The Hepatoprotective Effects of Moringa oleifera against Antiretroviral-Induced Cytotoxicity in HepG2 Cells: A Review
Abstract
:1. Introduction
- -
- Literature published in English.
- -
- Literature published at least 5 years ago.
- -
- African-based research study articles.
- -
- Published literature on HIV, antiretroviral therapy, oxidative stress, antioxidants, MO, and its bioactive compounds.
- -
- Published literature intended to investigate the underlying mechanism of action.
- -
- Published literature with rationale and scientific evidence.
- -
- Research studies identified through scientific databases such as Google Scholar, PubMed, and Science Direct.
- -
- Biorender.com for creating figures.
- -
- Conference papers.
- -
- Bioactive compounds not related to the antioxidant effect of MO.
- -
- Research studies that are not focusing on the HepG2 cell line.
2. Prevalence of HIV/AIDS
2.1. Antiretroviral Therapy
2.2. The Approved Standard Treatment for HIV
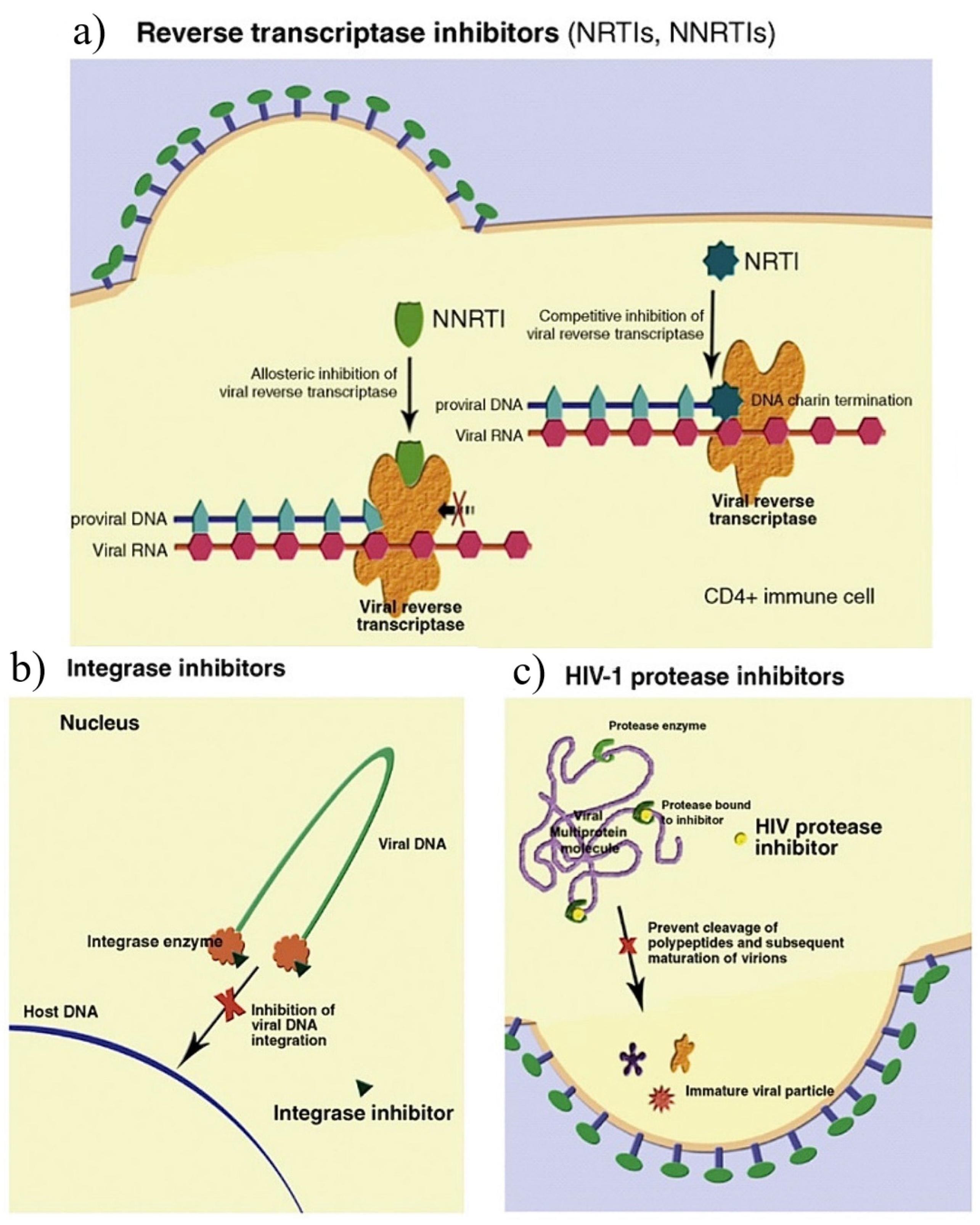
2.3. The Types of Antiretroviral Drugs
2.3.1. Tenofovir
2.3.2. Lamivudine
2.3.3. Dolutegravir
2.4. Antiretroviral Drugs’ Side-Effects
2.5. Oxidative Stress
2.6. Antioxidants
2.6.1. Superoxide Dismutase
2.6.2. Catalase
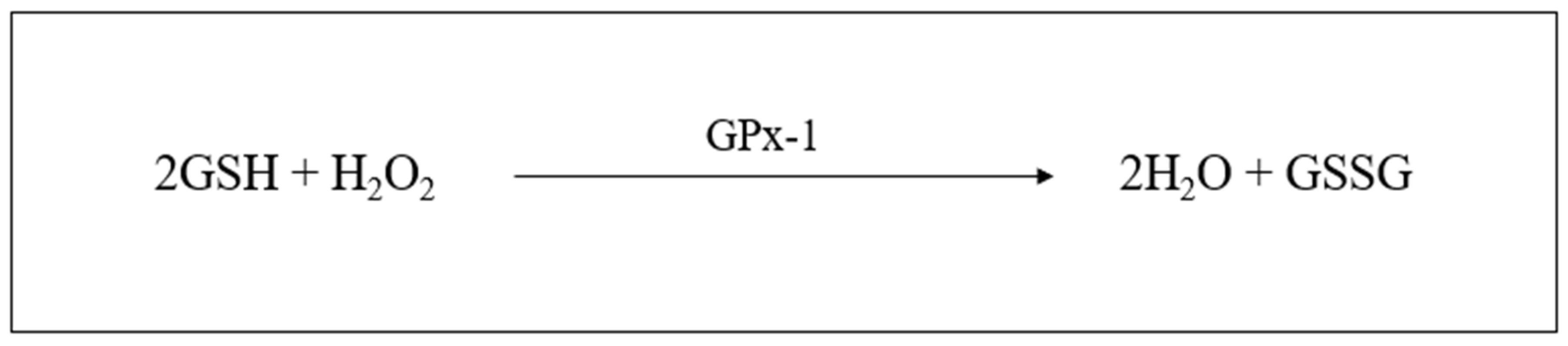
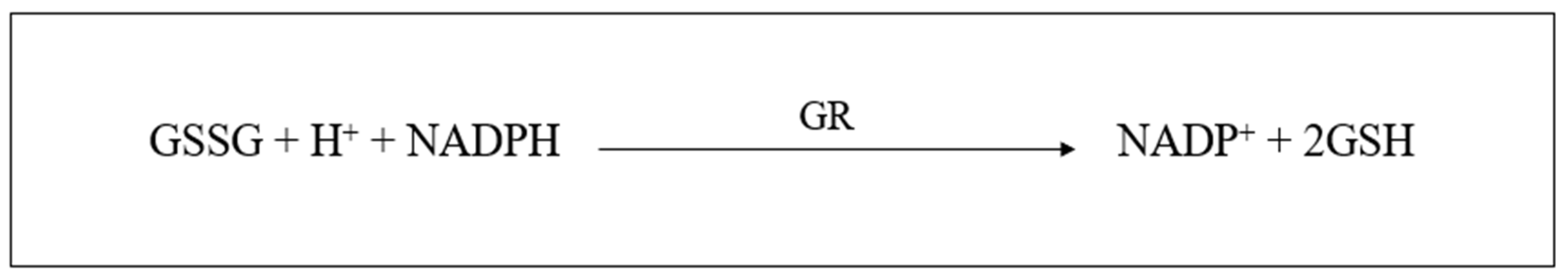
2.6.3. Glutathione
2.6.4. Nuclear-Factor-Erythroid-2-Related Factor 2
2.7. The Use of Traditional African Medicinal Plants
2.8. Moringa oleifera
2.8.1. Flavonoids
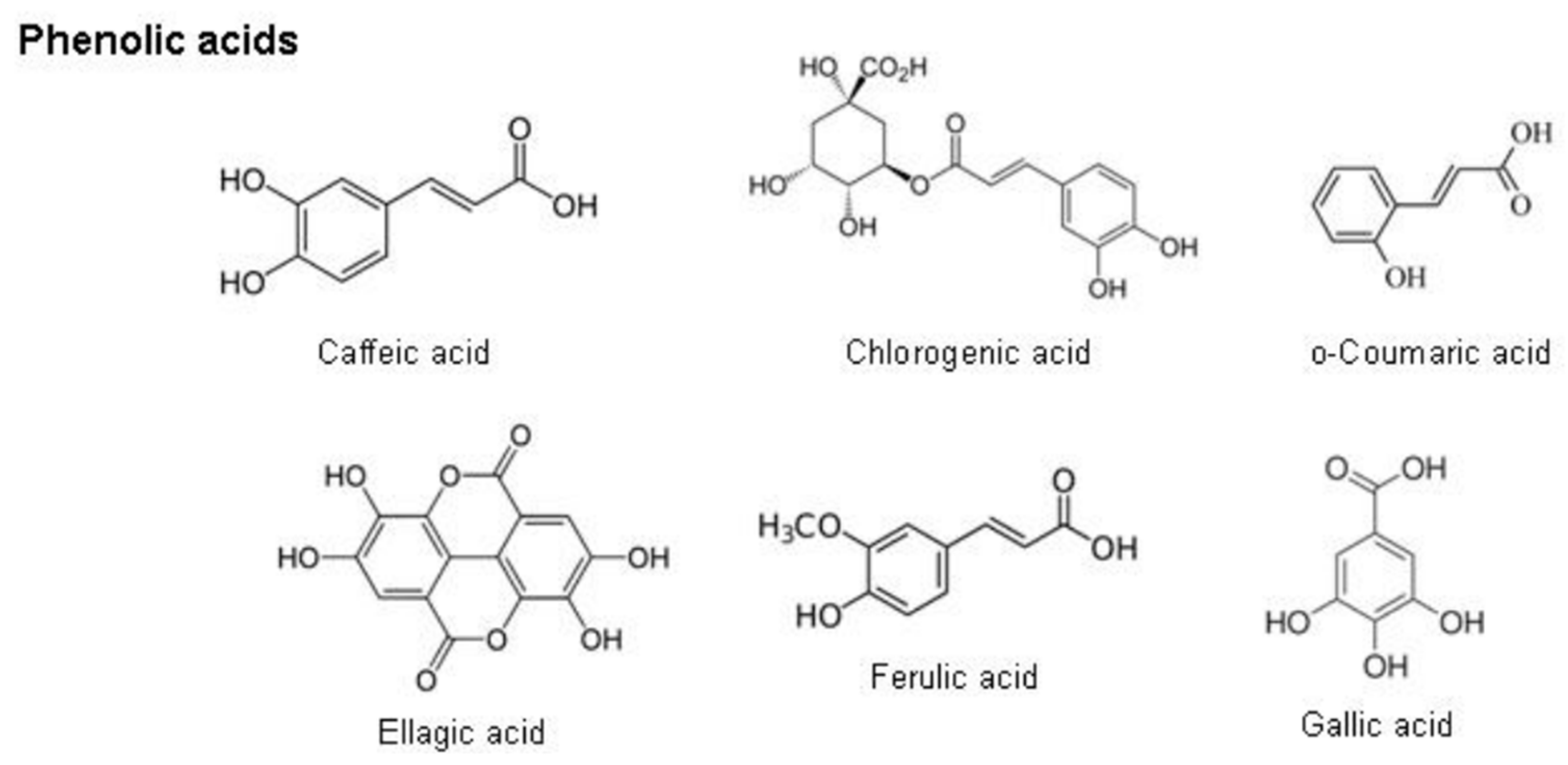
2.8.2. Phenolic Acids
2.8.3. Carotenoids
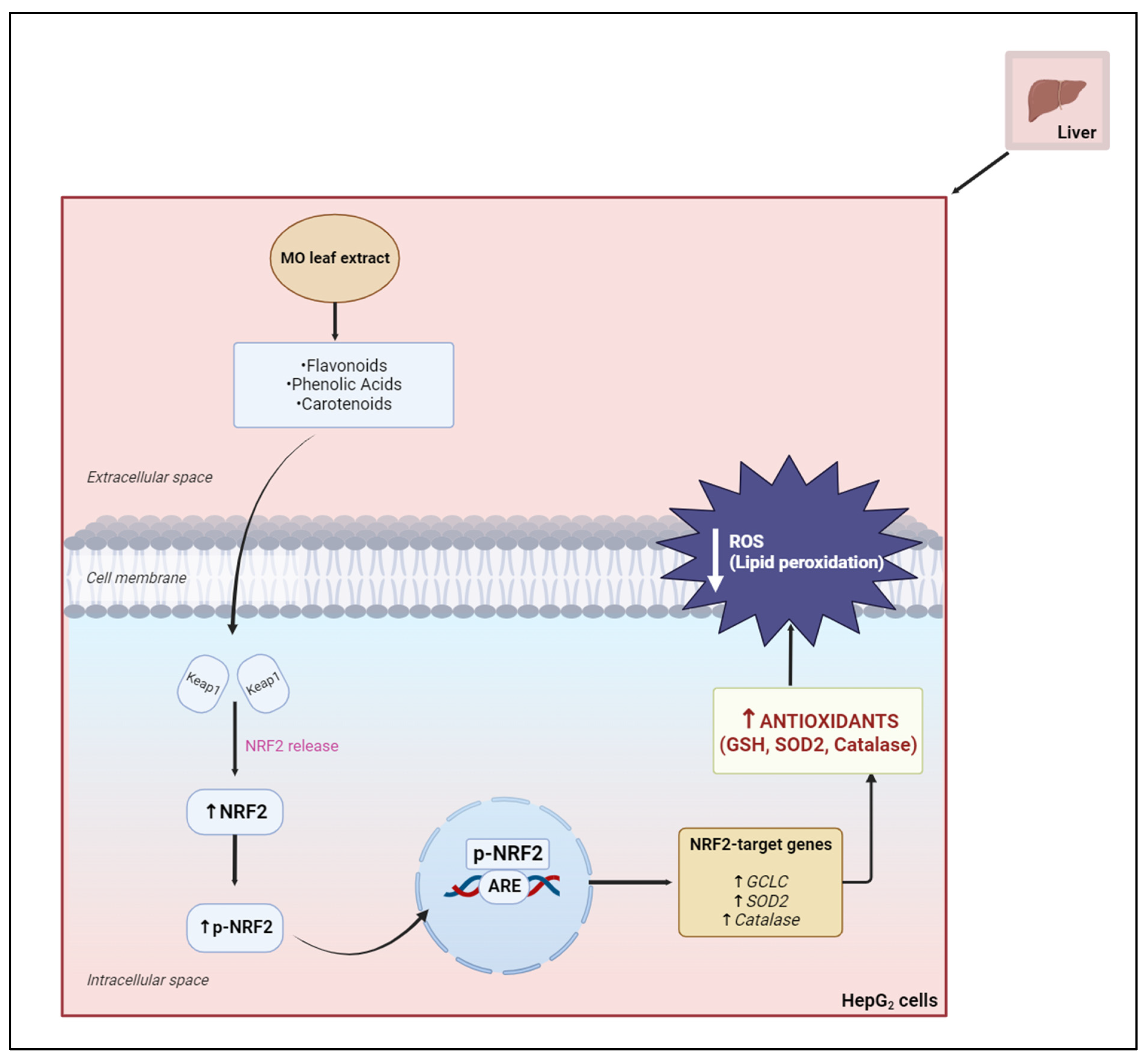
2.9. The Hepatoprotective Effects of Moringa oleifera against Oxidative Stress
2.10. The Use of Human HepG₂ Liver Cells In Vitro
3. Conclusions
4. Value of This Study
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Pillaye, J.N.; Marakalala, M.J.; Khumalo, N.; Spearman, W.; Ndlovu, H. Mechanistic insights into antiretroviral drug-induced liver injury. Pharmacol. Res. Perspect. 2020, 8, e00598. [Google Scholar] [CrossRef] [PubMed]
- Eilami, O.; Nazari, A.; Dousti, M.; Sayehmiri, F.; Ghasemi, M. Investigation of HIV/AIDS prevalence and associated risk factors among female sex workers from 2010 to 2017: A meta-analysis study. HIV/AIDS Res. Palliat. Care 2019, 11, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Spach, D.H. Antiretroviral Medications and Initial Therapy HIV Life Cycle and Antiretroviral Drug Targets; National HIV Curriculum, University of Washington: Seattle, WA, USA, 2020. [Google Scholar]
- Waters, L.; Mehta, V.; Gogtay, J.; Boffito, M. The Evidence for Using Tenofovir Disoproxil Fumarate Plus Lamivudine as a Nucleoside Analogue Backbone for the Treatment of HIV. J. Virus Erad. 2021, 7, 100028. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, T.P.; Peddiraju, K.; Fu, C.; Bakshi, K.; Yu, S.; Zhang, Z.; Tenorio, A.R.; Spancake, C.; Joshi, S.; Wolstenholme, A.; et al. Bioequivalence and Food Effect Assessment of 2 Fixed-Dose Combination Formulations of Dolutegravir and Lamivudine. Clin. Pharmacol. Drug Dev. 2020, 9, 189–202. [Google Scholar] [CrossRef]
- Umar, D.; Waziri, B.; Ndagi, U.; Mohammed, S.; Usman, N.; Abubakar-Muhammad, H. Impact of Tenofovir/Lamivudine/Dolutegravir (Tld) on the Health-Related Quality of Life and Clinical Outcomes of HIV/AIDS Patients at a Tertiary Health Facility in Niger State. 2020, pp. 1–18. Available online: https://www.researchsquare.com/article/rs-127277/v1 (accessed on 21 February 2021).
- Biswas, D.; Nandy, S.; Mukherjee, A.; Pandey, D.K.; Dey, A. Moringa oleifera Lam. and derived phytochemicals as promising antiviral agents: A review. S. Afr. J. Bot. 2020, 129, 272–282. [Google Scholar] [CrossRef]
- Salami, A.T.; Okonkwo, C.E.; Attah, F.A.; Olagoke, O.C. Bioactive Moringa olifera seed extracts attenuates cholesterol gall stones in hyperglycaemic Swiss mice. Comp. Clin. Pathol. 2021, 30, 207–216. [Google Scholar] [CrossRef]
- Rani, N.Z.A.; Husain, K.; Kumolosasi, E. Moringa genus: A review of phytochemistry and pharmacology. Front. Pharmacol. 2018, 9, 108. [Google Scholar] [CrossRef]
- Stohs, S.J.; Hartman, M.J. Review of the safety and efficacy of Moringa oleifera. Phytother. Res. 2015, 29, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Chen, S.; Guo, L. Chapter 8 Use of Liver-Derived Cell Lines for the Study. In Drug-Induced Liver Toxicity; Humana: New York, NY, USA, 2018. [Google Scholar]
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Bioactive components in Moringa oleifera leaves protect against chronic disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef]
- Nizioł-ŁUkaszewska, Z.; Furman-Toczek, D.; Bujak, T.; Wasilewski, T.; Hordyjewicz-Baran, Z. Moringa oleifera L. Extracts as Bioactive Ingredients That Increase Safety of Body Wash Cosmetics. Dermatol. Res. Pract. 2020, 2020, 8197902. [Google Scholar] [CrossRef]
- Sharp, P.M.; Hahn, B.H. The evolution of HIV-1 and the origin of AIDS. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2487–2494. [Google Scholar] [CrossRef]
- Heeney, J.L.; Dalgleish, A.G.; Weiss, R.A. Origins of HIV and the evolution of resistance to AIDS. Science 2006, 313, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Van Heuverswyn, F.; Peeters, M. The origins of HIV and implications for the global epidemic. Curr. Infect. Dis. Rep. 2007, 9, 338–346. [Google Scholar] [CrossRef] [PubMed]
- De Cock, K.M.; Jaffe, H.W.; Curran, J.W. The evolving epidemiology of HIV/AIDS. Aids 2012, 26, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Delpech, V.; Gahagan, J. The global epidemiology of HIV. Medicine 2009, 37, 317–320. [Google Scholar] [CrossRef]
- Ncube, S.; Madikizela, L.M.; Chimuka, L.; Nindi, M.M. Environmental fate and ecotoxicological effects of antiretrovirals: A current global status and future perspectives. Water Res. 2018, 145, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Bayer, W.; Ploquin, M.J.Y.; Kassiotis, G.; Hasenkrug, K.J.; Dittmer, U. Distinct roles of CD4+ T cell subpopulations in retroviral immunity: Lessons from the Friend virus mouse model. Retrovirology 2011, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.L.; McKinstry, K.K.; Strutt, T.M. Expanding roles for CD4+ T cells in immunity to viruses. Nat. Rev. Immunol. 2012, 12, 136–148. [Google Scholar] [CrossRef]
- Aavani, P.; Allen, L.J.S. The role of CD4 T cells in immune system activation and viral reproduction in a simple model for HIV infection. Appl. Math. Model. 2019, 75, 210–222. [Google Scholar] [CrossRef]
- Kirchhoff, F. HIV life cycle: Overview. Encycl. AIDS 2013, 1–9. [Google Scholar] [CrossRef]
- Pillay, Y.; Johnson, L. World AIDS day 2020: Reflections on global and South African progress and continuing challenges. S. Afr. J. HIV Med. 2021, 22, 1205. [Google Scholar] [CrossRef] [PubMed]
- Heyer, A.; Ogunbanjo, G.A. Adherence to HIV antiretroviral therapy. Part I: A review of factors that influence adherence. S. Afr. Fam. Pract. 2006, 48, 5–9. [Google Scholar] [CrossRef]
- Orton, P.M.; Sokhela, D.G.; Nokes, K.M.; Perazzo, J.D.; Webel, A.R. Factors related to functional exercise capacity amongst people with HIV in Durban, South Africa. Health SA Gesondheid 2021, 26, a1532. [Google Scholar] [CrossRef] [PubMed]
- Ramlagan, S.; Matseke, G.; Rodriguez, V.J.; Jones, D.L.; Peltzer, K.; Ruiter, R.A.C.; Sifunda, S. Determinants of disclosure and non-disclosure of HIV-positive status, by pregnant women in rural South Africa. Sahara J. 2018, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Mabaso, M.; Makola, L.; Naidoo, I.; Mlangeni, L.L.; Jooste, S.; Simbayi, L. HIV prevalence in South Africa through gender and racial lenses: Results from the 2012 population-based national household survey. Int. J. Equity Health 2019, 18, 167. [Google Scholar] [CrossRef] [PubMed]
- Loeliger, K.B.; Niccolai, L.M.; Mtungwa, L.N.; Moll, A.; Shenoi, S.V. “I have to push him with a wheelbarrow to the clinic”: Community health workers’ roles, needs, and strategies to improve HIV care in rural South Africa. AIDS Patient Care STDS 2016, 30, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Bessong, P.O.; Matume, N.D.; Tebit, D.M. Potential challenges to sustained viral load suppression in the HIV treatment programme in South Africa: A narrative overview. AIDS Res. Ther. 2021, 18, 1. [Google Scholar] [CrossRef]
- Torti, C.; Prosperi, M.; Motta, D.; Digiambenedetto, S.; Maggiolo, F.; Paraninfo, G.; Ripamonti, D.; Cologni, G.; Fabbiani, M.; Caputo, S.L.; et al. Factors influencing the normalization of CD4+ T-cell count, percentage and CD4+/CD8+ T-cell ratio in HIV-infected patients on long-term suppressive antiretroviral therapy. Clin. Microbiol. Infect. 2012, 18, 449–458. [Google Scholar] [CrossRef]
- Levi, J.; Raymond, A.; Pozniak, A.; Vernazza, P.; Kohler, P.; Hill, A. Can the UNAIDS 90-90-90 target be achieved? A systematic analysis of national HIV treatment cascades. BMJ Glob. Health 2016, 1, e000010. [Google Scholar] [CrossRef]
- AVERT. South Africa 90-90-90 Progress. 2020. Available online: https://www.avert.org/infographics/south-africa-90-90-90-progress (accessed on 14 October 2021).
- Moses, K.; Adefisayo, O.A.; Maryam, B.; Adeoye, A.; Oluwatosin, A.; Abiye, K.; Kent, K.; Iyiola, F.; Tolu, A.; Homsuk, S.; et al. Virologic Response among Key Populations Living with HIV following a Switch to Dolutegravir-Based Regimen in Southern Nigeria. Int. J. Virol. AIDS 2020, 7, 69. [Google Scholar] [CrossRef]
- Sibanda, M.; Manimbulu, M.N.; Naidoo, P. Concurrent use of Antiretroviral and African traditional medicines amongst people living with HIV/AIDS (PLWA) in the eThekwini Metropolitan area of KwaZulu Natal. Afr. Health Sci. 2016, 16, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Kis, O.; Robillard, K.; Chan, G.N.Y.; Bendayan, R. The complexities of antiretroviral drug-drug interactions: Role of ABC and SLC transporters. Trends Pharmacol. Sci. 2010, 31, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Max, B.; Sherer, R. Management of the adverse effects of antiretroviral therapy and medication adherence. Clin. Infect. Dis. 2000, 30, S96–S116. [Google Scholar] [CrossRef] [PubMed]
- Ha, B.; Larsen, K.P.; Zhang, J.; Fu, Z.; Montabana, E.; Jackson, L.N.; Chen, D.-H.; Puglisi, E.V. High-resolution view of HIV-1 reverse transcriptase initiation complexes and inhibition by NNRTI drugs. Nat. Commun. 2021, 12, 2500. [Google Scholar] [CrossRef] [PubMed]
- Temesgen, Z.; Warnke, D.; Kasten, M.J. Current status of antiretroviral therapy. Expert Opin. Pharmacother. 2006, 7, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- Eggleton, J.; Nagalli, S. Highly Active Antiretroviral Therapy (HAART); StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Weber, I.T.; Wang, Y.F.; Harrison, R.W. HIV protease: Historical perspective and current research. Viruses 2021, 13, 839. [Google Scholar] [CrossRef] [PubMed]
- Gabazana, Z.; Sitole, L. Raman-based metabonomics unravels metabolic changes related to a first-line tenofovir-based treatment in a small cohort of South African HIV-infected patients. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 248, 119256. [Google Scholar] [CrossRef]
- Kouanfack, C.; Mpoudi-Etame, M.; Bassega, O.; Eymard Duvernay, S.; Leroy, S.; Boyer, S.; Peeters, M.; Calmy, A.; Delaporte, E. Dolutegravir-Based or Low-Dose Efavirenz–Based Regimen for the Treatment of HIV-1. N. Engl. J. Med. 2019, 381, 816–826. [Google Scholar]
- Raffi, F.; Pozniak, A.L.; Wainberg, M.A. Has the time come to abandon efavirenz for first-line antiretroviral therapy? J. Antimicrob. Chemother. 2014, 69, 1742–1747. [Google Scholar] [CrossRef]
- Mendelsohn, A.S.; Ritchwood, T. COVID-19 and Antiretroviral Therapies: South Africa’s Charge Towards 90–90–90 in the Midst of a Second Pandemic. AIDS Behav. 2020, 24, 2754–2756. [Google Scholar] [CrossRef]
- Desta, A.; Biru, T.T.; Kefale, A.T. Health related quality of life of people receiving highly active antiretroviral therapy in Southwest Ethiopia. PLoS ONE 2020, 15, e0237013. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.L.; Kiser, J.J.; Gardner, E.M.; Rower, J.E.; Meditz, A.; Grant, R.M. Pharmacological considerations for tenofovir and emtricitabine to prevent HIV infection. J. Antimicrob. Chemother. 2011, 66, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Geboers, S.; Haenen, S.; Mols, R.; Brouwers, J.; Tack, J.; Annaert, P.; Augustijns, P. Intestinal behavior of the ester prodrug tenofovir DF in humans. Int. J. Pharm. 2015, 485, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Wassner, C.; Bradley, N.; Lee, Y. A Review and Clinical Understanding of Tenofovir: Tenofovir Disoproxil Fumarate versus Tenofovir Alafenamide. J. Int. Assoc. Provid. AIDS Care 2020, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cressey, T.R.; Siriprakaisil, O.; Kubiak, R.W.; Klinbuayaem, V.; Sukrakanchana, P.; Quame-Amaglo, J.; Okochi, H.; Tawon, Y.; Cressey, R.; Baeten, J.M.; et al. Plasma pharmacokinetics and urinary excretion of tenofovir following cessation in adults with controlled levels of adherence to tenofovir disoproxil fumarate. Int. J. Infect. Dis. 2020, 97, 365–370. [Google Scholar] [CrossRef] [PubMed]
- James, A.M.; Ofotokun, I.; Sheth, A.; Acosta, E.P.; King, J.R. Tenofovir: Once-daily dosage in the management of HIV infection. Clin. Med. Insights Ther. 2012, 4, 201–216. [Google Scholar] [CrossRef]
- Kearney, B.P.; Flaherty, J.F.; Shah, J. Tenofovir disoproxil fumarate: Clinical pharmacology and pharmacokinetics. Clin. Pharmacokinet. 2004, 43, 595–612. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Fernandez, B.; Montoya-Ferrer, A.; Sanz, A.B.; Sanchez-Niño, M.D.; Izquierdo, M.C.; Poveda, J.; Sainz-Prestel, V.; Ortiz-Martin, N.; Parra-Rodriguez, A.; Selgas, R.; et al. Tenofovir nephrotoxicity: 2011 update. AIDS Res. Treat. 2011, 2011, 354908. [Google Scholar] [CrossRef]
- Taylor, K.; Fritz, K.; Parmar, M. Lamivudine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Else, L.J.; Jackson, A.; Puls, R.; Hill, A.; Fahey, P.; Lin, E.; Amara, A.; Siccardi, M.; Watson, V.; Tjia, J.; et al. Pharmacokinetics of Lamivudine and Lamivudine-Triphosphate after Administration of 300 Milligrams and 150 Milligrams Once Daily to Healthy Volunteers: Results of the ENCORE 2 Study. Antimicrob. Agents Chemother. 2012, 56, 1427–1433. [Google Scholar] [CrossRef]
- Mohan, H.; Lenis, M.G.; Laurette, E.Y.; Tejada, O.; Sanghvi, T.; Leung, K.Y.; Cahill, L.S.; Sled, J.G.; Delgado-Olguín, P.; Greene, N.D.E.; et al. Dolutegravir in pregnant mice is associated with increased rates of fetal defects at therapeutic but not at supratherapeutic levels. eBioMedicine 2021, 63, 103167. [Google Scholar] [CrossRef]
- Kandel, C.E.; Walmsley, S.L. Dolutegravir—A review of the pharmacology, efficacy, and safety in the treatment of HIV. Drug Des. Dev. Ther. 2015, 9, 3547–3555. [Google Scholar] [CrossRef] [PubMed]
- Castellino, S.; Moss, L.; Wagner, D.; Borland, J.; Song, I.; Chen, S.; Lou, Y.; Min, S.S.; Goljer, I.; Culp, A.; et al. Metabolism, excretion, and mass balance of the HIV-1 integrase inhibitor dolutegravir in humans. Antimicrob. Agents Chemother. 2013, 57, 3536–3546. [Google Scholar] [CrossRef] [PubMed]
- Kaeni, M.C. Safety, Tolerability and Adherence of Dtg-Based Regimen among Adult HIV Patients Attending Kenyatta National Hospital; University of Nairobi: Nairobi, Kenya, 2020. [Google Scholar]
- Gambo, A.; Moodley, I.; Babashani, M.; Babalola, T.K.; Gqaleni, N. A double-blind, randomized controlled trial to examine the effect of Moringa oleifera leaf powder supplementation on the immune status and anthropometric parameters of adult HIV patients on antiretroviral therapy in a resource-limited setting. PLoS ONE 2021, 16, e0261935. [Google Scholar] [CrossRef] [PubMed]
- Damtie, D.; Yismaw, G.; Woldeyohannes, D.; Anagaw, B. Common opportunistic infections and their CD4 cell correlates among HIV-infected patients attending at antiretroviral therapy clinic of Gondar University Hospital, Northwest Ethiopia. BMC Res. Notes 2013, 6, 534. [Google Scholar] [CrossRef] [PubMed]
- Elias, A.; Nelson, B.; Oputiri, D.; Geoffrey, O.B.P. Antiretroviral toxicity and oxidative stress. Am. J. Pharmacol. Toxicol. 2013, 8, 187–196. [Google Scholar] [CrossRef]
- Paniagua, A.C.; Amariles, P. Hepatotoxicity by Drugs, Pharmacokinetics and Adverse Effects of Drugs—Mechanisms and Risks Factors; IntechOpen: London, UK, 2018. [Google Scholar]
- Singh, A.; Bhat, T.K.; Sharma, O.P. Clinical biochemistry of hepatotoxicity. J. Clinic. Toxicol. S. 2011, 4, 2161-0495. [Google Scholar]
- Chhatwani, C.; Purohit, J.; Vakil, A.; Khunt, S. Tenofovir Induced Severe Lactic Acidosis and Hepatitis. Natl. J. Med. Res. 2016, 6, 288–289. [Google Scholar]
- Smith, R.L.; de Boer, R.; Brul, S.; Budovskaya, Y.; van der Spek, H. Premature and accelerated aging: HIV or HAART? Front. Genet. 2013, 3, 328. [Google Scholar] [CrossRef]
- Akay, C.; Cooper, M.; Odeleye, A.; Jensen, B.K.; White, M.G.; Vassoler, F.; Gannon, P.J.; Mankowski, J.; Dorsey, J.L.; Buch, A.M.; et al. Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system. J. Neurovirol. 2014, 20, 39–53. [Google Scholar] [CrossRef]
- Warnke, D.; Barreto, J.; Temesgen, Z. Therapeutic review: Antiretroviral drugs. J. Clin. Pharmacol. 2007, 47, 1570–1579. [Google Scholar] [CrossRef]
- Kline, E.R.; Bassit, L.; Hernandez-Santiago, B.I.; Detorio, M.A.; Liang, B.; Kleinhenz, D.J.; Walp, E.R.; Dikalov, S.; Jones, D.P.; Schinazi, R.F.; et al. Long-term exposure to AZT, but not d4T, increases endothelial cell oxidative stress and mitochondrial dysfunction. Cardiovasc. Toxicol. 2009, 9, 1–12. [Google Scholar] [CrossRef]
- Ďuračková, Z. Some current insights into oxidative stress. Physiol. Res. 2010, 8408, 459–469. Available online: http://www.biomed.cas.cz/physiolres/pdf/59/59_459.pdf (accessed on 15 February 2021). [CrossRef] [PubMed]
- de Andrade Júnior, D.R.; Souza, R.B.; Santos, S.A. Oxygen free radicals and pulmonary disease. J. Bras. Pneumol. J. Bras. Pneumol. 2005, 31, 60–68. [Google Scholar]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.J.; Brand, M.D. Reactive oxygen species production by mitochondria. Methods Mol. Biol. 2009, 554, 165–181. [Google Scholar] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [PubMed]
- Andrés Juan, C.; Manuel Pérez de la Lastra, J.; Plou, F.J.; Pérez-Lebeña, E.; Reinbothe, S. Molecular Sciences The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Gupta, S.D. Chapter 11 Role of Free Radicals and Antioxidants in In Vitro. 2010. Available online: https://www.researchgate.net/profile/Snehasish-Dutta-Gupta/publication/260227296_Role_of_free_radicals_and_antioxidants_in_in_vitro_morphogenesis/links/0deec53832658c2751000000/Role-of-free-radicals-and-antioxidants-in-in-vitro-morphogenesis.pdf (accessed on 16 February 2021).
- Doshi, S.B.; Khullar, K.; Sharma, R.K.; Agarwal, A. Role of reactive nitrogen species in male infertility. Reprod. Biol. Endocrinol. 2012, 10, 109. [Google Scholar] [CrossRef]
- Martínez, C.M.; Andriantsitohaina, R. Reactive Nitrogen Species: Molecular Mechanisms and Potential Significance in Health and Disease. Antioxid. Redox Signal. 2009, 11, 669–702. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Review Article Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxidative Med. Cell. Longev 2019, 2019, 5080843. [Google Scholar]
- Mas-Bargues, C.; Escrivá, C.; Dromant, M.; Borrás, C.; Viña, J. Lipid peroxidation as measured by chromatographic determination of malondialdehyde. Human plasma reference values in health and disease. Arch. Biochem. Biophys. 2021, 709, 108941. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G.; Pizzimenti, S.; Daga, M.; Dianzani, C.; Arcaro, A.; Cetrangolo, G.P.; Giordano, G.; Cucci, M.A.; Graf, M.; Gentile, F. Lipid peroxidation-derived aldehydes, 4-hydroxynonenal and malondialdehyde in aging-related disorders. Antioxidants 2018, 7, 102. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Alkadi, H. A Review on Free Radicals and Antioxidants. Infect. Disord. Drug Targets 2018, 20, 16–26. [Google Scholar]
- Henriksen, E.J. Role of Oxidative Stress in the Pathogenesis of Insulin Resistance and Type 2 Diabetes. In Bioactive Food as Dietary Interventions for Diabetes; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–17. [Google Scholar]
- Kuciel-Lewandowska, J.M.; Pawlik-Sobecka, L.; Płaczkowska, S.; Kokot, I.; Paprocka-Borowicz, M. The assessment of the integrated antioxidant system of the body and the phenomenon of spa reaction in the course of radon therapy: A pilot study. Adv. Clin. Exp. Med. 2018, 27, 1341–1346. [Google Scholar] [PubMed]
- Matough, F.A.; Budin, S.B.; Hamid, Z.A.; Alwahaibi, N.; Mohamed, J. The role of oxidative stress and antioxidants in diabetic complications. Sultan. Qaboos Univ. Med. J. 2012, 12, 556–569. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Bohn, T. Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- de Vries, H.E.; Witte, M.; Hondius, D.; Rozemuller, A.J.M.; Drukarch, B.; Hoozemans, J.; van Horssen, J. Nrf2-induced antioxidant protection: A promising target to counteract ROS-mediated damage in neurodegenerative disease? Free. Radic. Biol. Med. 2008, 4, 1375–1383. [Google Scholar] [CrossRef]
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018, 12, 88–93. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29896077%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC5969776 (accessed on 2 March 2021).
- Pourvali, K.; Abbasi, M.; Mottaghi, A. Role of superoxide dismutase 2 gene Ala16Val polymorphism and total antioxidant capacity in diabetes and its complications. Avicenna J. Med. Biotechnol. 2016, 8, 48–56. [Google Scholar] [PubMed]
- Glorieux, C.; Zamocky, M.; Sandoval, J.M.; Verrax, J.; Calderon, P.B. Regulation of catalase expression in healthy and cancerous cells. Free. Radic. Biol. Med. 2015, 87, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Grigoras, A.G. Catalase immobilization—A review. Biochem. Eng. J. 2017, 117, 1–20. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Queval, G.; Mhamdi, A.; Chaouch, S.; Foyer, C.H. Glutathione. Arab. Book 2011, 9, e0142. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione Metabolism and Its Implications for Health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef]
- Franklin, C.C.; Backos, D.S.; Mohar, I.; White, C.C.; Forman, H.J.; Kavanagh, T.J. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol. Asp. Med. 2009, 30, 86–98. [Google Scholar] [CrossRef]
- Krejsa, C.M.; Franklin, C.C.; White, C.C.; Ledbetter, J.A.; Schieven, G.L.; Kavanagh, T.J. Rapid activation of glutamate cysteine ligase following oxidative stress. J. Biol. Chem. 2010, 285, 16116–16124. [Google Scholar] [CrossRef]
- Koide, S.I.; Kugiyama, K.; Sugiyama, S.; Nakamura, S.I.; Fukushima, H.; Honda, O.; Yoshimura, M.; Ogawa, H. Association of polymorphism in glutamate-cysteine ligase catalytic subunit gene with coronary vasomotor dysfunction and myocardial infarction. J. Am. Coll. Cardiol. 2003, 41, 539–545. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3143–3153. [Google Scholar] [CrossRef]
- Behnisch-Cornwell, S.; Wolff, L.; Bednarski, P.J. The effect of glutathione peroxidase-1 knockout on anticancer drug sensitivities and reactive oxygen species in haploid HAP-1 cells. Antioxidants 2020, 9, 1300. [Google Scholar] [CrossRef] [PubMed]
- Sarıkaya, E.; Doğan, S. Glutathione Peroxidase in Health and Diseases; IntechOpen: London, UK, 2020; Volume 49, Available online: https://www.researchgate.net/publication/339093247_Glutathione_Peroxidase_in_Health_and_Diseases (accessed on 3 March 2021).
- Guo, K.; Ge, J.; Zhang, C.; Lv, M.W.; Zhang, Q.; Talukder, M.; Li, J.-L. Cadmium induced cardiac inflammation in chicken (Gallus gallus) via modulating cytochrome P450 systems and Nrf2 mediated antioxidant defense. Chemosphere 2020, 249, 125858. [Google Scholar] [CrossRef] [PubMed]
- Nagiah, S.; Phulukdaree, A.; Chuturgoon, A. Mitochondrial and Oxidative Stress Response in HepG2 Cells Following Acute and Prolonged Exposure to Antiretroviral Drugs. J. Cell Biochem. 2015, 116, 1939–1946. [Google Scholar] [CrossRef] [PubMed]
- Holmström, K.M.; Kostov, R.V.; Dinkova-Kostova, A.T. The multifaceted role of Nrf2 in mitochondrial function. Curr. Opin. Toxicol. 2016, 2, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Gold, R.; Linker, R.A. Mechanisms of oxidative damage in multiple sclerosis and neurodegenerative diseases: Therapeutic modulation via fumaric acid esters. Int. J. Mol. Sci. 2012, 13, 11783–11803. [Google Scholar] [CrossRef]
- Sobiecki, J.F. The Intersection of Culture and Science in South African Traditional Medicine. Indo-Pac. J. Phenomenol. 2014, 14, 1–10. [Google Scholar] [CrossRef]
- Street, R.A.; Stirk, W.A.; Van Staden, J. South African traditional medicinal plant trade-Challenges in regulating quality, safety and efficacy. J. Ethnopharmacol. 2008, 119, 705–710. [Google Scholar] [CrossRef]
- Street, R.A.; Prinsloo, G. Commercially important medicinal plants of South Africa: A review. J. Chem. 2013, 2013, 205048. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Peltzer, K.; Preez, N.F.d.; Ramlagan, S.; Fomundam, H.; Anderson, J.; Chanetsa, L. Antiretrovirals and the Use of Traditional, Complementary and Alternative Medicine by HIV Patients in Kwazulu-Natal, South Africa: A longitudinal study. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 337–345. [Google Scholar] [CrossRef]
- Mills, E.; Cooper, C.; Seely, D.; Kanfer, I. African herbal medicines in the treatment of HIV: Hypoxis and Sutherlandia. An overview of evidence and pharmacology. Nutr. J. 2005, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Dukhi, N.; Taylor, M. A focus on four popular “functional foods” as part of a strategy to combat metabolic disease through the increased consumption of fruits and vegetables. Curr. Res. Nutr. Food Sci. 2018, 6, 294–306. [Google Scholar] [CrossRef]
- Luhlaza-ISS. Growing and Agro-Processing of Moringa Oleifera with Commercial Potential in South Africa; Industrial Development Corporation of South Africa (IDC); 2006; Volume 43, pp. 1–108. Available online: https://drive.google.com/file/d/1iZfoUMXG_2FZIucWnWxos0FdPnak_b1S/view (accessed on 23 October 2021).
- Monera-Penduka, T.G.; Maponga, C.C.; Wolfe, A.R.; Wiesner, L.; Morse, G.D.; Nhachi, C.F.B. Effect of Moringa oleifera Lam. leaf powder on the pharmacokinetics of nevirapine in HIV-infected adults: A one sequence cross-over study. AIDS Res. Ther. 2017, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Coppin, J. A Study of the Nutritional and Medicinal Values of Moringa oleifera Leaves from Sub-Saharan Africa: Ghana, Rwanda, Senegal and Zambia. Master’s Thesis, The State University of New Jersey, New Brunswick, NJ, USA, 2008. [Google Scholar]
- Razis, A.F.A.; Ibrahim, M.D.; Kntayya, S.B. Health benefits of Moringa oleifera. Asian Pac. J. Cancer Prev. 2014, 15, 8571–8576. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, B.; Warude, D.; Pushpangadan, P.; Bhatt, N. Ayurveda and traditional Chinese medicine: A comparative overview. Evid. Based Complement. Altern. Med. 2005, 2, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Banerjee, M.; Mandal, V.; Shukla, A.C.; Mandal, S.C. Modernization of Ayurveda: A brief overview of Indian initiatives. Nat. Prod. Commun. 2014, 9, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Cultivation, Genetic, Ethnopharmacology, Phytochemistry and Pharmacology of Moringa oleifera Leaves: An Overview. Int. J. Mol. Sci. 2015, 16, 12791–12835. [Google Scholar] [CrossRef]
- Tomar, M.; Subha, K.; Reetu, D.; Bhargavi, H.A. Moringa oleifera: A health food for animal and human consumption. Food Sci. Rep. 2020, 1, 11–14. [Google Scholar]
- Devkota, S.; Bhusal, K.K. Moringa oleifera: A miracle multipurpose tree for agroforestry and climate change mitigation from the Himalayas—A review. Cogent Food Agric. 2020, 6, 1805951. [Google Scholar] [CrossRef]
- Mashamaite, C.V.; Pieterse, P.J.; Mothapo, P.N.; Phiri, E.E. Moringa oleifera in South Africa: A review on its production, growing conditions and consumption as a food source. S. Afr. J. Sci. 2021, 117, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tiloke, C. The Antiproliferative and Apoptosis Inducing Effects of Moringa oleifera Aqueous Leaf Extract and Its Synthesised Gold Nanoparticles-Modulation of Oncogenes and Tumour Supppressor Genes in Human Cancer Cell Lines. Ph.D. Thesis, University of KwaZulu-Natal, Durban, South Africa, 2015. [Google Scholar]
- Paikra, B.K.; Dhongade, H.K.J.; Gidwani, B. Phytochemistry and pharmacology of Moringa oleifera Lam. J. Pharmacopunct. 2017, 20, 194–200. [Google Scholar]
- Trigo, C.; Castelló, M.L.; Ortolá, M.D.; García-Mares, F.J.; Soriano, M.D. Moringa oleifera: An Unknown Crop in Developed Countries with Great Potential for Industry and Adapted to Climate Change. Foods 2021, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Peñalver, R.; Martínez-Zamora, L.; Lorenzo, J.M.; Ros, G.; Nieto, G. Nutritional and Antioxidant Properties of Moringa oleifera Leaves in Functional Foods. Foods 2022, 11, 1107. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.; Kumar, S.; Riar, C.S.; Jindal, N.; Baniwal, P.; Guiné, R.P.F.; Correia, P.M.R.; Mehra, R.; Kumar, H. Recent Advances in Drumstick (Moringa oleifera) Leaves Bioactive Compounds: Composition, Health Benefits, Bioaccessibility, and Dietary Applications. Antioxidants 2022, 11, 402. [Google Scholar] [CrossRef] [PubMed]
- Safe, S.; Jayaraman, A.; Chapkin, R.S.; Howard, M.; Mohankumar, K.; Shrestha, R. Flavonoids: Structure–function and mechanisms of action and opportunities for drug development. Toxicol. Res. 2021, 37, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Zhang, J.; Chen, X. Bioactive flavonoids in Moringa oleifera and their health-promoting properties. J. Funct. Foods 2018, 47, 469–479. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, E47. [Google Scholar] [CrossRef]
- de la Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Phenolic compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2018; pp. 253–271. [Google Scholar]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef]
- Rungratanawanich, W.; Memo, M.; Uberti, D. Redox homeostasis and natural dietary compounds: Focusing on antioxidants of rice (Oryza sativa L.). Nutrients 2018, 10, 1605. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mezzomo, N.; Ferreira, S.R.S. Carotenoids functionality, sources, and processing by supercritical technology: A review. J. Chem. 2016, 2016, 3164312. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, G. Antioxidant protection from UV-and light-stress related to carotenoid structures. Antioxidants 2019, 8, 219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, L.; Doriya, K.; Kumar, D.S. Moringa oleifera: A review on nutritive importance and its medicinal application. Food Sci. Hum. Wellness 2016, 5, 49–56. [Google Scholar] [CrossRef]
- Brilhante, R.S.N.; Sales, J.A.; Pereira, V.S.; Castelo-Branco, D.d.S.C.M.; Cordeiro, R.d.A.; de Souza Sampaio, C.M.; Paiva, M.d.A.N.; dos Santos, J.B.F.; Sidrim, J.J.C.; Rocha, M.F.G. Research advances on the multiple uses of Moringa oleifera: A sustainable alternative for socially neglected population. Asian Pac. J. Trop Med. 2017, 10, 621–630. [Google Scholar] [CrossRef]
- Harjumäki, R.; Nugroho, R.W.N.; Zhang, X.; Lou, Y.R.; Yliperttula, M.; Valle-Delgado, J.J.; Österberg, M. Author Correction: Quantified forces between HepG2 hepatocarcinoma and WA07 pluripotent stem cells with natural biomaterials correlate with in vitro cell behavior. Sci. Rep. 2019, 9, 7354, Erratum in Sci. Rep. 2020, 10, 8803. [Google Scholar] [CrossRef]
- Donato, M.T.; Tolosa, L.; Gómez-Lechón, M.J. Culture and Functional Characterization of Human Hepatoma HepG2 Cells. In Protocols in In Vitro Hepatocyte Research; Humana Press: New York, NY, USA, 2015; pp. 77–93. [Google Scholar]
- Pinti, M.; Troiano, L.; Ferraresi, R.; Dobrucki, J.; Cossarizza, A. Hepatoma HepG2 cells as a model for in vitro studies on mitochondrial toxicity of antiviral drugs: Which correlation with the patient? J. Biol. Regul. Homeost. Agents 2003, 17, 166–171. [Google Scholar]
- Paemanee, A.; Sornjai, W.; Kittisenachai, S. Nevirapine induced mitochondrial dysfunction in HepG2 cells. Sci. Rep. 2017, 7, 9194. [Google Scholar] [CrossRef]
- Shamsabadi, A.A. Investigation into the Hepatotoxic Effects of Highly Active Anti-Retroviral Therapy (HAART) Medications. Master’s Thesis, University of Brighton, Brighton, UK, 2014. [Google Scholar]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saki, M.; De Villiers, H.; Ntsapi, C.; Tiloke, C. The Hepatoprotective Effects of Moringa oleifera against Antiretroviral-Induced Cytotoxicity in HepG2 Cells: A Review. Plants 2023, 12, 3235. https://doi.org/10.3390/plants12183235
Saki M, De Villiers H, Ntsapi C, Tiloke C. The Hepatoprotective Effects of Moringa oleifera against Antiretroviral-Induced Cytotoxicity in HepG2 Cells: A Review. Plants. 2023; 12(18):3235. https://doi.org/10.3390/plants12183235
Chicago/Turabian StyleSaki, Mbasakazi, Helena De Villiers, Claudia Ntsapi, and Charlette Tiloke. 2023. "The Hepatoprotective Effects of Moringa oleifera against Antiretroviral-Induced Cytotoxicity in HepG2 Cells: A Review" Plants 12, no. 18: 3235. https://doi.org/10.3390/plants12183235





