Solarplast®—An Enzymatically Treated Spinach Extract
Abstract
:1. Introduction
2. Results
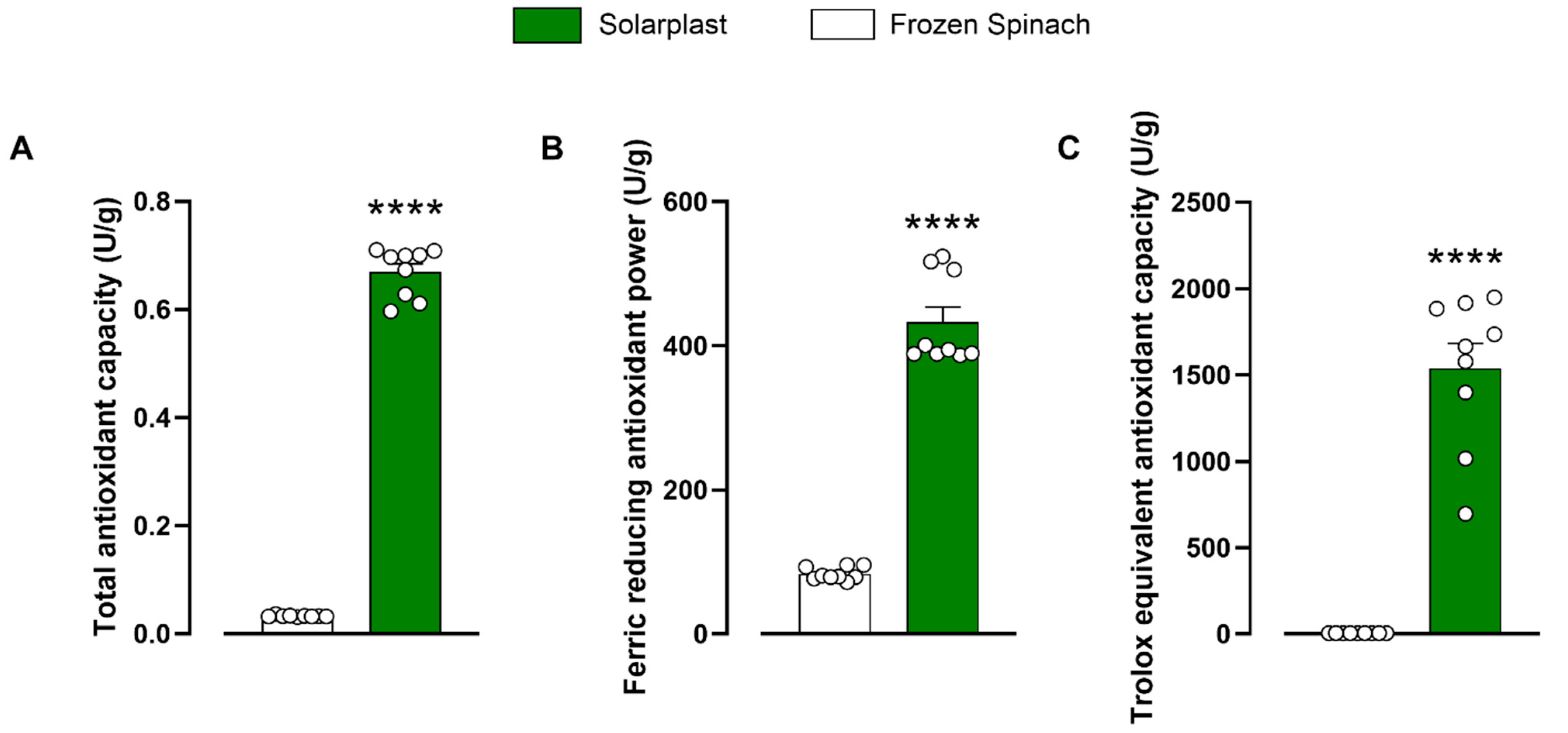
2.1. Total Antioxidant Capacity of Solarplast® and Frozen Spinach
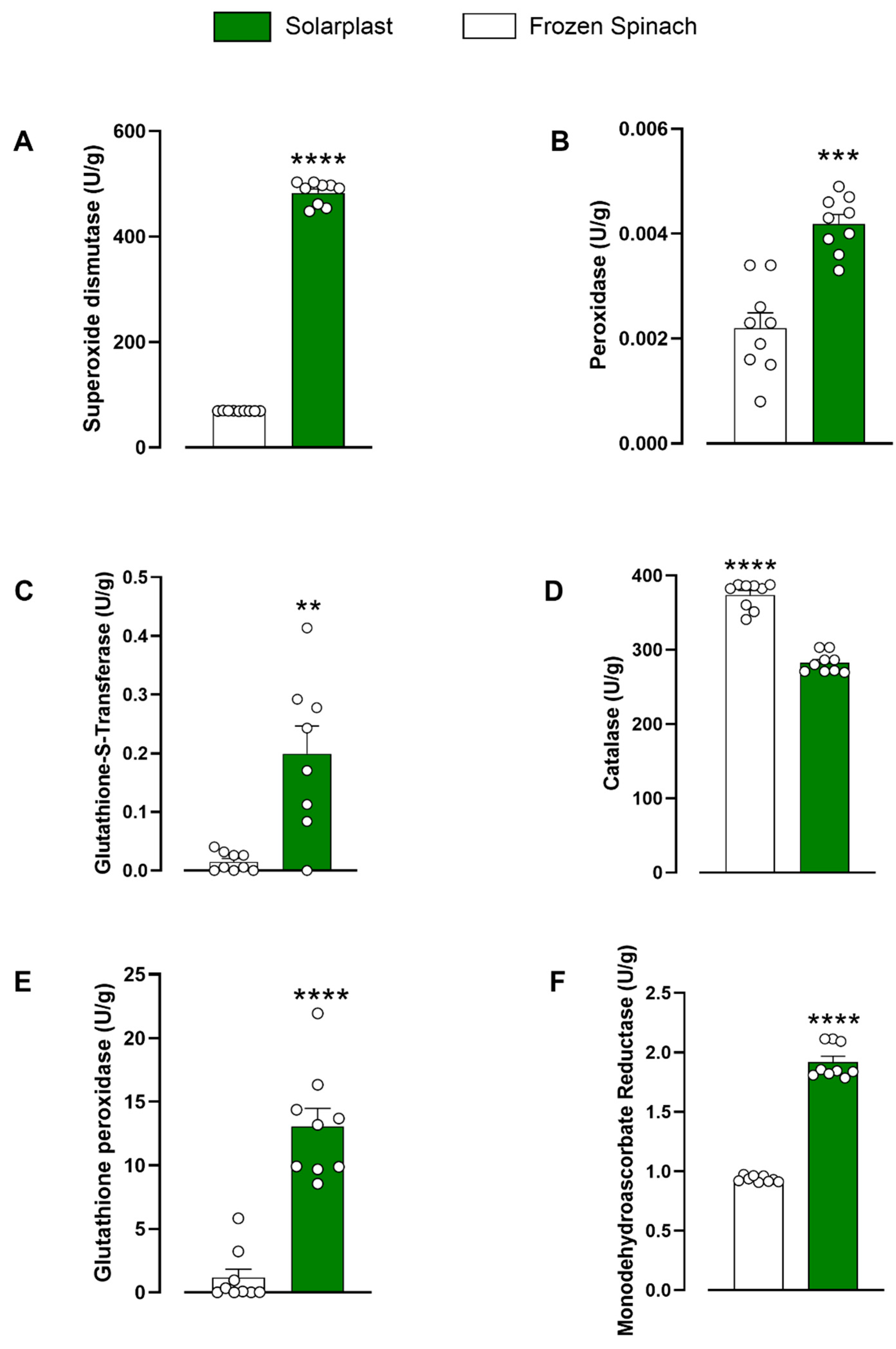
2.2. Solarplast® and Frozen Spinach Individual Antioxidant Activity
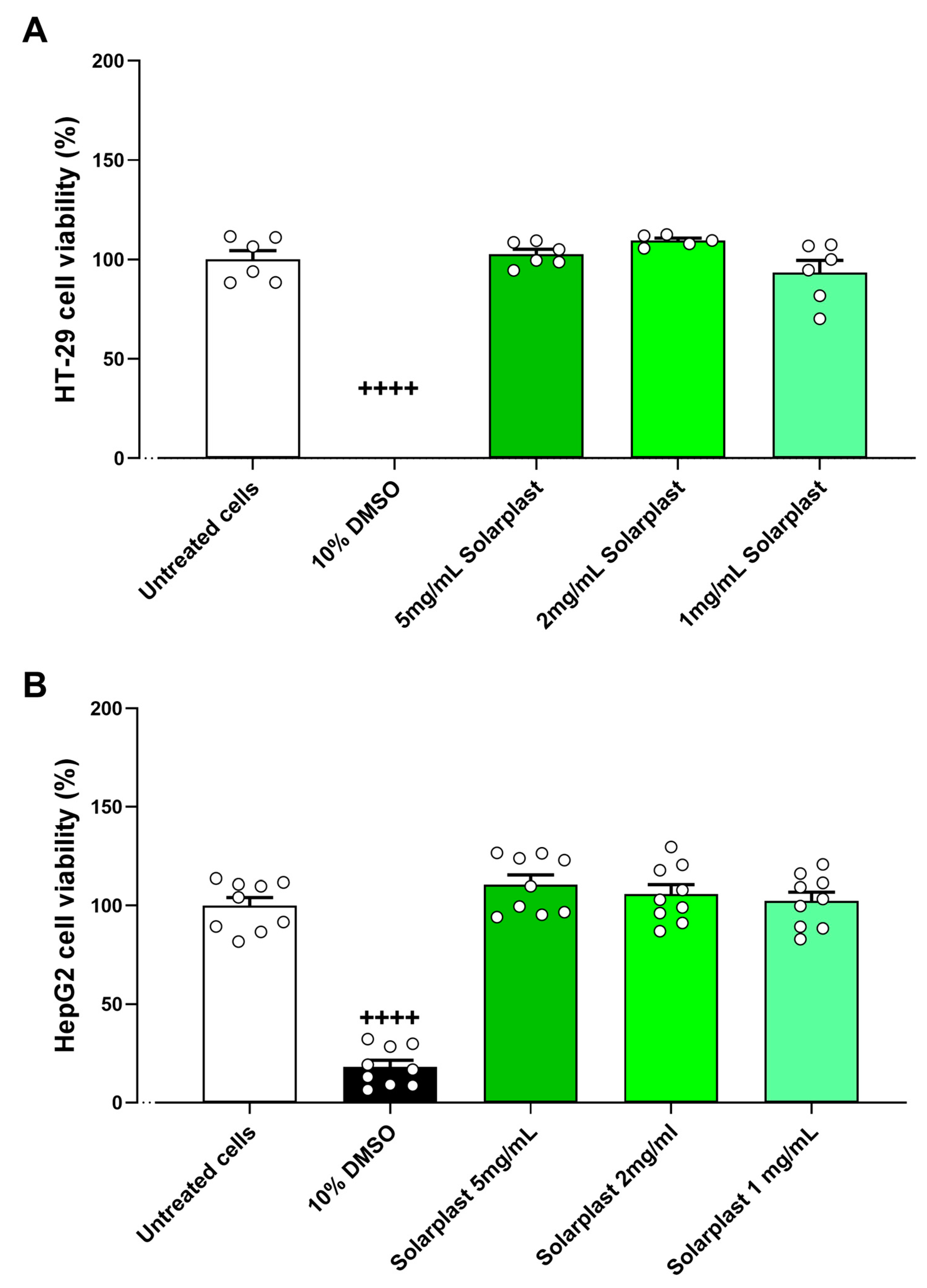
2.3. Solarplast® Safety in HT-29 and HepG2 Cell Lines
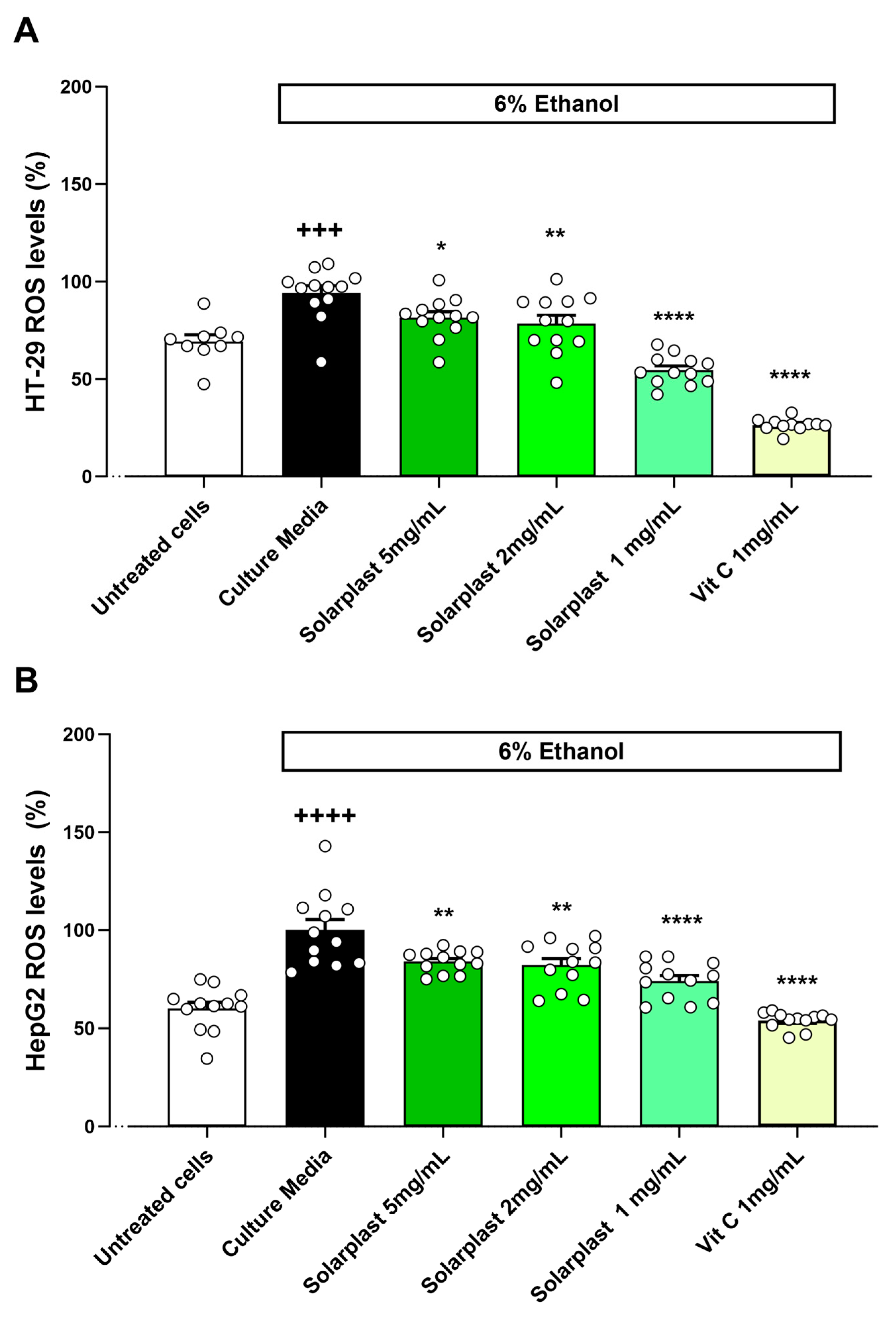
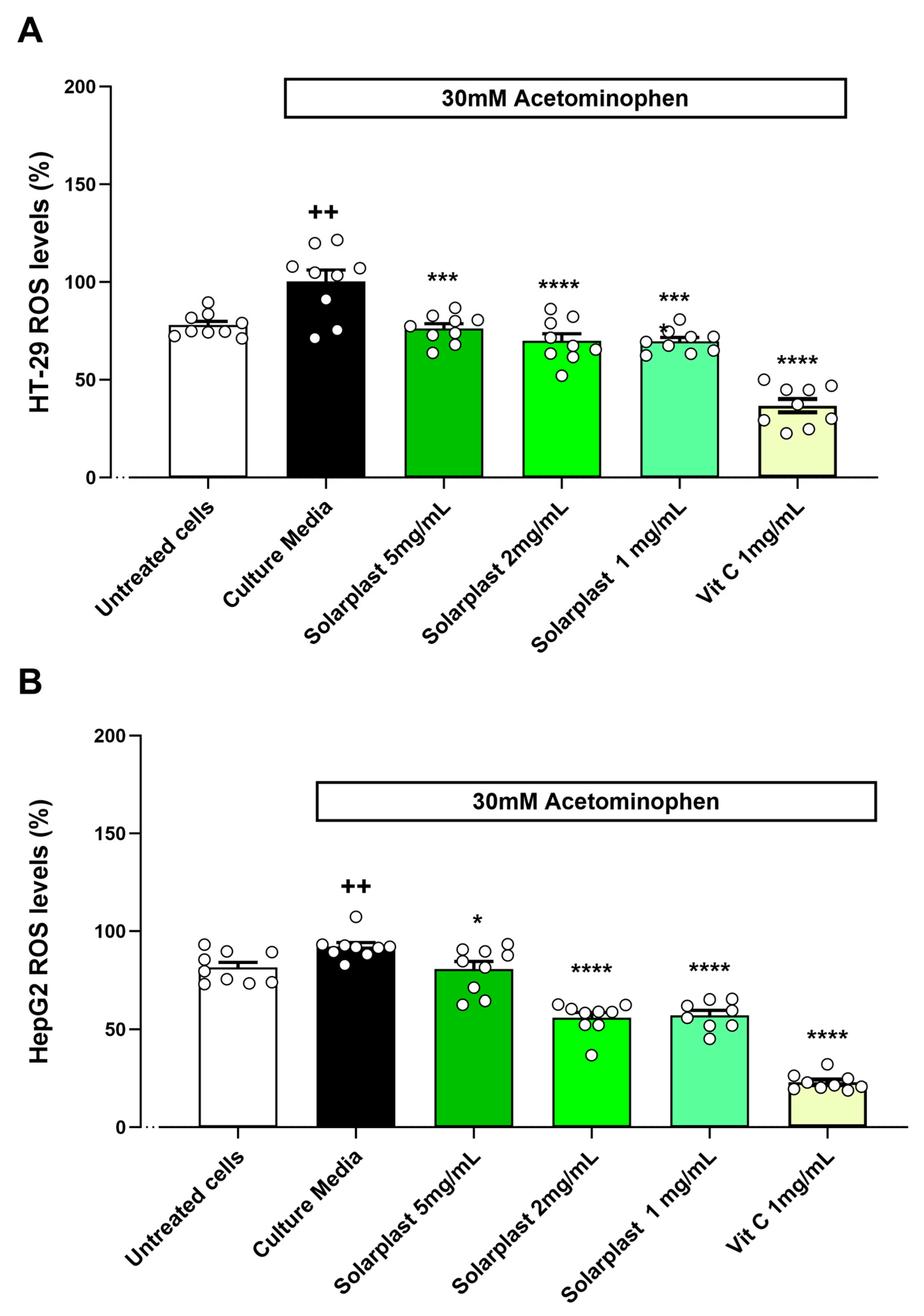
2.4. Solarplast® Antioxidant Capacity in HT-29 and HepG2 Cell Lines
3. Materials and Methods
3.1. Materials
3.2. Solarplast® and Frozen Spinach Sample Preparation
3.3. Enzymatic and Small Molecules Antioxidant Capacity in Solarplast® and Frozen Spinach
3.4. Total Antioxidant Assay
3.5. Superoxide Dismutase (SOD)
3.6. Catalase (CAT)
3.7. Peroxidase (POD)
3.8. Glutathione-S-Transferase (GST)
3.9. Monodehydroascorbate Reductase (MDHAR)
3.10. Glutathione Peroxidase (GPx)
3.11. Cell Culture Conditions
3.12. Solarplast® Extract Cytotoxicity in HT-29 and HepG2 Cell Lines
3.13. Solarplast® Antioxidant Capacity in HT-29 and HepG2 Cell Lines
3.14. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Halliwell, B.; Gutteridge, J.M.C. The Definition and Measurement of Antioxidants in Biological Systems. Free Radic. Biol. Med. 1995, 18, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Sarangarajan, R.; Meera, S.; Rukkumani, R.; Sankar, P.; Anuradha, G. Antioxidants: Friend or Foe? Asian Pac. J. Trop. Med. 2017, 10, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxid. Med. Cell. Longev. 2017, 2001, 6501046. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Keaney, J.F. Evolving Concepts of Oxidative Stress and Reactive Oxygen Species in Cardiovascular Disease. Curr. Atheroscler. Rep. 2012, 14, 476–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, J.L.; Moreau, R. Functional Properties of Spinach (Spinacia oleracea L.) Phytochemicals and Bioactives. Food Funct. 2016, 7, 3337–3353. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Li, F.; Duan, Y.; Wen, C.; Wang, W.; Zhang, L.; Huang, R.; Yin, Y. Oxidative Stress, Nutritional Antioxidants and Beyond. Sci. China Life Sci. 2020, 63, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Sáez, G.T.; Están-Capell, N. Antioxidant Enzymes. In Encyclopedia of Cancer; Schwab, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 288–294. [Google Scholar]
- Rogers, G. Final Report Developing a Nutrient and/or Health Claim Label for Packaged Baby Leaf Spinach and Rocket; Horticulutre Innovation Australia: Sydney, Australia, 2009; pp. 1–90. [Google Scholar]
- Assefi, M.; Lewandrowski, K.-U.; Nankali, S.; Sharafshah, A. Antioxidants Sources; IntechOpen: London, UK, 2023; Volume 1, pp. 1–43. [Google Scholar] [CrossRef]
- Ukom, A.; Albert, M.; Ojimelukwe, P.; Offia-Olua, B.; Nwanagba, L. Impact of Cooking Methods on the Chemical and Antioxidant Composition of Some Indigenous Vegetables Used in Different Food Dishes in Southeast Nigeria. J. Ethn. Foods 2023, 10, 6. [Google Scholar] [CrossRef]
- Ejiofor, E.U.; Onoja, S.O.; Agwamba, E.C.; Louis, H.; Onyedikachi, U.B.; Onwuasoanya, W.E.; Aguwamba, C.; Kube, M.T.; Nkume, P.I.; Ekeleme, M.N.; et al. Computational, Chemical Profile, in Vitro Antioxidant, Hypoglycaemic, and Anti-Inflammatory Activity of Hexane Extract of Some Selected Dark Green Vegetables. Proc. Indian Natl. Sci. Acad. 2023, 89, 386–400. [Google Scholar] [CrossRef]
- Gomes, L.M.; Freitas, A.M.; Dias, T.; Cavalcante, R.B. Vegetables and Their By-Products: Total Phenolic Compound Content, Total Flavonoids, and Antioxidant Activity. Hortic. Bras. 2023, 41, e2572. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total Antioxidant Capacity of Plant Foods, Beverages and Oils Consumed in Italy Assessed by Three Different in Vitro Assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergmana, M.; Varshavskya, L.; Gottliebb, H.E.; Grossmana, S. The Antioxidant Activity of Aqueous Spinach Extract: Chemical Identification of Active Fractions. Phytochemistry 2001, 58, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-H.; Park, J.-H.; Kim, S.-Y.; Lee, S.W.; Chun, S.-S.; Park, E. Antioxidant Effects of Spinach (Spinacia oleracea L.) Supplementation in Hyperlipidemic Rats. Prev. Nutr. Food Sci. 2014, 19, 19–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Verma, B.R. Protective Efficacy of Spinacia oleracea Extract against Sodium Arsenite Induced Oxidative Stress and Imbalance in the Level of Thyroid Hormones of Albino Rats. Int. J. Pharm. Res. Appl. 2023, 8, 2269. [Google Scholar] [CrossRef]
- Arru, L.; Mussi, F.; Forti, L.; Buschini, A. Biological Effect of Different Spinach Extracts in Comparison with the Individual Components of the Phytocomplex. Foods 2021, 10, 382. [Google Scholar] [CrossRef] [PubMed]
- Sani, H.A.; Rahmat, A.; Phd, M.I.; Phd, R.R.; Endrini, S. Potential Anticancer Effect of Red Spinach (Amaranthus gangeticus) Extract. Asia Pac. J. Clin. Nutr. 2004, 13, 396–400. [Google Scholar] [PubMed]
- Bohlooli, S.; Barmaki, S.; Khoshkhahesh, F.; Nakhostin-Roohi, B. The Effect of Spinach Supplementation on Exercise-Induced Oxidative Stress. J. Sports Med. Phys. Fit. 2015, 55, 609–614. [Google Scholar]
- Zurovsky, Y.; Eligal, Z.; Grossman, S. Unilateral Renal Ischemia Reperfusion in the Rat: Effect of Blood Volume Trapped in the Kidney, Sucrose Infusion, and Antioxidant Treatments. Exp. Toxicol. Pathol. 1995, 47, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Holland, C.; Ryden, P.; Edwards, C.H.; Grundy, M.M.L. Plant Cell Walls: Impact on Nutrient Bioaccessibility and Digestibility. Foods 2020, 9, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poljsak, B.; Kovač, V.; Milisav, I. Antioxidants, Food Processing and Health. Antioxidants 2021, 10, 433. [Google Scholar] [CrossRef] [PubMed]
- Duquenne, B.; Eeckhaut, T.; Werbrouck, S.; Van Huylenbroeck, J. Effect of Enzyme Concentrations on Protoplast Isolation and Protoplast Culture of Spathiphyllum and Anthurium. Plant Cell Tissue Organ Cult. 2007, 91, 165–173. [Google Scholar] [CrossRef]
- Jeong, Y.Y.; Lee, H.Y.; Kim, S.W.; Noh, Y.S.; Seo, P.J. Optimization of Protoplast Regeneration in the Model Plant Arabidopsis Thaliana. Plant Methods 2021, 17, 21. [Google Scholar] [CrossRef]
- Yoo, S.D.; Cho, Y.H.; Sheen, J. Arabidopsis Mesophyll Protoplasts: A Versatile Cell System for Transient Gene Expression Analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dal, S.; Sigrist, S. The Protective Effect of Antioxidants Consumption on Diabetes and Vascular Complications. Diseases 2016, 4, 24. [Google Scholar] [CrossRef] [Green Version]
- Hassan, A.S.; Morsy, N.M.; Aboulthana, W.M.; Ragab, A. In Vitro Enzymatic Evaluation of Some Pyrazolo[1,5-a]Pyrimidine Derivatives: Design, Synthesis, Antioxidant, Anti-diabetic, Anti-Alzheimer, and Anti-arthritic Activities with Molecular Modeling Simulation. Drug Dev. Res. 2023, 84, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jin, D.; Zhang, Z.; Zhang, Y.; Zhang, Y.; Kang, X.; Jiang, L.; Tong, X.; Lian, F. Effects of Antioxidants on Diabetic Kidney Diseases: Mechanistic Interpretations and Clinical Assessment. Chin. Med. 2023, 18, 3. [Google Scholar] [CrossRef]
- Kurniawan, R.; Nurkolis, F.; Taslim, N.A.; Subali, D.; Surya, R.; Ben Gunawan, W.; Alisaputra, D.; Mayulu, N.; Salindeho, N.; Kim, B. Carotenoids Composition of Green Algae Caulerpa Racemosa and Their Antidiabetic, Anti-Obesity, Antioxidant, and Anti-Inflammatory Properties. Molecules 2023, 28, 3267. [Google Scholar] [CrossRef] [PubMed]
- Basu, T.; Selman, A.; Reddy, A.P.; Reddy, P.H. Current Status of Obesity: Protective Role of Catechins. Antioxidants 2023, 12, 474. [Google Scholar] [CrossRef]
- Kwaśniewska, M.; Pikala, M.; Grygorczuk, O.; Waśkiewicz, A.; Stepaniak, U.; Pająk, A.; Kozakiewicz, K.; Nadrowski, P.; Zdrojewski, T.; Puch-Walczak, A.; et al. Dietary Antioxidants, Quality of Nutrition and Cardiovascular Characteristics among Omnivores, Flexitarians and Vegetarians in Poland—The Results of Multicenter National Representative Survey WOBASZ. Antioxidants 2023, 12, 222. [Google Scholar] [CrossRef]
- Amponsah-Offeh, M.; Diaba-Nuhoho, P.; Speier, S.; Morawietz, H. Oxidative Stress, Antioxidants and Hypertension. Antioxidants 2023, 12, 281. [Google Scholar] [CrossRef]
- Gil, M.I.; Ferreres, F.; Tomás-Barberán, F.A. Effect of Postharvest Storage and Processing on the Antioxidant Constituents (Flavonoids and Vitamin C) of Fresh-Cut Spinach. J. Agric. Food Chem. 1999, 47, 2213–2217. [Google Scholar] [CrossRef]
- Edenharder, R.; Keller, G.; Platt, K.L.; Unger, K.K. Isolation and Characterization of Structurally Novel Antimutagenic Flavonoids from Spinach (Spinacia oleracea). J. Agric. Food Chem. 2001, 49, 2767–2773. [Google Scholar] [CrossRef]
- Chu, Y.-F.; Sun, J.; Wu, X.; Liu, R.H. Antioxidant and Antiproliferative Activities of Common Vegetables. J. Agric. Food Chem. 2002, 50, 6910–6916. [Google Scholar] [CrossRef]
- Hunter, K.J.; Fletcher, J.M. The Antioxidant Activity and Composition of Fresh, Frozen, Jarred and Canned Vegetables. Innov. Food Sci. Emerg. Technol. 2002, 3, 399–406. [Google Scholar] [CrossRef]
- Mazzeo, T.; N’Dri, D.; Chiavaro, E.; Visconti, A.; Fogliano, V.; Pellegrini, N. Effect of Two Cooking Procedures on Phytochemical Compounds, Total Antioxidant Capacity and Colour of Selected Frozen Vegetables. Food Chem. 2011, 128, 627–633. [Google Scholar] [CrossRef]
- Da Silva Pereira, A.C.; Wurlitzer, N.J.; Dionísio, A.P.; Soares, M.V.L.; Bastos, M.D.S.R.; Alves, R.E.; Brasil, I.M. Synergistic, Additive and Antagonistic Effects of Fruit Mixtures on Total Antioxidant Capacities and Bioactive Compounds in Tropical Fruit Juices. Arch. Latinoam. Nutr. 2015, 65, 119–127. [Google Scholar]
- Bobrovskikh, A.; Zubairova, U.; Kolodkin, A.; Doroshkov, A. Subcellular Compartmentalization of the Plant Antioxidant System: An Integrated Overview. PeerJ 2020, 8, e9451. [Google Scholar] [CrossRef]
- Boonyanuphong, P.; Tobgay, U. Protective Effect of Two Thai Pigmented Rice Cultivars against H2O2-Induced Oxidative Damage in HT-29 Cell Culture. Food Res. 2022, 6, 27–33. [Google Scholar] [CrossRef]
- Kozics, K.; Klusová, V.; Srančíková, A.; Mučaji, P.; Slameňová, D.; Hunáková, Ľ.; Kusznierewicz, B.; Horváthová, E. Effects of Salvia Officinalis and Thymus Vulgaris on Oxidant-Induced DNA Damage and Antioxidant Status in HepG2 Cells. Food Chem. 2013, 141, 2198–2206. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Wang, Y.; Zhang, L.; Guo, Q. Oxidative Stress and Liver Disease. Hepatol. Res. 2012, 42, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Baliga, M.S.; Shivashankara, A.R.; Venkatesh, S.; Bhat, H.P.; Palatty, P.L.; Bhandari, G.; Rao, S. Phytochemicals in the Prevention of Ethanol-Induced Hepatotoxicity: A Revisit. In Dietary Interventions in Liver Disease; Academic Press: Cambridge, MA, USA, 2019; pp. 79–89. [Google Scholar] [CrossRef]
- Liao, J.; Lu, Q.; Li, Z.; Li, J.; Zhao, Q.; Li, J. Acetaminophen-Induced Liver Injury: Molecular Mechanism and Treatments from Natural Products. Front. Pharmacol. 2023, 14, 1122632. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simon, A.; Mazhar, S.; Khokhlova, E.; Leeuwendaal, N.; Phipps, C.; Deaton, J.; Rea, K.; Colom, J. Solarplast®—An Enzymatically Treated Spinach Extract. Plants 2023, 12, 2678. https://doi.org/10.3390/plants12142678
Simon A, Mazhar S, Khokhlova E, Leeuwendaal N, Phipps C, Deaton J, Rea K, Colom J. Solarplast®—An Enzymatically Treated Spinach Extract. Plants. 2023; 12(14):2678. https://doi.org/10.3390/plants12142678
Chicago/Turabian StyleSimon, Annie, Shahneela Mazhar, Ekaterina Khokhlova, Natasha Leeuwendaal, Christopher Phipps, John Deaton, Kieran Rea, and Joan Colom. 2023. "Solarplast®—An Enzymatically Treated Spinach Extract" Plants 12, no. 14: 2678. https://doi.org/10.3390/plants12142678



