Heavy Metal Induced Oxidative Stress Mitigation and ROS Scavenging in Plants
Abstract
:1. Introduction
2. Mechanism of ROS Production
3. Antioxidative Defense System in Plant Cell Components
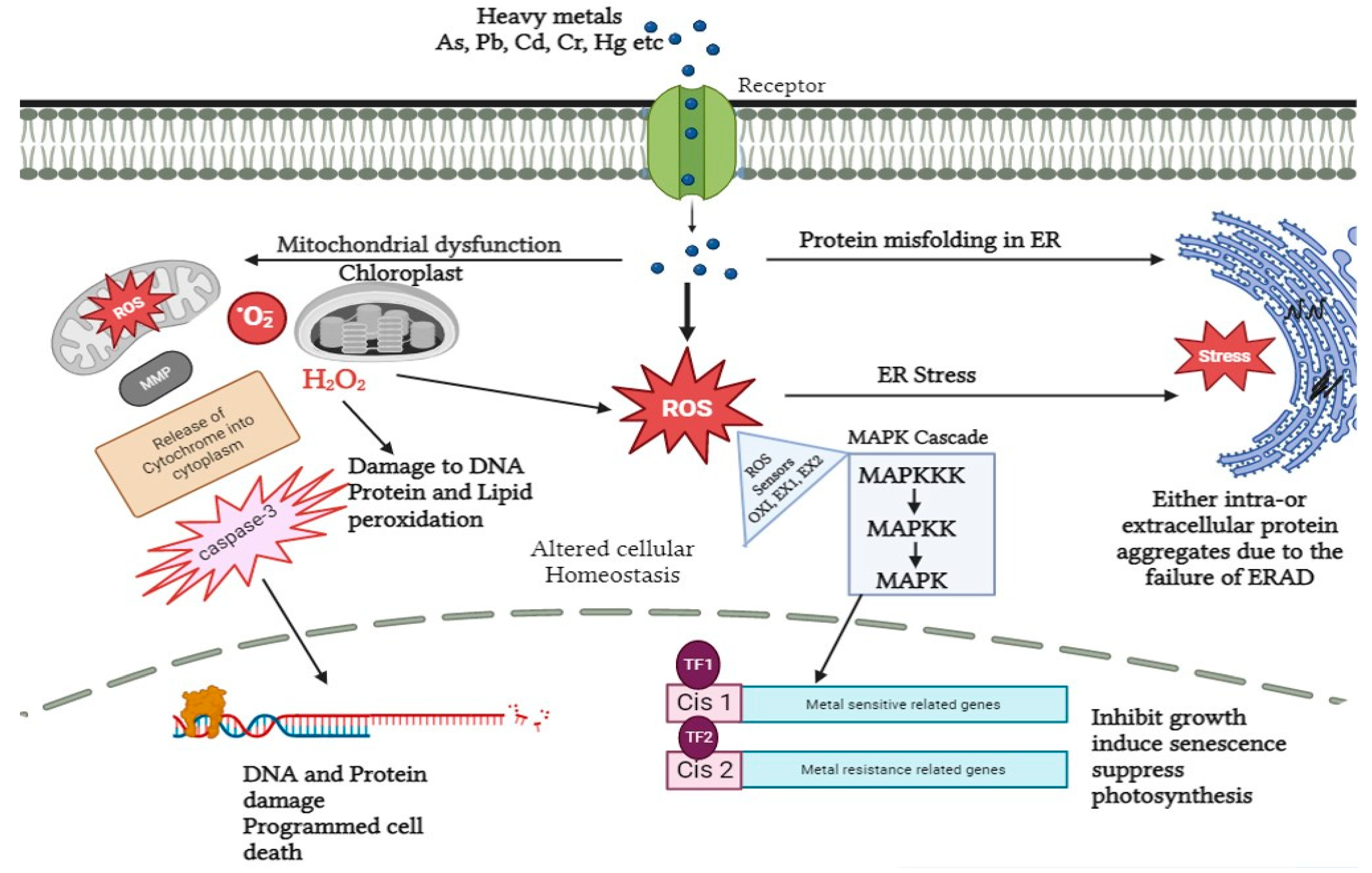
4. Heavy Metal Stress Signaling Events in Plants
5. Heavy Metal Mitigation Strategy
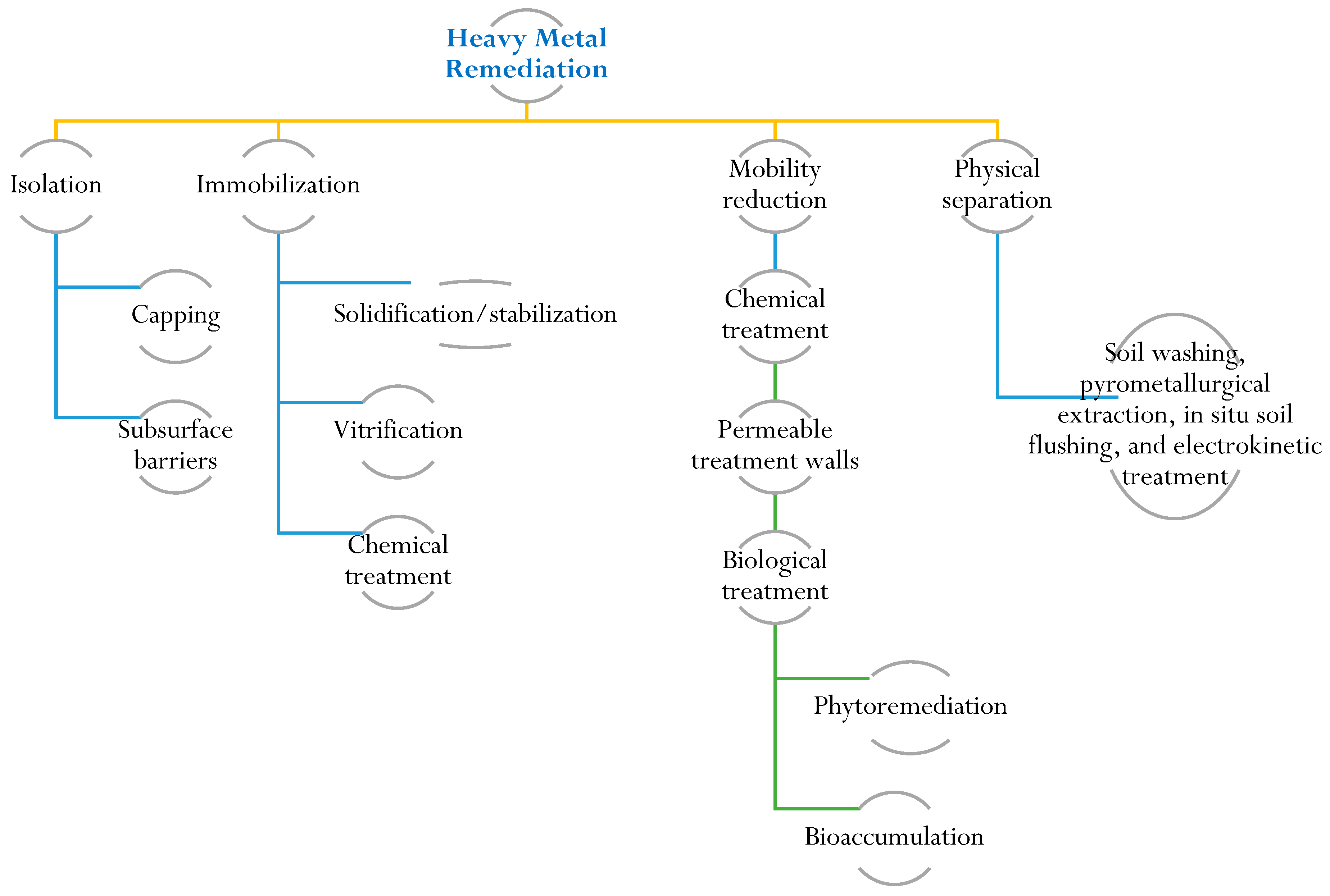
6. Heavy Metal Remediation for Plant Growth Improvement
7. Conclusions and Future
Author Contributions
Funding
Conflicts of Interest
References
- Gerschman, R.; Gilbert, D.L.; Nye, S.W.; Dwyer, P.; Fenn, W.O. Oxygen Poisoning and X-Irradiation: A Mechanism in Common. Science 1954, 119, 623–626. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, H.; Yin, K.; Guo, M.; Wang, Y.; Wang, D.; Zong, H.; Xing, M. The Protective Effect of Zn2+ on As3+ Toxicity in Common Carp: Resistance to Oxidative Stress, Inhibition of Endoplasmic Reticulum Stress, Apoptosis and Autophagy. Aquaculture 2022, 546, 737375. [Google Scholar] [CrossRef]
- Nathan, C.; Ding, A. SnapShot: Reactive Oxygen Intermediates (ROI). Cell 2010, 140, 951–951.e2. [Google Scholar] [CrossRef]
- Jan, B.; Bhat, T.A.; Sheikh, T.A.; Wani, O.A.; Bhat, M.A.; Nazir, A.; Fayaz, S.; Mushtaq, T.; Farooq, A.; Wani, S.; et al. Agronomic Bio-Fortification of Rice and Maize with Iron and Zinc: A Review. Int. Res. J. Pure Appl. Chem. 2020, 21, 28–37. [Google Scholar] [CrossRef]
- Mansoor, S.; Sharma, V.; Mir, M.A.; Mir, J.I.; Un Nabi, S.; Ahmed, N.; Alkahtani, J.; Alwahibi, M.S.; Masoodi, K.Z. Quantification of Polyphenolic Compounds and Relative Gene Expression Studies of Phenylpropanoid Pathway in Apple (Malus Domestica Borkh) in Response to Venturia Inaequalis Infection. Saudi J. Biol. Sci. 2020, 27, 3397–3404. [Google Scholar] [CrossRef]
- Mansoor, S.; Ali Wani, O.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive Oxygen Species in Plants: From Source to Sink. Antioxidants 2022, 11, 225. [Google Scholar] [CrossRef]
- Bartwal, A.; Mall, R.; Lohani, P.; Guru, S.K.; Arora, S. Role of Secondary Metabolites and Brassinosteroids in Plant Defense Against Environmental Stresses. J. Plant Growth Regul. 2013, 32, 216–232. [Google Scholar] [CrossRef]
- Bashir, S.S.; Hussain, A.; Hussain, S.J.; Wani, O.A.; Zahid Nabi, S.; Dar, N.A.; Baloch, F.S.; Mansoor, S. Plant Drought Stress Tolerance: Understanding Its Physiological, Biochemical and Molecular Mechanisms. Biotechnol. Biotechnol. Equip. 2021, 35, 1912–1925. [Google Scholar] [CrossRef]
- Khan, A.; Khan, S.; Khan, M.A.; Qamar, Z.; Waqas, M. The Uptake and Bioaccumulation of Heavy Metals by Food Plants, Their Effects on Plants Nutrients, and Associated Health Risk: A Review. Environ. Sci. Pollut. Res. 2015, 22, 13772–13799. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Hendrix, S.; Dard, A.; Meyer, A.J.; Reichheld, J.-P. Redox-Mediated Responses to High Temperature in Plants. J. Exp. Bot. 2023, 74, 2489–2507. [Google Scholar] [CrossRef]
- An, J.; Jeong, S.; Moon, H.S.; Jho, E.H.; Nam, K. Prediction of Cd and Pb Toxicity to Vibrio Fischeri Using Biotic Ligand-Based Models in Soil. J. Hazard. Mater. 2012, 203–204, 69–76. [Google Scholar] [CrossRef]
- Whiteside, J.R.; Box, C.L.; McMillan, T.J.; Allinson, S.L. Cadmium and Copper Inhibit Both DNA Repair Activities of Polynucleotide Kinase. DNA Repair 2010, 9, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Zhan, J.-C.; Yang, H.-R.; Huang, W.-D. Changes of Resveratrol and Antioxidant Enzymes during UV-Induced Plant Defense Response in Peanut Seedlings. J. Plant Physiol. 2010, 167, 95–102. [Google Scholar] [CrossRef]
- Álvarez, R.; Del Hoyo, A.; García-Breijo, F.; Reig-Armiñana, J.; Del Campo, E.M.; Guéra, A.; Barreno, E.; Casano, L.M. Different Strategies to Achieve Pb-Tolerance by the Two Trebouxia Algae Coexisting in the Lichen Ramalina Farinacea. J. Plant Physiol. 2012, 169, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Mangal, V.; Lal, M.K.; Tiwari, R.K.; Altaf, M.A.; Sood, S.; Kumar, D.; Bharadwaj, V.; Singh, B.; Singh, R.K.; Aftab, T. Molecular Insights into the Role of Reactive Oxygen, Nitrogen and Sulphur Species in Conferring Salinity Stress Tolerance in Plants. J. Plant Growth Regul. 2023, 42, 554–574. [Google Scholar] [CrossRef]
- Zhao, H.; Xia, B.; Fan, C.; Zhao, P.; Shen, S. Human Health Risk from Soil Heavy Metal Contamination under Different Land Uses near Dabaoshan Mine, Southern China. Sci. Total Environ. 2012, 417–418, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Foucault, Y.; Lévêque, T.; Xiong, T.; Schreck, E.; Austruy, A.; Shahid, M.; Dumat, C. Green Manure Plants for Remediation of Soils Polluted by Metals and Metalloids: Ecotoxicity and Human Bioavailability Assessment. Chemosphere 2013, 93, 1430–1435. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and Redox Signalling in the Response of Plants to Abiotic Stress: ROS and Redox Signalling in Plants. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef]
- Rhoads, D.M.; Umbach, A.L.; Subbaiah, C.C.; Siedow, J.N. Mitochondrial Reactive Oxygen Species. Contribution to Oxidative Stress and Interorganellar Signaling. Plant Physiol. 2006, 141, 357–366. [Google Scholar] [CrossRef]
- Hu, W.H.; Song, X.S.; Shi, K.; Xia, X.J.; Zhou, Y.H.; Yu, J.Q. Changes in Electron Transport, Superoxide Dismutase and Ascorbate Peroxidase Isoenzymes in Chloroplasts and Mitochondria of Cucumber Leaves as Influenced by Chilling. Photosynthetica 2008, 46, 581–588. [Google Scholar] [CrossRef]
- Carrasco-Gil, S.; Estebaranz-Yubero, M.; Medel-Cuesta, D.; Millán, R.; Hernández, L.E. Influence of Nitrate Fertilization on Hg Uptake and Oxidative Stress Parameters in Alfalfa Plants Cultivated in a Hg-Polluted Soil. Environ. Exp. Bot. 2012, 75, 16–24. [Google Scholar] [CrossRef]
- Chen, F.; Gao, J.; Zhou, Q. Toxicity Assessment of Simulated Urban Runoff Containing Polycyclic Musks and Cadmium in Carassius Auratus Using Oxidative Stress Biomarkers. Environ. Pollut. 2012, 162, 91–97. [Google Scholar] [CrossRef]
- Yadav, S.K. Heavy Metals Toxicity in Plants: An Overview on the Role of Glutathione and Phytochelatins in Heavy Metal Stress Tolerance of Plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Clemens, S. Safer Food through Plant Science: Reducing Toxic Element Accumulation in Crops. J. Exp. Bot. 2019, 70, 5537–5557. [Google Scholar] [CrossRef]
- Janků, M.; Luhová, L.; Petřivalský, M. On the Origin and Fate of Reactive Oxygen Species in Plant Cell Compartments. Antioxidants 2019, 8, 105. [Google Scholar] [CrossRef]
- Swanson, S.; Gilroy, S. ROS in Plant Development. Physiol. Plant. 2010, 138, 384–392. [Google Scholar] [CrossRef]
- Mansoor, S.; Sakina, A.; Mir, M.A.; Mir, J.I.; Wani, A.A.; un Nabi, S.; Alyemeni, M.N.; Chung, Y.S.; Masoodi, K.Z. Elucidating the role of reactive oxygen species metabolism and phenylpropanoid pathway during an incompatible interaction between apple-Venturia inaequalis host-pathosystem. S. Afr. J. Bot. 2023, 160, 428–436. [Google Scholar] [CrossRef]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.-F.; Lutts, S.; Cai, G.; Guerriero, G. Reactive Oxygen Species and Heavy Metal Stress in Plants: Impact on the Cell Wall and Secondary Metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive Oxygen Species Signalling in Plant Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Nadarajah, K.K. ROS Homeostasis in Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef]
- Choudhary, A.; Kumar, A.; Kaur, N. ROS and Oxidative Burst: Roots in Plant Development. Plant Divers. 2020, 42, 33–43. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Zulfiqar, F.; Raza, A.; Mohsin, S.; Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS Regulation of Plant Development and Stress Responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Corpas, F.J.; Gupta, D.K.; Palma, J.M. Production Sites of Reactive Oxygen Species (ROS) in Organelles from Plant Cells. In Reactive Oxygen Species and Oxidative Damage in Plants Under Stress; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–22. ISBN 978-3-319-20420-8. [Google Scholar]
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Abd_Allah, E.F.; Ahmad, P.; Corpas, F.J. Assessment of Subcellular ROS and NO Metabolism in Higher Plants: Multifunctional Signaling Molecules. Antioxidants 2019, 8, 641. [Google Scholar] [CrossRef]
- Nazir, F.; Fariduddin, Q.; Khan, T.A. Hydrogen Peroxide as a Signalling Molecule in Plants and Its Crosstalk with Other Plant Growth Regulators under Heavy Metal Stress. Chemosphere 2020, 252, 126486. [Google Scholar] [CrossRef] [PubMed]
- Locato, V.; De Gara, L. Programmed Cell Death in Plants: An Overview. In Plant Programmed Cell Death; De Gara, L., Locato, V., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2018; Volume 1743, pp. 1–8. ISBN 978-1-4939-7667-6. [Google Scholar]
- Ye, C.; Zheng, S.; Jiang, D.; Lu, J.; Huang, Z.; Liu, Z.; Zhou, H.; Zhuang, C.; Li, J. Initiation and Execution of Programmed Cell Death and Regulation of Reactive Oxygen Species in Plants. Int. J. Mol. Sci. 2021, 22, 12942. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Reichheld, J.-P.; Foyer, C.H. ROS-Related Redox Regulation and Signaling in Plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Del Río, L.A.; López-Huertas, E. ROS Generation in Peroxisomes and Its Role in Cell Signaling. Plant Cell Physiol. 2016, 57, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.-M.; Qian, P.; Xin, W.; Li, H.-Y.; Burritt, D.J.; Fujita, M.; Tran, L.-S.P. Hydrogen Peroxide Priming Modulates Abiotic Oxidative Stress Tolerance: Insights from ROS Detoxification and Scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef]
- You, J.; Chan, Z. ROS Regulation During Abiotic Stress Responses in Crop Plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef]
- Chmielowska-Bąk, J.; Izbiańska, K.; Ekner-Grzyb, A.; Bayar, M.; Deckert, J. Cadmium Stress Leads to Rapid Increase in RNA Oxidative Modifications in Soybean Seedlings. Front. Plant Sci. 2018, 8, 2219. [Google Scholar] [CrossRef]
- Huang, X.; Han, B. Natural Variations and Genome-Wide Association Studies in Crop Plants. Annu. Rev. Plant Biol. 2014, 65, 531–551. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Shen, C.; Wang, Y.; Huang, C.; Shi, J. New Insights into Regulation of Proteome and Polysaccharide in Cell Wall of Elsholtzia Splendens in Response to Copper Stress. PLoS ONE 2014, 9, e109573. [Google Scholar] [CrossRef]
- 47. Lin, Y.J.; Feng, X.H.; Feng, Y.X. Regulation of enzymatic and non-enzymatic antioxidants in rice seedlings against chromium stress through sodium hydrosulfide and sodium nitroprusside. Environ. Sci. Pollut. Res. 2023, 30, 25851–25862. [Google Scholar] [CrossRef] [PubMed]
- Chmielowska-Bąk, J.; Arasimowicz-Jelonek, M.; Izbiańska, K.; Frontasyeva, M.; Zinicovscaia, I.; Guiance-Varela, C.; Deckert, J. NADPH Oxidase Is Involved in Regulation of Gene Expression and ROS Overproduction in Soybean (Glycine Max L.) Seedlings Exposed to Cadmium. Acta Soc. Bot. Pol. 2017, 86, 3551. [Google Scholar] [CrossRef]
- Sharma, S.K.; Singh, D.; Pandey, H.; Jatav, R.B.; Singh, V.; Pandey, D. An Overview of Roles of Enzymatic and Nonenzymatic Antioxidants in Plant. In Antioxidant Defense in Plants; Aftab, T., Hakeem, K.R., Eds.; Springer Nature: Singapore, 2022; pp. 1–13. ISBN 9789811679803. [Google Scholar]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of Enzymatic and Nonenzymatic Antioxidants in Plants during Abiotic Stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Amareh, R.; Kaviani, B.; Sedaghathoor, S.; Allahyari, M.S. Assessment of Some Urban Ornamental Plants in Southern Iran Revealed That They Choose One of the Two Enzymatic or Non-Enzymatic Antioxidants Defensive Strategies against Heavy Metals. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Hameed, A.; Rasool, S.; Azooz, M.M.; Hossain, M.A.; Ahanger, M.A.; Ahmad, P. Heavy Metal Stress. In Plant Metal Interaction; Elsevier: Amsterdam, The Netherlands, 2016; pp. 557–583. ISBN 978-0-12-803158-2. [Google Scholar]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy Metal Stress and Some Mechanisms of Plant Defense Response. Sci. World J. 2015, 2015, 756120. [Google Scholar] [CrossRef]
- Aina, R.; Labra, M.; Fumagalli, P.; Vannini, C.; Marsoni, M.; Cucchi, U.; Bracale, M.; Sgorbati, S.; Citterio, S. Thiol-Peptide Level and Proteomic Changes in Response to Cadmium Toxicity in Oryza sativa L. Roots. Environ. Exp. Bot. 2007, 59, 381–392. [Google Scholar] [CrossRef]
- Anjum, N.A.; Umar, S.; Singh, S.; Nazar, R.; Khan, N.A. Sulfur Assimilation and Cadmium Tolerance in Plants. In Sulfur Assimilation and Abiotic Stress in Plants; Khan, N.A., Singh, S., Umar, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 271–302. ISBN 978-3-540-76325-3. [Google Scholar]
- Wang, Y.; Wang, Z.; Geng, S.; Du, H.; Chen, B.; Sun, L.; Wang, G.; Sha, M.; Dong, T.; Zhang, X.; et al. Identification of the GDP-L-Galactose Phosphorylase Gene as a Candidate for the Regulation of Ascorbic Acid Content in Fruits of Capsicum annuum L. Int. J. Mol. Sci. 2023, 24, 7529. [Google Scholar] [CrossRef]
- Tao, C.; Jin, X.; Zhu, L.; Xie, Q.; Wang, X.; Li, H. Genome-Wide Investigation and Expression Profiling of APX Gene Family in Gossypium Hirsutum Provide New Insights in Redox Homeostasis Maintenance during Different Fiber Development Stages. Mol. Genet. Genom. 2018, 293, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, P.; Munné-Bosch, S. Vitamin E in Plants: Biosynthesis, Transport, and Function. Trends Plant Sci. 2019, 24, 1040–1051. [Google Scholar] [CrossRef]
- Jańczak-Pieniążek, M.; Cichoński, J.; Michalik, P.; Chrzanowski, G. Effect of Heavy Metal Stress on Phenolic Compounds Accumulation in Winter Wheat Plants. Molecules 2022, 28, 241. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.N.; Tahjib-Ul-Arif, M.; Hannan, A.; Sultana, N.; Akhter, S.; Hasanuzzaman, M.; Akter, F.; Hossain, M.S.; Sayed, M.A.; Hasan, M.T.; et al. Melatonin Modulates Plant Tolerance to Heavy Metal Stress: Morphological Responses to Molecular Mechanisms. Int. J. Mol. Sci. 2021, 22, 11445. [Google Scholar] [CrossRef]
- Wang, L.; Feng, C.; Zheng, X.; Guo, Y.; Zhou, F.; Shan, D.; Liu, X.; Kong, J. Plant Mitochondria Synthesize Melatonin and Enhance the Tolerance of Plants to Drought Stress. J. Pineal Res. 2017, 63, e12429. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Fang, R.; Luo, L.; Yang, W.; Huang, Q.; Yang, C.; Hui, W.; Gong, W.; Wang, J. Potential Roles of Melatonin in Mitigating the Heavy Metals Toxicity in Horticultural Plants. Sci. Hortic. 2023, 321, 112269. [Google Scholar] [CrossRef]
- Keyster, M.; Niekerk, L.-A.; Basson, G.; Carelse, M.; Bakare, O.; Ludidi, N.; Klein, A.; Mekuto, L.; Gokul, A. Decoding Heavy Metal Stress Signalling in Plants: Towards Improved Food Security and Safety. Plants 2020, 9, 1781. [Google Scholar] [CrossRef]
- Ghori, N.-H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy Metal Stress and Responses in Plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Kumar, S.; Trivedi, P.K. Heavy Metal Stress Signaling in Plants. In Plant Metal Interaction; Elsevier: Amsterdam, The Netherlands, 2016; pp. 585–603. ISBN 978-0-12-803158-2. [Google Scholar]
- Mondal, S. Heavy Metal Stress–Induced Activation of Mitogen-Activated Protein Kinase Signalling Cascade in Plants. Plant Mol. Biol. Report. 2022, 41, 15–26. [Google Scholar] [CrossRef]
- Loix, C.; Huybrechts, M.; Vangronsveld, J.; Gielen, M.; Keunen, E.; Cuypers, A. Reciprocal Interactions between Cadmium-Induced Cell Wall Responses and Oxidative Stress in Plants. Front. Plant Sci. 2017, 8, 1867. [Google Scholar] [CrossRef]
- Afzal, Z.; Howton, T.; Sun, Y.; Mukhtar, M. The Roles of Aquaporins in Plant Stress Responses. J. Dev. Biol. 2016, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Kapilan, R.; Vaziri, M.; Zwiazek, J.J. Regulation of Aquaporins in Plants under Stress. Biol. Res. 2018, 51, 4. [Google Scholar] [CrossRef] [PubMed]
- Lamalakshmi Devi, E.; Kumar, S.; Basanta Singh, T.; Sharma, S.K.; Beemrote, A.; Devi, C.P.; Chongtham, S.K.; Singh, C.H.; Yumlembam, R.A.; Haribhushan, A.; et al. Adaptation Strategies and Defence Mechanisms of Plants During Environmental Stress. In Medicinal Plants and Environmental Challenges; Ghorbanpour, M., Varma, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 359–413. ISBN 978-3-319-68716-2. [Google Scholar]
- Ariani, A.; Barozzi, F.; Sebastiani, L.; Di Toppi, L.S.; Di Sansebastiano, G.P.; Andreucci, A. AQUA1 Is a Mercury Sensitive Poplar Aquaporin Regulated at Transcriptional and Post-Translational Levels by Zn Stress. Plant Physiol. Biochem. 2019, 135, 588–600. [Google Scholar] [CrossRef]
- De Caroli, M.; Furini, A.; DalCorso, G.; Rojas, M.; Di Sansebastiano, G.-P. Endomembrane Reorganization Induced by Heavy Metals. Plants 2020, 9, 482. [Google Scholar] [CrossRef]
- Kosakivska, I.V.; Babenko, L.M.; Romanenko, K.O.; Korotka, I.Y.; Potters, G. Molecular Mechanisms of Plant Adaptive Responses to Heavy Metals Stress. Cell Biol. Int. 2021, 45, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Parrotta, L.; Cresti, M. Organelle Trafficking, the Cytoskeleton, and Pollen Tube Growth: Organelle Trafficking in Pollen Tubes. J. Integr. Plant Biol. 2015, 57, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Mehlhorn, D.G.; Wallmeroth, N.; Asseck, L.Y.; Kar, R.; Voss, A.; Denninger, P.; Schmidt, V.A.F.; Schwarzländer, M.; Stierhof, Y.-D.; et al. Loss of GET Pathway Orthologs in Arabidopsis thaliana Causes Root Hair Growth Defects and Affects SNARE Abundance. Proc. Natl. Acad. Sci. USA 2017, 114, E1544–E1553. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, X.; Qu, Y.; Li, R.; Baluška, F.; Wan, Y. Mapping of Membrane Lipid Order in Root Apex Zones of Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 1151. [Google Scholar] [CrossRef]
- Fan, J.-L.; Wei, X.-Z.; Wan, L.-C.; Zhang, L.-Y.; Zhao, X.-Q.; Liu, W.-Z.; Hao, H.-Q.; Zhang, H.-Y. Disarrangement of Actin Filaments and Ca2+ Gradient by CdCl2 Alters Cell Wall Construction in Arabidopsis thaliana Root Hairs by Inhibiting Vesicular Trafficking. J. Plant Physiol. 2011, 168, 1157–1167. [Google Scholar] [CrossRef]
- Ge, W.; Jiao, Y.Q.; Sun, B.L.; Qin, R.; Jiang, W.S.; Liu, D.H. Cadmium-Mediated Oxidative Stress and Ultrastructural Changes in Root Cells of Poplar Cultivars. S. Afr. J. Bot. 2012, 83, 98–108. [Google Scholar] [CrossRef]
- Krzesłowska, M.; Lenartowska, M.; Samardakiewicz, S.; Bilski, H.; Woźny, A. Lead Deposited in the Cell Wall of Funaria Hygrometrica Protonemata Is Not Stable—A Remobilization Can Occur. Environ. Pollut. 2010, 158, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Tang, W.; Anderson, C.T.; Yang, Z. FERONIA’s Sensing of Cell Wall Pectin Activates ROP GTPase Signaling in Arabidopsis. Plant Biol. 2018, 22, 269647. [Google Scholar] [CrossRef]
- Rabęda, I.; Bilski, H.; Mellerowicz, E.J.; Napieralska, A.; Suski, S.; Woźny, A.; Krzesłowska, M. Colocalization of Low-Methylesterified Pectins and Pb Deposits in the Apoplast of Aspen Roots Exposed to Lead. Environ. Pollut. 2015, 205, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Offenborn, J.N.; Steinhorst, L.; Wu, X.N.; Xi, L.; Li, Z.; Jacquot, A.; Lejay, L.; Kudla, J.; Schulze, W.X. Plasma Membrane Calcineurin B-like Calcium-ion Sensor Proteins Function in Regulating Primary Root Growth and Nitrate Uptake by Affecting Global Phosphorylation Patterns and Microdomain Protein Distribution. New Phytol. 2021, 229, 2223–2237. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Ge, J.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Mur, L.A.J.; Jiang, D. The Different Root Apex Zones Contribute to Drought Priming Induced Tolerance to a Reoccurring Drought Stress in Wheat. Crop. J. 2021, 9, 1088–1097. [Google Scholar] [CrossRef]
- Mendoza-Cózatl, D.G.; Gokul, A.; Carelse, M.F.; Jobe, T.O.; Long, T.A.; Keyster, M. Keep Talking: Crosstalk between Iron and Sulfur Networks Fine-Tunes Growth and Development to Promote Survival under Iron Limitation. J. Exp. Bot. 2019, 70, 4197–4210. [Google Scholar] [CrossRef]
- Xuan, W.; Beeckman, T.; Xu, G. Plant Nitrogen Nutrition: Sensing and Signaling. Curr. Opin. Plant Biol. 2017, 39, 57–65. [Google Scholar] [CrossRef]
- Shi, S.; Li, S.; Asim, M.; Mao, J.; Xu, D.; Ullah, Z.; Liu, G.; Wang, Q.; Liu, H. The Arabidopsis Calcium-Dependent Protein Kinases (CDPKs) and Their Roles in Plant Growth Regulation and Abiotic Stress Responses. Int. J. Mol. Sci. 2018, 19, 1900. [Google Scholar] [CrossRef]
- Boro, P.; Chattopadhyay, S. Crosstalk between MAPKs and GSH under Stress: A Critical Review. J. Biosci. 2022, 47, 71. [Google Scholar] [CrossRef]
- Mazaheri-Tirani, M.; Dayani, S. In Vitro Effect of Zinc Oxide Nanoparticles on Nicotiana Tabacum Callus Compared to ZnO Micro Particles and Zinc Sulfate (ZnSO4). Plant Cell Tissue Organ Cult. 2020, 140, 279–289. [Google Scholar] [CrossRef]
- Peng, D.; Wang, W.; Liu, A.; Zhang, Y.; Li, X.; Wang, G.; Jin, C.; Guan, C.; Ji, J. Comparative Transcriptome Combined with Transgenic Analysis Reveal the Involvement of Salicylic Acid Pathway in the Response of Nicotiana Tabacum to Triclosan Stress. Chemosphere 2021, 270, 129456. [Google Scholar] [CrossRef]
- Xian, J.; Wang, Y.; Niu, K.; Ma, H.; Ma, X. Transcriptional Regulation and Expression Network Responding to Cadmium Stress in a Cd-Tolerant Perennial Grass Poa Pratensis. Chemosphere 2020, 250, 126158. [Google Scholar] [CrossRef]
- Valivand, M.; Amooaghaie, R.; Ahadi, A. Seed Priming with H2S and Ca2+ Trigger Signal Memory That Induces Cross-Adaptation against Nickel Stress in Zucchini Seedlings. Plant Physiol. Biochem. 2019, 143, 286–298. [Google Scholar] [CrossRef]
- Xu, Z.; Dong, M.; Peng, X.; Ku, W.; Zhao, Y.; Yang, G. New Insight into the Molecular Basis of Cadmium Stress Responses of Wild Paper Mulberry Plant by Transcriptome Analysis. Ecotoxicol. Environ. Saf. 2019, 171, 301–312. [Google Scholar] [CrossRef]
- Corpas, F.J.; Gupta, D.K.; Palma, J.M. (Eds.) Reactive Oxygen Species and Oxidative Damage in Plants Under Stress, 1st ed.; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-20421-5. [Google Scholar]
- Trchounian, A.; Petrosyan, M.; Sahakyan, N. Plant Cell Redox Homeostasis and Reactive Oxygen Species. In Redox State as a Central Regulator of Plant-Cell Stress Responses; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 25–50. ISBN 978-3-319-44080-4. [Google Scholar]
- Hasanuzzaman, M.; Fujita, M.; Oku, H.; Islam, M.T. (Eds.) Plant Tolerance to Environmental Stress: Role of Phytoprotectants, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019; ISBN 978-0-203-70531-5. [Google Scholar]
- Qadir, S.U.; Raja, V.; Siddiqi, W.A.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Heavy Metal Bioaccumulation by Selected Plants from Fly Ash–Contaminated Soils in Suburban Area. Arab. J. Geosci. 2021, 14, 116. [Google Scholar] [CrossRef]
- Blokhina, O. Antioxidants, Oxidative Damage and Oxygen Deprivation Stress: A Review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Maleki, M.; Ghorbanpour, M.; Kariman, K. Physiological and Antioxidative Responses of Medicinal Plants Exposed to Heavy Metals Stress. Plant Gene 2017, 11, 247–254. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. Role of Glutathione in Plant Abiotic Stress Tolerance. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants; Hasanuzzaman, M., Fotopoulos, V., Nahar, K., Fujita, M., Eds.; Wiley: New York, NY, USA, 2019; pp. 159–172. ISBN 978-1-119-46869-1. [Google Scholar]
- Khullar, S.; Sudhakara Reddy, M. Cadmium and Arsenic Responses in the Ectomycorrhizal Fungus Laccaria Bicolor: Glutathione Metabolism and Its Role in Metal(Loid) Homeostasis. Environ. Microbiol. Rep. 2019, 11, 53–61. [Google Scholar] [CrossRef]
- Maheshwari, R.; Dubey, R.S. Nickel-Induced Oxidative Stress and the Role of Antioxidant Defence in Rice Seedlings. Plant Growth Regul. 2009, 59, 37–49. [Google Scholar] [CrossRef]
- Gardner, P.R.; Fridovich, I. Superoxide Sensitivity of the Escherichia coli 6-Phosphogluconate Dehydratase. J. Biol. Chem. 1991, 266, 1478–1483. [Google Scholar] [CrossRef] [PubMed]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Shri, M.; Gupta, A.; Rani, V.; Chakrabarty, D. Toxicity and Detoxification of Heavy Metals during Plant Growth and Metabolism. Environ. Chem. Lett. 2018, 16, 1169–1192. [Google Scholar] [CrossRef]
- Fryzova, R.; Pohanka, M.; Martinkova, P.; Cihlarova, H.; Brtnicky, M.; Hladky, J.; Kynicky, J. Oxidative Stress and Heavy Metals in Plants. In Reviews of Environmental Contamination and Toxicology; De Voogt, P., Ed.; Reviews of Environmental Contamination and Toxicology; Springer International Publishing: Cham, Switzerland, 2017; Volume 245, pp. 129–156. ISBN 978-3-319-75036-1. [Google Scholar]
- Gjorgieva Ackova, D. Heavy Metals and Their General Toxicity for Plants. Plant Sci. Today 2018, 5, 14–18. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology; Luch, A., Ed.; Experientia Supplementum; Springer: Basel, Switzerland, 2012; Volume 101, pp. 133–164. ISBN 978-3-7643-8339-8. [Google Scholar]
- White, P.J.; Pongrac, P. Heavy-Metal Toxicity in Plants. In Plant Stress Physiology; Shabala, S., Ed.; CABI: Wallingford, UK, 2017; pp. 300–331. ISBN 978-1-78064-729-6. [Google Scholar]
- Zhao, Q.; Kaluarachchi, J.J. Risk Assessment at Hazardous Waste-Contaminated Sites with Variability of Population Characteristics. Environ. Int. 2002, 28, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Evanko, C.R.; Dzombak, D.A. Remediation of Metals-Contaminated Soils and Groundwater; Tech. Rep. TE-976-01, Pittsburgh, GWRTAC Series; Ground-water remediation technologies analysis center: Pittsburgh, PA, USA, 1997.
- Koźmińska, A.; Wiszniewska, A.; Hanus-Fajerska, E.; Muszyńska, E. Recent Strategies of Increasing Metal Tolerance and Phytoremediation Potential Using Genetic Transformation of Plants. Plant Biotechnol. Rep. 2018, 12, 1–14. [Google Scholar] [CrossRef]
- Mansoor, S.; Khan, N.F.; Farooq, I.; Kaur, N.; Manhas, S.; Raina, S.; Khan, I.F. Phytoremediation at Molecular Level. In Phytoremediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 65–90. ISBN 978-0-323-89874-4. [Google Scholar]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Mansoor, S.; Kour, N.; Manhas, S.; Zahid, S.; Wani, O.A.; Sharma, V.; Wijaya, L.; Alyemeni, M.N.; Alsahli, A.A.; El-Serehy, H.A.; et al. Biochar as a Tool for Effective Management of Drought and Heavy Metal Toxicity. Chemosphere 2021, 271, 129458. [Google Scholar] [CrossRef]
- Wang, H.; Lin, K.; Hou, Z.; Richardson, B.; Gan, J. Sorption of the Herbicide Terbuthylazine in Two New Zealand Forest Soils Amended with Biosolids and Biochars. J. Soils Sediments 2010, 10, 283–289. [Google Scholar] [CrossRef]
- Beesley, L.; Marmiroli, M. The Immobilisation and Retention of Soluble Arsenic, Cadmium and Zinc by Biochar. Environ. Pollut. 2011, 159, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhao, Y.; Sima, J.; Zhao, L.; Mašek, O.; Cao, X. Indispensable Role of Biochar-Inherent Mineral Constituents in Its Environmental Applications: A Review. Bioresour. Technol. 2017, 241, 887–899. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Köber, R.; Daus, B.; Ebert, M.; Mattusch, J.; Welter, E.; Dahmke, A. Compost-Based Permeable Reactive Barriers for the Source Treatment of Arsenic Contaminations in Aquifers: Column Studies and Solid-Phase Investigations. Environ. Sci. Technol. 2005, 39, 7650–7655. [Google Scholar] [CrossRef]
- Rzymski, P.; Tomczyk, K.; Rzymski, P.; Poniedziałek, B.; Opala, T.; Wilczak, M. Impact of Heavy Metals on the Female Reproductive System. Ann. Agric. Environ. Med. 2015, 22, 259–264. [Google Scholar] [CrossRef]
- Selvi, A.; Rajasekar, A.; Theerthagiri, J.; Ananthaselvam, A.; Sathishkumar, K.; Madhavan, J.; Rahman, P.K.S.M. Integrated Remediation Processes Toward Heavy Metal Removal/Recovery From Various Environments-A Review. Front. Environ. Sci. 2019, 7, 66. [Google Scholar] [CrossRef]
- Das, N.; Bhattacharya, S.; Maiti, M.K. Enhanced Cadmium Accumulation and Tolerance in Transgenic Tobacco Overexpressing Rice Metal Tolerance Protein Gene OsMTP1 Is Promising for Phytoremediation. Plant Physiol. Biochem. 2016, 105, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Kang, H.; Zhang, X.; Shao, H.; Chu, L.; Ruan, C. A Critical Review on the Bio-Removal of Hazardous Heavy Metals from Contaminated Soils: Issues, Progress, Eco-Environmental Concerns and Opportunities. J. Hazard. Mater. 2010, 174, 1–8. [Google Scholar] [CrossRef]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A Review on Drought Stress in Plants: Implications, Mitigation and the Role of Plant Growth Promoting Rhizobacteria. Resour. Environ. Sustain. 2021, 5, 100032. [Google Scholar] [CrossRef]
- Farooq Khan, N.; Rasool, A.; Mansoor, S.; Saleem, S.; Rehman Baba, T.; Maurifatul Haq, S.; Aafreen Rehman, S.; Oluwaseun Adetunji, C.; Mariana Popescu, S. Potential Applications of Rhizobacteria as Eco-Friendly Biological Control, Plant Growth Promotion and Soil Metal Bioremediation. In Sustainable Crop Production—Recent Advances; Singh Meena, V., Choudhary, M., Prakash Yadav, R., Kumari Meena, S., Eds.; IntechOpen: London, UK, 2022; ISBN 978-1-80355-696-3. [Google Scholar]
- Fasani, E.; Manara, A.; Martini, F.; Furini, A.; DalCorso, G. The Potential of Genetic Engineering of Plants for the Remediation of Soils Contaminated with Heavy Metals: Transgenic Plants for Phytoremediation. Plant Cell Environ. 2018, 41, 1201–1232. [Google Scholar] [CrossRef]
- Gupta, D.K.; Huang, H.G.; Corpas, F.J. Lead Tolerance in Plants: Strategies for Phytoremediation. Environ. Sci. Pollut. Res. 2013, 20, 2150–2161. [Google Scholar] [CrossRef] [PubMed]

| S. No | Enzymatic Antioxidants | Mode of Action |
|---|---|---|
| 1. | Superoxide Dismutase (SOD) | SOD is an enzyme that converts superoxide radicals (O2−) into hydrogen peroxide (H2O2) and oxygen (O2), preventing the accumulation of harmful superoxide radicals that can damage cells through oxidative stress. |
| 2. | Catalase | Catalase is an enzyme that transforms hydrogen peroxide (H2O2) into water and oxygen, effectively countering the potential toxicity of excess hydrogen peroxide, particularly in the presence of heavy metals. |
| 3. | Glutathione Peroxidase (GPx) | GPx is an enzyme that employs reduced glutathione (GSH) to convert hydrogen peroxide and lipid hydroperoxides into water and corresponding alcohols. Its vital role lies in safeguarding cells against oxidative damage triggered by heavy metals. |
| 4. | Peroxiredoxins | Peroxiredoxins are enzymes that neutralize peroxides, like hydrogen peroxide, using thiol groups in their active sites. They help detoxify ROS from heavy metal exposure. |
| 5. | Glutathione Reductase (GR) | GR is an enzyme that regulates reduced glutathione (GSH) levels by converting oxidized glutathione (GSSG) to its reduced form. This is crucial for upholding cellular redox equilibrium during heavy metal stress. |
| 6. | NAD(P)H Quinone Oxidoreductase 1 (NQO1) | NQO1 is an enzyme that detoxifies by reducing quinones and electrophilic substances, safeguarding cells from oxidative damage due to heavy metals and pollutants. |
| 7. | Selenium-Containing Enzymes | Selenium is in enzymes like glutathione peroxidases and thioredoxin reductases, crucial for antioxidant defense and redox regulation. They counter heavy-metal-triggered oxidative stress. |
| 8. | Cytochrome P450 Enzymes | Certain cytochrome P450 enzymes metabolize heavy metals, converting them into safer forms. This aids in detoxification and defending against heavy metal stress. |
| Non-Enzymatic Antioxidants | ||
| 9. | Glutathione (GSH) | Glutathione, a tripeptide (γ-glutamyl-cysteinyl-glycine), is a key intracellular antioxidant. It helps detoxify heavy metals by binding to them and aiding in their elimination. GSH also supports specific detoxification enzymes as a cofactor. |
| 10. | Ascorbic Acid (Vitamin C) | Vitamin C, a water-soluble antioxidant, neutralizes ROS, shielding cells from heavy-metal-triggered oxidative harm. It also indirectly boosts other antioxidants like GSH and vitamin E. |
| 11. | α-Tocopherol (Vitamin E) | Vitamin E, a lipid-soluble antioxidant, safeguards cell membranes by neutralizing lipid peroxyl radicals. It upholds membrane integrity during heavy metal stress. |
| 12. | Carotenoids | Carotenoids like β-carotene, lutein, and zeaxanthin are plant pigments with antioxidants. They counter ROS and shield cells from oxidative harm due to heavy metals. |
| 13. | Phenolic Compounds | Various phenolic compounds, such as flavonoids and polyphenols, are known for their antioxidant properties. They can scavenge ROS and chelate heavy metals, reducing their toxic effects. |
| 14. | Metal Chelators | Certain non-enzymatic antioxidants can bind to heavy metals, creating stable complexes that decrease reactivity and toxicity. Chelators like EDTA and citric acid, for instance, aid in trapping heavy metals and aiding their removal. |
| 15. | Selenium (Se) | Selenium, an essential trace element, functions as an antioxidant and can counteract heavy metal toxicity. Supplementation with selenium has been found to ease oxidative stress caused by heavy metals. |
| 16. | Melatonin | Melatonin, an indoleamine, functions as a potent antioxidant by scavenging ROS, safeguarding cells from oxidative harm due to heavy metals. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansoor, S.; Ali, A.; Kour, N.; Bornhorst, J.; AlHarbi, K.; Rinklebe, J.; Abd El Moneim, D.; Ahmad, P.; Chung, Y.S. Heavy Metal Induced Oxidative Stress Mitigation and ROS Scavenging in Plants. Plants 2023, 12, 3003. https://doi.org/10.3390/plants12163003
Mansoor S, Ali A, Kour N, Bornhorst J, AlHarbi K, Rinklebe J, Abd El Moneim D, Ahmad P, Chung YS. Heavy Metal Induced Oxidative Stress Mitigation and ROS Scavenging in Plants. Plants. 2023; 12(16):3003. https://doi.org/10.3390/plants12163003
Chicago/Turabian StyleMansoor, Sheikh, Asif Ali, Navneet Kour, Julia Bornhorst, Khadiga AlHarbi, Jörg Rinklebe, Diaa Abd El Moneim, Parvaiz Ahmad, and Yong Suk Chung. 2023. "Heavy Metal Induced Oxidative Stress Mitigation and ROS Scavenging in Plants" Plants 12, no. 16: 3003. https://doi.org/10.3390/plants12163003










