Precipitation Dominates the Allocation Strategy of Above- and Belowground Biomass in Plants on Macro Scales
Abstract
:1. Introduction
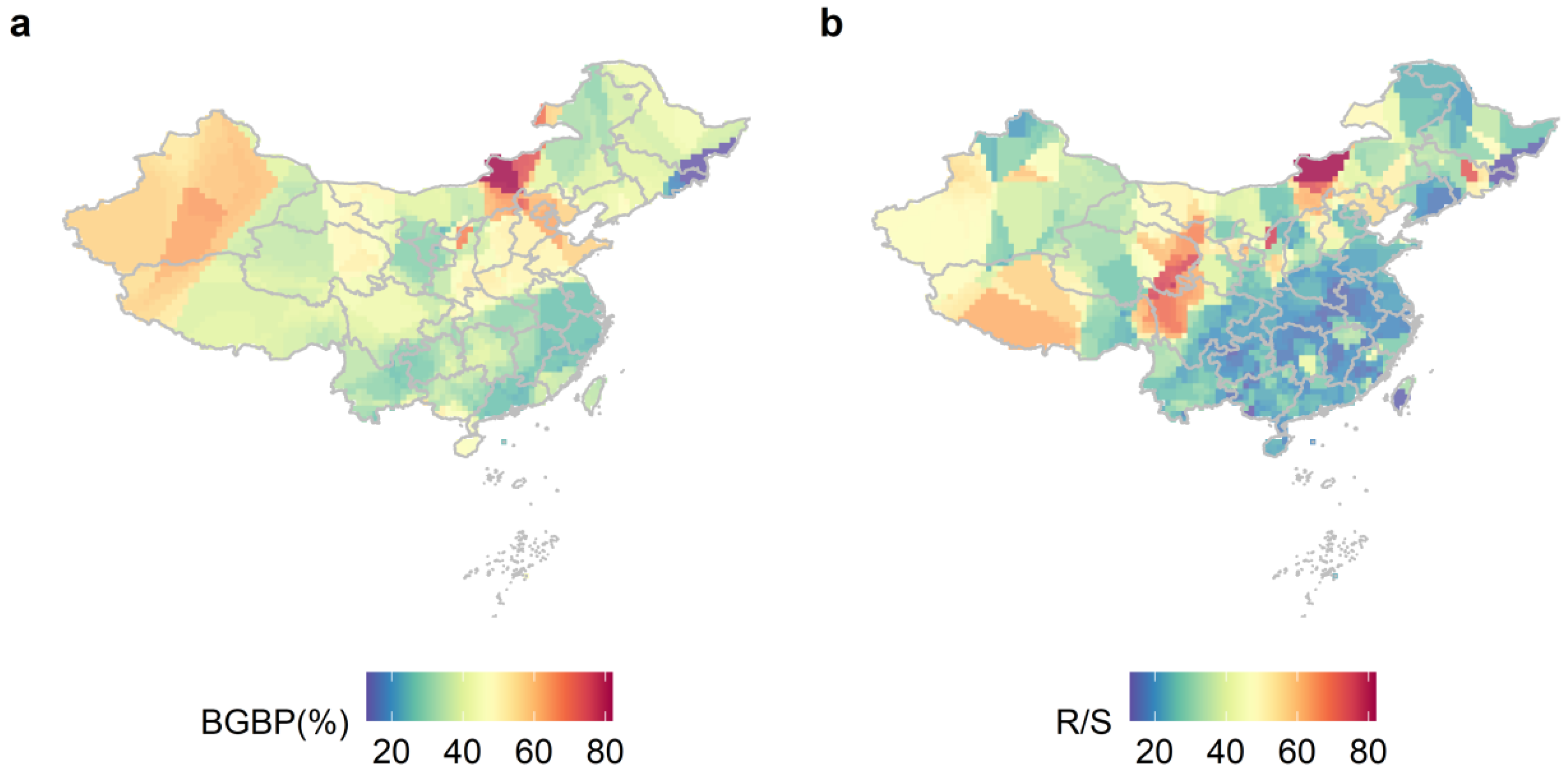
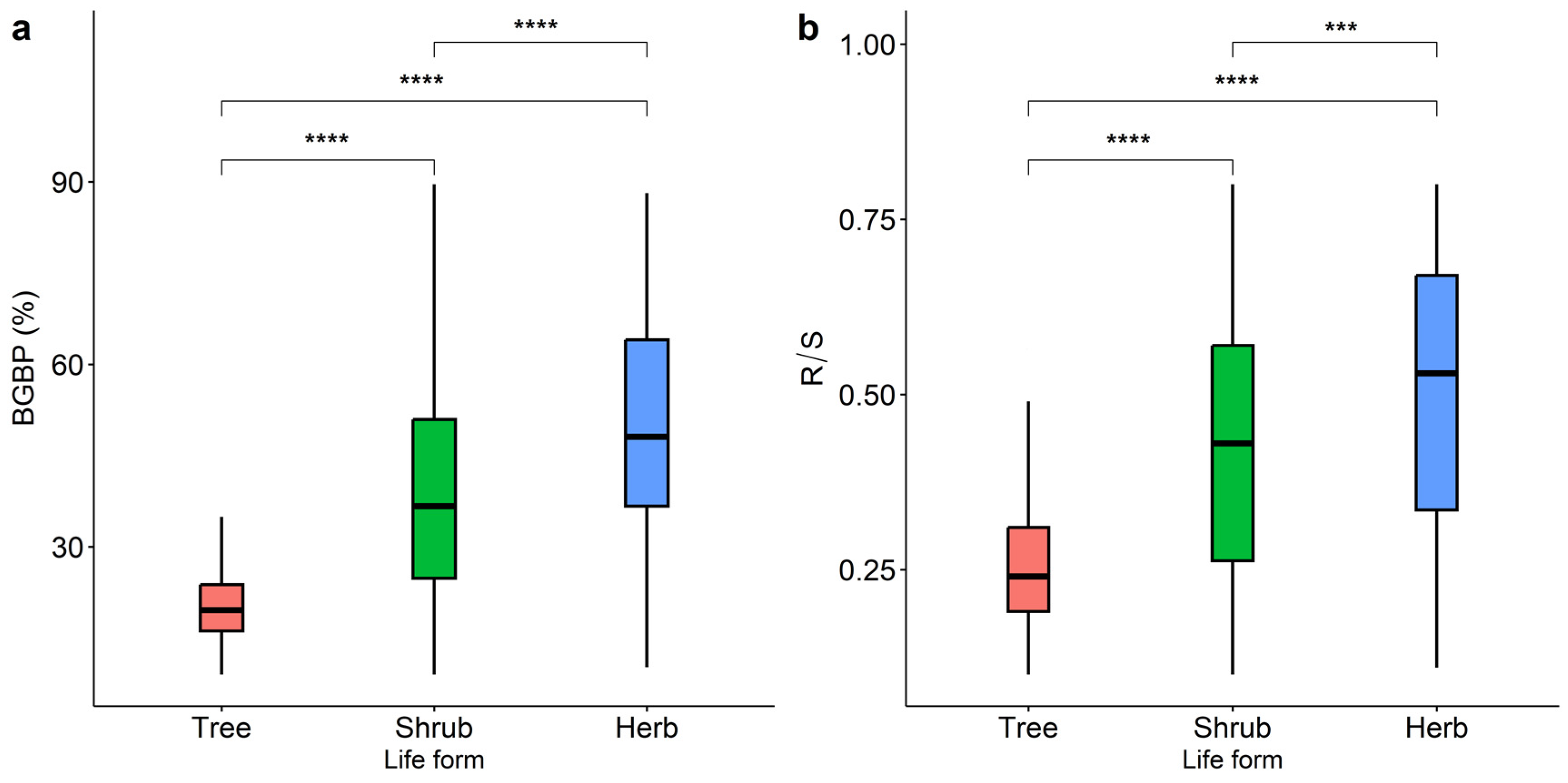
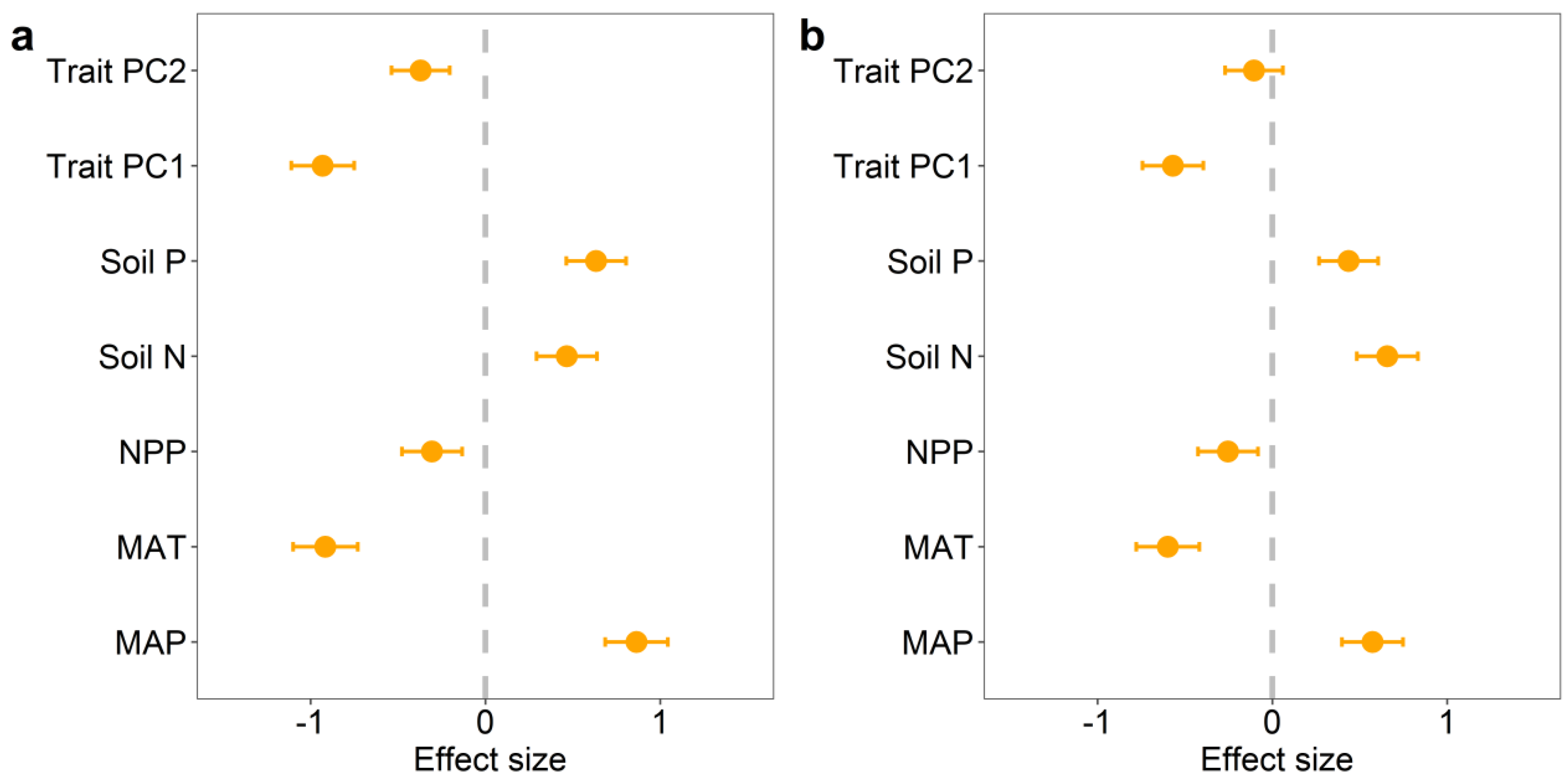
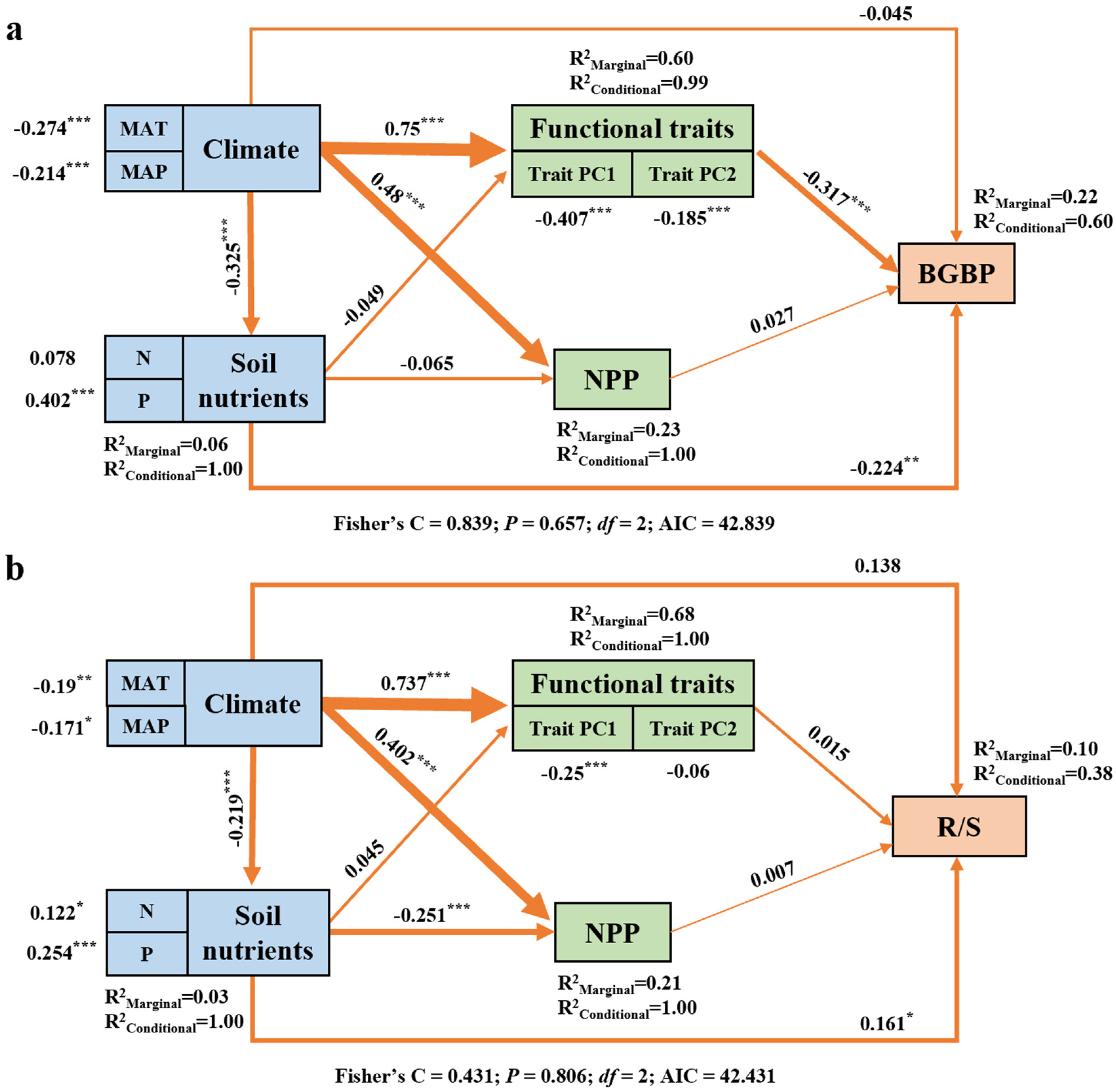
2. Results
3. Discussion
4. Conclusions
5. Methods
5.1. Data Collection
5.2. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bar-On, Y.M.; Phillips, R.; Milo, R. The biomass distribution on Earth. Proc. Natl. Acad. Sci. USA 2018, 115, 6506–6511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Post, A.K.; Knapp, A.K. The importance of extreme rainfall events and their timing in a semi-arid grassland. J. Ecol. 2020, 108, 2431–2443. [Google Scholar] [CrossRef]
- Ding, L.; Li, Z.; Shen, B.; Wang, X.; Xu, D.; Yan, R.; Yan, Y.; Xin, X.; Xiao, J.; Li, M. Spatial patterns and driving factors of aboveground and belowground biomass over the eastern Eurasian steppe. Sci. Total Environ. 2021, 803, 149700. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Belz, R.G.; Kitao, M.; Koike, T.; Calabrese, E.J. Does the root to shoot ratio show a hormetic response to stress? An ecological and environmental perspective. J. For. Res. 2019, 30, 1569–1580. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Hong, Y.; Li, C.; Lu, C.; He, Y.; Shao, J.; Sun, X.; Wang, C.; Liu, R.; Liu, H.; et al. Responses of biomass allocation to multi-factor global change: A global synthesis. Agric. Ecosyst. Environ. 2020, 304, 107115. [Google Scholar] [CrossRef]
- Sun, Y.; Wan, H.; Zhao, Y.; Chen, S.; Bai, Y. Spatial patterns and drivers of root turnover in grassland ecosystems in China. Chin. J. Plant Ecol. 2018, 42, 337–348. [Google Scholar]
- Gounand, I.; Little, C.J.; Harvey, E.; Altermatt, F.; Hickler, T. Global quantitative synthesis of ecosystem functioning across climatic zones and ecosystem types. Glob. Ecol. Biogeogr. 2020, 29, 1139–1176. [Google Scholar] [CrossRef]
- Wilcox, K.R.; Blumenthal, D.M.; Kray, J.A.; Mueller, K.E.; Derner, J.D.; Ocheltree, T.; Porensky, L.M. Plant traits related to precipitation sensitivity of species and communities in semiarid shortgrass prairie. New Phytol. 2021, 229, 2007–2019. [Google Scholar] [CrossRef]
- Gong, H.; Gao, J. Soil and climatic drivers of plant SLA (specific leaf area). Resour. Conserv. Recycl. 2019, 20, e00696. [Google Scholar] [CrossRef]
- Reich, P.B.; Luo, Y.; Bradford, J.B.; Poorter, H.; Perry, C.H.; Oleksyn, J. Temperature drives global patterns in forest biomass distribution in leaves, stems, and roots. Proc. Natl. Acad. Sci. USA 2014, 111, 13721–13726. [Google Scholar] [CrossRef]
- Erb, K.H.; Kastner, T.; Plutzar, C.; Bais, A.L.S.; Carvalhais, N.; Fetzel, T.; Gingrich, S.; Haberl, H.; Lauk, C.; Niedertscheider, M. Unexpectedly large impact of forest management and grazing on global vegetation biomass. Nature 2018, 553, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liu, C.; Qian, S.S.; Luo, Y.; Zhou, R.; Tang, J.; Bao, W. Large-scale patterns of understory biomass and its allocation across China’s forests. Sci. Total Environ. 2022, 804, 150169. [Google Scholar] [CrossRef] [PubMed]
- Eziz, A.; Yan, Z.B.; Tian, D.; Han, W.X.; Tang, Z.Y.; Fang, J.Y. Drought effect on plant biomass allocation: A meta-analysis. Ecol. Evol. 2017, 7, 11002–11010. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Leppälammi-Kujansuu, J.; Helmisaari, H.-S. Fine root longevity and below- and aboveground litter production in a boreal Betula pendula forest. For. Ecol. Manag. 2019, 431, 17–25. [Google Scholar] [CrossRef]
- Zadworny, M.; Mucha, J.; Bagniewska-Zadworna, A.; Żytkowiak, R.; Mąderek, E.; Danusevičius, D.; Oleksyn, J.; Wyka, T.P.; McCormack, M.L. Higher biomass partitioning to absorptive roots improves needle nutrition but does not alleviate stomatal limitation of northern Scots pine. Glob. Chang. Biol. 2021, 27, 3859–3869. [Google Scholar] [CrossRef]
- Kaiser, C.; Franklin, O.; Dieckmann, U.; Richter, A. Microbial community dynamics alleviate stoichiometric constraints during litter decay. Ecol. Lett. 2014, 17, 680–690. [Google Scholar] [CrossRef] [Green Version]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Hao, H.M.; Lu, R.; Liu, Y.; Fang, N.F.; Wu, G.L.; Shi, Z.H. Effects of shrub patch size succession on plant diversity and soil water content in the water-wind erosion crisscross region on the Loess Plateau. Catena 2016, 144, 177–183. [Google Scholar] [CrossRef]
- Yan, B.G.; Ji, Z.H.; Fan, B.; Wang, X.M.; He, G.X.; Shi, L.T.; Liu, G.C. Plants adapted to nutrient limitation allocate less biomass into stems in an arid-hot grassland. New Phytol. 2016, 211, 1232–1240. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Huang, L.; Zhang, X.; Wang, M.; Tan, D. Root Morphology and Biomass Allocation of 50 Annual Ephemeral Species in Relation to Two Soil Condition. Plants 2022, 11, 2495. [Google Scholar] [CrossRef]
- Liu, S.B.; He, F.K.; Kuzyakov, Y.; Xiao, H.X.; Hoang, D.T.T.; Pu, S.Y.; Razavi, B.S. Nutrients in the rhizosphere: A meta-analysis of content, availability, and influencing factors. Sci. Total Environ. 2022, 826, 153908. [Google Scholar] [CrossRef] [PubMed]
- Freschet, G.T.; Swart, E.M.; Cornelissen, J.H. Integrated plant phenotypic responses to contrasting above- and below-ground resources: Key roles of specific leaf area and root mass fraction. New Phytol. 2015, 206, 1247–1260. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.D.; Song, W.C.; Wang, J.F.; Wang, X.X.; Ji, Y.H.; Zhang, X.Y.; Gao, J. Climate factors affect forest biomass allocation by altering soil nutrient availability and leaf traits. J. Integr. Plant Biol. 2023. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Tariq, A.; Zeng, F.J.; Graciano, C.; Zhang, B. Nitrogen application mitigates drought-induced metabolic changes in Alhagi sparsifolia seedlings by regulating nutrient and biomass allocation patterns. Plant Physiol. Biochem. 2020, 155, 828–841. [Google Scholar] [CrossRef]
- Ochoa-Hueso, R.; Collins, S.L.; Delgado-Baquerizo, M.; Hamonts, K.; Pockman, W.T.; Sinsabaugh, R.L.; Smith, M.D.; Knapp, A.K.; Power, S.A. Drought consistently alters the composition of soil fungal and bacterial communities in grasslands from two continents. Glob. Chang. Biol. 2018, 24, 2818–2827. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Sack, L. Pitfalls and possibilities in the analysis of biomass allocation patterns in plants. Front. Plant Sci. 2012, 3, 259. [Google Scholar] [CrossRef] [Green Version]
- Mackay, D.S.; Savoy, P.R.; Grossiord, C.; Tai, X.; Pleban, J.R.; Wang, D.R.; McDowell, N.G.; Adams, H.D.; Sperry, J.S. Conifers depend on established roots during drought: Results from a coupled model of carbon allocation and hydraulics. New Phytol. 2020, 225, 679–692. [Google Scholar] [CrossRef]
- Rixen, C.; Wipf, S.; Rumpf, S.B.; Millen, J.; Morgan, J.W.; Freschet, G.T.; Kurzbck, C.; Li, J.; Pan, H.; Wang, X. Intraspecific trait variation in alpine plants relates to their elevational distribution. J. Ecol. 2022, 110, 860–875. [Google Scholar] [CrossRef]
- Wang, J.F.; Wang, X.X.; Ji, Y.H.; Gao, J. Climate factors determine the utilization strategy of forest plant resources at large scales. Front. Plant Sci. 2022, 13, 990441. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Yan, E.R.; Chang, S.X.; Cheng, J.Y.; Liu, X.Y. Community-weighted mean of leaf traits and divergence of wood traits predict aboveground biomass in secondary subtropical forests. Sci. Total Environ. 2017, 574, 654–662. [Google Scholar] [CrossRef]
- Mommer, L.; Weemstra, M. The role of roots in the resource economics spectrum. New Phytol. 2012, 195, 725–727. [Google Scholar] [CrossRef] [PubMed]
- Myers-Smith, I.H.; Thomas, H.J.D.; Bjorkman, A.D. Plant traits inform predictions of tundra responses to global change. New Phytol. 2019, 221, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Freschet, G.T.; Violle, C.; Bourget, M.Y.; Scherer-Lorenzen, M.; Fort, F. Allocation, morphology, physiology, architecture: The multiple facets of plant above- and below-ground responses to resource stress. New Phytol. 2018, 219, 1338–1352. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Tian, T.; Han, X.; Xu, J.; Chai, Y.; Mo, J.; Lei, M.; Wang, L.; Yue, M. The relationships between biomass allocation and plant functional trait. Ecol. Indic. 2019, 102, 302–308. [Google Scholar] [CrossRef]
- Husáková, I.; Weiner, J.; Münzbergová, Z. Species traits and shoot-root biomass allocation in 20 dry-grassland species. J. Plant Ecol. 2018, 11, 273–285. [Google Scholar] [CrossRef]
- Shibata, R.; Kurokawa, H.; Shibata, M.; Tanaka, H.; Iida, S.; Masaki, T.; Nakashizuka, T.; Sayer, E. Relationships between resprouting ability, species traits and resource allocation patterns in woody species in a temperate forest. Funct. Ecol. 2016, 30, 1205–1215. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.; Guo, X.; Du, Y.; Ke, X.; Cao, Y.; Li, Y.; Cao, G.; Zhang, F. Thirteen-year variation in biomass allocation under climate change in an alpine Kobresia meadow, northern Qinghai–Tibetan Plateau. Grass Forage Sci. 2019, 74, 476–485. [Google Scholar] [CrossRef]
- Gao, Y.Z.; Chen, Q.; Lin, S.; Giese, M.; Brueck, H. Resource manipulation effects on net primary production, biomass allocation and rain-use efficiency of two semiarid grassland sites in Inner Mongolia, China. Oecologia 2011, 165, 855–864. [Google Scholar] [CrossRef]
- Wang, X.X.; Wang, R.; Gao, J. Precipitation and soil nutrients determine the spatial variability of grassland productivity at large scales in China. Front. Plant Sci. 2022, 13, 996313. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, G.; Shi, F.; Mao, R. Biomass allocation between leaf and stem regulates community-level plant nutrient resorption efficiency response to nitrogen and phosphorus additions in a temperate wetland of Northeast China. J. Plant Ecol. 2021, 14, 58–66. [Google Scholar] [CrossRef]
- Tateno, R.; Hishi, T.; Takeda, H. Above- and belowground biomass and net primary production in a cool-temperate deciduous forest in relation to topographical changes in soil nitrogen. For. Ecol. Manag. 2004, 193, 297–306. [Google Scholar] [CrossRef]
- Gao, J.; Ji, Y.; Zhang, X. Net primary productivity exhibits a stronger climatic response in planted versus natural forests. For. Ecol. Manag. 2023, 529, 120722. [Google Scholar] [CrossRef]
- Liu, L.B.; Yang, H.M.; Xu, Y.; Guo, Y.M.; Ni, J. Forest Biomass and Net Primary Productivity in Southwestern China: A Meta-Analysis Focusing on Environmental Driving Factors. Forests 2016, 7, 173. [Google Scholar] [CrossRef] [Green Version]
- Falster, D.S.; Brännström, Å.; Dieckmann, U.; Westoby, M. Influence of four major plant traits on average height, leaf-area cover, net primary productivity, and biomass density in single-species forests: A theoretical investigation. J. Ecol. 2010, 99, 148–164. [Google Scholar] [CrossRef]
- Feng, S.Y.; Ding, J.Y.; Zhan, T.; Zhao, W.; Pereira, P. Plant biomass allocation is mediated by precipitation use efficiency in arid and semiarid ecosystems. Land Degrad. Dev. 2022, 34, 221–233. [Google Scholar] [CrossRef]
- Boonman, C.C.F.; van Langevelde, F.; Oliveras, I.; Couédon, J.; Luijken, N.; Martini, D.; Veenendaal, E.M. On the importance of root traits in seedlings of tropical tree species. New Phytol. 2020, 227, 156–167. [Google Scholar] [CrossRef] [Green Version]
- Yue, Y.; Yan, D.; Yue, Q.; Ji, G.; Wang, Z. Future changes in precipitation and temperature over the Yangtze River Basin in China based on CMIP6 GCMs. Atmos. Res. 2021, 264, 105828. [Google Scholar] [CrossRef]
- Qi, Y.; Wei, W.; Chen, C.; Chen, L. plant root-shoot biomass allocation over diverse biomes: A global synthesis. Glob. Ecol. Conserv. 2019, 18, e00606. [Google Scholar] [CrossRef]
- Chai, Y.; Zhong, J.; Zhao, J.; Guo, J.; Yue, M.; Guo, Y.; Wang, M.; Wan, P. Environment and plant traits explain shrub biomass allocation and species composition across ecoregions in North China. J. Veg. Sci. 2021, 32, e13080. [Google Scholar] [CrossRef]
- Mueller, K.E.; LeCain, D.R.; McCormack, M.L.; Pendall, E.; Mary, C.; Blumenthal, D.M. Root responses to elevated CO2, warming and irrigation in a semi-arid grassland: Integrating biomass, length and life span in a 5-year field experiment. J. Ecol. 2018, 106, 2176–2189. [Google Scholar] [CrossRef]
- Kim, J.; Kug, J.S.; Jeong, S.J.; Huntzinger, D.N.; Michalak, A.M.; Schwalm, C.R.; Wei, Y.; Schaefer, K. Reduced North American terrestrial primary productivity linked to anomalous Arctic warming. Nat. Geosci. 2017, 10, 572–576. [Google Scholar] [CrossRef]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root Exudation of Primary Metabolites: Mechanisms and Their Roles in Plant Responses to Environmental Stimuli. Front. Plant Sci. 2019, 10, 157. [Google Scholar] [CrossRef] [Green Version]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.Q.; Lv, X.H.; Li, Y.G.; Wang, Z.K.; Zhang, W.; Zhuang, L. Future N deposition and precipitation changes will be beneficial for the growth of Haloxylon ammodendron in Gurbantunggut Desert, northwest China. Sci. Rep. 2019, 9, 4001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.W.; Guo, Y.; Tang, Z.; Feng, Y.; Fang, J. Patterns of nitrogen and phosphorus pools in terrestrial ecosystems in China. Earth Syst. Sci. Data 2021, 13, 5337–5351. [Google Scholar] [CrossRef]
- Zhou, H.; Gao, Y.; Jia, X.; Wang, M.; Wu, B. Network analysis reveals the strengthening of microbial interaction in biological soil crust development in the Mu Us Sandy Land, northwestern China. Soil Biol. Biochem. 2020, 144, 107782. [Google Scholar] [CrossRef]
- Tian, P.; Liu, S.; Zhao, X.; Sun, Z.; Yao, X.; Niu, S.; Crowther, T.W.; Wang, Q. Past climate conditions predict the influence of nitrogen enrichment on the temperature sensitivity of soil respiration. Commun. Earth Environ. 2021, 2, 251. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Chen, X.; Xu, J.; Ji, Y.; Du, X.; Gao, J. Precipitation Dominates the Allocation Strategy of Above- and Belowground Biomass in Plants on Macro Scales. Plants 2023, 12, 2843. https://doi.org/10.3390/plants12152843
Wang X, Chen X, Xu J, Ji Y, Du X, Gao J. Precipitation Dominates the Allocation Strategy of Above- and Belowground Biomass in Plants on Macro Scales. Plants. 2023; 12(15):2843. https://doi.org/10.3390/plants12152843
Chicago/Turabian StyleWang, Xianxian, Xiaohong Chen, Jiali Xu, Yuhui Ji, Xiaoxuan Du, and Jie Gao. 2023. "Precipitation Dominates the Allocation Strategy of Above- and Belowground Biomass in Plants on Macro Scales" Plants 12, no. 15: 2843. https://doi.org/10.3390/plants12152843





