, GA, IAA, AP2/ERF, ARF2, GST, and ERD4. | [77] |
| Azospirillum brasiliense Sp245 Ochrobactum cytisi IPA7.2 | Solanum tuberosum | ↑ Cytokinin and auxin in the stems and leaves | [78] |
| Bradyrhizobium japonicum | Arabidopsis thaliana | ↑ RD20, CAT3, and MDAR2 genes in roots; ↓ DHAR, MSD1, MYC2, and RD22 in shoots and ADC2, ANACO55, DHAR, GTR1, RD20, RD29B, VSP1, and VSP2 in roots | [79] |
| Nutrients uptake | |||
| Kosakonia radicincitans KR-17 | Raphanus sativus L. | ↑ N, P, K+, Ca2+, Mg, Zn, Fe, Cu, and Na+ | [80] |
| Pseudomonas sp. M30-35 | Chenopodium quinoa | Maintaining P content and homeostasis | [81] |
| Priestia endophytica SK1 | Trigonella foenum-graecum | ↑ N and P | [82] |
| Glutamicibacter sp. Pseudomonas sp. | Suaeda fruticose | ↓ Na+ and Cl− in shoots | [83] |
| Pseudomonas sp. | Suaeda fruticose | ↑ K+ and Ca2+, activity of urease, ß-glucosidase, and dehydrogenase | [83] |
| Arthrobacter nitroguajacolicus | Wheat | ↑ Genes related to metal binding and involved in iron acquisition | [84] |
| Bacillus altitudinis WR10 | Wheat | ↓ Na+; ↑ K+, P, and Ca2+, H+-ATPase, P-solubilizing activity and biofilm production | [85] |
| Biofilm production | |||
| Pantoea alhagi NX-11 | Zea mays | ↑ OsXTH25 | [86] |
| Bacillus tequilensis | Cicer arietinum | Bacterial flocculation directly related to EPS production and biofilm formation traits | [87] |
| Glutamicibacter halophytocola KLBMP 518 | - | Antioxidant activity in vitro against superoxide anion and hydroxyl radical | [88] |
| Volatile organic compounds (VOCs) | |||
| Rahnella aquatilis JZ-GX1 | Robinia pseudoacacia | Changes in chlorophyll content and root morphology; ↑ Proline and antioxidant activity; ↓ MDA, O2•−, and H2O2; ↓ intracellular Na+; ↑ RpNHX1 | [89] |
| Bacillus amyloliquefaciens FZB42 | Arabidopsis thaliana | ↑ Chlorophyll content and total soluble sugars; ↑ CAT, SOD, POD, and ROS scavenging capacity; ↑ HKT1 and NHX1; alleviate salt-tolerance-regulating JA signaling pathways | [90] |
| Burkholderia pyrrocinia CNUC9 | Arabidopsis thaliana | ↓ ROS, MDA, and proline | [91] |
| Paraburkholderia phytofirmans PsJN | Arabidopsis thaliana | Long-term effects resulting in salinity tolerance | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannelli, G.; Potestio, S.; Visioli, G. The Contribution of PGPR in Salt Stress Tolerance in Crops: Unravelling the Molecular Mechanisms of Cross-Talk between Plant and Bacteria. Plants 2023, 12, 2197. https://doi.org/10.3390/plants12112197
Giannelli G, Potestio S, Visioli G. The Contribution of PGPR in Salt Stress Tolerance in Crops: Unravelling the Molecular Mechanisms of Cross-Talk between Plant and Bacteria. Plants. 2023; 12(11):2197. https://doi.org/10.3390/plants12112197
Chicago/Turabian StyleGiannelli, Gianluigi, Silvia Potestio, and Giovanna Visioli. 2023. "The Contribution of PGPR in Salt Stress Tolerance in Crops: Unravelling the Molecular Mechanisms of Cross-Talk between Plant and Bacteria" Plants 12, no. 11: 2197. https://doi.org/10.3390/plants12112197






