An Insight into Citrus medica Linn.: A Systematic Review on Phytochemical Profile and Biological Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Methodological Quality Assessment
3. Results and Discussion
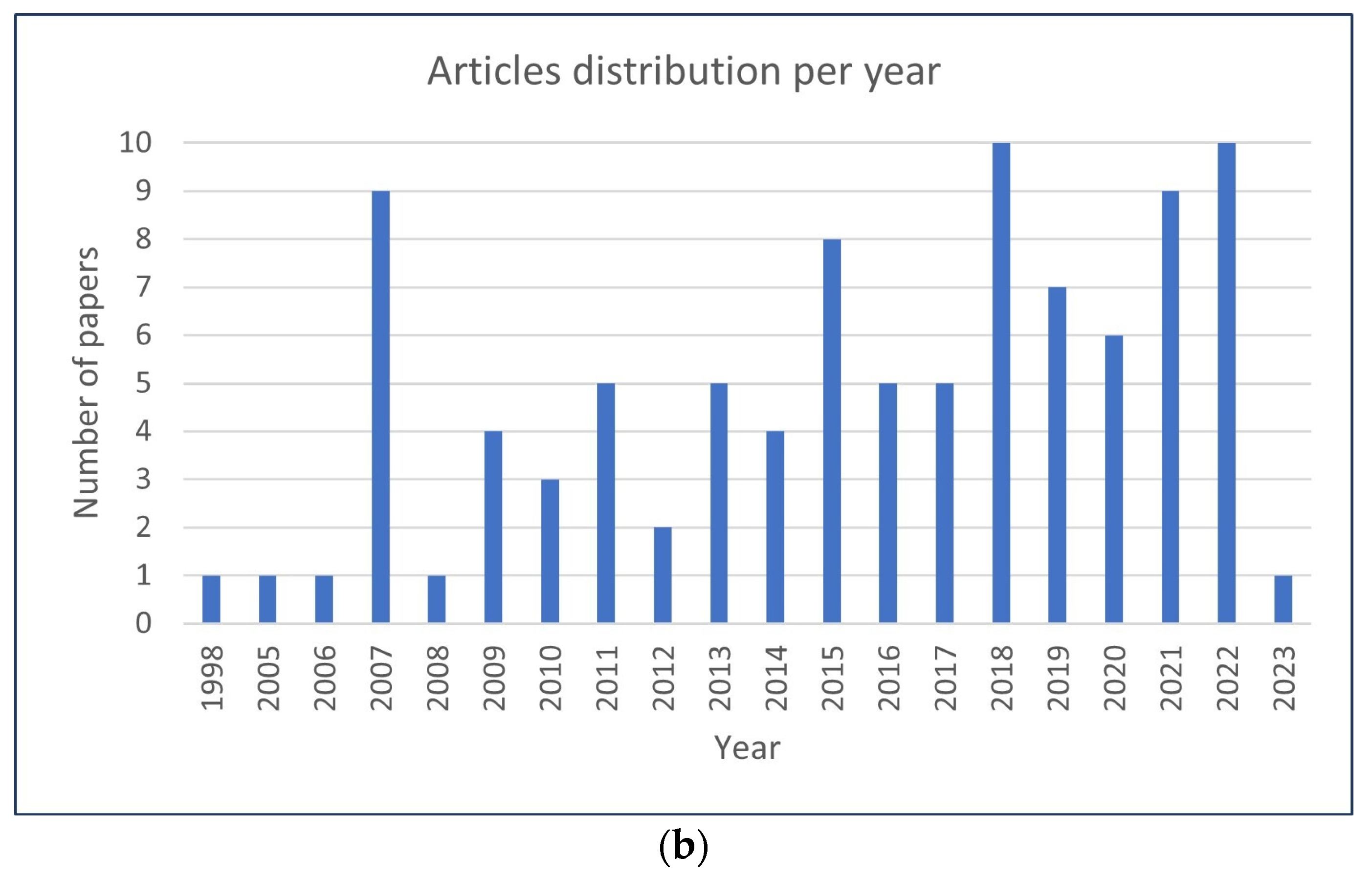
3.1. Study Characteristics
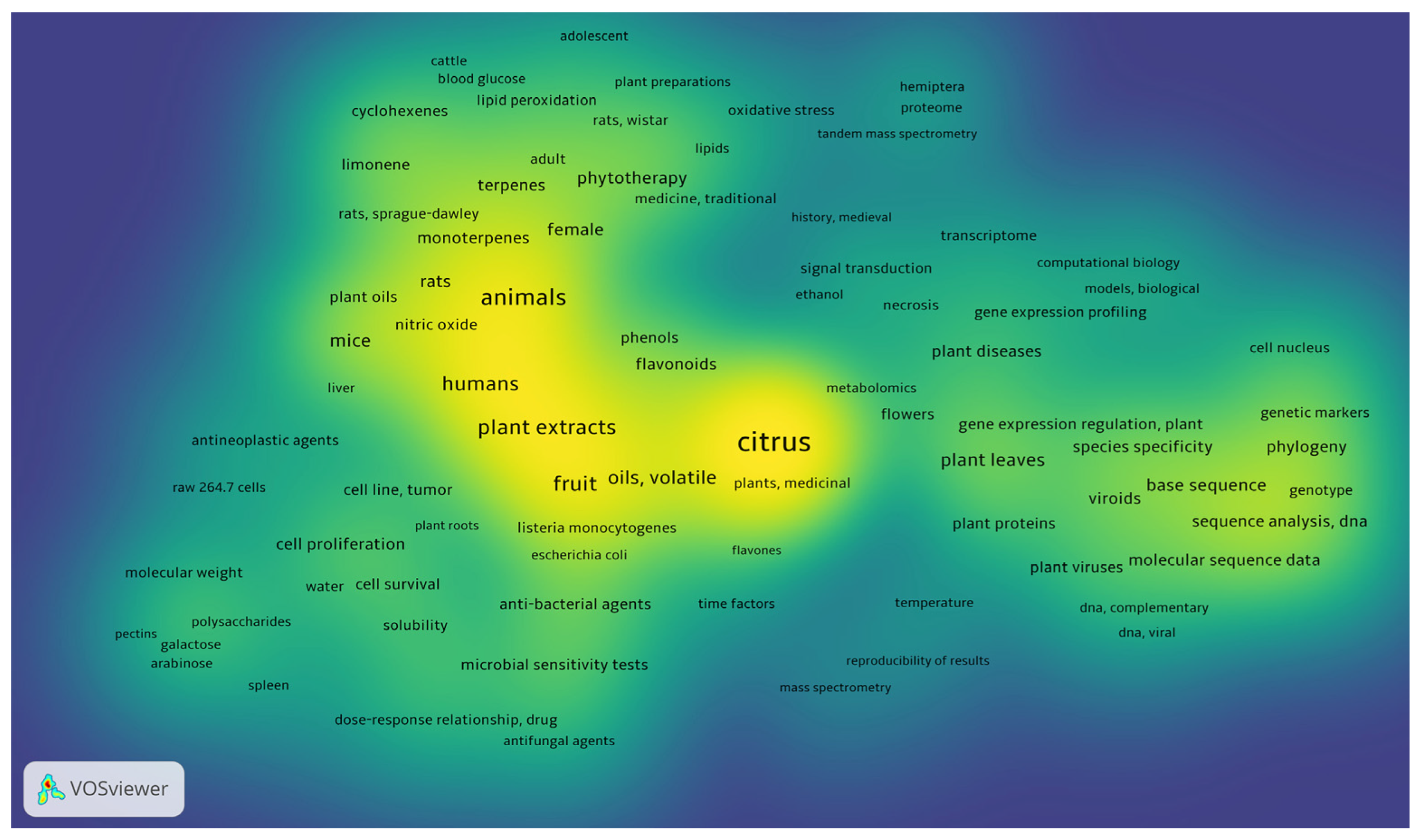
3.2. Phytochemistry
3.2.1. Macronutrients and Micronutrients
3.2.2. Polyphenols, Flavonoids, and Phenolic Acids
3.2.3. Terpenes
3.2.4. Coumarins
3.3. Other Compounds
3.4. Biological Activity
3.4.1. Antioxidant Activity
3.4.2. Antibacterial, Antiviral, and Antifungal Activity
3.4.3. Cytotoxic Activity
3.4.4. Anti-Inflammatory and Analgesic Activity
3.4.5. Other Activities
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
| APE | Alginate extract and pectin filler |
| COSY | Correlation spectroscopy |
| CPE | Continuous phase-transition extraction |
| C-RDI | Contribution reference daily intake |
| DLA | Dalton’s lymphoma ascites |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| DW | Dry weight |
| EC50 | Half maximal effective concentration |
| EAC | Ehrlich’s ascites carcinoma |
| EtOH | Ethanol |
| EI-MS | Electron ionization–mass spectrometry |
| EO | Essential oil |
| ERK | Extracellular signal-regulated kinase |
| FW | FW |
| GAE | Gallic acid equivalent |
| GC–MS | Gas chromatography–mass spectrometry |
| GC–MS–SPME | Gas chromatography–mass spectrometry–solid-phase microextraction |
| HeLa | Henrietta Lacks |
| HL60 | Human leukemia cell line |
| HMQC | Heteronuclear multiple quantum coherence |
| HMBC | Heteronuclear multiple bond coherence |
| HPGPC | High-performance gel-permeation chromatography |
| HPLC | High-performance liquid chromatography |
| HPLC–PDA–MS | High-performance liquid chromatography–photodiode array–mass spectrometry |
| HPLC–QTOF–MS | High-performance liquid chromatography–quadruple time of flight–mass spectrometry |
| HRGC–MS | High-resolution gas chromatography–mass spectrometry |
| HR–ESI–MS | High-resolution–electrospray ionization–mass spectrometry |
| HR–EI–MS | High-resolution–electron ionization–mass spectrometry |
| HR–MAS–NMR | High-resolution–magic angle sinning–nuclear magnetic resonance |
| HY | Hydrolat |
| IC50 | Half-maximal inhibitory concentration |
| IkB-α: | Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha |
| IL-6 | Interleukin 6 |
| IL-1β | Interleukin 1β |
| IL-10 | Interleukin 10 |
| JNK | c-Jun N-terminal kinase |
| LPS | Lipopolysaccharide |
| MAPKs | Mitogen-activated protein kinase |
| MeOH | Methanol |
| MIC | Minimum inhibitory concentration |
| NF-kB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | Nitric oxide |
| NOESY | Nuclear Overhauser effect spectroscopy |
| PC-3 | Human prostate adenocarcinoma cell line |
| PLE | Pressure liquid extraction |
| RAW 264.7 | Macrophage cell line |
| RSA | Radical-scavenging activity |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| SCF–CO2 | Super-critical fluid–carbon bioxide |
| SH-SY5Y | Human neuroblastoma cell line |
| TFC | Total flavonoid content |
| TNF-α | Tumor necrosis factor alpha |
| TPC | Total phenolic content |
| UV | Ultraviolet |
| UAHD | Hydro-distillation ultrasound-assisted extraction |
| UAE | Ultrasound-assisted extraction |
| UHPLC–QTOF–IMS | Ultra-performance liquid chromatography–quadruple time of flight–mass spectrometry |
| Var. | Variety |
References
- Ramadugu, C.; Keremane, M.L.; Hu, X.; Karp, D.; Federici, C.T.; Kahn, T.; Roose, M.L.; Lee, R.F. Genetic analysis of citron (Citrus medica L.) using simple sequence repeats and single nucleotide polymorphisms. Sci. Hortic. 2015, 195, 124–137. [Google Scholar] [CrossRef] [Green Version]
- Hayat, K. Citrus molecular phylogeny antioxidant properties and medicinal uses. Nova. Sci. 2014, 3, 235. [Google Scholar]
- Dey, P.; Hoque, M.; Islam, M.; Monalisa, K. Kinetics of antioxidants degradation and quality changes of citron (Citrus medica) fruit juice concentrate during storage. J. Microbiol. Biotechnol. Food Sci. 2021, 2021, 230–234. [Google Scholar]
- Pieroni, A. Medicinal plants and food medicines in the folk traditions of the upper Lucca Province, Italy. J. Ethnopharmacol. 2000, 70, 235–273. [Google Scholar] [CrossRef] [PubMed]
- Secundus, C.P. Naturalis Historiae, Libri XXXVII; Lipsiae, in Aedibus B. G. Teubneri; Nabu Press: Charleston, SC, USA, 1865; Volume 6. [Google Scholar]
- Pieroni, A.; Quave, C.L.; Villanelli, M.L.; Mangino, P.; Sabbatini, G.; Santini, L.; Boccetti, T.; Profili, M.; Ciccioli, T.; Rampa, L.G. Ethnopharmacognostic survey on the natural ingredients used in folk cosmetics, cosmeceuticals and remedies for healing skin diseases in the inland Marches, Central-Eastern Italy. J. Ethnopharmacol. 2004, 91, 331–344. [Google Scholar] [CrossRef]
- Sahoo, C.R.; Sahoo, J.; Mahapatra, M.; Lenka, D.; Sahu, P.K.; Dehury, B.; Padhy, R.N.; Paidesetty, S.K. Coumarin derivatives as promising antibacterial agent (s). Arab. J. Chem. 2021, 14, 102922. [Google Scholar] [CrossRef]
- Haridas, M.; Sasidhar, V.; Nath, P.; Abhithaj, J.; Sabu, A.; Rammanohar, P. Compounds of Citrus medica and Zingiber officinale for COVID-19 inhibition: In silico evidence for cues from Ayurveda. Future J. Pharm. Sci. 2021, 7, 13. [Google Scholar] [CrossRef]
- Zarshenas, M.M.; Mahmoodian, R.; Moein, M. Colorimetric determination of flavonoids in Citrus medica peel traditional medicinal oil. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 696–700. [Google Scholar]
- Jafarpour, M.; Yousefi, G.; Hamedi, A.; Shariat, A.; Salehi, A.; Heydari, M. Effect of a traditional syrup from Citrus medica L. fruit juice on migraine headache: A randomized double blind placebo controlled clinical trial. J. Ethnopharmacol. 2016, 179, 170–176. [Google Scholar] [CrossRef]
- Kalariya, M.; Prajapati, R.; Chavda, J. Pharmacological potential of Citrus medica: A review. Pharma Sci. Monit. 2019, 10, 66–81. [Google Scholar]
- Karp, D.; Hu, X. The citron (Citrus medica L.) in China. Hortic. Rev. 2018, 45, 143–196. [Google Scholar]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, 14651858. [Google Scholar] [CrossRef] [Green Version]
- Ponticelli, M.; Lela, L.; Moles, M.; Mangieri, C.; Bisaccia, D.; Faraone, I.; Falabella, R.; Milella, L. The healing bitterness of Gentiana lutea L., phytochemistry and biological activities: A systematic review. Phytochemistry 2022, 206, 113518. [Google Scholar] [CrossRef] [PubMed]
- Dadwal, V.; Joshi, R.; Gupta, M. A comparative metabolomic investigation in fruit sections of Citrus medica L. and Citrus maxima L. detecting potential bioactive metabolites using UHPLC-QTOF-IMS. Food Res. Int. 2022, 157, 111486. [Google Scholar] [CrossRef]
- Hasan, M.M.; Roy, P.; Alam, M.; Hoque, M.M.; Zzaman, W. Antimicrobial activity of peels and physicochemical properties of juice prepared from indigenous citrus fruits of Sylhet region, Bangladesh. Heliyon 2022, 8, e09948. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, A.A.; Al-Ansi, W.; Ahmed, M.I.; Xiaoyun, C.; Mohammed, J.K.; Sulieman, A.A.; Mushtaq, B.S.; Harimana, Y.; Wang, H. Microwave assisted extraction of the bioactive compounds from peel/pulp of Citrus medica L. var. sarcodactylis swingle along with its nutritional profiling. J. Food Meas. Charact. 2020, 14, 283–292. [Google Scholar] [CrossRef]
- Khomdram, S.; Barthakur, S.; Devi, G.S. Biochemical and molecular analysis of wild endemic fruits of the Manipur region of India. Int. J. Fruit Sci. 2014, 14, 253–266. [Google Scholar] [CrossRef]
- Madhavi, N.; Jyothi, B. Colorimetric determination of vitamin C in fresh and dilute fruit juices and effect of thermal exposure on concentration at various stages. Int. J. Pharma Bio. Sci. 2016, 7, 197–211. [Google Scholar]
- Pallavi, M.; Ck, R.; Krishna, V.; Parveen, S. Quantitative phytochemical analysis and antioxidant activities of some citrus fruits of South India. Asian J. Pharm. Clin. Res. 2017, 10, 198–205. [Google Scholar]
- Wang, E.; Li, Y.; Maguy, B.L.; Lou, Z.; Wang, H.; Zhao, W.; Chen, X. Separation and enrichment of phenolics improved the antibiofilm and antibacterial activity of the fractions from Citrus medica L. var. sarcodactylis in vitro and in tofu. Food Chem. 2019, 294, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Mucci, A.; Parenti, F.; Righi, V.; Schenetti, L. Citron and lemon under the lens of HR-MAS NMR spectroscopy. Food Chem. 2013, 141, 3167–3176. [Google Scholar] [CrossRef]
- Bellavite, P.; Donzelli, A. Hesperidin and SARS-CoV-2: New light on the healthy function of citrus fruits. Antioxidants 2020, 9, 742. [Google Scholar] [CrossRef]
- Malleshappa, P.; Kumaran, R.C.; Venkatarangaiah, K.; Parveen, S. Peels of citrus fruits: A potential source of anti-inflammatory and anti-nociceptive agents. Pharmacogn. J. 2018, 10, s172–s178. [Google Scholar] [CrossRef] [Green Version]
- Adham, A.N. Comparative antimicrobrial activity of peel and juice extract of citrus fruits growing in Kurdistan/Iraq. Am. J. Microbiol. Re. 2015, 5, 155–159. [Google Scholar]
- Taghvaeefard, N.; Ghani, A.; Hosseinifarahi, M. Comparative study of phytochemical profile and antioxidant activity of flavedo from two Iranian citron fruit (Citrus medica L.). J. Food Meas. Charact. 2021, 15, 2821–2830. [Google Scholar] [CrossRef]
- Menichini, F.; Loizzo, M.R.; Bonesi, M.; Conforti, F.; De Luca, D.; Statti, G.A.; de Cindio, B.; Menichini, F.; Tundis, R. Phytochemical profile, antioxidant, anti-inflammatory and hypoglycemic potential of hydroalcoholic extracts from Citrus medica L. cv Diamante flowers, leaves and fruits at two maturity stages. Food Chem. Toxicol. 2011, 49, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Adham, A.N. Qualitative and quantitative estimation of hesperidin in peel and juice of citrus fruits by RP-HPLC method growing in Kurdistan region/Iraq. Int. J. Pharm. Sci. Rev. Res. 2015, 33, 220–224. [Google Scholar]
- Choi, I.-W.; Choi, S.-Y.; Ji, J.-R. Flavonoids and functional properties of germinated citron (Citrus junos Sieb. ex TANAKA) shoots. Food Sci. Biotechnol. 2009, 18, 1224–1229. [Google Scholar]
- Tahir, T.; Ashfaq, M.; Shahzad, M.I.; Bukhari, S.T. Recent Approaches in the Extraction of Citrus Metabolites. Curr. Biotechnol. 2019, 8, 85–95. [Google Scholar]
- Govindarajan, D.K.; Viswalingam, N.; Meganathan, Y.; Devaraj, B.S.; Sivamani, S.; Sivarajasekar, N. Response surface methodology optimization for extraction of pectin from waste rinds of Citrus medica. AIP Conf. Proc. 2022, 2446, 020013. [Google Scholar]
- Ma, Q.-G.; Wei, R.-R.; Yang, M.; Huang, X.-Y.; Wang, F.; Dong, J.-H.; Sang, Z.-P. Isolation and characterization of neolignan derivatives with hepatoprotective and neuroprotective activities from the fruits of Citrus medica L. var. Sarcodactylis Swingle. Bioorg. Chem. 2021, 107, 104622. [Google Scholar] [CrossRef]
- Menichini, F.; Tundis, R.; Loizzo, M.R.; Bonesi, M.; D’Angelo, D.; Lombardi, P.; Mastellone, V. Citrus medica L. cv Diamante (Rutaceae) peel extract improves glycaemic status of Zucker diabetic fatty (ZDF) rats and protects against oxidative stress. J. Enzyme Inhib. 2016, 31, 1270–1276. [Google Scholar] [CrossRef] [Green Version]
- Fratianni, F.; Cozzolino, A.; De Feo, V.; Coppola, R.; Ombra, M.N.; Nazzaro, F. Polyphenols, antioxidant, antibacterial, and biofilm inhibitory activities of peel and pulp of Citrus medica L., Citrus bergamia, and Citrus medica cv. Salò cultivated in southern Italy. Molecules 2019, 24, 4577. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Duan, L.; Guo, L.; Dou, L.-L.; Dong, X.; Zhou, P.; Li, P.; Liu, E.-H. Chemical and biological comparison of the fruit extracts of Citrus wilsonii Tanaka and Citrus medica L. Food Chem. 2015, 173, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.-Y.; Li, C.-H.; Shen, Y.-C.; Wu, T.-S. Anti-inflammatory principles from the stem and root barks of Citrus medica. Chem. Pharm. Bull. 2010, 58, 61–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fomani, M.; Happi, E.N.; Francois-Hugues, P.; Lallemand, M.-C.; Waffo, A.F.K.; Wansi, J.D. A prenylated acridone alkaloid and ferulate xanthone from barks of Citrus medica (Rutaceae). ZNB 2015, 70, 71–75. [Google Scholar] [CrossRef]
- Hetta, M.; El-Alfy, T.; Yassin, N.; Abdel-Rahman, R.; Kadry, E. Phytochemical and antihyperglycemic studies on Citrus medica L. leaves (Etrog) growing in Egypt. Int. J. Pharmacogn. Phytochem. Res. 2013, 5, 271–277. [Google Scholar]
- Roowi, S.; Crozier, A. Flavonoids in tropical citrus species. J. Agric. Food Chem. 2011, 59, 12217–12225. [Google Scholar] [CrossRef]
- Chu, J.; Li, S.-L.; Yin, Z.-Q.; Ye, W.-C.; Zhang, Q.-W. Simultaneous quantification of coumarins, flavonoids and limonoids in Fructus Citri Sarcodactylis by high performance liquid chromatography coupled with diode array detector. J. Pharm. Biomed. Anal. 2012, 66, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Wang, J.; Chen, H.; Zhou, A.; Song, M.; Zhong, Q.; Chen, H.; Cao, Y. Identification of flavoanoids from finger citron and evaluation on their antioxidative and antiaging activities. Front. Nutr. 2020, 207, 584900. [Google Scholar] [CrossRef]
- Mondal, M.; Saha, S.; Sarkar, C.; Hossen, M.S.; Hossain, M.S.; Khalipha, A.B.R.; Islam, M.F.; Wahed, T.B.; Islam, M.T.; Rauf, A. Role of Citrus medica L. Fruits Extract in Combatting the Hematological and Hepatic Toxic Effects of Carbofuran. Chem. Res. Toxicol. 2021, 34, 1890–1902. [Google Scholar] [CrossRef]
- Chan, Y.-Y.; Hwang, T.-L.; Kuo, P.-C.; Hung, H.-Y.; Wu, T.-S. Constituents of the Fruits of Citrus medica L. var. sarcodactylis and the Effect of 6, 7-Dimethoxy-coumarin on Superoxide Anion Formation and Elastase Release. Molecules 2017, 22, 1454. [Google Scholar] [CrossRef] [Green Version]
- Belletti, N.; Kamdem, S.S.; Patrignani, F.; Lanciotti, R.; Covelli, A.; Gardini, F. Antimicrobial activity of aroma compounds against Saccharomyces cerevisiae and improvement of microbiological stability of soft drinks as assessed by logistic regression. Appl. Environ. Microbiol. 2007, 73, 5580–5586. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Li, H.; Yang, Y.; Zhan, Y.; Tu, D. Variation in the components and antioxidant activity of Citrus medica L. var. sarcodactylis essential oils at different stages of maturity. Ind. Crops Prod. 2013, 46, 311–316. [Google Scholar] [CrossRef]
- Guo, J.-J.; Gao, Z.-P.; Xia, J.-L.; Ritenour, M.A.; Li, G.-Y.; Shan, Y. Comparative analysis of chemical composition, antimicrobial and antioxidant activity of citrus essential oils from the main cultivated varieties in China. LWT 2018, 97, 825–839. [Google Scholar] [CrossRef]
- Jing, L.; Lei, Z.; Zhang, G.; Pilon, A.C.; Huhman, D.V.; Xie, R.; Xi, W.; Zhou, Z.; Sumner, L.W. Metabolite profiles of essential oils in citrus peels and their taxonomic implications. Metabolomics 2015, 11, 952–963. [Google Scholar] [CrossRef]
- Poiana, M.; Sicari, V.; Mincione, B. A comparison between the chemical composition of the oil, solvent extract and supercritical carbon dioxide extract of Citrus medica cv. Diamante. J. Essent. Oil Res. 1998, 10, 145–152. [Google Scholar] [CrossRef]
- Gabriele, B.; Fazio, A.; Dugo, P.; Costa, R.; Mondello, L. Essential oil composition of Citrus medica L. Cv. Diamante (Diamante citron) determined after using different extraction methods. J. Sep. Sci. 2009, 32, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Statti, G.A.; Tundis, R.; Loizzo, M.R.; Menichini, F. In vitro activities of Citrus medica L. cv. Diamante (Diamante citron) relevant to treatment of diabetes and Alzheimer’s disease. Phytother. Res. 2007, 21, 427–433. [Google Scholar] [CrossRef]
- Xing, C.; Qin, C.; Li, X.; Zhang, F.; Linhardt, R.J.; Sun, P.; Zhang, A. Chemical composition and biological activities of essential oil isolated by HS-SPME and UAHD from fruits of bergamot. LWT 2019, 104, 38–44. [Google Scholar] [CrossRef]
- Wei, L.; Yu, X.; Li, H.; Zhu, M.; Pu, D.; Lu, Q.; Bao, Y.; Zu, Y. Optimization of solvent-free microwave extraction of essential oil from the fresh peel of Citrus medica L. var. arcodactylis Swingle by response surface methodology, chemical composition and activity characterization. Sci. Hortic. 2023, 309, 111663. [Google Scholar] [CrossRef]
- Peng, C.-H.; Ker, Y.-B.; Weng, C.-F.; Peng, C.-C.; Huang, C.-N.; Lin, L.-Y.; Peng, R.Y. Insulin secretagogue bioactivity of finger citron fruit (Citrus medica L. var. Sarcodactylis Hort, Rutaceae). J. Agric. Food Chem. 2009, 57, 8812–8819. [Google Scholar] [CrossRef] [PubMed]
- Vitalini, S.; Iriti, M.; Ovidi, E.; Laghezza Masci, V.; Tiezzi, A.; Garzoli, S. Detection of Volatiles by HS-SPME-GC/MS and Biological Effect Evaluation of Buddha’s Hand Fruit. Molecules 2022, 27, 1666. [Google Scholar] [CrossRef] [PubMed]
- Fanciullino, A.-L.; Dhuique-Mayer, C.; Luro, F.; Casanova, J.; Morillon, R.; Ollitrault, P. Carotenoid diversity in cultivated citrus is highly influenced by genetic factors. J. Agric. Food Chem. 2006, 54, 4397–4406. [Google Scholar] [CrossRef]
- Lim, J.Y.; Yoon, H.-S.; Kim, K.-Y.; Kim, K.-S.; Noh, J.G.; Song, I.G. Optimum conditions for the enzymatic hydrolysis of citron waste juice using response surface methodology (RSM). Food Sci. Biotechnol. 2010, 19, 1135–1142. [Google Scholar] [CrossRef]
- Belletti, N.; Ndagijimana, M.; Sisto, C.; Guerzoni, M.E.; Lanciotti, R.; Gardini, F. Evaluation of the antimicrobial activity of citrus essences on Saccharomyces cerevisiae. J. Agric. Food Chem. 2004, 52, 6932–6938. [Google Scholar] [CrossRef]
- Kim, K.-N.; Ko, Y.-J.; Yang, H.-M.; Ham, Y.-M.; Roh, S.W.; Jeon, Y.-J.; Ahn, G.; Kang, M.-C.; Yoon, W.-J.; Kim, D. Anti-inflammatory effect of essential oil and its constituents from fingered citron (Citrus medica L. var. sarcodactylis) through blocking JNK, ERK and NF-κB signaling pathways in LPS-activated RAW 264.7 cells. Food Chem. Toxicol. 2013, 57, 126–131. [Google Scholar] [CrossRef]
- Gancel, A.L.; Ollitrault, P.; Froelicher, Y.; Tomi, F.; Jacquemond, C.; Luro, F.; Brillouet, J.M. Citrus somatic allotetraploid hybrids exhibit a differential reduction of leaf sesquiterpenoid biosynthesis compared with their parents. Flavour. Fragr. J. 2005, 20, 626–632. [Google Scholar] [CrossRef]
- Wang, S.F.; Ju, Y.; Chen, X.G.; De Hu, Z. Separation and determination of coumarins in the root bark of three citrus plants by micellar electrokinetic capillary chromatography. Planta Med. 2003, 69, 483–486. [Google Scholar]
- Peng, B.; Yang, J.; Huang, W.; Peng, D.; Bi, S.; Song, L.; Wen, Y.; Zhu, J.; Chen, Y.; Yu, R. Structural characterization and immunoregulatory activity of a novel heteropolysaccharide from bergamot (Citrus medica L. var. sarcodactylis) by alkali extraction. Ind. Crops Prod. 2019, 140, 111617. [Google Scholar] [CrossRef]
- Peng, B.; Luo, Y.; Hu, X.; Song, L.; Yang, J.; Zhu, J.; Wen, Y.; Yu, R. Isolation, structural characterization, and immunostimulatory activity of a new water-soluble polysaccharide and its sulfated derivative from Citrus medica L. var. sarcodactylis. Int. J. Biol. Macromol. 2019, 123, 500–511. [Google Scholar] [CrossRef]
- Gao, Y.; Peng, B.; Xu, Y.; Yang, J.-n.; Song, L.-y.; Bi, S.-x.; Chen, Y.; Zhu, J.-h.; Wen, Y.; Yu, R.-m. Structural characterization and immunoregulatory activity of a new polysaccharide from Citrus medica L. var. sarcodactylis. RSC Adv. 2019, 9, 6603–6612. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Liang, F.; Zhang, Y.; Pan, Y. Water-soluble polysaccharides from finger citron fruits (Citrus medica L. var. sarcodactylis). Carbohydr. Res. 2014, 388, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Shen, C.-Y.; Jiang, J.-G. Structural characterization of novel arabinoxylan and galactoarabinan from citron with potential antitumor and immunostimulatory activities. Carbohydr. Polym. 2021, 269, 118331. [Google Scholar] [CrossRef]
- Luo, B.; Lv, J.; Li, K.; Liao, P.; Chen, P. Structural Characterization and Anti-inflammatory Activity of a Galactorhamnan Polysaccharide From Citrus medica L. var. sarcodactylis. Front. Nutr. 2022, 9, 916976. [Google Scholar] [CrossRef]
- Wu, Z.; Li, H.; Tu, D.; Yang, Y.; Zhan, Y. Extraction optimization, preliminary characterization, and in vitro antioxidant activities of crude polysaccharides from finger citron. Ind. Crops Prod. 2013, 44, 145–151. [Google Scholar] [CrossRef]
- Castillo, S.; Dávila-Aviña, J.; Heredia, N.; Garcia, S. Antioxidant activity and influence of Citrus byproduct extracts on adherence and invasion of Campylobacter jejuni and on the relative expression of cadF and ciaB. Food Sci. Biotechnol. 2017, 26, 453–459. [Google Scholar] [CrossRef] [PubMed]
- El Hawary, S.S.E.; Hashim, F.A.; Ibrahim, N.A.; Matloub, A.A.; ElSayed, A.M.; Farid, M.A.; Kutkat, O.; El-Abd, E.A.; Ali, M.; Shata, H.M. A Comparative study of Chemical Compositions, Antimicrobial and Antiviral Activities of Essential Oils for Citrus medica var. sarcodactylis Swingle Fruits and Leaves along with Limonia acidissima L. Leaves. Egypt J. Chem. 2022, 65, 2–4. [Google Scholar] [CrossRef]
- Belletti, N.; Lanciotti, R.; Patrignani, F.; Gardini, F. Antimicrobial efficacy of citron essential oil on spoilage and pathogenic microorganisms in fruit-based salads. J. Food Sci. 2008, 73, M331–M338. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Jin, R.; Bao, X.; Yu, G.; Yi, F. Potential roles of essential oils from the flower, fruit and leaf of Citrus medica L. var. sarcodactylis in preventing spoilage of Chinese steamed bread. Food Biosci. 2021, 43, 101271. [Google Scholar] [CrossRef]
- Khorsandi, A.; Ziaee, E.; Shad, E.; Razmjooei, M.; Eskandari, M.H.; Aminlari, M. Antibacterial effect of essential oils against spoilage bacteria from vacuum-packed cooked cured sausages. J. Food Prot. 2018, 81, 1386–1393. [Google Scholar] [CrossRef]
- Li, Z.-H.; Cai, M.; Liu, Y.-S.; Sun, P.-L.; Luo, S.-L. Antibacterial activity and mechanisms of essential oil from Citrus medica L. var. sarcodactylis. Molecules 2019, 24, 1577. [Google Scholar] [CrossRef] [Green Version]
- Mitropoulou, G.; Nikolaou, A.; Santarmaki, V.; Sgouros, G.; Kourkoutas, Y. Citrus medica and Cinnamomum zeylanicum essential oils as potential biopreservatives against spoilage in low alcohol wine products. Foods 2020, 9, 577. [Google Scholar] [CrossRef]
- Chen, W.F.; Wong, L.L.; Zhang, X.; Zhou, F.X.; Xia, F.; Tang, L.P.; Li, X. New 4, 5-seco-20 (10→5)-abeo-Abietane Diterpenoids with Anti-Inflammatory Activity from Isodon lophanthoides var. graciliflorus (Benth.) H. Hara. Chem. Biodivers. 2019, 16, e1900206. [Google Scholar] [CrossRef]
- Al-Mariri, A.; Safi, M. In vitro antibacterial activity of several plant extracts and oils against some gram-negative bacteria. Iran. J. Med. Sci. 2014, 39, 36. [Google Scholar] [PubMed]
- Mitropoulou, G.; Fitsiou, E.; Spyridopoulou, K.; Tiptiri-Kourpeti, A.; Bardouki, H.; Vamvakias, M.; Panas, P.; Chlichlia, K.; Pappa, A.; Kourkoutas, Y. Citrus medica essential oil exhibits significant antimicrobial and antiproliferative activity. LWT 2017, 84, 344–352. [Google Scholar] [CrossRef]
- Zhang, H.; Lou, Z.; Chen, X.; Cui, Y.; Wang, H.; Kou, X.; Ma, C. Effect of simultaneous ultrasonic and microwave assisted hydrodistillation on the yield, composition, antibacterial and antibiofilm activity of essential oils from Citrus medica L. var. sarcodactylis. J. Food Eng. 2019, 244, 126–135. [Google Scholar] [CrossRef]
- Wang, F.; You, H.; Guo, Y.; Wei, Y.; Xia, P.; Yang, Z.; Ren, M.; Guo, H.; Han, R.; Yang, D. Essential oils from three kinds of fingered citrons and their antibacterial activities. Ind. Crops Prod. 2020, 147, 112172. [Google Scholar] [CrossRef]
- Suryavanshi, P.; Pandit, R.; Gade, A.; Derita, M.; Zachino, S.; Rai, M. Colletotrichum sp.-mediated synthesis of sulphur and aluminium oxide nanoparticles and its in vitro activity against selected food-borne pathogens. LWT 2017, 81, 188–194. [Google Scholar] [CrossRef]
- Li, Z.-H.; Cai, M.; Liu, Y.-s.; Sun, P.-L. Development of finger citron (Citrus medica L. var. sarcodactylis) essential oil loaded nanoemulsion and its antimicrobial activity. Food Control 2018, 94, 317–323. [Google Scholar] [CrossRef]
- Keerthana, P.; Vijayakumar, S.; Vidhya, E.; Punitha, V.; Nilavukkarasi, M.; Praseetha, P. Biogenesis of ZnO nanoparticles for revolutionizing agriculture: A step towards anti-infection and growth promotion in plants. Ind. Crops Prod. 2021, 170, 113762. [Google Scholar]
- Adham, A.N. Phytochemical analysis and evaluation antibacterial activity of Citrus medica peel and juice growing in Kurdistan/Iraq. J. Appl. Pharm. Sci. 2015, 5, 136–141. [Google Scholar] [CrossRef] [Green Version]
- Sah, A.N.; Juyal, V.; Melkani, A.B. Antimicrobial activity of six different parts of the plant Citrus medica Linn. Pharmacogn. J. 2011, 3, 80–83. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Verma, S.; Rana, S.; Rana, A. Rapid screening and quantification of major organic acids in citrus fruits and their bioactivity studies. J. Food Sci. Technol. 2018, 55, 1339–1349. [Google Scholar] [CrossRef]
- Castillo, S.; Heredia, N.; Arechiga-Carvajal, E.; García, S. Citrus extracts as inhibitors of quorum sensing, biofilm formation and motility of Campylobacter jejuni. Food Biotechnol. 2014, 28, 106–122. [Google Scholar] [CrossRef]
- Shende, S.; Ingle, A.P.; Gade, A.; Rai, M. Green synthesis of copper nanoparticles by Citrus medica Linn.(Idilimbu) juice and its antimicrobial activity. World J. Microbiol. Biotechnol. 2015, 31, 865–873. [Google Scholar] [CrossRef]
- Selvaraju, N.; Ganesh, P.S.; Palrasu, V.; Venugopal, G.; Mariappan, V. Evaluation of Antimicrobial and Antibiofilm Activity of Citrus medica Fruit Juice Based Carbon Dots against Pseudomonas aeruginosa. ACS Omega 2022, 7, 36227–36234. [Google Scholar] [CrossRef]
- Nair, A.; Kurup Sr, R.; Nair, A.S.; Baby, S. Citrus peels prevent cancer. Phytomedicine 2018, 50, 231–237. [Google Scholar] [CrossRef]
- Ajikumaran, N.S.; Rajani Kurup, S.; Akhila, S.; Sabulal, B. Citrus peels prevent cancer. Phytomedicine 2018, 50, 231–237. [Google Scholar]
- Dahiya, R.; Kumar, A. Synthetic and biological studies on a cyclopolypeptide of plant origin. J. Zhejiang Univ. Sci. B 2008, 9, 391–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tundis, R.; Calabria, U.; Bonesi, M.; Calabria, U.; Calabria, U. Chemical composition and bioactivity of Citrus medica L. cv. Diamante essential oil obtained by hydrodistillation. Nat. Prod. Res. 2011, 25, 789–799. [Google Scholar]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, J.; Li, Y.; Guo, W. Effects of high temperature on photosynthesis, chlorophyll fluorescence, chloroplast ultrastructure, and antioxidant activities in fingered citron. Russ. J. Plant Physiol. 2012, 59, 732–740. [Google Scholar] [CrossRef]
- Ebrahimi, Y.; Hasanvand, A.; Valibeik, A.; Ebrahimi, F.; Abbaszadeh, S. Natural antioxidants and medicinal plants effective on hyperlipidemia. Res. J. Pharm. Technol. 2019, 12, 1457–1462. [Google Scholar] [CrossRef]
- Jayaprakasha, G.; Patil, B.S. In vitro evaluation of the antioxidant activities in fruit extracts from citron and blood orange. Food Chem. 2007, 101, 410–418. [Google Scholar] [CrossRef]
- Pallavi, M.; Ramesh, C.; Siddesha, J.; Krishna, V.; Kavitha, G.; Nethravathi, A.; Parveen, S.; KM, A.K. Anti-hyperlipidemic effects of citrus fruit peel extracts against high fat diet-induced hyperlipidemia in rats. Int. J. Res. Pharm. Sci. 2021, 12, 2226–2232. [Google Scholar] [CrossRef]
- Ghani, A.; Taghvaeefard, N.; Hosseinifarahi, M.; Dakhlaoui, S.; Msaada, K. Essential oil composition and antioxidant activity of citron fruit (Citrus medica var. macrocarpa Risso.) peel as relation to ripening stages. Int. J. Environ. Health Res. 2022, 1–11. [Google Scholar] [CrossRef]
- Dadwal, V.; Joshi, R.; Gupta, M. Formulation, characterization and in vitro digestion of polysaccharide reinforced Ca-alginate microbeads encapsulating Citrus medica L. phenolics. LTW 2021, 152, 112290. [Google Scholar] [CrossRef]
- Wu, Z. Effect of different drying methods on chemical composition and bioactivity of finger citron polysaccharides. Int. J. Biol. Macromol. 2015, 76, 218–223. [Google Scholar] [CrossRef]
- Mengsa, H.; Kun, X.; Pei, L.; Jun, L. Five Rutaceae family ethanol extracts alleviate H2O2 and LPS-induced inflammation via NF-κB and JAK-STAT3 pathway in HaCaT cells. Chin. J. Nat. Med. 2022, 20, 937–947. [Google Scholar]
- Hanafy, S.M.; Abd El-Shafea, Y.M.; Saleh, W.D.; Fathy, H.M. Chemical profiling, in vitro antimicrobial and antioxidant activities of pomegranate, orange and banana peel-extracts against pathogenic microorganisms. J. Genet. Eng. Biotechnol. 2021, 19, 80. [Google Scholar] [CrossRef]
- Herman, A.; Tambor, K.; Herman, A. Linalool affects the antimicrobial efficacy of essential oils. Curr. Microbiol. 2016, 72, 165–172. [Google Scholar] [CrossRef]
- Nagy, M.M.; Al-Mahdy, D.A.; Abd El Aziz, O.M.; Kandil, A.M.; Tantawy, M.A.; El Alfy, T.S. Chemical composition and antiviral activity of essential oils from Citrus reshni hort. ex Tanaka (Cleopatra mandarin) cultivated in Egypt. J. Essent. Oil-Bear. Plants 2018, 21, 264–272. [Google Scholar] [CrossRef]
- Punitha, V.; Vijayakumar, S.; Nilavukkarasi, M.; Vidhya, E.; Praseetha, P. Fruit peels that unlock curative potential: Determination of biomedical application and bioactive compounds. S. Afr. J. Bot. 2022, 150, 1051–1060. [Google Scholar] [CrossRef]
- Thi, T.U.D.; Nguyen, T.T.; Thi, Y.D.; Thi, K.H.T.; Phan, B.T.; Pham, K.N. Green synthesis of ZnO nanoparticles using orange fruit peel extract for antibacterial activities. RSC Adv. 2020, 10, 23899–23907. [Google Scholar]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Nyrop, K.A.; Damone, E.M.; Deal, A.M.; Wheeler, S.B.; Charlot, M.; Reeve, B.B.; Basch, E.; Shachar, S.S.; Carey, L.A.; Reeder-Hayes, K.E.; et al. Patient-reported treatment toxicity and adverse events in Black and White women receiving chemotherapy for early breast cancer. Breast Cancer Res. Treat. 2022, 191, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Cirmi, S.; Maugeri, A.; Ferlazzo, N.; Gangemi, S.; Calapai, G.; Schumacher, U.; Navarra, M. Anticancer potential of citrus juices and their extracts: A systematic review of both preclinical and clinical studies. Front. Pharmacol. 2017, 8, 420. [Google Scholar] [CrossRef] [Green Version]
- Aliberti, L.; Caputo, L.; De Feo, V.; De Martino, L.; Nazzaro, F.; Souza, L.F. Chemical composition and in vitro antimicrobial, cytotoxic, and central nervous system activities of the essential oils of Citrus medica L. cv.‘Liscia’and C. medica cv.‘Rugosa’cultivated in Southern Italy. Molecules 2016, 21, 1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawat, A.; Reddy, A.V.B. Recent advances on anticancer activity of coumarin derivatives. Eur. J. Med. Chem. 2022, 5, 100038. [Google Scholar] [CrossRef]
- Xiao, S.; Liu, W.; Bi, J.; Liu, S.; Zhao, H.; Gong, N.; Xing, D.; Gao, H.; Gong, M. Anti-inflammatory effect of hesperidin enhances chondrogenesis of human mesenchymal stem cells for cartilage tissue repair. J. Inflamm. 2018, 15, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sood, S.; Bansal, S.; Muthuraman, A.; Gill, N.; Bali, M. Therapeutic potential of Citrus medica L. peel extract in carrageenan induced inflammatory pain in rat. J. Med. Plant Res. 2009, 3, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Marouf, B.H.; Hussain, S.A.; Ali, Z.S.; Ahmmad, R.S. Resveratrol supplementation reduces pain and inflammation in knee osteoarthritis patients treated with meloxicam: A randomized placebo-controlled study. J. Med. Food 2018, 21, 1253–1259. [Google Scholar] [CrossRef]
- Awosika, T.O.; Aluko, R.E. Inhibition of the in vitro activities of α-amylase, α-glucosidase and pancreatic lipase by yellow field pea (Pisum sativum L.) protein hydrolysates. Int. J. Food Sci. Technol. 2019, 54, 2021–2034. [Google Scholar] [CrossRef] [Green Version]
- Tolmie, M.; Bester, M.J.; Apostolides, Z. Inhibition of α-glucosidase and α-amylase by herbal compounds for the treatment of type 2 diabetes: A validation of in silico reverse docking with in vitro enzyme assays. J. Diabetes 2021, 13, 779–791. [Google Scholar] [CrossRef]
- Abdou, H.M.; Hamaad, F.A.; Ali, E.Y.; Ghoneum, M.H. Antidiabetic efficacy of Trifolium alexandrinum extracts hesperetin and quercetin in ameliorating carbohydrate metabolism and activating IR and AMPK signaling in the pancreatic tissues of diabetic rats. Biomed. Pharmacother. 2022, 149, 112838. [Google Scholar] [CrossRef] [PubMed]
- Wahyuono, R.A.; Hesse, J.; Hipler, U.-C.; Elsner, P.; Böhm, V. In vitro lipophilic antioxidant capacity, antidiabetic and antibacterial activity of citrus fruits extracts from Aceh, Indonesia. Antioxidants 2017, 6, 11. [Google Scholar]
- Dehghan, M.; Fathinejad, F.; Farzaei, M.H.; Barzegari, E. In silico unraveling of molecular anti-neurodegenerative profile of Citrus medica flavonoids against novel pharmaceutical targets. Chem. Pap. 2022, 77, 595–610. [Google Scholar] [CrossRef]
- Figueiredo, R.T.; Bittencourt, V.C.B.; Lopes, L.C.L.; Sassaki, G.; Barreto-Bergter, E. Toll-like receptors (TLR2 and TLR4) recognize polysaccharides of Pseudallescheria boydii cell wall. Carbohydr. Res. 2012, 356, 260–264. [Google Scholar] [CrossRef]






| Checklist for Assessment of Risks of Bias in Pre-Clinical Studies |
|---|
| Are the hypothesis and objective of the study clearly described? |
| Are the main outcomes to be measured clearly described? |
| Are the main findings of the study clearly described? |
| Are the samples size calculations reported? |
| Are the animals randomly housed during the experiment? |
| Are the investigators blinded from knowledge which treatment used? |
| Are the outcome assessors blinded? |
| Is the dose/route of administration of Citrus medica L. properly reported? |
| Is the dose/route of administration of the drug in co-treatment properly reported? |
| Is the frequency of treatments adequately described? |
| Nutrient Compounds | Part of Plant | Quantitative | References |
|---|---|---|---|
| Minerals | |||
| Calcium (Ca) | peel, pulp | 107.39–195.91 mg/100 g FW | [17] |
| Copper (Cu) | peel, pulp | 0.061–0.45 mg/100 g FW | [17] |
| Iron (Fe) | peel, pulp | 0.82–2.92 mg/100 g FW | [17] |
| Magnesium (Mg) | peel, pulp | 5.86–16.29 mg/100 g FW | [17] |
| Manganese (Mn) | peel, pulp | 0.052–0.266 mg/100 g FW | [17] |
| Potassium (K) | peel, pulp | 126.04–263.27 mg/100 g FW | [17] |
| Sodium (Na) | peel, pulp | 6.74–27.92 mg/100 g FW | [17] |
| Zinc (Zn) | peel, pulp | 0.24–0.51 mg/100 g FW | [17] |
| Vitamins | |||
| Ascorbic acid (vitamin C) | peel, pulp, exocarp, mesocarp, endocarp, seeds | 0.23–2.39 mg/100 g FW | [17] |
| 2.33–7.95 mg/100 g DW | [15] | ||
| fructus | 11.61 ± 2.50 mg/100 g FW | [18] | |
| peel | - | [19] | |
| peel | - | [20] | |
| fructus | - | [21] | |
| juice | 18.49 ± 0.52 mg/100 g FW | [3] | |
| Niacin (vitamin B3) | peel, pulp | 0.05–0.63 mg/100 g FW | [17] |
| Pyridoxine (vitamin B6) | peel, pulp | 0.75–10.12 mg/100 g FW | [17] |
| Riboflavin (vitamin B2) | peel, pulp, exocarp, mesocarp, endocarp, seeds | 0.37–1.16 mg/100 g FW | [17] |
| 1.85–6.38 mg/100 g DW | [15] | ||
| Thiamin (vitamin B1) | peel, pulp, exocarp, endocarp | 1.32–3.65 mg/100 g FW | [17] |
| 0.18–0.40 mg/100 g DW | [15] | ||
| Essential amino acids | |||
| Histidine | peel, pulp | 7.68–38.04 mg/100 g FW | [17] |
| Isoleucine | peel, pulp | 16.14–81.95 mg/100 g FW | [17] |
| Leucine | peel, pulp | 30.05–126.24 mg/100 g FW | [17] |
| Lysine | peel, pulp | 27.37–94.46 mg/100 g FW | [17] |
| - | [22] | ||
| Methionine | peel, pulp | 1.63–11.53 mg/100 g FW | [17] |
| Phenylalanine | peel, pulp, exocarp, endocarp, mesocarp, seeds | 19.21–89.44 mg/100 g FW | [17] |
| - | [15] | ||
| Threonine | albedo, pulp | - | [22] |
| Valine | peel, pulp, albedo, pulp | 29.64–121.92 mg/100 g FW | [17] |
| - | [22] | ||
| Non-essential amino acids | |||
| Alanine | peel, pulp | 57.55–153.99 mg/100 g FW | [17] |
| albedo, pulp | [22] | ||
| Arginine | peel, pulp | 18.64–90.62 mg/100 g FW | [17] |
| Asparagine | peel, oil glands, albedo, pulp | - | [22] |
| Aspartic acid | peel, pulp | 232.86–637.32 mg/100 g FW | [17] |
| Cystine | peel, pulp | 1.76–1.82 mg/100 g FW | [17] |
| Glutamic acid | peel, pulp | 71.47–227.50 mg/100 g FW | [17] |
| Glycine | peel, pulp | 21.15–108.48 mg/100 g FW | [17] |
| Proline | peel, pulp | 55.22–150.18 mg/100 g FW | [17] |
| - | [15] | ||
| - | [22] | ||
| Serine | peel, pulp | 22.45–78.84 mg/100 g FW | [17] |
| Tryptophan | exocarp, endocarp, mesocarp | - | [17] |
| Tyrosine | peel, pulp | 12.51–53.74 mg/100 g FW | [17] |
| Macronutrients | |||
| Moisture content | peel, pulp | 81.78–86.03 g/100 g FW | [17] |
| Fat | peel, pulp | 0.39–0.56 g/100 g FW | [17] |
| Protein | peel, pulp | 0.80–2.99 g/100 g FW | [17] |
| Ash | peel, pulp | 0.44–1.23 g/100 g FW | [17] |
| Carbohydrates | peel, pulp | 9.19–16.60 g/100 g FW | [17] |
| Energy | peel, pulp | 53.74–73.06 g/100 g FW | [17] |
| Glucose | peel, pulp | 0.92–2.27 g/100 g FW | [17] |
| Fructose | peel, pulp | 1.60–2.95 g/100 g FW | [17] |
| Sucrose | peel, pulp | 0.27–1.03 g/100 g FW | [17] |
| Compounds | Formula | Structure | Extraction Method | Chemical Analysis | Part of the Plant | Quantitative | References |
|---|---|---|---|---|---|---|---|
| Apigenin | C15H12O5 | 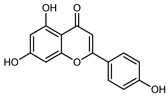 | Maceration 70% EtOH | HPLC | flavedo | 62.80 mg/kg FW | [33] |
| Exhaustive maceration 70% EtOH | HPLC | flowers, leaves, mesocarp, endocarp | 58.00–941.00 mg/kg FW | [27] | |||
| Maceration 100% EtOH | UPLC–DAD | peel and pulp | 24.26 ± 1.67 µg/g FW | [34] | |||
| Apigenin-6,8-di-C-glucoside | C27H30O15 |  | UAE 50%MeOH | HPLC–Q/TOF–MS | fructus | - | [35] |
| Atalantoflavon | C21H18O4 | 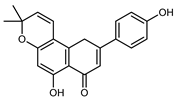 | Maceration Acetone | COSY, NOESY, HMQC, HMBC, HR–ESI–MS | root bark, stem bark | - | [36] |
| Maceration MeOH | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] | |||
| Catechin | C15H14O6 |  | UAE EtOH 80% | UHPLC–QTOF–IMS | mesocarp, endocarp, seeds | 5.14–57.87 mg/100 g DW | [15] |
| Maceration 100% EtOH | UPLC–DAD | flavedo, pulp | 4.34–68.78 µg/g FW | [34] | |||
| Dihydrokaem-pferide | C16H14O6 | 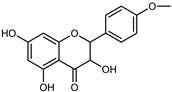 | Maceration 70% MeOH | UV, MS, NMR | leaves | - | [38] |
| Dihydroquercetin | C15H12O7 |  | UAE EtOH 80% | UHPLC–QTOF–IMS | exocarp, endocarp, seeds | - | [15] |
| Epicatechin | C15H14O6 | 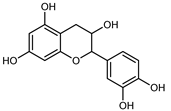 | Maceration 100% EtOH | UPLC–DAD | flavedo, pulp | 9.85–105.10 µg/g FW | [34] |
| Eriocitrin (Eriodictyol-7-O-rutinoside) | C27H32O15 | 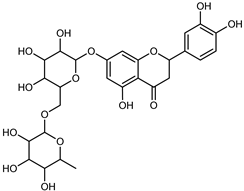 | Maceration MeOH and 0.1% HCl | HPLC–PDA–MS | fructus | - | [39] |
| Herbacetin | C15H10O7 | 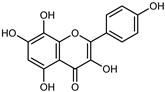 | UAE EtOH 80% | UHPLC–QTOF–IMS | exocarp, mesocarp, seeds | - | [15] |
| Hesperetin | C16H14O6 | 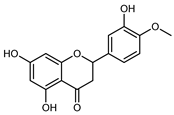 | Dynamic maceration 70% EtOH | HPLC | flavedo | 0.39–1.82 mg/g DW | [26] |
| UAE EtOH 80% | UHPLC–QTOF–IMS | exocarp, endocarp, mesocarp, seeds | - | [15] | |||
| Maceration 70% EtOH | HPLC | flavedo | 50.4 mg/kg FW | [33] | |||
| Exhaustive maceration 70% EtOH | HPLC | flowers, leaves, mesocarp, endocarp | 203.80 mg/kg FW | [27] | |||
| Hesperetin-7-O-rutinoside | C28H34O15 | 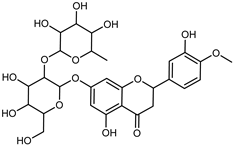 | Maceration MeOH and 0.1% HCl | HPLC–PDA–MS | fructus | - | [39] |
| Hesperidin | C28H34O15 | 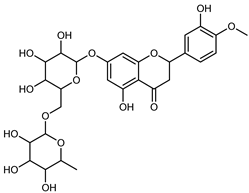 | PLE MeOH | HPLC–DAD | fructus | 30.36 µg/mL | [40] |
| Dynamic maceration with 70% EtOH | HPLC | flavedo | 1.86–2.77 mg/g DW | [26] | |||
| UAE 80% EtOH | UHPLC–QTOF–IMS | exocarp, mesocarp, endocarp, seeds | 383.02–3307.25 mg/100 g DW | [15] | |||
| Exhaustive maceration 70% EtOH | HPLC | flowers, leaves, mesocarp, endocarp | 9.00–224.30 mg/kg FW | [27] | |||
| UAE 50% MeOH | HPLC–Q/TOF–MS | fructus | 0.84–1.84 mg/g DW | [35] | |||
| Kaempferol 3-O-rutinoside | C27H32O15 |  | Dynamic maceration 70% EtOH | HPLC | flavedo | - | [26] |
| Limocitrol 3-α-l-arabinopyranosyl-(1->3) -galactoside | C29H34O18 | 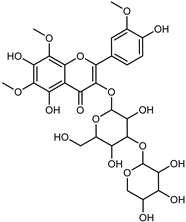 | CPE 85% EtOH | UPLC–QTOF–MS/MS | fructus | - | [41] |
| Lonchocarpol A | C25H28O5 | 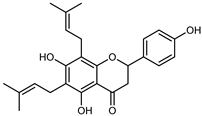 | Maceration Acetone | COSY, NOESY, HMQC, HMBC, HR–ESI–MS | root bark, stem bark | - | [36] |
| Naringenin 7-O-glucoside | C21H22O10 |  | UAE 80% EtOH | UHPLC–QTOF–IMS | exocarp, mesocarp, seeds | - | [15] |
| Naringin | C27H32O14 |  | Exhaustive extraction 70% EtOH | HPLC | fructus | 556.00 mg/kg FW | [27] |
| UAE 80% EtOH | UHPLC–QTOF–IMS | exocarp, mesocarp, endocarp, seeds | 36.82–295.15 mg/100 g DW | [15] | |||
| UAE 80% EtOH | HPLC–QTOF–MS | fructus | 0.43–0.61 mg/g DW | [35] | |||
| Maceration 70% EtOH | HPLC | flavedo | 18.60 mg/kg FW | [33] | |||
| Neodiosmin (Diosmetin-7-O-neoheseridoside) | C28H32O15 | 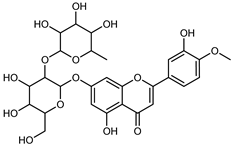 | CPE 85% EtOH | UPLC–QTOF–MS/MS | fructus | - | [41] |
| Diosmin | Exhaustively maceration 70% EtOH | HPLC | flowers, leaves, mesocarp, endocarp | 18.20–372.50 mg/kg FW | [27] | ||
| Neohesperidin (hesperetin-7-O-neohesperidoside) | C28H34O15 |  | Maceration MeOH and 0.1% HCl | HPLC–PDA–MS | fructus | - | [39] |
| Nobiletin | C21H22O8 | 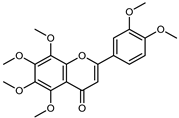 | UAE EtOH 80% | UHPLC–QTOF–IMS | exocarp, mesocarp, endocarp, seeds | 25.63–94.32 mg/100 g DW | [15] |
| Phloretin-3′, 5′-di-C-glucoside | C27H34O15 |  | Maceration MeOH and 0.1%HCl | HPLC–PDA–MS | fructus | - | [39] |
| Quercetin | C15H10O7 | 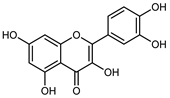 | Soxhlet MeOH 65 °C | HPLC | fructus 2 | 0.025 mg/g DW | [42] |
| Maceration 70% EtOH | HPLC | flavedo | 18.20 mg/kg FW | [33] | |||
| Dynamic maceration 70% EtOH | HPLC | flavedo | 1.62–3.01 mg/g DW | [26] | |||
| Exhaustive maceration 70% EtOH | HPLC | flowers, leaves, mesocarp, endocarp | 11.00–580.80 mg/kg FW | [27] | |||
| Rutin | C27H30O16 | 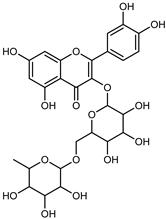 | Maceration 100% EtOH | UPLC–DAD | flavedo and pulp | 19.39–115.47 µg/g FW | [34] |
| Dynamic maceration 70% EtOH | HPLC | flavedo | 0.20–0.42 mg/g DW | [26] | |||
| UAE EtOH 80% | UHPLC–QTOF–IMS | exocarp, mesocarp, endocarp | 74.08–328.82 mg/100 g DW | [15] | |||
| 70% MeOH | UV, MS, NMR | leaves | - | [38] | |||
| Sakuranetin | C16H14O5 |  | Maceration on cold 70% MeOH | UV, MS, NMR | leaves | - | [38] |
| Stachannin Scutellarein 4′-methyl ether 7-glucoside | C22H22011 | 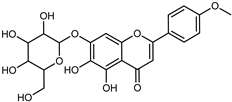 | UAE EtOH 80% | UHPLC–QTOF–IMS | exocarp, endocarp, seeds | - | [15] |
| Tangeritin | C20H20O7 | 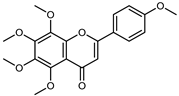 | UAE EtOH 80% | UHPLC–QTOF–IMS | exocarp, mesocarp, endocarp, seeds | 18.96–164.88 mg/100 g DW | [15] |
| Vitexin | C21H20010 |  | UAE EtOH 80% | UHPLC–QTOF–IMS | exocarp, endocarp, seeds | - | [15] |
| Vitexin-2-rhamnoside | C27H30O14 | 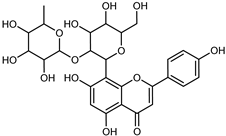 | PLE MeOH | HPLC–DAD | fructus | - | [40] |
| 3,5,6-Trihydroxy-3′,4′,7-trimethoxyflavone | C18H16O8 | 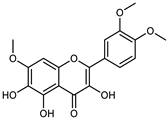 | UAE EtOH 80% | UHPLC–QTOF–IMS | exocarp, mesocarp, endocarp, seeds | - | [15] |
| 5,7-Dihydroxy-3′, 4′, 5′-trimethoxyflavone | C18H16O7 | 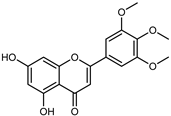 | UAE EtOH 80% | UHPLC–QTOF–IMS | exocarp, seeds | - | [15] |
| 5-Demethylnobiletin | C20H20O8 | 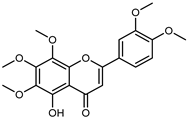 | Dynamic maceration 70% EtOH | HPLC | flavedo | - | [26] |
| 6,8-di-C-glucosyldiosmetin | C28H32016 |  | PLE MeOH | HPLC–DAD | fructus | 13.51 µg/mL | [40] |
| 7-O-Methyl-aromadendrin | C16H14O6 | 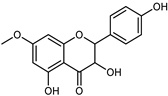 | Maceration on cold 70% MeOH | UV, MS, NMR | leaves | - | [38] |
| Scoparin (Chrysoeriol 8-C-glucoside) | C22H22O11 | 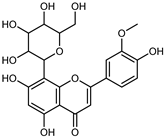 | MeOH under reflux | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| Phenolic acids | |||||||
| Benzoic acid | C7H6O2 | 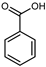 | Soxhlet with MeOH | HPLC | fructus | 0.00103 mg/g DW | [42] |
| Caffeic acid | C9H8O4 |  | UAE EtOH 80% | UHPLC–QTOF–IMS | exocarp, mesocarp, endocarp, seeds | 36.38–122.88 mg/100 g DW | [15] |
| 100% EtOH for 24 h | UPLC–DAD | flavedo, pulp | 6.97–7.11 µg/g FW | [34] | |||
| Chlorogenic acid | C16H18O9 | 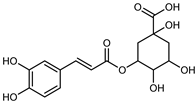 | UAE EtOH 80% | UHPLC–QTOF–IMS | mesocarp, endocarp, seeds | 66.66–109.85 mg/100 g DW | [15] |
| Gallic acid | C7H605 | 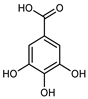 | UAE EtOH 80% | UHPLC–QTOF–IMS | exocarp, mesocarp, endocarp | 13.51–26.36 mg/100 g DW | [15] |
| 100% EtOH | UPLC–DAD | flavedo, pulp | 16.84–39.02 µg/g FW | [34] | |||
| Soxhlet with MeOH | HPLC | fructus | 0.30 mg/g DW | [42] | |||
| p-Coumaric acid | C9H803 | 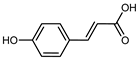 | UAE EtOH 80% | UHPLC–QTOF–IMS | exocarp, mesocarp, endocarp, seeds | 3.90–28.09 mg/100 g DW | [15] |
| Methyl-4-hydroxycinnamate | C10H10O3 | 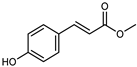 | MeOH under reflux | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| Salicylic acid | C7H6O3 | 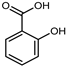 | Soxhlet with MeOH | HPLC | fructus | 0.16 mg/g DW | [42] |
| trans-Cinnamic acid | C9H8O2 | 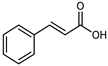 | UAE EtOH 80% | UHPLC–QTOF–IMS | mesocarp, endocarp, seeds | 0.42–13.06 mg/100 g DW | [15] |
| trans-Ferulic acid | C10H10O4 | 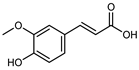 | UAE EtOH 80% | UHPLC–QTOF–IMS | exocarp, mesocarp. Endocarp, seeds | 19.85–96.79 mg/100 g DW | [15] |
| 100% EtOH | UPLC–DAD | flavedo and pulp | 106.36–295.97 µg/g FW | [34] | |||
| Dynamic maceration 70% EtOH | HPLC | flavedo | 0.21–1.08 mg/g DW | [26] | |||
| Neolignans | |||||||
| (7E)-1-Allyl alcohol-5,6-(11-isopropyl)-furanyl-3′,5′-dimethoxy-4′-glycerol-9′-isovalerate-3,4,7′,8′-benzodioxane neolignan | C33H40O11 |  | reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| 2(7E,10′E,11E)-1-(9-Methoxyl)-propenyl-5-hydroxy-6-prenyl-8′-methylol-11′,16′-dihydroxy-15′,17′-dimethoxy-10′-phenylallyl alcohol-3,4,7′,8′-benzodioxane neolignan | C35H38O10 |  | reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| (7E,11E)-1-(9-Methoxyl)-propenyl-5-hydroxy-6-geranyl-16′-hydroxy-15′,17′-dimethoxyphenyl-8′,11′-dimethylol-benzofuranyl 3,4,7′,8′-benzodioxane neolignan | C39H44O11 | 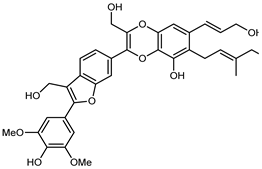 | reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| 1-(18,19-Dimethyl)-propanol-4-hydroxyl-5,6-(13-hydroxyl-12-methoxyl)-phenylethyl-7′-(4′-hydroxyl-5′-methoxy)-phenyl-9′-O-β-D glucopyranosyl-phenanthrofuran neolignan | C36H42O13 | 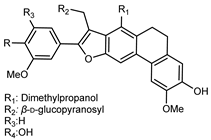 | reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| 1-(17-Furanyl)-ethyl-4-hydroxyl-5,6-(13-hydroxyl-12-methoxyl)- phenylethyl-7′-(3′,4′,5′-trimethoxy)-phenyl-9′-O-β-D-glucopyranosyl- phenanthrofuran neolignan | C39H42O14 | 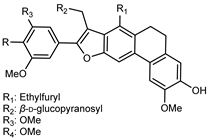 | reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| 1-(17Z)-Methyl-butanol-4-hydroxyl-5,6-(13-hydroxyl-12-methoxyl)- phenylethyl-7′-(4′-hydroxy-3′,5′-dimethoxy)-phenyl-9′-O-β-D-glucopyranosyl-phenanthrofuran neolignan | C37H42O14 |  | reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| (9′E)-4,5-(11,12-Dimethyl)- pyranyl-7′-(4′-hydroxy)- phenyl-4′-propenyl-8′-methylol-furanyl-6′-acetyl-1′,6-biphenyl-7-ketone | C32H28O6 | 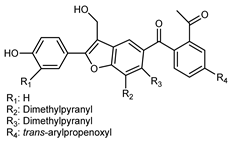 | reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| (9E,9′E)-5-Isopentenyl-7′-(4′-hydroxy-5′-methoxy)-phenyl-4′-propenylketone-8′-methylol-furanyl-6′-acetyl-1′,6-biphenyl-7-ketone | C33H30O8 | 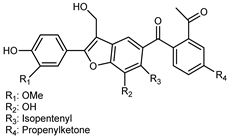 | reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| (7E,10E)-4,5′-Dihydroxy-5-isopentenol-6-(7,8-trans allyl)-alcohol7′-(4′-hydroxy-3′,5′-dimethoxyl)-phenyl-9′,9′-dimethylol-1′,7′- bineolignan | C34H34O11 | 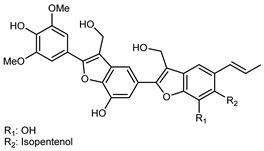 | reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| (7E)-5′-Hydroxy-4,5-(13,14-dimethyl)-pyranyl-6-allyl alcohol-7′-(4′-hydroxy-3′,5′-dimethoxyl)-phenyl-9′,9′-dimethylol-1′,7′-bineolignan | C34H32O10 |  | reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| (7S,8R)-9′,3-Dimethoxyl isoamericanol | C20H22O7 | 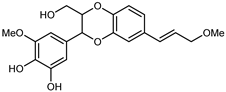 | reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| (7S,8R,7′S,8′R)-7,8–7′,8′-trans-7′,8′-Z-Sesquiverniciasin A | C27H25O9 | 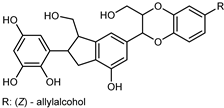 | reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| (7S,8R,7′S,8′R)-7,8–7′,8′-trans-7′,8′-E-Sesquiverniciasin A | C27H25O9 |  | reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| Selamoellenin B | C21H24O7 |  | reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| Dendronbibisline A | C30H26O7 | 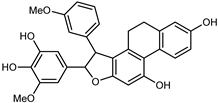 | reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| Dendronbibisline B | C25H24O7 |  | reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| Dendronbibisline C | C32H32O8 | 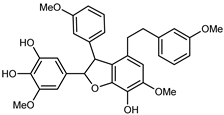 | reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| Dendronbibisline D | C33H34O8 | 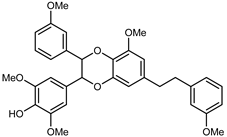 | reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| Herpetiosol B | C30H34O9 |  | reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| Herpetosiols C | C31H34O9 |  | reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| Silychristin A | C25H22O10 | 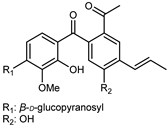 | reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| Silychristin B | C25H22O10 |  | reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| (7S,8R)-threo-1′-[3′-Hydroxy-7-(4-hydroxy-3- methoxyphenyl)-8-hydroxymethyl-7,8-dihydrobenzofuran]acryl-aldehyde | C19H18O6 | 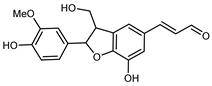 | reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| (-)-(7R,8S,7′E)-4-Hydroxy-3,5,5′,9′-tetramethoxy-4′,7-epoxy-8,3′-neolign-7′-en-9-ol | C22H26O7 | 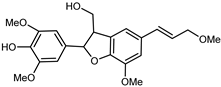 | reflux 95% EtOH | NMR, HR–ESI–MS | fructus | - | [32] |
| Compounds | Formula | Structure | Extraction Method * | Analysis | Part of the Plant | Abundance | References |
|---|---|---|---|---|---|---|---|
| Monoterpenes | |||||||
| ɑ-Thujene | C10H16 | 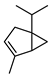 | I | GC–MS | flavedo | 0.2% | [50] |
| II, III, IV | HRGC–MS | flavedo | 0.28–0.59% | [49] | |||
| V, VI, VII | GC–MS | flavedo | 0.2–0.9% | [48] | |||
| VIII | GC–MS–SPME | industrial essence | - | [57] | |||
| X | GC–MS | fructus | 1.20–1.29% | [53] | |||
| XI | GC–MS | fructus | 0.87% | [58] | |||
| XII | HR–MAS–NMR | oil glands | - | [22] | |||
| XV | GC–MS | exocarp, mesocarp | 0.4–0.5% | [54] | |||
| ɑ-Thujone | C10H16O |  | XIII | GC–MS | fresh fructus | 4.29–5.05% | [45] |
| Ꞵ-Thujene | C10H16 | 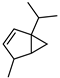 | VIII | GC–MS–SPME | industrial essence | 0.78% | [57] |
| ɑ-Pinene | C10H16 | 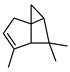 | I | GC–MS | flavedo | 0.49% | [50] |
| II, III, IV | HRGC–MS | flavedo | 0.69–1.46% | [49] | |||
| V, VI, VII | GC–MS | flavedo | 0.6–2.1% | [48] | |||
| X | GC–MS | fructus | 2.92–3.40% | [53] | |||
| XI | GC–MS | fructus | 1.99% | [58] | |||
| XII | HR–MAS–NMR | oil glands | - | [22] | |||
| XIII | GC–MS | fresh fructus | 6.38–7.73% | [45] | |||
| XV | GC–MS | exocarp, mesocarp | 1.4–1.6% | [54] | |||
| Sabinene | C10H16 | 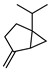 | I | GC–MS | flavedo | 0.64% | [50] |
| II, III, IV | HRGC–MS | flavedo | 0.14–0.22% | [49] | |||
| V, VI, VII | GC–MS | flavedo | 0.1–0.3% | [48] | |||
| XII | HR–MAS–NMR | oil glands | - | [22] | |||
| Camphene | C10H16 | 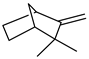 | II, III, IV | HRGC–MS | flavedo | 0.01% | [49] |
| V, VI, VII | GC–MS | flavedo | trace | [48] | |||
| VIII | GC–MS–SPME | industrial essence | 0.04% | [57] | |||
| X | GC–MS | fructus | 0.02–0.03% | [53] | |||
| XIII | GC–MS | fresh fruit | 0.22–0.29% | [45] | |||
| cis-Sabinene hydrate | C10H18O |  | II, III, IV | HRGC–MS | flavedo | 0.04–0.06% | [49] |
| V, VI, VII | GC–MS | flavedo | trace | [48] | |||
| trans-Sabinene hydrate | C10H18O | 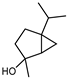 | VIII | GC–MS–SPME | industrial essence | - | [57] |
| Ꞵ-Pinene | C10H16 | 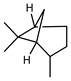 | I | GC–MS | flavedo | 0.63% | [50] |
| II, III, IV | HRGC–MS | flavedo | 0.69–1.47% | [49] | |||
| V, VI, VII | GC–MS | flavedo | 1.0–2.0% | [48] | |||
| VIII | GC–MS–SPME | industrial essence | 20.07% | [57] | |||
| X | GC–MS | fructus | 2.48–2.88% | [53] | |||
| XI | GC–MS | fructus | 2.02% | [58] | |||
| XII | HR–MAS–NMR | flavedo, oil glands | - | [22] | |||
| XIII | GC–MS | fresh fructus | 2.64–3.18% | [45] | |||
| XIV | GC–MS | exocarp, mesocarp | 2.4–2.5% | [54] | |||
| Myrcene | C10H16 | 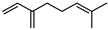 | I | GC–MS | flavedo | 0.89 | [50] |
| II, III, IV | HRGC–MS | flavedo | 1.13–1.47% | [49] | |||
| V, VI, VII | GC–MS | flavedo | 0.8–1.6% | [48] | |||
| XII | HR–MAS–NMR | oil glands | - | [22] | |||
| VIII | GC–MS–SPME | industrial essence | 2.24% | [57] | |||
| X | GC–MS | fructus | 1.64–1.76% | [53] | |||
| XI | GC–MS | fructus | 1.25% | [58] | |||
| Limonene | C10H16 | 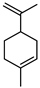 | I | GC–MS | flavedo | 15.20% | [50] |
| II, III, IV | HRGC–MS | flavedo | 25.70–60.30 g/100 g DW | [49] | |||
| V, VI, VII | GC–MS | flavedo | 34.6–60.8% | [48] | |||
| VIII | GC–MS–SPME | industrial essence | 41.07% | [57] | |||
| X | GC–MS | fructus | 51.24–57.63% | [53] | |||
| XI | GC–MS | fructus | 52.44% | [58] | |||
| XII | HR–MAS–NMR | flavedo, oil glands, albedo | - | [22] | |||
| XIII | GC–MS | fresh fructus | 32.07–36.37% | [45] | |||
| XIV | UHPLC–QTOF–IMS | exocarp, mesocarp, endocarp, seeds | - | [15] | |||
| XV | GC–MS | exocarp, mesocarp | 75.8–76.2% | [54] | |||
| Decane | C10H22 |  | II, III, IV | HRGC–MS | flavedo | trace | [49] |
| Decanal | C10H20O | 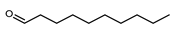 | II, III, IV | HRGC–MS | flavedo | 0.04–0.07 | [49] |
| V, VI, VII | GC–MS | flavedo | 0.1% | [48] | |||
| VIII | GC–MS–SPME | industrial essence | 0.27% | [57] | |||
| Octyl acetate | C10H20O2 |  | II, III, IV | HRGC–MS | flavedo | 0.01% | [49] |
| VIII | GC–MS | industrial essence | 0.16% | [57] | |||
| Citronellol | C10H20O | 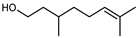 | II, III, IV | HRGC–MS | flavedo | 0.03–0.11% | [49] |
| cis-Limonene oxide | C10H16O |  | II, III, IV | HRGC–MS | flavedo | 0.01% | [49] |
| VIII | GC–MS–SPME | industrial essence | - | [57] | |||
| trans-Limonene oxide | C10H16O | 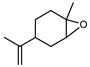 | II, III, IV | HRGC–MS | flavedo | trace | [49] |
| VIII | GC–MS | industrial essence | 0.28% | [57] | |||
| trans-Carveol | C10H16O | 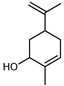 | V, VI, VII | GC–MS | flavedo | 0.1% | [48] |
| Carveol | C10H16O | 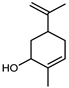 | XV | GC–MS | mesocarp | 0.1% | [54] |
| Camphor | C10H16O | 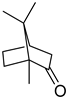 | II, III, IV | HRGC–MS | flavedo | 0.01% | [49] |
| Citronellal | C10H18O |  | II, III, IV | HRGC–MS | flavedo | 0.04–0.06% | [49] |
| V, VI, VII | GC–MS | flavedo | 0.1–0.2% | [48] | |||
| VIII | GC–MS–SPME | industrial essence | 0.27% | [57] | |||
| XII | HR–MAS–NMR | oil glands | - | [22] | |||
| XIII | GC–MS | fresh fructus | 0.11% | [45] | |||
| Borneol | C10H18O | 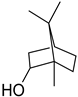 | II, III, IV | HRGC–MS | flavedo | 0.01% | [49] |
| (Z)-Ꞵ-Ocimene | C10H16 | 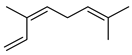 | II, III, IV | HRGC–MS | flavedo | 0.75–1.19% | [49] |
| V, VI, VII | GC–MS | flavedo | 1.4–1.5% | [48] | |||
| VIII | GC–MS–SPME | industrial essence | - | [57] | |||
| XI | GC–MS | fructus | 0.94% | [58] | |||
| (E)-Ꞵ-Ocimene | C10H16 |  | II, III, IV | HRGC–MS | flavedo | 1.10–1.74% | [49] |
| V, VI, VII | GC–MS | flavedo | 1.9–2.1% | [48] | |||
| VIII | GC–MS–SPME | industrial essence | 0.07% | [57] | |||
| XIII | GC–MS | fresh fructus | 0.55–0.99% | [45] | |||
| X | GC–MS | fructus | 0.23–0.93% | [53] | |||
| XI | GC–MS | fructus | 0.65% | [58] | |||
| XV | GC–MS | exocarp, mesocarp | 1.1–1.2% | [54] | |||
| Citral | C10H16O | 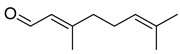 | VIII | GC–MS–SPME | industrial essence | - | [57] |
| X | GC–MS | fructus | 1.96–2.34% | [53] | |||
| XII | HR–MAS–NMR | flavedo | - | [22] | |||
| XV | GC–MS | mesocarp | 0.1% | [54] | |||
| Octanal | C8H16O |  | II, III, IV | HRGC–MS | flavedo | 0.01% | [49] |
| V, VI, VII | GC–MS | flavedo | - | [48] | |||
| VIII | GC–MS–SPME | industrial essence | 0.31% | [57] | |||
| ɑ-Phellandrene | C10H16 |  | II, III, IV | HRGC–MS | flavedo | 0.04–0.05% | [49] |
| XV | GC–MS | mesocarp | trace | [54] | |||
| V, VI, VII | GC–MS | flavedo | 0.1% | [48] | |||
| X | GC–MS | fructus | 0.1% | [53] | |||
| δ-3-Carene | C10H16 |  | I | GC–MS | flavedo | 2.30% | [50] |
| II, III, IV | HRGC–MS | flavedo | trace | [49] | |||
| 3-Carene | C10H16 |  | XIII | GC–MS | fresh fructus | 8.15–9.01% | [45] |
| 4-Carene | C10H16 | 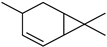 | VIII | GC–MS–SPME | industrial essence | 0.10% | [57] |
| γ-Terpinene | C10H16 |  | I | GC–MS | flavedo | 10.27% | [50] |
| II, III, IV | HRGC–MS | flavedo | 21.19–23.44% | [49] | |||
| V, VI, VII | GC–MS | flavedo | 22.1–24.6% | [48] | |||
| VIII | GC–MS–SPME | industrial essence | 8.35% | [57] | |||
| X | GC–MS | fructus | 27.01–33.71% | [53] | |||
| XI | GC–MS | fructus | 28.41% | [58] | |||
| XII | HR–MAS–NMR | flavedo, oil glands | - | [22] | |||
| XIII | GC–MS | fresh fructus | 22.44–25.23% | [45] | |||
| XV | GC–MS | exocarp, mesocarp | 15.0–16.5% | [54] | |||
| ɑ-Terpinene | C10H16 | 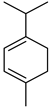 | II, III, IV | HRGC–MS | flavedo | 0.35–0.41% | [49] |
| V, VI, VII | GC–MS | flavedo | trace | [48] | |||
| X | GC–MS | fructus | 1.28% | [53] | |||
| XI | GC–MS | fructus | 0.81% | [58] | |||
| Terpinolene | C10H16 | 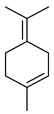 | I | GC–MS | flavedo | 0.91% | [50] |
| II, III, IV | HRGC–MS | flavedo | 0.87–1.00% | [49] | |||
| V, VI, VII | GC–MS | flavedo | 1.0–1.2% | [48] | |||
| VIII | GC–MS–SPME | industrial essence | 0.33% | [57] | |||
| X | GC–MS | fructus | 1.25–1.54% | [53] | |||
| XIII | GC–MS | industrial essence | - | [45] | |||
| XV | GC–MS | exocarp, mesocarp | 0.2–0.6% | [54] | |||
| Linalool | C10H18O |  | I | GC–MS | flavedo | 1.15% | [50] |
| II, III, IV | HRGC–MS | flavedo | 0.10–0.20 g/100 g DW | [47] | |||
| V, VI, VII | GC–MS | flavedo | 0.1–0.3% | [48] | |||
| VIII | GC–MS–SPME | industrial essence | 1.73% | [57] | |||
| XIII | GC–MS | fresh fructus | 0.16–0.18% | [45] | |||
| Linalool oxide | C10H18O2 | 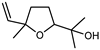 | VIII | GC–MS–SPME | industrial essence | 0.28% | [57] |
| Allocimene | C10H16 | 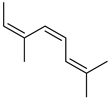 | I | GC–MS | flavedo | 0.70% | [50] |
| Terpinen-4-ol | C10H18O | 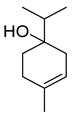 | I | GC–MS | flavedo | 1.02% | [50] |
| II, III, IV | HRGC–MS | flavedo | 0.04–0.06% | [49] | |||
| V, VI, VII | GC–MS | flavedo | 0.1–0.2% | [48] | |||
| X | GC–MS | fructus | 0.34–0.51% | [53] | |||
| VIII | GC–MS–SPME | industrial essence | 0.31% | [57] | |||
| XIII | GC–MS | fresh fructus | 0.69–0.88% | [45] | |||
| ɑ-Terpineol | C10H18O |  | I | GC–MS | flavedo | 2.64% | [50] |
| V, VI, VII | GC–MS | flavedo | 0.1–0.3% | [48] | |||
| VIII | GC–MS–SPME | industrial essence | 0.10% | [57] | |||
| X | GC–MS | fructus | 0.48–0.58% | [53] | |||
| XIII | GC–MS | fresh fruit | 1.17–1.61% | [45] | |||
| XV | GC–MS | exocarp, mesocarp | 0.1–0.4% | [54] | |||
| Nerol | C10H18O |  | I | GC–MS | flavedo | 4.69% | [50] |
| V, VI, VII | GC–MS | flavedo | 0.1–0.3% | [48] | |||
| XIII | GC–MS | fresh fructus | 0.9–1.53% | [45] | |||
| Neral | C10H16O | 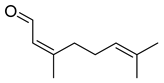 | II, III, IV | HRGC–MS | flavedo | 1.20–9.40 g/100 g DW | [49] |
| V, VI, VII | GC–MS | flavedo | trace | [48] | |||
| VIII | GC–MS–SPME | industrial essence | 2.49% | [57] | |||
| X | GC–MS | fructus | 0.45% | [53] | |||
| XII | HR–MAS–NMR | flavedo | - | [22] | |||
| XIII | GC–MS | fresh fructus | 1.04–1.60% | [45] | |||
| p-Cymen-8-ol | C10H14O | 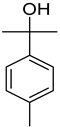 | II, III, IV | HRGC–MS | flavedo | 0.01% | [47] |
| p-Cymene | C10H14 | 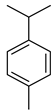 | V, VI, VII | GC–MS | flavedo | 0.4–0.6% | [48] |
| XIV | GC–MS | exocarp, mesocarp | 0.2–0.7% | [54] | |||
| VIII | GC–MS–SPME | industrial essence | 5.92% | [57] | |||
| XIII | GC–MS | fresh fructus | 1.64–2.77% | [45] | |||
| Geraniol | C10H18O | 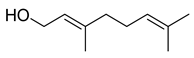 | I | GC–MS | flavedo | 4.63% | [50] |
| II, III, IV | HRGC–MS | flavedo | 0.10–8.50 g/100 g DW | [49] | |||
| V, VI, VII | GC–MS | flavedo | 0.1–0.7% | [48] | |||
| X | GC–MS | fructus | 0.55–0.58% | [53] | |||
| VIII | GC–MS-SPME | industrial essence | 0.27% | [57] | |||
| XIII | GC–MS | fresh fructus | 1.18–2.02% | [45] | |||
| Perillal | C10H14O | 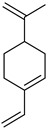 | VIII | GC–MS–SPME | industrial essence | 0.10% | [57] |
| Cuminol | C10H14O | 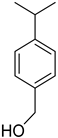 | VIII | GC–MS–SPME | industrial essence | 0.03% | [57] |
| Carvacrol | C10H14O | 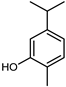 | II, III, IV | HRGC–MS | flavedo | trace | [49] |
| Perilla aldehyde | C10H14O | 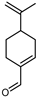 | II, III, IV | HRGC–MS | flavedo | 0.01–0.02% | [49] |
| Sesquiterpenes | |||||||
| δ-Elemene | C15H24 |  | II, III, IV | HRGC–MS | flavedo | 0.06–0.16% | [49] |
| V, VI, VII | GC–MS | flavedo | 0.1% | [48] | |||
| Ꞵ-Elemene | C15H24 | 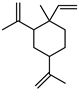 | I | GC–MS | flavedo | 0.1% | [50] |
| II, III, IV | HRGC–MS | flavedo | 0.1% | [49] | |||
| V, VI, VII | GC–MS | flavedo | 0.1% | [48] | |||
| X | GC–MS | flavedo | 0.1% | [53] | |||
| Copaene | C15H24 |  | X | GC–MS | fructus | 0.02% | [53] |
| trans-Caryophyllene | C15H24 |  | I | GC–MS | flavedo | 0.41% | [50] |
| ɑ-Bisabolol | C15H26O |  | V, VI, VII | GC–MS | flavedo | 0.2% | [48] |
| ɑ-Bergamotene | C15H24 |  | I | GC–MS | flavedo | 1.09% | [50] |
| XV | GC–MS | exocarp, mesocarp | 0.3–0.6% | [54] | |||
| X | GC–MS | fructus | 0.07% | [53] | |||
| V, VI, VII | GC–MS | flavedo | 0.2–1.7% | [48] | |||
| ɑ-Himachalene | C15H24 | 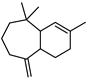 | XV | GC–MS | exocarp, mesocarp | 0.1–0.6% | [54] |
| γ-Gurjunene | C15H24 | 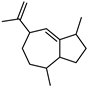 | XV | GC–MS | mesocarp | trace | [54] |
| ɑ-Humulene | C15H24 | 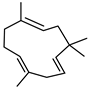 | I | GC–MS | flavedo | 0.13% | [50] |
| II, III, IV | HRGC–MS | flavedo | 0.1% | [49] | |||
| V, VI, VII | GC–MS | flavedo | 0.1% | [48] | |||
| X | GC–MS | fructus | - | [53] | |||
| (Z)-β-Farnesene | C15H24 | 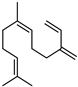 | I | GC–MS | flavedo | 0.14% | [50] |
| II, III, IV | HRGC–MS | flavedo | trace | [49] | |||
| V, VI, VII | GC–MS | flavedo | 0.1% | [48] | |||
| α-Bisabolene | C15H24 | 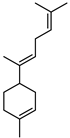 | I | GC–MS | flavedo | 0.10% | [50] |
| IX | GC–MS | leaves | - | [59] | |||
| β-Bisabolene | C15H24 | 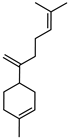 | I | GC–MS | flavedo | 1.39% | [50] |
| II, III, IV | HRGC–MS | flavedo | 0.03–0.05% | [49] | |||
| V, VI, VII | GC–MS | flavedo | 0.2–2.6% | [48] | |||
| VIII | GC–MS–SPME | industrial essence | 0.30% | [57] | |||
| Spathulenol | C15H24O | 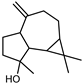 | I | GC–MS | flavedo | 0.1% | [50] |
| V, VI, VII | GC–MS | flavedo | 0.1% | [48] | |||
| α-cis-Bergamotene | C15H24 | 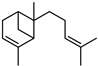 | II, III, IV | HRGC–MS | flavedo | 0.02–0.03% | [49] |
| E-β-Caryophyllene | C15H24 |  | II, III, IV | HRGC–MS | flavedo | 0.10 g/100 g DW | [49] |
| VIII | GC–MS | industrial essence | 0.23% | [57] | |||
| IX | GC–MS | leaves | - | [59] | |||
| X | GC–MS | fructus | 0.06% | [53] | |||
| XIII | GC–MS | fresh fructus | 0.27–0.46% | [45] | |||
| XIV | UHPLC–QTOF–IMS | mesocarp | - | [15] | |||
| α-trans-Bergamotene | C15H24 | 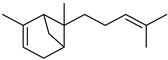 | II, III, IV | HRGC–MS | flavedo | 0.29–0.45% | [49] |
| V, VI, VII | GC–MS | flavedo | 0.2–1.7% | [48] | |||
| IX | GC–MS | leaves | - | [59] | |||
| (E)-β-Farnesene | C15H24 | 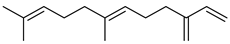 | II, III, IV | HRGC–MS | flavedo | trace | [49] |
| XV | GC–MS | exocarp | 0.2% | [54] | |||
| IX | GC–MS | leaves | - | [59] | |||
| (Z)-β-Santalene | C15H24 |  | II, III, IV | HRGC–MS | flavedo | 0.01% | [49] |
| Valencene | C15H24 |  | II, III, IV | HRGC–MS | flavedo | 0.03–0.07% | [49] |
| Bicyclogermacrene | C15H24 | 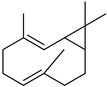 | II, III, IV | HRGC–MS | flavedo | 0.03–0.04% | [49] |
| (Z)-α-Bisabolene | C15H24 |  | II, III, IV | HRGC–MS | flavedo | 0.03–0.05% | [49] |
| β-Cadinene | C15H24 |  | XIII | GC–MS | fresh fructus | 0.74–1.09% | [45] |
| α-Cedrene | C15H24 |  | XIII | GC–MS | fresh fructus | 0.55–0.64% | [45] |
| (E,E)-α-Farnesene | C15H24 | 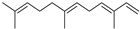 | V, VI, VII | GC–MS | flavedo | trace | [48] |
| (Z)-α-Farnesene | C15H24 | 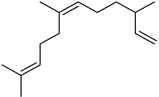 | XV | GC–MS | exocarp | 0.6% | [54] |
| α-Farnesene | C15H24 | 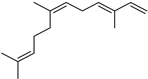 | X | GC–MS | fructus | 0.1% | [53] |
| XV | GC–MS | mesocarp | 0.2% | [54] | |||
| (Z)-γ-Bisabolene | C15H24 | 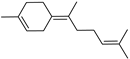 | II, III, IV | HRGC–MS | flavedo | trace | [49] |
| Germacrene B | C15H24 | 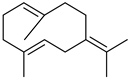 | V, VI, VII | GC–MS | flavedo | 0.1–0.3% | [48] |
| X | GC–MS | fructus | - | [53] | |||
| Gemacrene D | C15H24 | 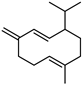 | IX | GC–MS | leaves | - | [59] |
| X | GC–MS | fructus | 0.15–0.19% | [53] | |||
| Bicyclogermacrene | C15H24 | 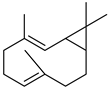 | IX | GC–MS | leaves | - | [59] |
| X | GC–MS | fructus | 0.06% | [53] | |||
| (E)-Nerolidol | C15H26O | 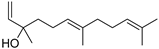 | V, VI, VII | GC–MS | flavedo | 0.1–0.3% | [48] |
| IX | GC–MS | leaves | - | [59] | |||
| Β-Bisabolene | C15H24 |  | II, III, IV | HRGC–MS | flavedo | 0.40–0.67% | [49] |
| Farnesol | C15H26O | 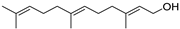 | V, VI, VII | GC–MS | flavedo | trace | [48] |
| Farnesal | C15H24O | 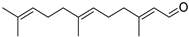 | V, VI, VII | GC–MS | flavedo | trace | [48] |
| Triterpenoids (Limonoids) | |||||||
| Limonyl acetate | C28H34O9 | 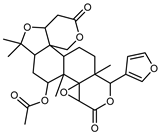 | XIV | UHPLC–QTOF–IMS | exocarp, seeds | - | [15] |
| Limonin | C26H30O8 | 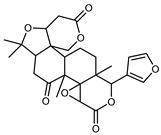 | XVII | HPLC | citron waste | 3.08 mg/100 g DW | [56] |
| XVI | EI–MS, HR–EI–MS | fresh fructus | - | [43] | |||
| XIV | HPLC–Q/TOF–MS | fructus | 0.45–0.86 mg/g DW | [35] | |||
| XVIII | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] | |||
| Nomilin | C28H34O9 | 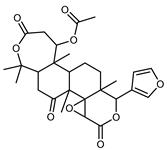 | XVII | HPLC | citron waste | 0.87 mg/100 g DW | [56] |
| XVI | EI–MS, HR–EI–MS | fresh fructus | - | [43] | |||
| XIV | HPLC–Q/TOF–MS | fructus | 1.97–3.84 mg/g DW | [35] | |||
| Citrusin | C28H34O11 | 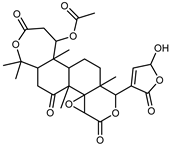 | XVI | EI–MS, HR–EI–MS | fresh fructus | - | [43] |
| Obacunone | C26H30O7 | 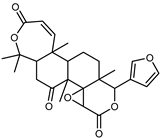 | XVI | EI–MS, HR–EI–MS | fresh fructus | - | [43] |
| XIV | HPLC–Q/TOF–MS | fructus | 0.15–0.36 mg/g DW | [35] | |||
| Nomilinic acid | C28H36O10 | 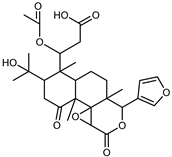 | XIV | UHPLC–QTOF–IMS | exocarp, seeds | - | [15] |
| Terpenoids | |||||||
| Geranyl acetate | C12H20O2 | 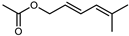 | I | GC–MS | flavedo | 0.75% | [50] |
| Citronellyl acetate | C12H22O2 |  | II, III, IV | HRGC–MS | flavedo | 0.10 g/100 g DW | [49] |
| V, VI, VII | GC–MS | flavedo | 0.1–0.2% | [48] | |||
| Dihydrolinalyl acetate | C12H22O2 |  | II, III, IV | HRGC–MS | flavedo | trace | [49] |
| β-lonone | C13H20O | 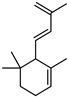 | XIII | GC–MS | fresh fructus | 0.20–0.49% | [45] |
| Linalyl acetate | C12H20O2 |  | VIII | GC–MS | industrial essence | 1.82% | [57] |
| Compounds | Formula | Structure | Extraction Method | Method Analyses | Part of the Plant | Quantitative | References |
|---|---|---|---|---|---|---|---|
| Oxypeucedanin hydrate | C16H16O6 |  | MeOH under reflux | EI–MS, HR–EI–MS | fresh fruit | 2.03–21.30 g/100 g DW | [43] |
| Scoparone (6,7-dimethoxycoumarin) | C11H10O4 | 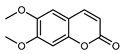 | MeOH under reflux | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| PLE MeOH | HPLCDAD | fructus | 38.79 µg/mL | [40] | |||
| UAE | HPLC–Q/TOF–MS | fructus | - | [35] | |||
| Skimmin | C15H16O8 | 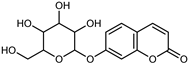 | MeOH under reflux | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| Haploperoside A | C22H28O13 | 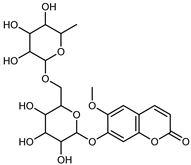 | MeOH under reflux | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| Leptodactylone | C11H10O5 |  | MeOH under reflux | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| Herniarin (7-methoycoumarin) | C10H8O3 | 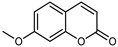 | MeOH under reflux | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| Isomeranzin | C15H16O4 | 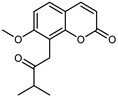 | UAE | HPLC–Q/TOF–MS | fructus | - | [35] |
| Scopoletin | C10H8O4 |  | MeOH under reflux | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| PLE MeOH | HPLC–DAD | fructus | 53.56 µg/mL | [40] | |||
| Isoscopoletin | C10H8O4 | 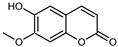 | PLE MeOH | HPLC–DAD | fructus | 63.06 µg/mL | [40] |
| Umbelliferone (7-hydroxycoumarin) | C9H6O3 |  | MeOH under reflux | EI-MS, HR–EI–MS | fresh fruit | - | [43] |
| PLE MeOH | HPLC–DAD | fructus | 40.23 µg/mL | [40] | |||
| Nordentatin | C19H20O4 |  | MeOH under reflux | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| Maceration in acetone | COSY, NOESY, HMQC, HMBC, HR–ESI–MS | root bark, stem bark | - | [36] | |||
| 2-pyrone | C5H4O2 | 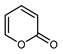 | Maceration and UAE MeOH | GC–MS | exocarp, mesocarp | 23.4–33.1% | [54] |
| Citropten (5,7-dimethoxycoumarin) | C11H10O4 |  | PLE MeOH | HPLC–DAD | fructus | 106.47 µg/mL | [40] |
| MeOH under reflux | HR–EI–MS1 | fresh fruits | 0.16–0.45 mg/g DW | [43] | |||
| Maceration of peel with n-hexane at room temperature | GC–MS | fructus | 12.64% | [50] | |||
| UAE | HPLC–Q/TOF–MS | fructus | 0.18–0.45 mg/g DW | [35] | |||
| Bergapten | C12H8O4 | 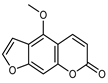 | PLE MeOH | HPLC–DAD | fructus | 35.07 µg/mL | [40] |
| UAE | HPLC–Q/TOF–MS | fructus | - | [35] | |||
| Maceration MeOH | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] | |||
| Citrumedin-B | C24H28O4 | 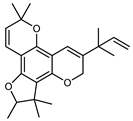 | Acetone at room temperature | COSY, NOESY, HMQC, HMBC, HR–ESI–MS | root bark, stem bark | - | [36] |
| Xanthyletin | C14H12O3 | 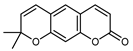 | PLE MeOH | HPLC–DAD | fructus | - | [40] |
| UAE with CHCl3 | MEKC (micellar electrokinetic capillary chromatography) | root bark | - | [60] | |||
| Xanthoxyletin | C15H14O4 | 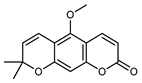 | UAE with CHCl3 | MEKC (micellar electrokinetic capillary chromatography) | root bark | - | [60] |
| 5,8-dimethoxhypsoralene | C12H8O4 |  | Maceration MeOH | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] |
| Compounds | Formula | Structure | * Extraction Method | Method Analyses | Part of the Plant | Abundance | References |
|---|---|---|---|---|---|---|---|
| Alkaloids | |||||||
| 1,2,3,4-Tetrahydro-beta-carboline-3-carboxylic acid | C12H12N2O2 | 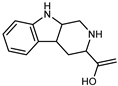 | IX | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| Acridine derivatives | |||||||
| Medicacridone | C20H21NO4 | 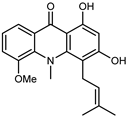 | VIII | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] |
| Citracridone-I | C20H19NO5 |  | VIII | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] |
| citracridone-III | C19H17NO5 | 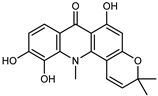 | VIII | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] |
| 5-hydroxynoracronycine 3 | C19H17NO4 | 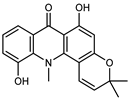 | VIII | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] |
| Xanthones | |||||||
| Medicaxanthone | C51H75O8 | 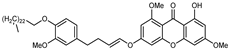 | VIII | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] |
| Lichenxanthone | C15H12O6 |  | VIII | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] |
| Glycol | |||||||
| 2,3-Butanediol | C4H10O2 |  | X | GC–MS | exocarp, mesocarp | 23.7% | [54] |
| Furan derivatives | |||||||
| Furfural | C5H4O2 | 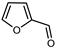 | X | GC–MS | exocarp, mesocarp | 3.9% | [54] |
| 2(3H)-Furanone, 5-methyl | C8H12O2 | 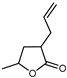 | X | GC–MS | exocarp, mesocarp | 0.9% | [54] |
| 5- 5-Hydroxymethylfurfural | C6H6O3 | 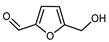 | X | GC–MS | exocarp, mesocarp | 1.9% | [54] |
| Hydrocarbons | |||||||
| 1,3-Cyclopentadiene | C5H6 | 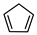 | XIII | GC–MS | fresh fructus | 1.75–2.36% | [45] |
| Benzene | C6H6 |  | XI | GC–MS | fructus | 1.67% | [58] |
| Eicosane | C20H42 | 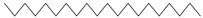 | I | GC–MS | flavedo | 0.10% | [50] |
| Nonacosane | C29H60 |  | I | GC–MS | flavedo | 0.10% | [50] |
| Mono or polyunsaturated aldehyde | |||||||
| Undecanal | C11H22O |  | V, VI, VII | GC–MS | flavedo | 0.1–0.2% | [48] |
| II, III, IV | HRGC–MS | flavedo | 0.03–0.06% | [49] | |||
| Dodecanal | C12H24O |  | II, III, IV | HRGC–MS | flavedo | 0.02–0.03% | [49] |
| V, VI, VII | GC–MS | flavedo | 0.1% | [48] | |||
| 9,17-octadecadienal | C18H32O | 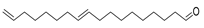 | I | GC–MS | flavedo | 9.29% | [50] |
| 16-Octadecenal | C18H34O |  | I | GC–MS | flavedo | 0.10% | [50] |
| Nonanal | C9H18O |  | II, III, IV | HRGC–MS | flavedo | 0.04–0.07% | [49] |
| Tetradecanal | C14H28O |  | V, VI, VII | GC–MS | flavedo | 0.1% | [48] |
| Pentadecanal | C15H30O |  | V, VI, VII | GC–MS | flavedo | 0.1% | [48] |
| Phenylpropanoids | |||||||
| Coniferin | C16H22O8 | 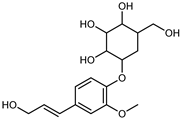 | IX | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| Syringin | C17H24O9 |  | IX | EI–MS, HR–EI-MS | fresh fruit | - | [43] |
| Phytosterols | |||||||
| Lupeol | C26H32O7 | 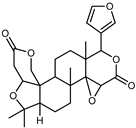 | VIII | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] |
| Stigmasterol | C29H48O |  | VIII | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] |
| Ꞵ-Sitosterol | C29H50O |  | VIII | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] |
| Fatty acids and their esters | |||||||
| Lauric acid | C12H24O2 | 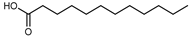 | I | GC–MS | flavedo | 0.11% | [50] |
| Myristic acid | C14H28O2 |  | I | GC–MS | flavedo | 0.23% | [50] |
| Palmitic acid | C16H32O2 |  | I | GC–MS | flavedo | 5.17% | [50] |
| Hexadecanal | C16H32O |  | XIII | GC–MS | mesocarp | 1.6% | [54] |
| V, VI, VII | GC–MS | flavedo | 0.1% | [48] | |||
| Pentadecanoic acid methyl ester | C16H32O2 | 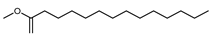 | I | GC–MS | flavedo | 0.22% | [50] |
| Palmitoleic acid | C17H32O2 |  | I | GC–MS | flavedo | 0.19% | [50] |
| Heptadecanoic acid | C17H34O2 | 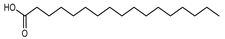 | I | GC–MS | flavedo | 0.20% | [50] |
| Stearic acid | C18H36O2 |  | I | GC–MS | flavedo | 0.18% | [50] |
| 16-Octadecenal | C18H34O |  | I | GC–MS | flavedo | 0.10% | [50] |
| Linoleic acid, methyl ester | C19H34O2 |  | I | GC–MS | flavedo | 0.19% | [50] |
| Linolenic acid, methyl ester | C19H32O2 | 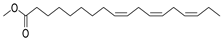 | I | GC–MS | flavedo | 0.41%0.30% | [50] |
| Stearic acid, methyl ester | C19H38O2 | 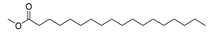 | I | GC–MS | flavedo | 0.30% | [50] |
| Benzoates | |||||||
| Methyl vanillate methyl ester | C9H10O4 | 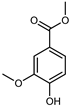 | IX | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| Methyl benzoate | C8H8O2 | 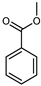 | IX | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| Methyl paraben | C8H8O3 | 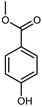 | IX | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| Compounds | Molecular Weight | Extraction Method | Method Analyses | Part of the Plant | Abundance | References |
|---|---|---|---|---|---|---|
| Polysaccharides | ||||||
| CMSPB80-1 | 103 kDa | Alkali extraction | HPGPC, FT–IR, methylation analysis, GC–MS, NMR | fructus | - | [61] |
| CMSPW90-1 | 18.8 kDa | Hot water | HPGPC, FT–IR, methylation analysis, GC–MS, NMR | pulp | - | [62] |
| CMSPW90-M1 | 75.4 kDa | Hot water | HPGPC, FT–IR, methylation analysis, GC–MS, NMR | pulp | - | [62] |
| CMSPA90-1 | 17.6 kDa | Acid extraction | HPGPC, FT–IR, methylation analysis, GC–MS, NMR | fructus | 97.77% ± 1.4% (w/w) DW | [63] |
| FCp-1, FCp-2, FCp-3, and FCp-4 | 113.9, 32.6, 140.3, and 177.1 kDa respectively | Hot water | acid hydrolysis, methylation, IR, GC–MS, and NMR | fructus | - | [64] |
| CM-1 and CM-2 | 21.520 kDa, 22.303 kDa respectively | Hot water | Monosaccharide composition, linkage, and NMR | fructus | - | [65] |
| K-CLMP | 3.76 × 103 kDa | Hot water | methylation analysis, NMR | fructus | 5.81% | [66] |
| Crude polysaccharides (FCPs) | - | Hot water | FT–IR | fructus | 3.19 ± 0.10% | [67] |
| Test/Model | Concentration/Dosage Tested/Results | Part of the Plant | Extraction Method | Reference |
|---|---|---|---|---|
| Antioxidant activity | ||||
| ABTS | RSA 87.94% 0.2 mg/mL | fructus | CPE | [41] |
| DPPH | RSA 89.86% at 0.8 mg/mL | |||
| ORAC | 928.64 µmol TE/g | |||
| DPPH | 112.18 μg ascorbic acid/ mL | fructus | Soxhlet | [42] |
| NO | 112.18 μg ascorbic acid/ mL | |||
| TPC | 177.50 ± 4.95 mgGAE/g | |||
| TFC | 165.52 ± 0.65 mgQUE/g | |||
| TPC | 227.45 ± 1.04 mg GAE/100 g FW | peel | MAE | [17] |
| 88.76 ± 1.38 mg GAE/100 g FW | pulp | |||
| DPPH | 22.79 ± 0.12 IC50 μg GAE/mL | peel | ||
| 22.79 ± 0.12 IC50 μg GAE/mL | pulp | |||
| ABTS | 214.81 ± 1.45 mg TE/100 g FW | peel | ||
| 71.53 ± 0.84 mg TE/100 g FW | pulp | |||
| DPPH | EC50 827.26 µg/mL | peel | Maceration | [20] |
| TPC | 66.36 μg GAE/mg | peel | ||
| 51.21 μg GAE/mg | pulp | |||
| TFC | 40.17μg cathecol equivalent/mg | peel | ||
| 37.9μg cathecol equivalent/mg | pulp | |||
| DPPH | 0.80 ± 0.07 (IC50 mg/mL) | peel | Maceration | [33] |
| ABTS | 3.48 ± 1.0 (IC50 mg/mL) | |||
| BCB | 0.23 ± 0.002 (IC50 mg/mL) | |||
| FRAP | 3.9 ± 0.5 P (µm Fe (II)/g) | |||
| DPPH | 147 ± 1.23 IC50 µg/mL | peel | Maceration | [50] |
| BCB | 3 ± 0.05 IC50 µg/mL at 30 min | |||
| Bovine brain peroxidation assay | 2472 ± 4.19 IC50 µg/mL | |||
| DPPH | 72.00 ± 0.82% scavenging activity | juice | Maceration | [3] |
| TPC | 309.08 ± 3.06 mgGAE/g | |||
| DPPH | EC50 102.9 µg/mL | leaves | Maceration | [38] |
| TPC | 398.0 ± 3.2 mg/100 g FW | flowers | Exhaustive maceration | [27] |
| 401.6 ± 5.1 mg/100 g FW | leaves | |||
| 181.3 ± 3.1 mg/100 g FW | immature mesocarp | |||
| 262.6 ± 3.7 mg/100 g FW | immature endocarp | |||
| 123.1 ± 6.5 mg/100 g FW | mature mesocarp | |||
| 109.4 ± 2.9 mg/100 g FW | mature endocarp | |||
| TFC | 266.9 ± 7.2 mg QUE/100 g FW | flowers | ||
| 97.5 ± 2.8 mg QUE/100 g FW | leaves | |||
| 95.7 ± 3.2 mg QUE/100 g FW | immature mesocarp | |||
| 64.9 ± 3.2 mg QUE/100 g FW | immature endocarp | |||
| 43.1 ± 1.2 mg QUE/100 g FW | mature mesocarp | |||
| 37.5 ± 1.6 mg QUE/100 g FW | mature endocarp | |||
| DPPH | 425.0 ± 2.95 µg Ascorbic acid/mL | flowers | ||
| 502.0 ± 3.01 µg Ascorbic acid/mL | leaves | |||
| 382.0 ± 2.45 µg Ascorbic acid/mL | immature mesocarp | |||
| >1000 µg Ascorbic acid/mL | immature endocarp | |||
| >1000 µg Ascorbic acid/mL | mature mesocarp | |||
| >1000 µg Ascorbic acid/mL | mature endocarp | |||
| BCB | 2.8 ± 0.002 µg/mL at 30 min | flowers | ||
| >100 µg/mL at 30 min | leaves | |||
| 3.7 ± 0.007 µg/mL at 30 min | immature mesocarp | |||
| 4.1 ± 0.009 µg/mL at 30 min | immature endocarp | |||
| 36.6 ± 0.075 µg/mL at 30 min | mature mesocarp | |||
| 3.5 ± 0.008 µg/mL at 30 min | mature endocarp | |||
| TPC | 227.45 mg GAE/100 g FW | peel | Maceration 70% MeOH | [26] |
| 88.76 mg GAE/100 g FW | pulp | |||
| DPPH | IC50 22.79 μg GAE/ml | peel | ||
| IC50 54.74 μg GAE/mL | pulp | |||
| TPC | 2.52 ± 0.07 mg GAE/g | exocarp | UAE | [54] |
| 1.74 ± 0.02 mg GAE/g | mesocarp | |||
| TFC | 2.20 ± 0.26 mg QE/g | exocarp | ||
| 1.50 ± 0.06 mg QE/g | mesocarp | |||
| ABTS | 55.8 ± 5.4% RSA | exocarp | ||
| 52.0 ± 0.4% RSA | mesocarp | |||
| 54.1 ± 0.2% RSA | EO | Hydro-distillation | ||
| 3.1 ± 0.2% RSA | Hy | |||
| DPPH | 55.7 ± 1.20% RSA | exocarp | UAE | |
| 46.7 ± 0.82% RSA | mesocarp | |||
| 26.4 ± 0.74% RSA | EO | Hydro-distillation | ||
| 2.5 ± 0.3% RSA | Hy | |||
| DPPH | 77.2% RSA | EO | Hydro-distillation | [46] |
| TPC | 2.74 ± 1.12 mg GAE/g | fructus | UAE | [35] |
| TFC | 2.41 ± 2.03 mg QUE/g | fructus | ||
| DPPH | 1.48 ± 1.82 TE mM/g | fructus | ||
| ABTS | 0.92 ± 2.08 TE mM/g | fructus | ||
| FRAP | 0.38 ± 1.98 FeII mM/g | fructus | ||
| TPC | 31.60 ± 0.35 mg GAE/g | fructus | Maceration and UAE | [15] |
| TFC | 15.38 ± 0.02 mg RE/g | |||
| DPPH | EC50 78.00% μg/mL | |||
| DPPH | 47.45% (3.2 mg/mL) | fructus | Maceration in 95% ethanol and 0.3 mol/L of NaOH solution overnight | [61] |
| ABTS | 49.58% (3.2 mg/mL) | |||
| DPPH | 90.24% at 1.0 mg/mL | fructus | CPE | [41] |
| ORAC | 928.64 µmol TE/g | |||
| Hydroxyl RSA | 81.5% at 0.8 mg/mL | fructus | Digestion | [45] |
| Superoxide anion radical scavenging activity | 7.7 to 73.5% at 0.05 to 0.8 mg/mL | |||
| TPC | 25.8 ± 2.8 mg GAE/g of DW | by-products | Maceration 96% EtOH | [68] |
| DPPH | 43.8 ± 0.3% | |||
| Antimicrobial, antiviral and antifungal activity | ||||
| MTT | 95% inhibition at 0.5 µg/µL on Madin Darby canine kidney (MDCK) cell line with Avian influenza A virus (H5N1 | EO | Hydro-distillation | [69] |
| Agar diffusion assay | MIC: C. albicans 3 µg/mL, B. subtilis 25 µg/mL, K. pneumonia 25 µg/mL | fructus | Hydro-distillation | [41] |
| Inibition zone (mm): B. subtilis 13, B. cereus 21, S. aureus 12, K. pneumonia 15, C. albicans 27, A. niger 11 | leaves | |||
| Plaque reduction assay | 50% at 0.504 µg/µl | fructus | ||
| 95% at 0.5 µg/µl | leaves | |||
| Plate count analysis | Saccharomyces cerevisiae: 3 min at 500 ppm | fructus | Hydro-distillation | [44] |
| Plate count analysis | Bacteria survival: E. coli (600 ppm) 1 log decrease at day 3, S. Enteritidis (600 ppm) 3 log decrease at day 3, L. monocytogenes (600 ppm) 4 log decrease at day 3 | fructus | Hydro-distillation | [70] |
| Disc diffusion test | Inibition zone (mm): mold growth on bread from 8.54 ± 1.27 mm to 15.26 ± 2.16 mm | flower and fructus | Hydro-distillation | [71] |
| Inibition zone (mm): mold growth on bread > 90 mm | leaves | Hydro-distillation | ||
| Agar diffusion assay | MIC (µL/mL): Lactobacillus curvatus, Weissella viridescens, Leuconostoc mesenteroides, Enterococcus faecium, Lactobacillus reuteri, Lactobacillus dextrinicus, Lactobacillus sakei, and Pediococcus dextrinicus from 7.33 ± 0.57 to 9.00 ± 0.00 | fructus | Hydro-distillation | [72] |
| Agar diffusion assay | MIC (mg/mL): Gram-positive from 0.625 to 1.25; Gram-negative bacteria 2.5 | fructus | Hydro-distillation | [73] |
| Plate count analysis | MIC (mg/mL): Gluconobacter cerinus, Dekkera bruxellensis, Candida zemplinina, Hanseniaspora uvarum, Pichia guilliermondii, and Zygosaccharomyces bailii from 530 to 4240 | fructus | Hydro-distillation | [74] |
| Oxford cup method | MIC (mg/mL): Fusarium oxysporum 9.38, Fusarium solani 12.05, and Cylindrocarpon destructans 8.44 | fructus | Hydro-distillation | [75] |
| Plate count analysis | Yersinia enterocolitica O9, Proteus spp., Klebsiella pneumoniae, and E. coli: not effective | aerial parts | Hydro-distillation | [76] |
| Agar diffusion assay | MIC (mg/L): Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, Listeria monocytogenes, Salmonella Enteritidis, Salmonella Typhimurium, Pseudomonas fragi, Saccharomyces cerevisiae, and Aspergillus niger < 2000 | fructus | Hydro-distillation | [77] |
| Agar diffusion assay | MIC (v/v%): Escherichia coli, Pseudomonas Aeruginosa, Salmonella paratyphi B, Listeria monocytogenes, Staphylococcus aureus, Bacillus subtilis, Candida albicans, and Aspergillus flavus from 1 and 4% v/v | peel | Hydro-distillation | [46] |
| Biofilm formation | Inhibition of biofilm formation (%): Staphylococcus aureus 100% at 0.75 mg/mL | fructus | Ultrasonic/microwave assisted hydro-distillation | [78] |
| Inhibition of biofilm formation (%): Staphylococcus aureus 83l% at 0.75 mg/mL | Maceration | |||
| Agar diffusion assay | Gold fingered citron MIC (mg/mL): Bacillus subtilis, Streptococcus pneumoniae, Enterococcus faecalis, Escherichia coli, and Staphylococcus aureus from 0.00 to 10.82 ± 02 | fructus | ultrasonic | [79] |
| Cantonese fingered citron MIC (mg/mL): Bacillus subtilis, Streptococcus pneumoniae, Enterococcus faecalis, Escherichia coli, and Staphylococcus aureus from 0.00 to 9.81 ± 0.20 | ||||
| Sichuan fingered citron MIC (mg/mL): Bacillus subtilis, Streptococcus pneumoniae, Enterococcus faecalis, Escherichia coli, and Staphylococcus aureus from 0.00 to 10.83 ± 0.24 | ||||
| Agar diffusion assay | Sulphur nanoparticles MIC (µg/mL): Listeria monocytogenes, Salmonella typhi, Chromobacterium violaceum, Fusarium oxysporum, and Aspergillus flavus from 250 ± 1.21 to 700 ± 1.88 | leaves | Hydro-distillation | [80] |
| Aluminium oxide nanoparticles MIC (µg/mL): Listeria monocytogenes, Salmonella typhi, Chromobacterium violaceum, Fusarium oxysporum, and Aspergillus flavus from 150 ± 2.77 to 1000 ± 1.1 | ||||
| Tetrazolium microplate Assay | Nanoemulsions MIC (μL/mL): Escherichia coli, Bacillus subtilis, and Staphylococcus aureus from 0.16 to >2.5 | commercial EO | Commercial EO | [81] |
| Mycelial growth assay | Nanoemulsions mycelial growth inhibition (%): Penicillium citrinum and Aspergillus niger from 3.6 ± 0.6 to 27.0 ± 1.1 | |||
| Agar diffusion assay | ZnO nanoparticles inhibition zone (mm): Streptomyces sannanesis, Bacillus subtilis, Pseudomonas aeruginosa, Salmonella enterica, Candida albicans, and Aspergillus niger from 22 to 25 | peel | Maceration | [82] |
| Agar diffusion assay | Ethyl acetate and ethanolic extract MIC (mg/mL): Staphylococcus auricularis not active, Streptococcus mitis not active, Streptococcus pneumoniae not active, Klebsiella pneumoniae, and Escherichia coli from 12.5 to 25. | peel | Reflex extraction | [83] |
| MIC (mg/mL): Staphylococcus auricularis, Streptococcus mitis, Streptococcus pneumoniae, and Klebsiella pneumoniae from 1.5625 to 6.25; Escherichia coli not active | juice | Hand squeezing | ||
| Agar diffusion assay | Zone of inhibition (mm): Bacillus subtilis, Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Proteus vulgaris, Aspergillus flavus, A. niger, and Candida albicans from 0 to 23 | root, leaf, bark, peel, pulp | Maceration | [84] |
| juice | Hand squeezing | |||
| Agar diffusion assay | Bacillus subtilis, Staphylococcus aureus, and Escherichia coli; Klebsiella pneumonia not active | juice | Hand squeezing | [85] |
| Pour plate | (At 2.0 mg/mL) MIC: P. aeruginosa 82.8%, S. aureus 100% | fructus | Maceration | [21] |
| Microtiter-plate | E. coli, L. monocytegenes, P. carotovorum, Ps. aeruginosa, and S. aureus MIC (mg/mL): 7–10 | peel | Maceration | [34] |
| pulp | ||||
| Adherence and invasion in HeLA cells | Adherence (50.8 to 91%), invasion (85.1 to 94.8%) in C. jejuni strain | by-products | Maceration | [68] |
| Motility | Inhibition C. jejuni: 35–50% | peles, seeds, bagasse | Maceration | [86] |
| Biofilm formation | Inhibition C. jejuni: 60–75%, | |||
| Disk diffusion | E. coli, K. pneumoniae, P. aeruginosa, Propionibacterium acnes, Salmonella typhi, and fungi Fusarium culmorum, F. oxysporum, and F. graminearum; zone of inhibition:10–30 mm | fructus | Squeezing | [87] |
| Crystal violet staining | MIC = 1.25% (v/v) | fructus | Carbon-quantum-dots synthesis | [88] |
| Cytotoxic activity | ||||
| MTT | Growth inhibition 56.5 ± 3.6% 50 µg/mL | peel | Hydro-distillation | [89] |
| Growth inhibition 30.2 ± 2.2% and 73.3 ± 2.6% cell death at 25 and 50 μg/mL | [90] | |||
| EC50 1.24 ± 0.42 EO EC50 2.97 ± 0.07 Hy | fructus | [54] | ||
| EC50 1.76 ± 0.32 | exocarp | Maceration and UAE | [54] | |
| 50% growth inhibition at 7.80 and 9.50 μmol/L | peel | Semisynthesis | [91] | |
| IC50 from 60.5 to 80.0 μM | bark | Maceration | [37] | |
| Anti-inflammatory and analgesic activity | ||||
| NO | 56% after 12 h, 83% after 24 h at 0.063 mg/mL | fructus | Hydro-distillation | [77] |
| IC50 17.0 mg/mL | peel | [92] | ||
| 10.87–82.77% at 500 mg (60–300 min) | [24] | |||
| 250–500 mg at 30–120 min | ||||
| Clinical study | > than placebo in reduction in headache-attack intensity | juice | Syrup | [10] |
| Hypoglycemic activity | ||||
| α-amylase | IC50 625 ± 8.53 µg/mL | peel | Maceration | [50] |
| >1000 IC50 µg/mL | flowers | [27] | ||
| 438.5 ± 5.2 IC50 µg/mL | leaves | |||
| 702.2 ± 5.7 IC50 µg/mL | immature mesocarp | |||
| 702.2 ± 5.7 IC50 µg/mL | immature endocarp | |||
| 707.4 ± 5.6 IC50 µg/mL | mature mesocarp | |||
| 426.0 ± 4.4 IC50 µg/mL | mature endocarp | |||
| α -glucosidase | >1000 IC50 µg/mL | flowers | ||
| 777.8 ± 5.4 IC50 µg/mL | leaves | |||
| 539.7 ± 6.4 IC50 µg/mL | immature mesocarp | |||
| 472.9 ± 4.7 IC50 µg/mL | immature endocarp | |||
| 633.1 ± 3.4 IC50 µg/mL | mature mesocarp | |||
| 574.1 ± 5.8 IC50 µg/mL | mature endocarp | |||
| Plasma glucose level | Glucose (mg/dL): from 213 (60 min) to 155 (120 min) | epicarp | Hydro-distillation | [53] |
| Glucose (mg/dL): from 228 (60 min) to 216(120 min) | pulp | |||
| From glucose level (mg/dL) 106.8 ± 5.87 to 105.2 ± 8.35 (after 1 month) at 200 mg/kg/day; from glucose level (mg/dL) 109.3 ± 5.04 to 87.4 ± 6.30 (after 1 month) at 400 mg/kg/day | leaves | Maceration | [38] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benedetto, N.; Carlucci, V.; Faraone, I.; Lela, L.; Ponticelli, M.; Russo, D.; Mangieri, C.; Tzvetkov, N.T.; Milella, L. An Insight into Citrus medica Linn.: A Systematic Review on Phytochemical Profile and Biological Activities. Plants 2023, 12, 2267. https://doi.org/10.3390/plants12122267
Benedetto N, Carlucci V, Faraone I, Lela L, Ponticelli M, Russo D, Mangieri C, Tzvetkov NT, Milella L. An Insight into Citrus medica Linn.: A Systematic Review on Phytochemical Profile and Biological Activities. Plants. 2023; 12(12):2267. https://doi.org/10.3390/plants12122267
Chicago/Turabian StyleBenedetto, Nadia, Vittorio Carlucci, Immacolata Faraone, Ludovica Lela, Maria Ponticelli, Daniela Russo, Claudia Mangieri, Nikolay T. Tzvetkov, and Luigi Milella. 2023. "An Insight into Citrus medica Linn.: A Systematic Review on Phytochemical Profile and Biological Activities" Plants 12, no. 12: 2267. https://doi.org/10.3390/plants12122267





