Do Silicon and Salicylic Acid Attenuate Water Deficit Damage in Talisia esculenta Radlk Seedlings?
Abstract
:1. Introduction
2. Results
2.1. Gas Exchange
2.2. Relative Water Content in the Leaves (RWC)
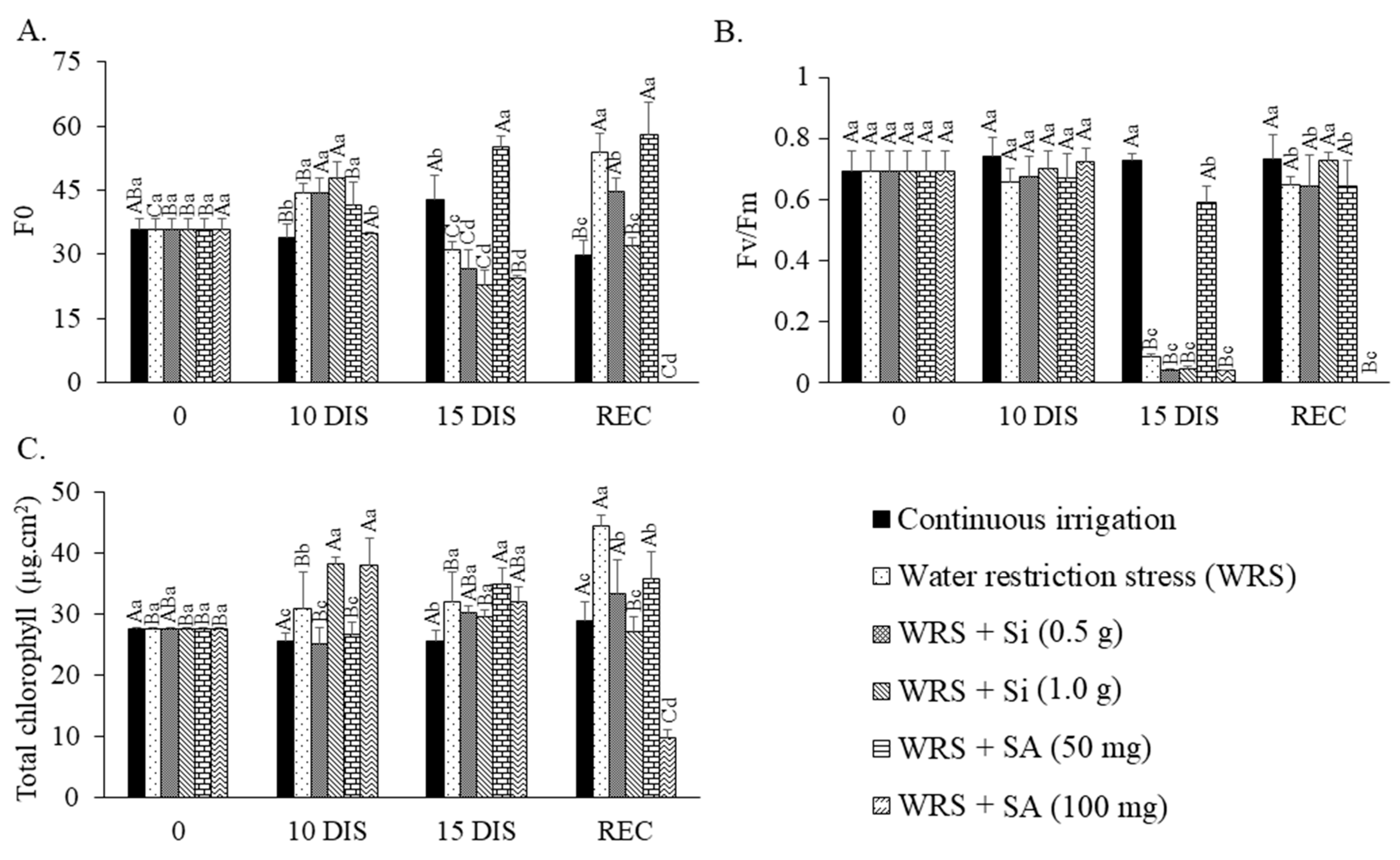
2.3. Chlorophyll a Fluorescence and Chlorophyll Index
2.4. Leaf and Root Proline Content
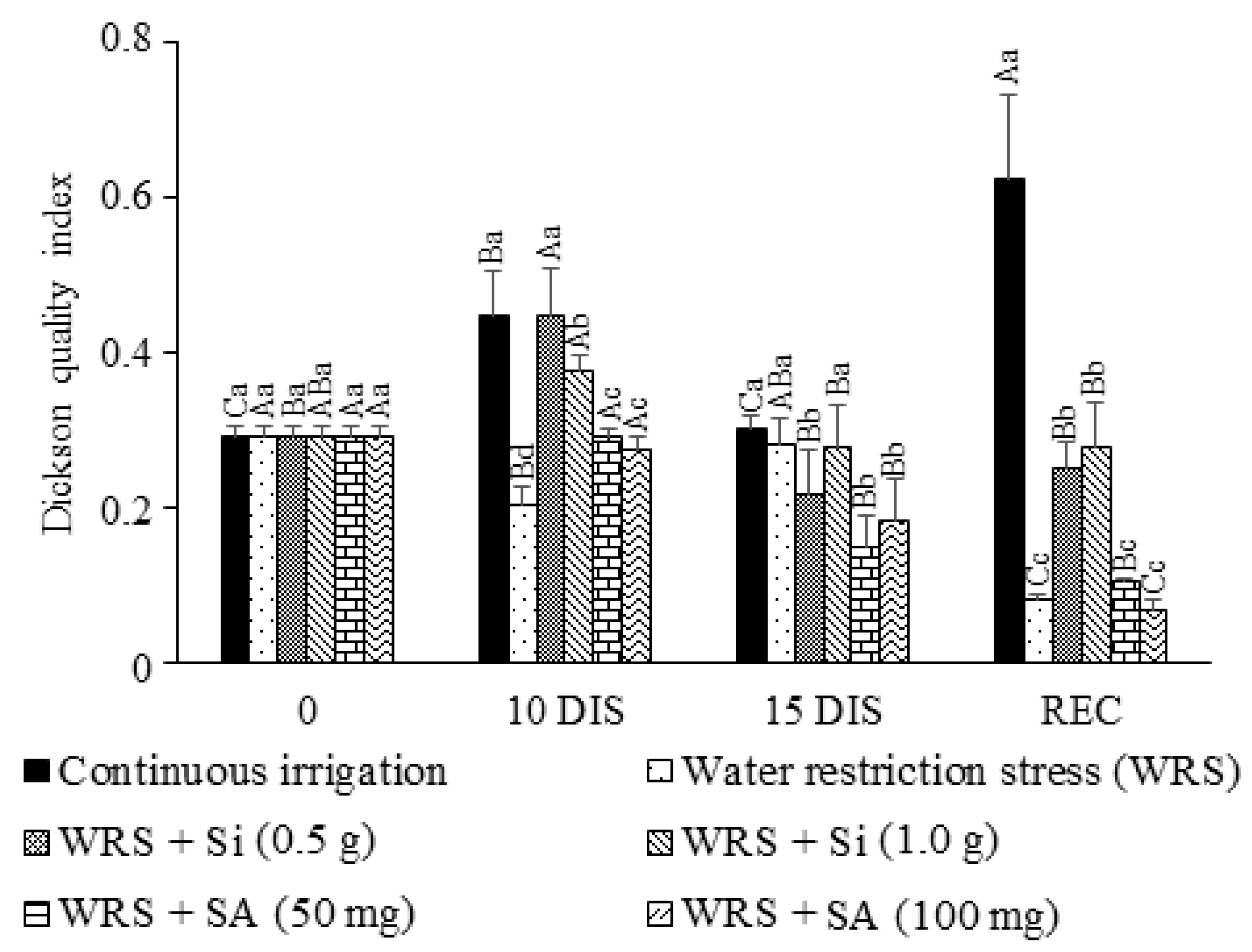
2.5. Seedling Quality
2.6. Ecological Resilience Potential
3. Discussion
4. Materials and Methods
4.1. Plant Material, Growth Conditions, and Treatments
4.2. Analyses
4.2.1. Gas Exchange
4.2.2. Relative Water Content in the Leaves (RWC)
4.2.3. Chlorophyll a Fluorescence and Chlorophyll Index
4.2.4. Leaf and Root Proline Content
4.2.5. Seedling Quality
4.2.6. Ecological Resilience Potential
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigues, S.; Brito, E.S.; Silva, E.O. Pitomba—Talisia esculenta. In Frutas Exóticas; Academic Press: Cambridge, MA, USA, 2018; pp. 351–354. [Google Scholar]
- Lorenzi, H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas do Brasil, 7th ed.; Instituto Plantarum de Estudos da Flora: São Paulo, Brazil, 2016; Volume 1, p. 384. [Google Scholar]
- Foresti, A.C.; Reis, L.C.; Scalon, S.P.Q.; Dresch, D.M.; Santos, C.C.; Jesus, M.V. Salicylic acid mitigating damage to the photosynthetic apparatus and quality of Eugenia myrcianthes seedlings under water deficit. Rodriguésia 2022, 73, e00872021. [Google Scholar] [CrossRef]
- Santos, C.C.; Scalon, S.P.Q.; Foresti, A.C.; Reis, L.C.; Dresch, D.M. The role of silicon in the mitigation of water stress in Eugenia myrcianthes Nied. seedlings. Braz. J. Biol. 2022, 82, e260420. [Google Scholar] [CrossRef]
- Silva, M.S.; Scalon, S.P.Q.; Santos, C.C.; Silverio, J.M.; Santos, J.K.V.; Dresch, D.M. Does silicon help to alleviate water deficit stress and in the recovery of Dipteryx alata seedlings? Braz. J. Biol. 2022, 82, e259016. [Google Scholar] [CrossRef]
- Guirra, B.S.; Silva, J.A.; Leal, C.C.P.; Torres, S.B.; Silva, J.E.S.B.; Guirra, K.S.; Pereira, K.T.O. Growth and metabolism of Pityrocarpa moniliformis Benth. seedlings under water deficit. Ciênc. Florest. 2022, 32, 923–938. [Google Scholar] [CrossRef]
- Queiroz, T.B.; Rocha, S.M.G.; Fonseca, F.S.A.; Martins, E.R.; Alvarenga, I.C.A. Efeitos do déficit hídrico no cultivo de mudas de Eucalipto. Irriga 2017, 22, 659–674. [Google Scholar] [CrossRef]
- Silva, D.C.; Melo, A.S.; Melo, Y.L.; Andrade, W.L.; Lima, L.M.; Santos, A.R. Silicon foliar application attenuates the effects of water suppression on cowpea cultivars. Cienc. Agrotec. 2019, 43, e023019. [Google Scholar] [CrossRef]
- Santos, C.C.; Basso Júnior, I.J.; Navarro, V.L.; Silva, W.C.; Silverio, J.M.; Scalon, S.P.Q. Silicon alleviates damages on photosynthetic apparatus and increases resilience in young Inga vera exposed to water deficit. J. Soil Sci. Plant Nutr 2023, 1–13. [Google Scholar] [CrossRef]
- Ferminiano, A.P.; Kaseker, J.F.; Nohatto, M.A.; Oliveira, J.D.; Rosa, E.D.F.F.; Nunes, D.H. Aplicação de ácido salicílico em plantas de arroz submetidas a competição com arroz-vermelho. Agropecuária Cient. Semiárido 2018, 14, 198–203. [Google Scholar] [CrossRef]
- Gastl Filho, J.; Bonetti, L.L.S.; Araujo, R.S.; Santi, S.L.; Nascimento, V.A.; Vilarinho, M.S. Ácido salicílico e potencial germinativo na germinação de sementes de pepino. Rev. Inova Ciênc. Tecnol./Innov. Sci. Technol. J. 2017, 3, 7–12. [Google Scholar]
- Zhang, Y.; Yu, S.H.I.; Gong, H.J.; Zhao, H.L.; Li, H.L.; Hu, Y.H.; Wang, Y.C. Beneficial effects of silicon on photosynthesis of tomato seedlings under water stress. J. Int. Agric. 2018, 17, 2151–2159. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef]
- Fatima, R.T.; Jesus, E.G.; Guerrero, A.C.; Rocha, J.L.A.; Brito, M.E.B. Adubação silicatada como atenuante do estresse hídrico no crescimento e trocas gasosas da alface. Rev. Eng. Agric. 2019, 27, 170–178. [Google Scholar] [CrossRef]
- Junglos, F.S.; Junglos, M.S.; Dresch, D.M.; Pereira, N.S.; Kodama, F.M.; Scalon, S.P.Q. Recovery of the photosynthetic capacity of Campomanesia adamantium (Myrtaceae) after water deficit. Braz. J. Bot. 2016, 39, 541–546. [Google Scholar] [CrossRef]
- Bartieres, E.M.; Scalon, S.P.Q.; Dresch, D.M.; Cardoso, E.A.; Jesus, M.V.; Pereira, Z.V. Shading as a means of mitigating water deficit in seedlings of Campomanesia xanthocarpa (Mart.) O. Berg. Not. Bot. Horti Agrobot. 2020, 48, 234–244. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal, 6th ed.; Artmed: Porto Alegre, Brazil, 2017; 858p. [Google Scholar]
- Brito, C.; Dinis, L.T.; Meijón, M.; Ferreira, H.; Pinto, G.; Moutinho-Pereira, J.; Correia, C. Salicylic acid modulates olive tree physiological and growth responses to drought and rewatering events in a dose dependent manner. J. Plant Physiol. 2018, 230, 21–32. [Google Scholar] [CrossRef]
- Santos, C.C.; Silverio, J.M.; Scalon, S.P.Q.; Vieira, M.C. Hydrogel and water regimes in the chlorophyll-a fluorescence and growth of Campomanesia xanthocarpa seedlings. Eng. Agric. 2021, 3, 330–337. [Google Scholar] [CrossRef]
- Rosa, D.B.C.J.; Scalon, S.P.Q.; Cremon, T.; Dresch, D.M. Shading for water stress mitigation in Copaifera langsdorffii Desf. Seedlings. S. Afr. J. Bot. 2021, 140, 240–248. [Google Scholar] [CrossRef]
- Bartieres, E.M.M.; Dresch, D.M.; Reis, L.C.; Pereira, Z.V.; Mussury, R.M.; Scalon, S.P.Q. Sombreamento minimiza o efeito do déficit hídrico em mudas de Campomanesia xanthocarpa (Mart.) O. Berg. Braz. J. Biol. 2021, 83, e244718. [Google Scholar] [CrossRef]
- Moura, A.R.; Nogueira, R.M.C.; Silva, J.A.A.; Lima, T.V.D. Relações hídricas e solutos orgânicos em plantas jovens de Jatropha curcas L. sob diferentes regimes hídricos. Cienc. Florest. 2016, 26, 345–354. [Google Scholar] [CrossRef]
- Poór, P.; Borbély, P.; Bódi, N.; Bagyánszki, M. Effects of salicylic acid on photosynthetic activity and chloroplast morphology under light and prolonged darkness. Photosynthetica 2019, 57, 367–376. [Google Scholar] [CrossRef]
- Alvarez, M.E.; Savouré, A.; Szabados, L. Proline metabolism as regulatory hub. Trends Plant Sci. 2022, 27, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Furlan, A.L.; Bianucci, E.; Giordano, W.; Castro, S.; Becker, D.F. Proline metabolic dynamics and implications in drought tolerance of peanut plants. Plant Physiol. Bichem. 2020, 151, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Reis, S.M.; Marimon-Júnior, B.H.; Morandi, P.S.; Santos, C.O.; Oliveira, B.D.; Marimon, B.S. Desenvolvimento inicial e qualidade de mudas de Copaifera langsdorffii Desf. sob diferentes níveis de sombreamento. Cienc. Florest. 2016, 26, 11–20. [Google Scholar] [CrossRef]
- Gomes, S.H.M.; Gonçalves, F.B.; Ferreira, R.A.; Pereira, F.R.M.; Ribeiro, M.M.J. Avaliação dos parâmetros morfológicos da qualidade de mudas de Paubrasilia echinata (pau-brasil) em viveiro florestal. Sci. Plena 2019, 15, 11701. [Google Scholar] [CrossRef]
- Stotz, G.C.; Salgado-Luarte, C.; Escobedo, V.M.; Valladares, F.; Gianoli, E. Global trends in phenotypic plasticity of plants. Ecol. Lett. 2021, 24, 2267–2281. [Google Scholar] [CrossRef]
- Valladares, F.; Sanchez-Gomez, D.; Zavala, M.A. Quantitative estimation of phenotypic plasticity: Bridging the gap between the evolutionary concept and its ecological applications. J. Ecol. 2006, 94, 1103–1116. [Google Scholar] [CrossRef]
- Souza, C.C.; Oliveira, F.A.; Silva, I.F.; Amorim-Neto, M.S. Avaliação de métodos de determinação de água disponível e manejo da irrigação em terra roxa sob cultivo de algodoeiro herbáceo. Rev. Bras. Eng. Agric. Ambient. 2000, 4, 338–342. [Google Scholar] [CrossRef]
- Capitulino, J.D.; Lima, G.S.; Azevedo, C.A.V.; Silva, A.A.R.; Soares, L.A.; Arruda, T.F.L.; Sousa, V.D.; Gheyi, H.R. Morphophysiology of soursop under salt stress and H2O2 application in the pre-flowering phase. Rev. Bras. Eng. Agric. Ambient. 2023, 27, 948–957. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.A.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Colton-Gagnon, K.; Ali-Benali, M.A.; Mayer, B.F.; Dionne, R.; Bertrand, A.; Carmo, S.; Charron, J.B. Comparative analysis of the cold acclimation and freezing tolerance capacities of seven diploid Brachypodium distachyon accessions. Ann. Bot. 2014, 113, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Dickson, A.; Leaf, A.L.; Hosner, J.F. Quality appraisal of white spruce and white pine seedling stock in nurseries. For. Chron. 1960, 36, 10–13. [Google Scholar] [CrossRef]
- Ferreira, D.F. Sisvar: A computer analysis system to fixed effects Split plot type designs. Rev. Bras. Biom. 2019, 37, 529–535. [Google Scholar] [CrossRef]



| Treatments | PPI (0.00 a 1.00) | |||
|---|---|---|---|---|
| A | A/Ci | Fv/Fm | DQI | |
| Water restriction stress (WRS) | 0.909061 | 0.900543 | 0.883402 | 0.069024 |
| WRS + Si (0.5 g) | 0.931068 | 0.923442 | 0.948788 | 0.278752 |
| WRS + Si (1.0 g) | 0.902589 | 0.851954 | 0.938272 | 0.278752 |
| WRS + SA (50 mg) | 0.925566 | 0.917749 | 0.190672 | 0.499342 |
| WRS + SA (100 mg) | 0.888673 | 0.663373 | 0.947417 | 0.388007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueiredo, V.M.d.A.; Scalon, S.d.P.Q.; Santos, C.C.; Linné, J.A.; Silverio, J.M.; Cerqueira, W.M.; de Almeida, J.L.d.C.S. Do Silicon and Salicylic Acid Attenuate Water Deficit Damage in Talisia esculenta Radlk Seedlings? Plants 2023, 12, 3183. https://doi.org/10.3390/plants12183183
Figueiredo VMdA, Scalon SdPQ, Santos CC, Linné JA, Silverio JM, Cerqueira WM, de Almeida JLdCS. Do Silicon and Salicylic Acid Attenuate Water Deficit Damage in Talisia esculenta Radlk Seedlings? Plants. 2023; 12(18):3183. https://doi.org/10.3390/plants12183183
Chicago/Turabian StyleFigueiredo, Vanda Maria de Aquino, Silvana de Paula Quintão Scalon, Cleberton Correia Santos, Jéssica Aline Linné, Juliana Milene Silverio, Wállas Matos Cerqueira, and João Lucas da Costa Santos de Almeida. 2023. "Do Silicon and Salicylic Acid Attenuate Water Deficit Damage in Talisia esculenta Radlk Seedlings?" Plants 12, no. 18: 3183. https://doi.org/10.3390/plants12183183








