Plant Growth Regulators and Activated Charcoal Selectively Affect Phenylethanoid and Flavone Glycoside Accumulation in Sideritis scardica Griseb. Tissue Culture
Abstract
:1. Introduction
2. Results and Discussion
2.1. In Vitro Seed Germination
2.2. Effect of In Vitro Culture Treatments on the Developmental Patterns of S. scardica
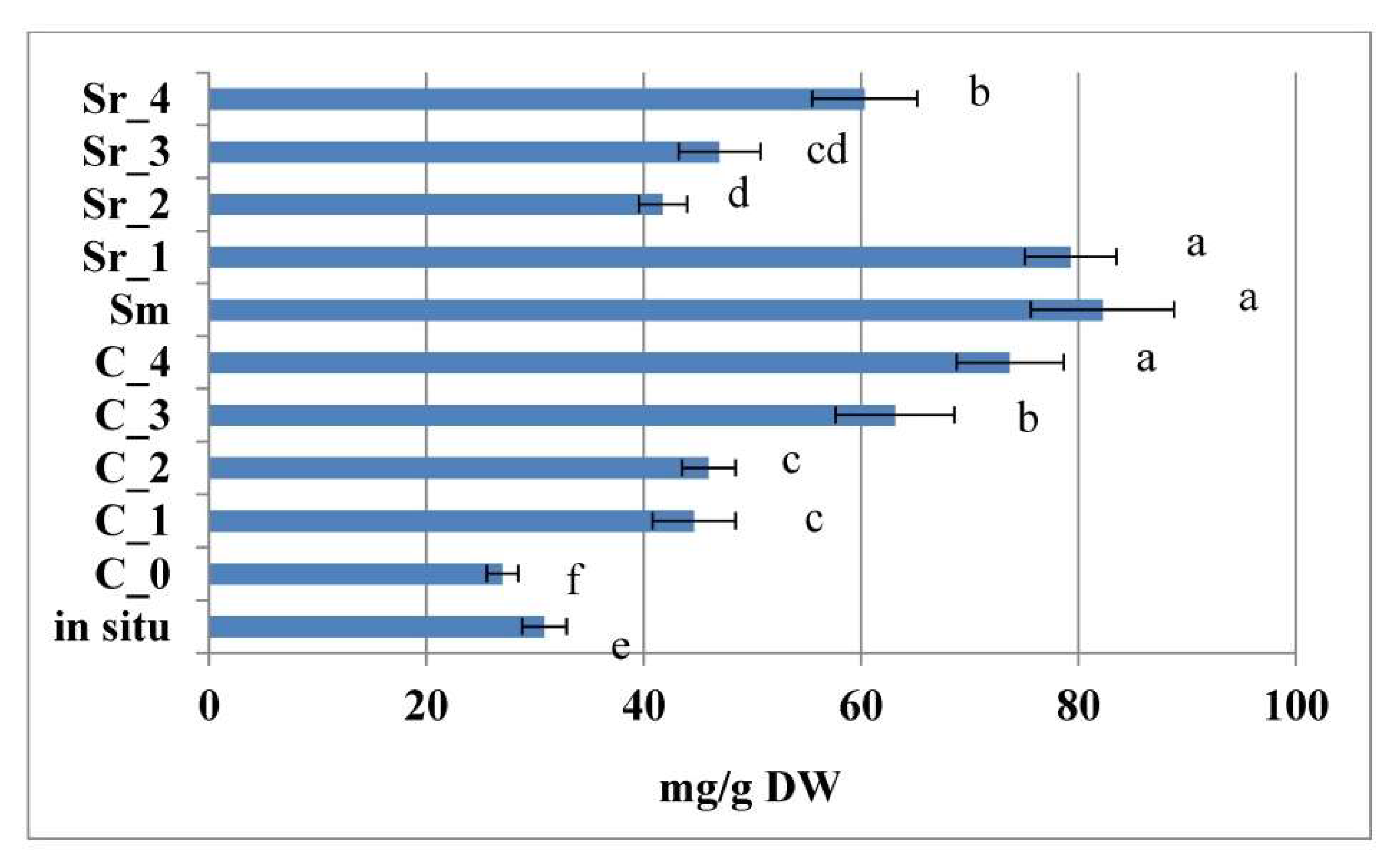
2.3. Effect of PGRs and AC on 5-Caffeoylquinic Acid Content In Vitro and In Situ
2.4. Effect of PGRs and AC on Phenylethanoid Glycosides In Vitro
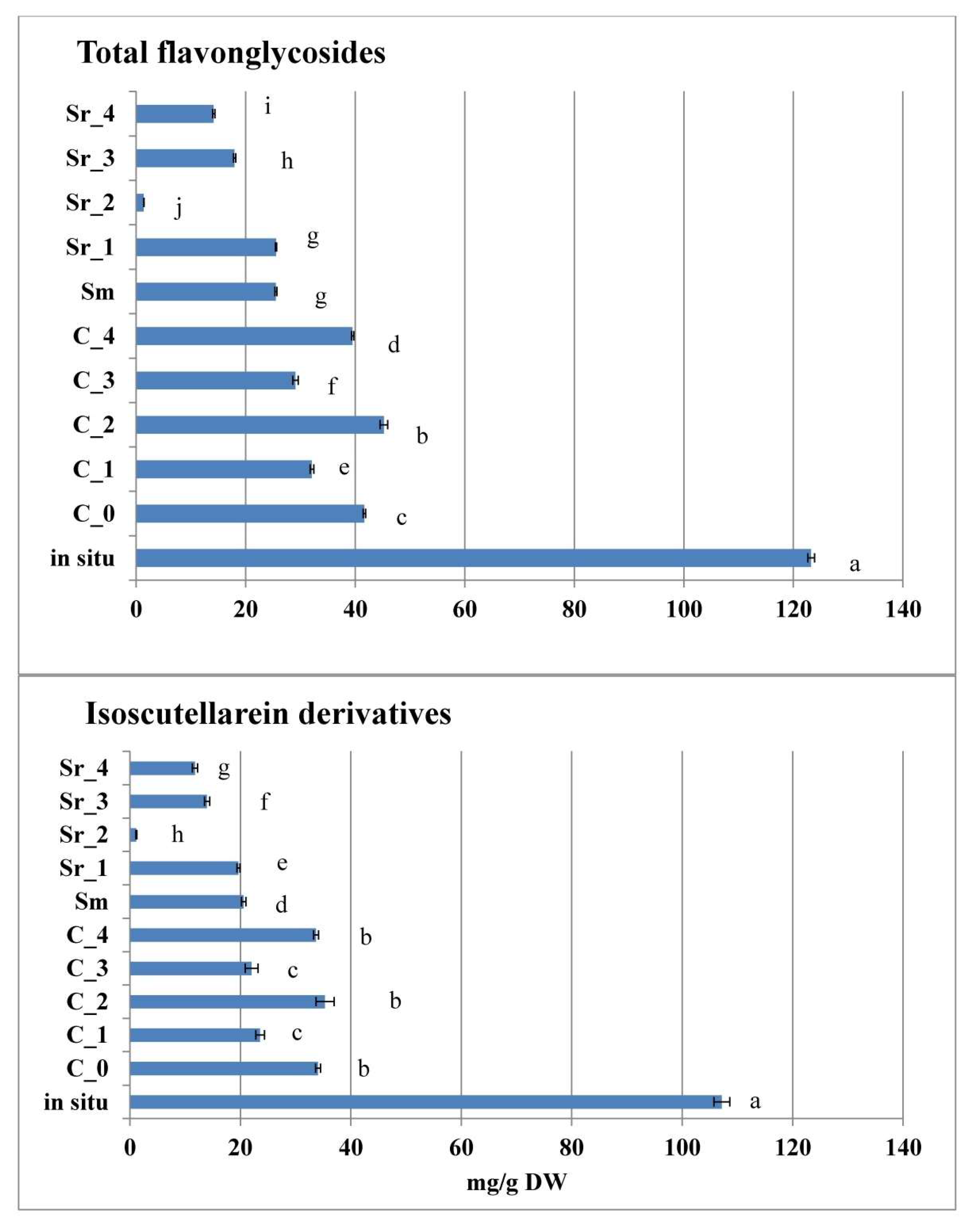
2.5. Effect of PGRs and AC on Flavone Glycosides In Vitro
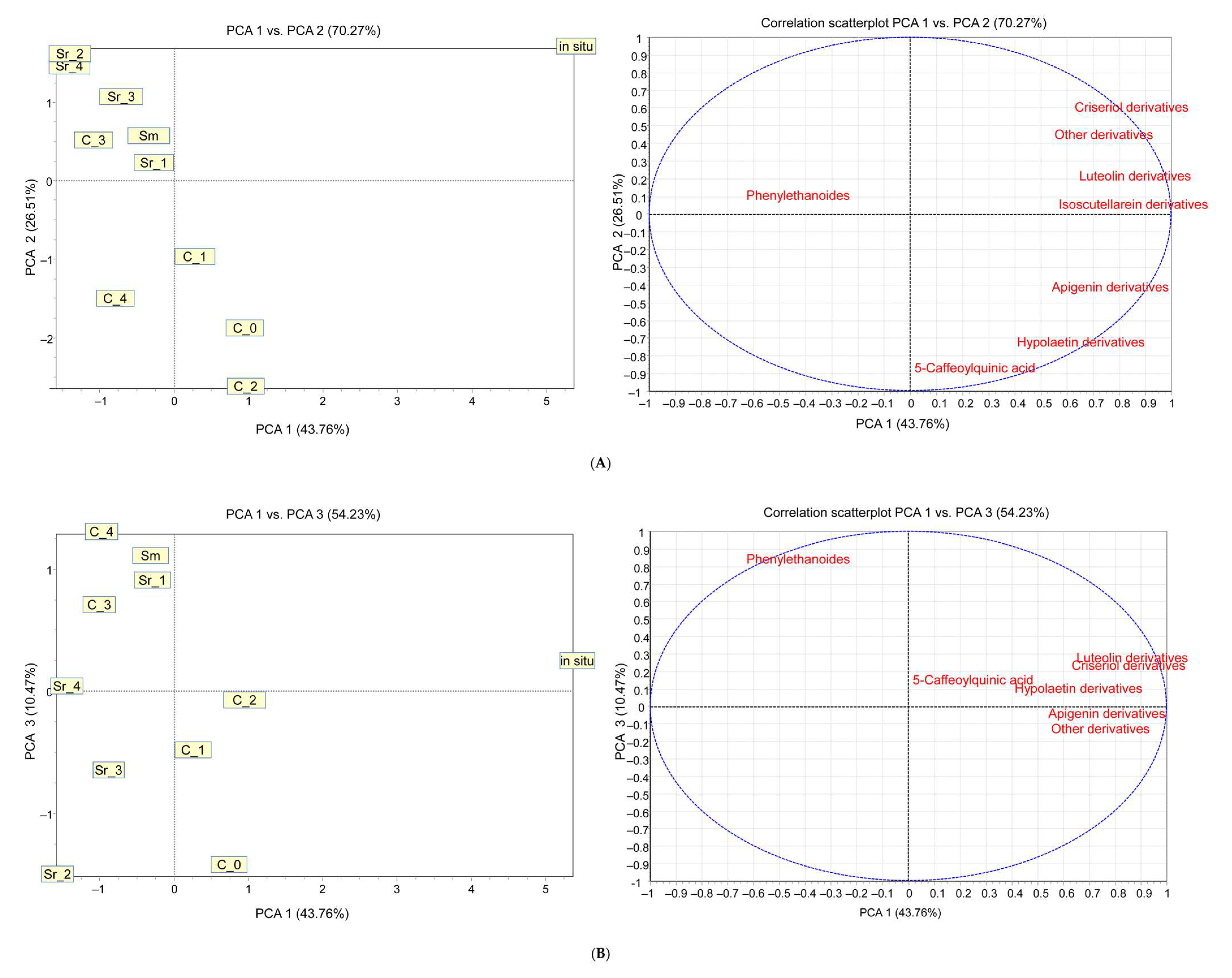
2.6. Principal Component Analysis of Obtained Data of the Phytochemical Studies
3. Materials and Methods
3.1. Plant Material
3.2. Tissue Culture Initiation
3.3. Tissue Culture Experiment
3.4. Extraction of Plant Material
3.5. LC/MS Analyses
3.6. Identification and Quantification of Phenolic Compounds
3.7. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fraga, B.M. Phytochemistry and chemotaxonomy of Sideritis species from the Mediterranean region. Phytochemistry 2012, 76, 7–24. [Google Scholar] [CrossRef]
- Font Quer, P. Plantas Medicinales: El Dioscórides Renovado; Ediciones Península: Barcelona, Spain, 2000. [Google Scholar]
- González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. Sideritis spp.: Uses, chemical composition and pharmacological activities—A review. J. Ethnopharmacol. 2011, 135, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Aneva, I.; Zhelev, P.; Kozuharova, E.; Danova, K.; Nabavi, S.F.; Behzad, S. Genus Sideritis, section Empedoclia in southeastern Europe and Turkey—Studies in ethnopharmacology and recent progress of biological activities. DARU J. Pharm. Sci. 2019, 27, 407–421. [Google Scholar] [CrossRef]
- Bondi, M.; Bruno, M.; Piozzi, F.; Baser, K.H.C.; Simmonds, M. Diversity and antifeedant activity of diterpenes from Turkish species of Sideritis. Biochem. Syst. Ecol. 2000, 28, 299–303. [Google Scholar] [CrossRef]
- Bruno, M.; Rosselli, S.; Pibiri, I.; Piozzia, F.; Luisa, M.; Simmonds, M. Semisynthetic derivatives of ent-kauranes and their antifeedant activity. Phytochemistry 2001, 58, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.; Rosselli, S.; Pibiri, I.; Kilgore, N.; Lee, K.H. Anti-HIVagents from the ent-kaurane diterpenoid linearol. J. Nat. Prod. 2002, 65, 1594–1597. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.P.; Bharate, S.B.; Bhutani, K.K. Anti-HIV natural products. Curr. Sci. 2005, 89, 269–290. [Google Scholar]
- Heiner, F.; Feistel, B.; Wink, M. Sideritis scardica extracts inhibit aggregation and toxicity of amyloid-β in Caenorhabditis elegans used as a model for Alzheimer’s disease. PeerJ 2018, 6, e4683. [Google Scholar] [CrossRef] [Green Version]
- Todorova, M.; Trendafilova, A. Sideritis scardica Griseb., an endemic species of Balkan peninsula: Traditional uses, cultivation, chemical composition, biological activity. J. Ethnopharmacol. 2014, 152, 256–265. [Google Scholar] [CrossRef]
- Jeremic, I.; Tadic, V.; Isakovic, A.; Trajkovic, V.; Markovic, I.; Redzic, Z.; Isakovic, A. The mechanisms of in vitro cytotoxicity of mountain tea, Sideritis scardica, against the C6 glioma cell line. Planta Med. 2013, 79, 1516–1524. [Google Scholar] [CrossRef]
- Feistel, B.; Wegener, T.; Rzymski, P.; Pischel, I. Assessment of the acute and subchronic toxicity and mutagenicity of Sideritis scardica Griseb. extracts. Toxins 2018, 10, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanoeva, J.P.; Stefova, M.; Stefkov, G.; Kulevanova, S.; Alipieva, K.; Bankova, V.; Aneva, I.; Evstatieva, L.N. Chemotaxonomic contribution to the Sideritis species dilemma on the Balkans. Biochem. Syst. Ecol. 2015, 61, 477–487. [Google Scholar] [CrossRef]
- Shtereva, L.; Vassilevska-Ivanova, R.D.; Kraptchev, B.V. In vitro cultures for micropropagation, mass multiplication and preservation of an endangered medicinal plant Sideritis scardica Griseb. Bot. Serbica 2015, 39, 111–120. [Google Scholar]
- Koleva, P.; Petrova, N.; Krumova, S.; Velikova, V.; Aneva, I.; Evstatieva, L.; Wolfram, E.; Danova, K. Effect of activated charcoal on the developmental patterns, polyphenolics productivity and photosynthetic activity of Sideritis scardica in vitro. Planta Med. 2016, 82, S1–S381. [Google Scholar] [CrossRef]
- Danova, K.; Markovska, Y.; Aneva, I. Physiological factors affecting polyphenolics production of in vitro cultivated Balkan endemic Sideritis scardica. Bulg. Chem. Commun. 2019, 51, 113–118. [Google Scholar]
- Sarropoulou, V.; Maloupa, E. In vitro culture and ex situ conservation of the endangered and medicinal mountain tea plant species Sideritis raeseri Boiss & Heldr., endemic to Greece under the influence of activated charcoal, salicylic acid and α- & β-cyclodextrins. Int. J. Bot. Stud. 2017, 2, 93–104. [Google Scholar]
- Bertsouklis, K.; Theodorou, P.; Aretaki, P.-E. In Vitro Propagation of the Mount Parnitha Endangered Species Sideritis raeseri subsp. Attica. Horticulturae 2022, 8, 1114. [Google Scholar] [CrossRef]
- Sevindik, B.; Tütüncü, M.; İzgü, T.; Tagipur, E.M.; Çürük, P.; Kaynak, G.; Yilmaz, Ö.; Mendi, Y.Y. Micropropogation of Sideritis pisidica Boiss. et Heldr. Apud Bentham. Acta Hortic. 2019, 1242, 581–586. [Google Scholar] [CrossRef]
- Pan, M.; van Staden, J. The use of charcoal in in vitro culture—A review. Plant Growth Regul. 1998, 26, 155–163. [Google Scholar] [CrossRef]
- Yam, T.Y.; Ernst, R.; Arditti, J.; Nair, H.; Weatherhead, M.A. Charcoal in orchid seed germination and tissue culture media: A review. Lindleyana 1990, 5, 256–265. [Google Scholar]
- Thomas, T.D. The role of activated charcoal in plant tissue culture. Biotechnol. Adv. 2008, 26, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Fridborg, G.; Eriksson, T. Effects of activated charcoal on morphogenesis in plant tissue cultures. Physiol. Plant 1975, 34, 306–308. [Google Scholar] [CrossRef]
- Collins, M.T.; Dixon, K.W. Micropropagation of an Australian orchid Diuris longzfia R. Br. Aust. J. Exp. Agric. 1992, 32, 131–135. [Google Scholar] [CrossRef]
- Mohamed-Yasseen, Y.; Splittstoesseer, W.E. Regeneration of soybean (Glycine max L. Merr.) from the seedling apex, stem node, cotyledonary node and cotyledons. Plant Growth Regul. Soc. Am. 1990, 18, 203–210. [Google Scholar]
- Tisserat, B. Propagation of data palm (Phoenix dactylifera L.) in vitro. J. Exp. Bot. 1979, 30, 1275–1283. [Google Scholar] [CrossRef]
- Ernst, R. Studies in asymbiotic culture of orchids. Am. Orchid Soc. Bull. 1975, 44, 12–18. [Google Scholar]
- Druart, P.; De Wuif, O. Activated charcoal catalyses sucrose hydrolysis during autoclaving. Plant Cell Tissue Org. Cult. 1993, 32, 97–99. [Google Scholar] [CrossRef]
- Wann, S.R.; Veazey, R.L.; Kaphammer, J. Activated charcoal does not catalyze sucrose hydrolysis in tissue culture media during autoclaving. Plant Cell Tissue Org. Cult. 1997, 50, 221–224. [Google Scholar] [CrossRef]
- Srividya, A.; Dharavath, R. Activated charcoal: An effective enhancer of in vitro seed germination and growth in Nothapodytes foetida—A threatened medicinal plant. Vegetos 2022. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Chang, J.-C. Development of an Improved Micropropagation Protocol for Red-Fleshed Pitaya ‘Da Hong’ with and without Activated Charcoal and Plant Growth Regulator Combinations. Horticulturae 2022, 8, 104. [Google Scholar] [CrossRef]
- Dong, F.S.; Lv, M.Y.; Wang, J.P.; Shi, X.P.; Liang, X.X.; Liu, Y.W.; Yang, F.; Zhao, H.; Chai, J.F.; Zhou, S. Transcriptome analysis of activated charcoal-induced growth promotion of wheat seedlings in tissue culture. BMC Genet. 2020, 21, 69. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.Y.; Li, M.X.; Lin, T.; Qiu, Y.; Zhu, Y.T.; Li, X.L.; Tao, W.D.; Wang, P.; Ren, X.X.; Chen, L.P. A review on the structure and pharmacological activity of phenylethanoid glycosides. Eur. J. Med. Chem. 2021, 209, 112563. [Google Scholar] [CrossRef] [PubMed]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Zürn, M.; Tóth, G.; Ausbüttel, T.; Mucsi, Z.; Horváti, K.; Bősze, S.; Sütöri-Diószegi, M.; Pályi, B.; Kis, Z.; Noszál, B.; et al. Tissue-Specific Accumulation and isomerization of valuable phenylethanoid glycosides from Plantago and Forsythia plants. Int. J. Mol. Sci. 2021, 22, 3880. [Google Scholar] [CrossRef]
- Ellis, B. Production of hydroxyphenylethanol glycosides in suspension cultures of Syringa vulgaris. Phytochemistry 1983, 22, 1941–1943. [Google Scholar] [CrossRef]
- Yang, Y.; Xi, D.; Wu, Y.; Liu, T. Complete biosynthesis of the phenylethanoid glycoside verbascoside. Plant Commun. 2023, 4, 100592. [Google Scholar] [CrossRef]
- Guo, Z.G.; Yu, J.; Liu, R.Z.; Ju, Y.; Xiao, Q. Studies on culture of Cistanche salsa callus and synthesis of phenylethanoid glycosides. Chin. Tradit. Herb. Drugs 1994, 90–93. [Google Scholar]
- Xu, L.S.; Xue, X.F.; Fu, C.; Jin, Z.; Chen, Y.Q.; Zhao, D.X. Effects of methyl jasmonate and salicylic acid on phenylethanoid glycosides synthesis in suspension cultures of Cistanche deserticola. Sheng Wu Gong Cheng Xue Bao 2005, 21, 402–406. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutreint requerments of suspension culture of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Petreska, J.; Stefkov, G.; Kulevanova, S.; Alipieva, K.; Bankova, V.; Stefova, M. Phenolic compounds of mountain tea from the Balkans: LC/DAD/ESI/MSn profile and content. Nat. Prod. Commun. 2011, 6, 21–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petreska, J.; Stefova, M.; Ferreres, F.; Moreno, D.A.; Tomás-Barberán, F.A.; Stefkov, G.; Kulevanova, S.; Gil-Izquierdo, A. Potential bioactive phenolics of Macedonian Sideritis species used for medicinal “Mountain Tea”. Food Chem. 2011, 125, 13–20. [Google Scholar] [CrossRef]





| Medium Abbreviation | Shoot Length, cm | IC, Leaf Couples per cm | Number Axillary Shoots |
|---|---|---|---|
| C_0 | 5.90 ± 0.50 a | 1.40 ± 0.05 f | 9.00 ± 0.80 f |
| Sm | 1.83 ± 0.05 f | 3.21 ± 0.05 a | 8.67 ± 0.69 f |
| Sr_1 | 3.90 ± 0.20 bc | 1.70 ± 0.10 d | 10.00 ± 0.80 ef |
| Sr_2 | 4.00 ± 0.17 bc | 1.60 ± 0.07 de | 16.50 ± 0.70 d |
| Sr_3 * | 3.40 ± 0.51 cd | 1.50 ± 0.09 e | 35 ± 1.20 a |
| Sr_4 | 3.00 ± 1.50 bcd | 1.75 ± 0.50 de | 25 ± 1.50 c |
| C_1 | 2.90 ± 0.10 d | 2.90 ± 0.20 b | 10 ± 0.90 e |
| C_2 | 4.30 ± 0.30 b | 1.60 ± 0.04 d | 32 ± 1.30 b |
| C_3 ** | 2.50 ± 0.30 e | 3.10 ± 0.30 ab | 15 ± 1.20 d |
| C_4 *** | 3.83 ± 0.50 bc | 2.47 ± 0.10 c | 9 ± 0.90 e |
| Medium Abbreviation | PGR Supplementation | Medium Abbreviation | AC Supplementation |
|---|---|---|---|
| C_0 | PGR-free | C_0 | AC-free |
| Sm | 0.2 mg/L BA + 0.02 mg/L NAA | C_1 | 0.02 g/L |
| Sr_1 | 0.2 mg/L BA + 0.5 mg/L NAA | C_2 | 0.05 g/L |
| Sr_2 | 0.2 mg/L BA + 1.0 mg/L NAA | C_3 | 0.2 g/L |
| Sr_3 | 0.5 mg/L BA + 0.5 mg/L NAA | C_4 | 0.5 g/L |
| Sr_4 | 0.5 mg/L BA + 1.0 mg/L NAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danova, K.; Stanoeva, J.P.; Aneva, I.; Alipieva, K.; Stefova, M. Plant Growth Regulators and Activated Charcoal Selectively Affect Phenylethanoid and Flavone Glycoside Accumulation in Sideritis scardica Griseb. Tissue Culture. Plants 2023, 12, 2541. https://doi.org/10.3390/plants12132541
Danova K, Stanoeva JP, Aneva I, Alipieva K, Stefova M. Plant Growth Regulators and Activated Charcoal Selectively Affect Phenylethanoid and Flavone Glycoside Accumulation in Sideritis scardica Griseb. Tissue Culture. Plants. 2023; 12(13):2541. https://doi.org/10.3390/plants12132541
Chicago/Turabian StyleDanova, Kalina, Jasmina Petreska Stanoeva, Ina Aneva, Kalina Alipieva, and Marina Stefova. 2023. "Plant Growth Regulators and Activated Charcoal Selectively Affect Phenylethanoid and Flavone Glycoside Accumulation in Sideritis scardica Griseb. Tissue Culture" Plants 12, no. 13: 2541. https://doi.org/10.3390/plants12132541






