Chamaecyparis lawsoniana Leaf Essential Oil as a Potential Anticancer Agent: Experimental and Computational Studies
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of CLLEO
2.2. Antioxidant Activity
2.3. In Vitro Cytotoxic Potential and Selectivity of CLLEO
2.4. In Silico Molecular Docking Study
2.4.1. Docking with Epidermal Growth Factor Receptor (EGFR)
2.4.2. Docking with Induced Myeloid Leukemia Cell Differentiation (Mcl-1)
2.4.3. Docking with Caspase-8
3. Discussion
4. Material and Methods
4.1. Chemicals
4.2. Plant Material
4.3. Essential Oil Extraction
4.4. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
4.5. Antioxidant Activity Assays
4.6. In Vitro Anticancer Assay
4.6.1. Cell Line Propagation
4.6.2. Cytotoxicity Evaluation Using Viability Assay
4.6.3. Statistical Analysis
4.6.4. Calculation of Selectivity Index (SI)
4.7. In Silico Molecular Docking Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maiko, I.; Shinichiro, H.; Lyle, E.C. Role of Medicinal and Aromatic Plants: Past, Present, and Future. In Pharmacognosy; Shagufta, P., Areej, A.-T., Eds.; IntechOpen: Rijeka, Croatia, 2019; Chapter 2. [Google Scholar]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Lang, G.; Buchbauer, G. A review on recent research results (2008–2010) on essential oils as antimicrobials and antifungals. A review. Flavour Fragr. J. 2012, 27, 13–39. [Google Scholar] [CrossRef]
- Camele, I.; Gruľová, D.; Elshafie, H.S. Chemical composition and antimicrobial properties of Mentha × piperita cv.‘Kristinka’essential oil. Plants 2021, 10, 1567. [Google Scholar] [CrossRef] [PubMed]
- Abd-ElGawad, A.M.; Bonanomi, G.; Al-Rashed, S.A.; Elshamy, A.I. Persicaria lapathifolia essential oil: Chemical constituents, antioxidant activity, and allelopathic effect on the weed Echinochloa colona. Plants 2021, 10, 1798. [Google Scholar] [CrossRef]
- Mohamed, M.E.; Mohafez, O.M.; Khalil, H.E.; Alhaider, I.A. Essential oil from myrtle leaves growing in the eastern part of Saudi Arabia: Components, anti-inflammatory and cytotoxic activities. J. Essent. Oil Bear. Plants 2019, 22, 877–892. [Google Scholar] [CrossRef]
- Bharti, K.; Sharma, M.; Vyas, G.K.; Sharma, S. A Review on Phytochemical Pharmacological and Biological Activities of Thuja Occidentalis. Asian J. Pharm. Res. Dev. 2022, 10, 111–115. [Google Scholar] [CrossRef]
- Mazari, K.; Bendimerad, N.; Bekhechi, C.; Fernandez, X. Chemical composition and antimicrobial activity of essential oils isolated from Algerian Juniperus phoenicea L. and Cupressus sempervirens L. J. Med. Plants Res. 2010, 4, 959–964. [Google Scholar]
- Ben Nouri, A.; Dhifi, W.; Bellili, S.; Ghazghazi, H.; Aouadhi, C.; Chérif, A.; Hammami, M.; Mnif, W. Chemical composition, antioxidant potential, and antibacterial activity of essential oil cones of Tunisian Cupressus sempervirens. J. Chem. 2015, 2015, 538929. [Google Scholar] [CrossRef] [Green Version]
- Galovičová, L.; Čmiková, N.; Schwarzová, M.; Vukic, M.D.; Vukovic, N.L.; Kowalczewski, P.Ł.; Bakay, L.; Kluz, M.I.; Puchalski, C.; Obradovic, A.D. Biological Activity of Cupressus sempervirens Essential Oil. Plants 2023, 12, 1097. [Google Scholar] [CrossRef]
- Boufares, K.; Hassani, A.; Alem, A.S. Essential oil composition and antimicrobial activities of some Cupressaceae species from Algeria against two phytopathogenic microorganisms. J. Crop. Prot. 2019, 8, 223–234. [Google Scholar]
- Bajes, H.R.; Oran, S.A.; Bustanji, Y.K. Chemical composition and antiproliferative and antioxidant activities of essential oil from Juniperus phoenicea L. cupressaceae. Res. J. Pharm. Technol. 2022, 15, 153–159. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Lee, S.-G.; Oh, T.-J.; Lim, S.R.; Kim, S.-H.; Lee, H.J.; Kim, Y.-S.; Choi, H.-K. Antiproliferative and apoptotic activity of Chamaecyparis obtusa leaf extract against the HCT116 human colorectal cancer cell line and investigation of the bioactive compound by gas chromatography-mass spectrometry-based metabolomics. Molecules 2015, 20, 18066–18082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.-M.; Xu, J.; Xiao, L.; Zeng, G.-Z.; Sun, Z.-H.; Tan, N.-H. A New Phenolic Glycoside from Chamaecyparis obtusa var. breviramea f. crippsii. Molecules 2013, 18, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Khanavi, M.; Enayati, A.; Shams Ardekani, M.; Akbarzadeh, T.; Karimpour Razkenari, E.; Eftekhari, M. Cytotoxic activity of Juniperus excelsa M. Bieb. Leaves Essent. Oil Breast Cancer Cell Lines. Res. J. Pharm. 2019, 6, 1–7. [Google Scholar]
- Jlizi, S.; Lahmar, A.; Zardi-Bergaoui, A.; Ascrizzi, R.; Flamini, G.; Harrath, A.H.; Chekir-Ghedira, L.; Ben Jannet, H. Chemical composition and cytotoxic activity of the fractionated trunk bark essential oil from Tetraclinis articulata (Vahl) Mast. growing in Tunisia. Molecules 2021, 26, 1110. [Google Scholar] [CrossRef]
- Elsharkawy, E.; Aljohar, H.; Donia, A. Comparative study of antioxidant and anticancer activity of Thuja orientalis growing in Egypt and Saudi Arabia. Br. J. Pharm. Res. 2017, 15, 1–9. [Google Scholar] [CrossRef]
- Elshafie, H.; Caputo, L.; De Martino, L.; Gruľová, D.; Zheljazkov, V.; De Feo, V.; Camele, I. Biological investigations of essential oils extracted from three Juniperus species and evaluation of their antimicrobial, antioxidant and cytotoxic activities. J. Appl. Microbiol. 2020, 129, 1261–1271. [Google Scholar] [CrossRef]
- Fayed, S.A. Chemical composition, antioxidant, anticancer properties and toxicity evaluation of leaf essential oil of Cupressus sempervirens. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 320–326. [Google Scholar] [CrossRef] [Green Version]
- Rathi, A.; Baburaj, D.; Sundaram, E.; Kumar, S.; Khurana, A.; Manchanda, R. Pharmacognostic study of Chamaecyparis lawsoniana (Murr.) Parl.: A drug used in Homoeopathy. Indian J. Res. Homoeopath. 2015, 9, 20–24. [Google Scholar] [CrossRef]
- Palá-Paúl, J.; Usano-Alemany, J.; Granda, E.; Soria, A.-C. Antifungal and antibacterial activity of the essential oil of Chamaecyparis lawsoniana from Spain. Nat. Prod. Commun. 2012, 7, 1934578X1200701036. [Google Scholar] [CrossRef] [Green Version]
- De Pooter, H.; Vermeesch, J.; De Buyck, L.; Huang, Q.-L.; Schamp, N.; De Bruyn, A. Volatile Fractions of Chamaecyparis lawsoniana (A. Murray) Parl, and Chamaecyparis pisifera (Sieb. et Zucc.) Endl. var. filifera. Identification of Oplopanonyl Acetate. J. Essent. Oil Res. 1991, 3, 1–6. [Google Scholar] [CrossRef]
- Emami, S.A.; Massoomi, H.; Moghadam, M.S.; Asili, J. Identification of volatile oil components from aerial parts of Chamaecyparis lawsoniana by GC-MS and 13C-NMR methods. J. Essent. Oil Bear. Plants 2009, 12, 661–665. [Google Scholar] [CrossRef]
- Giatropoulos, A.; Pitarokili, D.; Papaioannou, F.; Papachristos, D.P.; Koliopoulos, G.; Emmanouel, N.; Tzakou, O.; Michaelakis, A. Essential oil composition, adult repellency and larvicidal activity of eight Cupressaceae species from Greece against Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 2013, 112, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, B.M.; Milanović, S.D.; Milenković, I.L.; Todosijević, M.M.; Đorđević, I.Ž.; Brkić, M.Z.; Mitić, Z.S.; Marin, P.D.; Tešević, V.V. Bioactivity of Chamaecyparis lawsoniana (A. Murray) Parl. and Thuja plicata Donn ex D. Don essential oils on Lymantria dispar (Linnaeus, 1758) (Lepidoptera: Erebidae) larvae and Phytophthora de Bary 1876 root pathogens. Ind. Crop. Prod. 2022, 178, 114550. [Google Scholar] [CrossRef]
- Smith, E.C.; Williamson, E.M.; Wareham, N.; Kaatz, G.W.; Gibbons, S. Antibacterials and modulators of bacterial resistance from the immature cones of Chamaecyparis lawsoniana. Phytochemistry 2007, 68, 210–217. [Google Scholar] [CrossRef]
- Zazharskyi, V.; Davydenko, P.; Kulishenko, O.; Borovik, I.; Kabar, A.; Brygadyrenko, V. Antibacterial and fungicidal effect of ethanol extracts from Juniperus sabina, Chamaecyparis lawsoniana, Pseudotsuga menziesii and Cephalotaxus harringtonia. Regul. Mech. Biosyst. 2020, 11, 105–109. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Fernald, K.; Kurokawa, M. Evading apoptosis in cancer. Trends Cell Biol. 2013, 23, 620–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, B.; Singh, S.; Skvortsova, I.; Kumar, V. Promising targets in anti-cancer drug development: Recent updates. Curr. Med. Chem. 2017, 24, 4729–4752. [Google Scholar] [CrossRef]
- Abdullaev, F. Pharmacology and therapeutics in the new millennium. In New Delhi: Narosa Puplising House. Plant-Derived Agents against Cancer; Springer: Berlin/Heidelberg, Germany, 2001; pp. 345–354. [Google Scholar]
- Sarker, S.D.; Nahar, L.; Miron, A.; Guo, M. Anticancer natural products. In Annual Reports in Medicinal Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; Volume 55, pp. 45–75. [Google Scholar]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. IJBS 2008, 4, 89. [Google Scholar]
- Mani, S. Production of reactive oxygen species and its implication in human diseases. In Free Radicals in Human Health and Disease; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–15. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 5th ed.; Texensis Publishing: Gruver, TX, USA, 2017. [Google Scholar]
- Kasali, A.A.; Ekundayo, O.; Paul, C.; König, W.A. epi-Cubebanes from Solidago canadensis. Phytochemistry 2002, 59, 805–810. [Google Scholar] [CrossRef]
- Sacchetti, G.; Medici, A.; Maietti, S.; Radice, M.; Muzzoli, M.; Manfredini, S.; Braccioli, E.; Bruni, R. Composition and functional properties of the essential oil of Amazonian basil, Ocimum micranthum Willd., Labiatae in comparison with commercial essential oils. J. Agric. Food Chem. 2004, 52, 3486–3491. [Google Scholar] [CrossRef]
- Asuming, W.A.; Beauchamp, P.S.; Descalzo, J.T.; Dev, B.C.; Dev, V.; Frost, S.; Ma, C.W. Essential oil composition of four Lomatium Raf. species and their chemotaxonomy. Biochem. Syst. Ecol. 2005, 33, 17–26. [Google Scholar] [CrossRef]
- Yaşar, A.; Üçüncü, O.; Güleç, C.; İnceer, H.; Ayaz, S.; Yayl, N. GC-MS Analysis of Chloroform Extracts in Flowers, Stems, and Roots of Tripleurospermum callosum. Pharm. Biol. 2005, 43, 108–112. [Google Scholar] [CrossRef] [Green Version]
- Gooré, S.G.; Ouattara, Z.A.; Yapi, A.T.; Békro, Y.-A.; Bighelli, A.; Paoli, M.; Tomi, F. Chemical composition of the leaf oil of Artabotrys jollyanus from Côte d’Ivoire. Rev. Bras. Farmacogn. 2017, 27, 414–418. [Google Scholar] [CrossRef]
- Duquesnoy, E.; Marongiu, B.; Castola, V.; Piras, A.; Porcedda, S.; Casanova, J. Combined analysis by GC (RI), GC-MS and 13C NMR of the supercritical fluid extract of Abies alba twigs. Nat. Prod. Commun. 2010, 5, 1934578X1000501235. [Google Scholar] [CrossRef] [Green Version]
- Isidorov, V.A.; Lech, P.; Żółciak, A.; Rusak, M.; Szczepaniak, L. Gas chromatographic–mass spectrometric investigation of metabolites from the needles and roots of pine seedlings at early stages of pathogenic fungi Armillaria ostoyae attack. Trees 2008, 22, 531–542. [Google Scholar] [CrossRef]
- Stashenko, E.E.; Cervantes, M.; Combariza, Y.; Fuentes, H.; Martínez, J.R. HRGC/FID and HRGC/MSD analysis of the secondary metabolites obtained by different extraction methods from Lepechinia schiedeana, and in vitro evaluation of its antioxidant activity. J. High Resolut. Chromatogr. 1999, 22, 343–349. [Google Scholar] [CrossRef]
- Veličković, D.T.; Ranđelović, N.V.; Ristić, M.S.; Veličković, A.S.; Šmelcerović, A.A. Chemical constituents and antimicrobial activity of the ethanol extracts obtained from the flower, leaf and stem of Salvia officinalis L. J. Serb. Chem. Soc. 2003, 68, 17–24. [Google Scholar] [CrossRef]
- Stamos, J.; Sliwkowski, M.X.; Eigenbrot, C. Structure of the epidermal growth factor receptor kinase domain alone and in complex with a 4-anilinoquinazoline inhibitor. J. Biol. Chem. 2002, 277, 46265–46272. [Google Scholar] [CrossRef] [Green Version]
- Czabotar, P.E.; Lee, E.F.; van Delft, M.F.; Day, C.L.; Smith, B.J.; Huang, D.C.; Fairlie, W.D.; Hinds, M.G.; Colman, P.M. Structural insights into the degradation of Mcl-1 induced by BH3 domains. Proc. Natl. Acad. Sci. USA 2007, 104, 6217–6222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanchard, H.; Donepudi, M.; Tschopp, M.; Kodandapani, L.; Wu, J.C.; Grütter, M.G. Caspase-8 specificity probed at subsite S4: Crystal structure of the caspase-8-Z-DEVD-cho complex. J. Mol. Biol. 2000, 302, 9–16. [Google Scholar] [CrossRef]
- Karawya, M.S. Volatile oil of Chamaecyparis lawsoniana Parl. Egypt. J. Pharm. Sci. 1986, 27, 341–346. [Google Scholar]
- Barbosa, L.C.A.; Filomeno, C.A.; Teixeira, R.R. Chemical variability and biological activities of Eucalyptus spp. essential oils. Molecules 2016, 21, 1671. [Google Scholar] [CrossRef] [Green Version]
- Yoewono, J.R.; Purwanto, R.O.; Woolley, C.L.; Chang, C.; Shih, W. Antioxidant activities and oral toxicity studies of Chamaecyparis formosensis and Cymbopogon nardus essential oils. Int. J. Adv. Sci. Res. Manag 2016, 1, 47–58. [Google Scholar]
- Eltayeb, L.M.; Yagi, S.; Mohamed, H.M.; Zengin, G.; Shariati, M.A.; Rebezov, M.; Uba, A.I.; Lorenzo, J.M. Essential Oils Composition and Biological Activity of Chamaecyparis obtusa, Chrysopogon nigritanus and Lavandula coronopifolia Grown Wild in Sudan. Molecules 2023, 28, 1005. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Hong, C.; Gwak, K.; Yeo, H.; Lee, J.; Choi, I. Whitening and antioxidant activities of essential oils from Cryptomeria japonica and Chamaecyparis obtusa. Mokchae Konghak = J. Korean Wood Sci. Technol. 2011, 39, 291–302. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Shupe, T.F.; Hse, C.Y.; Eberhardt, T.L. Antioxidant activity of extracts from the bark of Chamaecyparis lawsoniana (A. Murray) Parl. De Gruyter 2006, 60, 459–462. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-González, C.; Pérez-Ramos, J.; Méndez-Cuesta, C.A.; Serrano-Vega, R.; Martell-Mendoza, M.; Pérez-Gutiérrez, S. Cytotoxic activity of essential oils of some species from Lamiaceae family. In Cytotoxicity: Definition, Identification, and Cytotoxic Compounds; Istifli, E.S., Ila, H.B., Eds.; IntechOpen: Rijeka, Croatia, 2019; pp. 29–43. [Google Scholar]
- Capello, T.M.; Martins, E.G.; de Farias, C.F.; Figueiredo, C.R.; Matsuo, A.L.; Passero, L.F.D.; Oliveira-Silva, D.; Sartorelli, P.; Lago, J.H.G. Chemical composition and in vitro cytotoxic and antileishmanial activities of extract and essential oil from leaves of Piper cernuum. Nat. Prod. Commun. 2015, 10, 1934578X1501000217. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zeng, G.-Z.; Liu, Y.-M.; Chen, K.-L.; Sun, Z.-H.; Zhang, Y.-M.; Tan, N.-H. Sesquiterpenoids and diterpenes from Chamaecyparis obtusa var. breviramea f. crippsii. Z. Nat. B 2014, 69, 362–368. [Google Scholar] [CrossRef]
- Wee, P.; Wang, Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers 2017, 9, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ongko, J.; Setiawan, J.V.; Feronytha, A.G.; Juliana, A.; Effraim, A.; Wahjudi, M.; Antonius, Y. In-silico screening of inhibitor on protein epidermal growth factor receptor (EGFR). In Proceedings of the IOP Conference Series: Earth and Environmental Science, Qingdao, China, 16–18 July 2022; p. 012075. [Google Scholar]
- Mady, A. Development and Characterization of Novel Mcl-1 Inhibitors for Treatment of Cancer. Ph.D. Thesis, University of Michigan, Ann Arbor, MI, USA, 2016. [Google Scholar]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [Green Version]
- Negi, A.; Murphy, P.V. Development of Mcl-1 inhibitors for cancer therapy. Eur. J. Med. Chem. 2021, 210, 113038. [Google Scholar] [CrossRef]
- Wei, S.-H.; Dong, K.; Lin, F.; Wang, X.; Li, B.; Shen, J.-j.; Zhang, Q.; Wang, R.; Zhang, H.-Z. Inducing apoptosis and enhancing chemosensitivity to gemcitabine via RNA interference targeting Mcl-1 gene in pancreatic carcinoma cell. Cancer Chemother. Pharmacol. 2008, 62, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Guoan, X.; Hanning, W.; Kaiyun, C.; Hao, L. Adenovirus-mediated siRNA targeting Mcl-1 gene increases radiosensitivity of pancreatic carcinoma cells in vitro and in vivo. Surgery 2010, 147, 553–561. [Google Scholar] [CrossRef]
- Mnich, K.; Koryga, I.; Pakos-Zebrucka, K.; Thomas, M.; Logue, S.E.; Eriksson, L.A.; Gorman, A.M.; Samali, A. The stressosome, a caspase-8-activating signalling complex assembled in response to cell stress in an ATG5-mediated manner. J. Cell. Mol. Med. 2021, 25, 8809–8820. [Google Scholar] [CrossRef]
- Fulda, S.; Debatin, K.-M. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006, 25, 4798–4811. [Google Scholar] [CrossRef] [Green Version]
- Elkomy, N.M.; El-Shaibany, A.; Elnagar, G.M.; Abdelkhalek, A.S.; Al-Mahbashi, H.; Elaasser, M.M.; Raweh, S.M.; Aldiyarbi, M.A.; Raslan, A.E. Evaluation of acute oral toxicity, anti-diabetic and antioxidant effects of Aloe vera flowers extract. J. Ethnopharmacol. 2023, 309, 116310. [Google Scholar] [CrossRef]
- Ling, L.T.; Yap, S.-A.; Radhakrishnan, A.K.; Subramaniam, T.; Cheng, H.M.; Palanisamy, U.D. Standardised Mangifera indica extract is an ideal antioxidant. Food Chem. 2009, 113, 1154–1159. [Google Scholar] [CrossRef]
- Elaasser, M.; Morsi, M.; Galal, S.; Abd El-Rahman, M.; Katry, M. Antioxidant, anti-inflammatory and cytotoxic activities of the unsaponifiable fraction of extra virgin olive oil. Grasas Aceites 2020, 71, e386. [Google Scholar] [CrossRef]
- Gomha, S.M.; Riyadh, S.M.; Mahmmoud, E.A.; Elaasser, M.M. Synthesis and anticancer activity of arylazothiazoles and 1,3,4-thiadiazoles using chitosan-grafted-poly(4-vinylpyridine) as a novel copolymer basic catalyst. Chem. Heterocycl. Compd. 2015, 51, 1030–1038. [Google Scholar] [CrossRef]
- Yousefbeyk, F.; Dabirian, S.; Ghanbarzadeh, S.; Eghbali Koohi, D.; Yazdizadeh, P.; Ghasemi, S. Green synthesis of silver nanoparticles from Stachys byzantina K. Koch: Characterization, antioxidant, antibacterial, and cytotoxic activity. Part. Sci. Technol. 2022, 40, 219–232. [Google Scholar] [CrossRef]
- Mansour, K.A.; Elbermawi, A.; Al-Karmalawy, A.A.; Lahloub, M.-F.; El-Neketi, M. Cytotoxic effects of extracts obtained from plants of the Oleaceae family: Bio-guided isolation and molecular docking of new secoiridoids from Jasminum humile. Pharm. Biol. 2022, 60, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Chemical Computing Group. Molecular Operating Environment; Chemical Computing Group Inc.: Montreal, QC, Canada, 2008. [Google Scholar]
- Mohamed, M.E.; Tawfeek, N.; Elbaramawi, S.S.; Elbatreek, M.H.; Fikry, E. Agathis robusta Bark Extract Protects from Renal Ischemia-Reperfusion Injury: Phytochemical, In Silico and In Vivo Studies. Pharmaceuticals 2022, 15, 1270. [Google Scholar] [CrossRef] [PubMed]
- RCSB Protein Data Bank (PDB). Available online: https://www.rcsb.org/ (accessed on 24 March 2023).




| Peak | Compound Name | Chemical Class | RIExp. a | RILit. b | Area% | Identification c |
|---|---|---|---|---|---|---|
| 1 | α-Thujene | Bicyclic monoterpene hydrocarbon | 922 | 924 | 0.13 | MS, RI |
| 2 | α-Pinene | Bicyclic monoterpene hydrocarbon | 931 | 932 | 6.20 | MS, RI |
| 3 | Sabinene | Bicyclic monoterpene hydrocarbon | 968 | 969 | 0.13 | MS, RI |
| 4 | β-Pinene | Bicyclic monoterpene hydrocarbon | 971 | 974 | 0.06 | MS, RI |
| 5 | Myrcene | Acyclic monoterpene hydrocarbon | 987 | 988 | 0.24 | MS, RI |
| 6 | α-Terpinene | Monocyclic monoterpene hydrocarbon | 1012 | 1014 | 0.15 | MS, RI |
| 7 | p-Cymene | Aromatic monoterpene hydrocarbon | 1020 | 1020 | 0.03 | MS, RI |
| 8 | Limonene | Monocyclic monoterpene hydrocarbon | 1024 | 1024 | 0.14 | MS, RI |
| 9 | γ-Terpinene | Monocyclic monoterpene hydrocarbon | 1055 | 1054 | 0.36 | MS, RI |
| 10 | Terpinolene | Monocyclic monoterpene hydrocarbon | 1084 | 1086 | 0.15 | MS, RI |
| 11 | trans-Sabinene hydrate | Monocyclic monoterpene alcohol | 1117 | 1098 | 0.02 | MS |
| 12 | Terpinen-4-ol | Monocyclic monoterpene alcohol | 1176 | 1174 | 1.20 | MS, RI |
| 13 | α-Terpineol | Monocyclic monoterpene alcohol | 1188 | 1186 | 0.21 | MS, RI |
| 14 | Bornyl acetate | Bicyclic monoterpene ester | 1282 | 1284 | 0.02 | MS, RI |
| 15 | α-Terpinyl acetate | Monocyclic monoterpene ester | 1346 | 1346 | 0.12 | MS, RI |
| 16 | α-Ionol | Monocyclic sesquiterpene alcohol | 1376 | 1376 | 0.30 | MS, RI |
| 17 | β-Elemene | Monocyclic sesquiterpene hydrocarbon | 1390 | 1389 | 0.12 | MS, RI |
| 18 | β-Caryophyllene | Bicyclic sesquiterpene hydrocarbon | 1418 | 1417 | 0.15 | MS, RI |
| 19 | 6-epi-β-Cubebene | Bicyclic sesquiterpene hydrocarbon | 1448 | 1449 | 3.16 | MS, RI |
| 20 | α-Humulene | Monocyclic sesquiterpene hydrocarbon | 1454 | 1452 | 0.14 | MS, RI |
| 21 | δ-Muurolene (cis-muurola-4(14)-diene) | Bicyclic sesquiterpene hydrocarbon | 1467 | 1467 | 8.57 | MS, RI |
| 22 | γ-Muurolene | Bicyclic sesquiterpene hydrocarbon | 1477 | 1478 | 0.09 | MS, RI |
| 23 | Germacrene D | Bicyclic sesquiterpene hydrocarbon | 1483 | 1480 | 1.32 | MS, RI |
| 24 | β-Selinene | Bicyclic sesquiterpene hydrocarbon | 1487 | 1489 | 0.03 | MS, RI |
| 25 | cis-Cadina-1,4-diene | Bicyclic sesquiterpene hydrocarbon | 1494 | 1495 | 0.17 | MS, RI |
| 26 | γ-Amorphene | Bicyclic sesquiterpene hydrocarbon | 1500 | 1495 | 4.10 | MS, RI |
| 27 | γ-Cadinene | Bicyclic sesquiterpene hydrocarbon | 1514 | 1513 | 0.08 | MS, RI |
| 28 | δ-Cadinene | Bicyclic sesquiterpene hydrocarbon | 1524 | 1522 | 3.71 | MS, RI |
| 29 | α-Cadinene | Bicyclic sesquiterpene hydrocarbon | 1537 | 1537 | 0.05 | MS, RI |
| 30 | α-Calacorene | Aromatic bicyclic sesquiterpene hydrocarbon | 1543 | 1544 | 0.03 | MS, RI |
| 31 | Elemol | Monocyclic sesquiterpene alcohol | 1550 | 1548 | 0.59 | MS, RI |
| 32 | trans-Nerolidol | Acyclic sesquiterpene alcohol | 1561 | 1561 | 0.04 | MS, RI |
| 33 | Caryophyllene oxide | Bicyclic sesquiterpene oxide | 1584 | 1582 | 0.19 | MS, RI |
| 34 | Humulene epoxide II | Monocyclic sesquiterpene epoxide | 1611 | 1608 | 0.04 | MS, RI |
| 35 | 1,10-di-epi-Cubenol | Bicyclic sesquiterpene alcohol | 1617 | 1618 | 1.52 | MS, RI |
| 36 | Junenol | Bicyclic sesquiterpene alcohol | 1621 | 1618 | 0.04 | MS, RI |
| 37 | γ-Eudesmol | Bicyclic sesquiterpene alcohol | 1634 | 1630 | 0.14 | MS, RI |
| 38 | epi-α-Cadinol (tau-cadinol) | Bicyclic sesquiterpene alcohol | 1644 | 1638 | 1.74 | MS, RI |
| 39 | Cubenol | Bicyclic sesquiterpene alcohol | 1650 | 1645 | 0.21 | MS, RI |
| 40 | β-Eudesmol | Bicyclic sesquiterpene alcohol | 1654 | 1649 | 0.22 | MS, RI |
| 41 | α-Cadinol | Bicyclic sesquiterpene alcohol | 1661 | 1660 | 8.84 | MS, RI |
| 42 | Germacra-4(15),5,10(14)-trien-1-α-ol | Monocyclic sesquiterpene alcohol | 1690 | 1685 | 0.44 | MS, RI |
| 43 | cis-14-nor-Muurol-5-en-4-one | Bicyclic sesquiterpene ketone | 1692 | 1688 | 0.15 | MS, RI |
| 44 | 6-Isopropenyl-4,8a-dimethyl-1,2,3,5,6,7,8, 8a-octahydronaphthalene-2-ol | Bicyclic sesquiterpene alcohol | 1710 | 1714 | 0.08 | MS, RI |
| 45 | 7-Hydroxycalamenene | Bicyclic sesquiterpene alcohol | 1798 | 1803 | 0.07 | MS, RI |
| 46 | Isopimara-9(11),15-diene | Tricyclic diterpene hydrocarbon | 1915 | 1905 | 0.10 | MS, RI |
| 47 | Pimaradiene | Tricyclic diterpene hydrocarbon | 1949 | 1948 | 0.04 | MS, RI |
| 48 | Sandaracopimara-8(14),15-diene (13-isopimaradiene) | Tricyclic diterpene hydrocarbon | 1969 | 1968 | 2.05 | MS, RI |
| 49 | Kaur-15-ene | Tetracyclic diterpene hydrocarbon | 1997 | 1997 | 0.43 | MS, RI |
| 50 | 13-epi-Manool oxide | Tricyclic diterpene oxide | 2019 | 2009 | 0.04 | MS, RI |
| 51 | Abietatriene | Tricyclic diterpene hydrocarbon | 2061 | 2055 | 1.89 | MS, RI |
| 52 | Abietadiene | Tricyclic diterpene hydrocarbon | 2087 | 2087 | 0.07 | MS, RI |
| 53 | cis-Abienol | Bicyclic diterpene alcohol | 2120 | 2112 | 23.43 | MS, RI |
| 54 | Sandaracopimarinal | Tricyclic diterpene aldehyde | 2195 | 2184 | 0.83 | MS |
| 55 | Pimara-7,15-dien-3-one | Tricyclic diterpene ketone | 2225 | 2227 | 1.65 | MS, RI |
| 56 | Sandaracopimarinol | Tricyclic diterpene alcohol | 2282 | 2269 | 0.73 | MS |
| 57 | trans-Totarol | Tricyclic diterpene alcohol | 2291 | 2290 | 1.27 | MS, RI |
| 58 | trans-Ferruginol | Tricyclic diterpene alcohol | 2325 | 2331 | 14.31 | MS, RI |
| 59 | cis-Ferruginol | Tricyclic diterpene alcohol | 2337 | 2340 | 0.44 | MS, RI |
| Total identified | 92.77 | |||||
| Monoterpenes hydrocarbons | 7.59 | |||||
| Oxygenated monoterpenes | 1.57 | |||||
| Sesquiterpene hydrocarbons | 21.72 | |||||
| Oxygenated sesquiterpenes | 14.61 | |||||
| Diterpene hydrocarbons | 4.58 | |||||
| Oxygenated diterpenes | 42.70 |
| IC50 ± SD (µg/mL) | |||
|---|---|---|---|
| DPPH | ABTS | FRAP | |
| CLLEO | 116.91 ± 5.73 | 73.02 ± 4.06 | 218.64 ± 8.41 |
| Ascorbic acid | 10.22 ± 0.56 | 10.66 ± 0.89 | 20.89± 1.25 |
| MRC-5 | MCF-7 | HCT-116 | A-549 | HepG-2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| CC50 | IC50 | SI | IC50 | SI | IC50 | SI | IC50 | SI | |
| CLLEO | 95.17 ± 3.71 | 23.74 ± 1.72 | 4.01 | 28.27 ± 2.13 | 3.37 | 25.79 ± 1.95 | 3.69 | 15.34 ± 0.96 | 6.20 |
| Cisplatin | 19.43 ± 3.66 | 5.69 ± 0.37 | 3.41 | 2.51 ± 0.67 | 7.74 | 7.51 ± 0.82 | 2.59 | 3.68 ± 0.24 | 5.28 |
| Component | S Score Kcal/mol | 3D Protein-Component Interactions |
|---|---|---|
| cis-Abienol | −5.5184 | 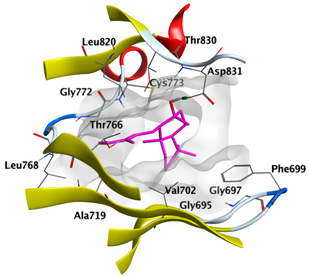 |
| trans-Ferruginol | −5.7955 | 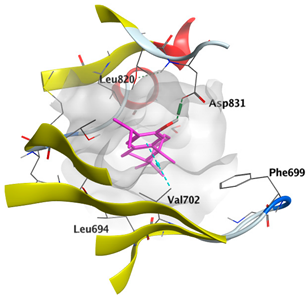 |
| α-Cadinol | −5.1630 | 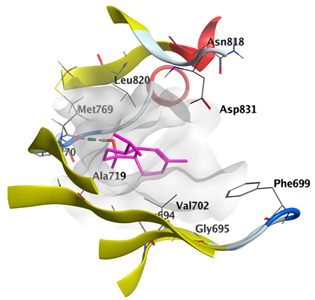 |
| δ-Muurolene | −5.7461 |  |
| α-Pinene | −4.9882 | 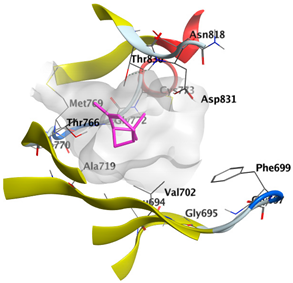 |
| Component | S Score Kcal/mol | 3D Protein-Component Interactions |
|---|---|---|
| cis-Abienol | −5.3337 | 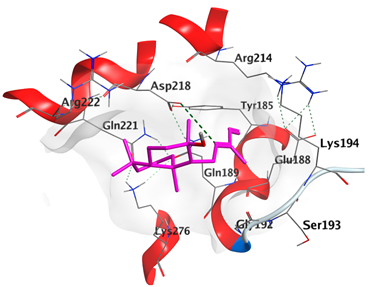 |
| trans-Ferruginol | −5.2220 | 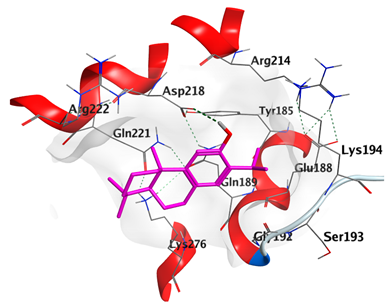 |
| α-Cadinol | −4.5189 | 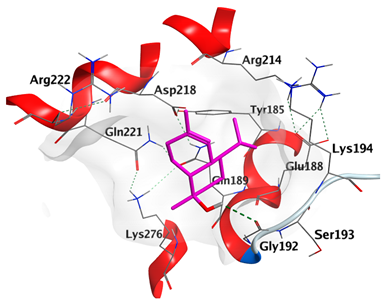 |
| δ-Muurolene | −4.4583 |  |
| α-Pinene | −4.1318 | 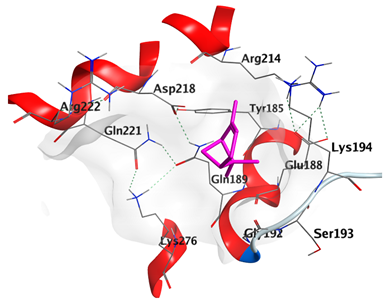 |
| Component | S Score Kcal/mol | 3D Protein-Component Interactions |
|---|---|---|
| cis-Abienol | −5.3517 | 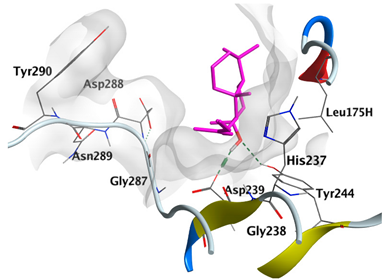 |
| trans-Ferruginol | −5.4981 | 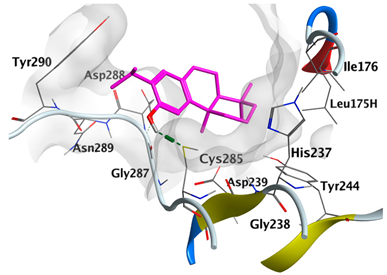 |
| α-Cadinol | −5.0823 | 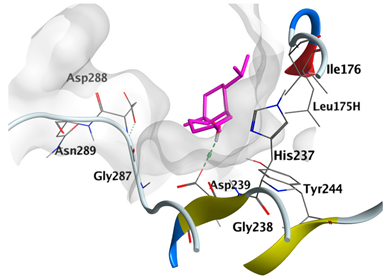 |
| δ-Muurolene | −4.7582 | 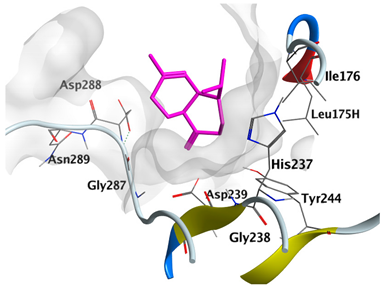 |
| α-Pinene | −4.2315 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fikry, E.; Orfali, R.; Elbaramawi, S.S.; Perveen, S.; El-Shafae, A.M.; El-Domiaty, M.M.; Tawfeek, N. Chamaecyparis lawsoniana Leaf Essential Oil as a Potential Anticancer Agent: Experimental and Computational Studies. Plants 2023, 12, 2475. https://doi.org/10.3390/plants12132475
Fikry E, Orfali R, Elbaramawi SS, Perveen S, El-Shafae AM, El-Domiaty MM, Tawfeek N. Chamaecyparis lawsoniana Leaf Essential Oil as a Potential Anticancer Agent: Experimental and Computational Studies. Plants. 2023; 12(13):2475. https://doi.org/10.3390/plants12132475
Chicago/Turabian StyleFikry, Eman, Raha Orfali, Samar S. Elbaramawi, Shagufta Perveen, Azza M. El-Shafae, Maher M. El-Domiaty, and Nora Tawfeek. 2023. "Chamaecyparis lawsoniana Leaf Essential Oil as a Potential Anticancer Agent: Experimental and Computational Studies" Plants 12, no. 13: 2475. https://doi.org/10.3390/plants12132475






