Genome-Wide Identification and Characterization of the NAC Gene Family and Its Involvement in Cold Response in Dendrobium officinale
Abstract
:1. Introduction
2. Results
2.1. Identification of the NAC Gene Family in D. officinale
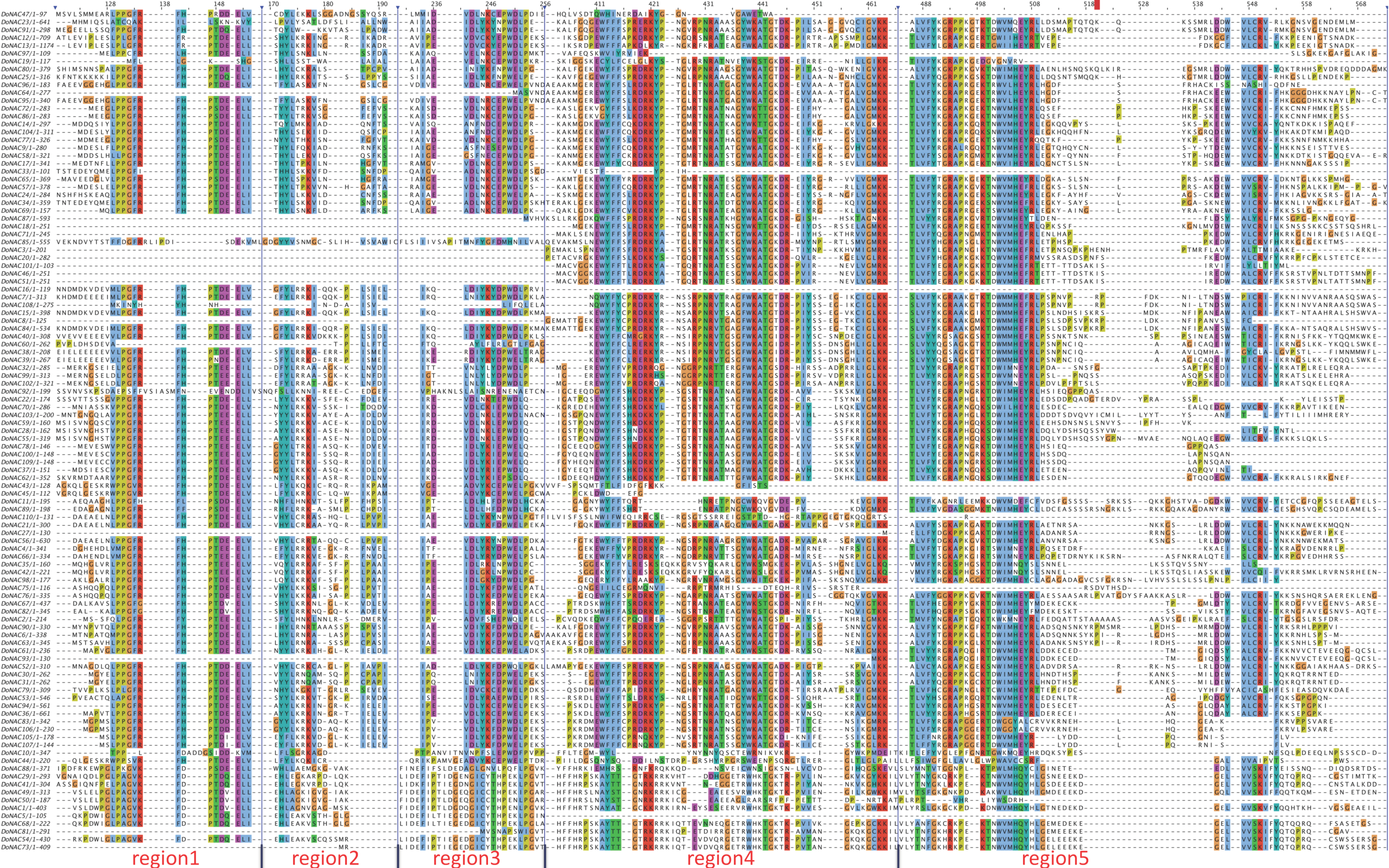
2.2. Phylogenetic and Conserved Motif Analysis of DoNACs
2.3. Cis-Element Analysis of DoNACs
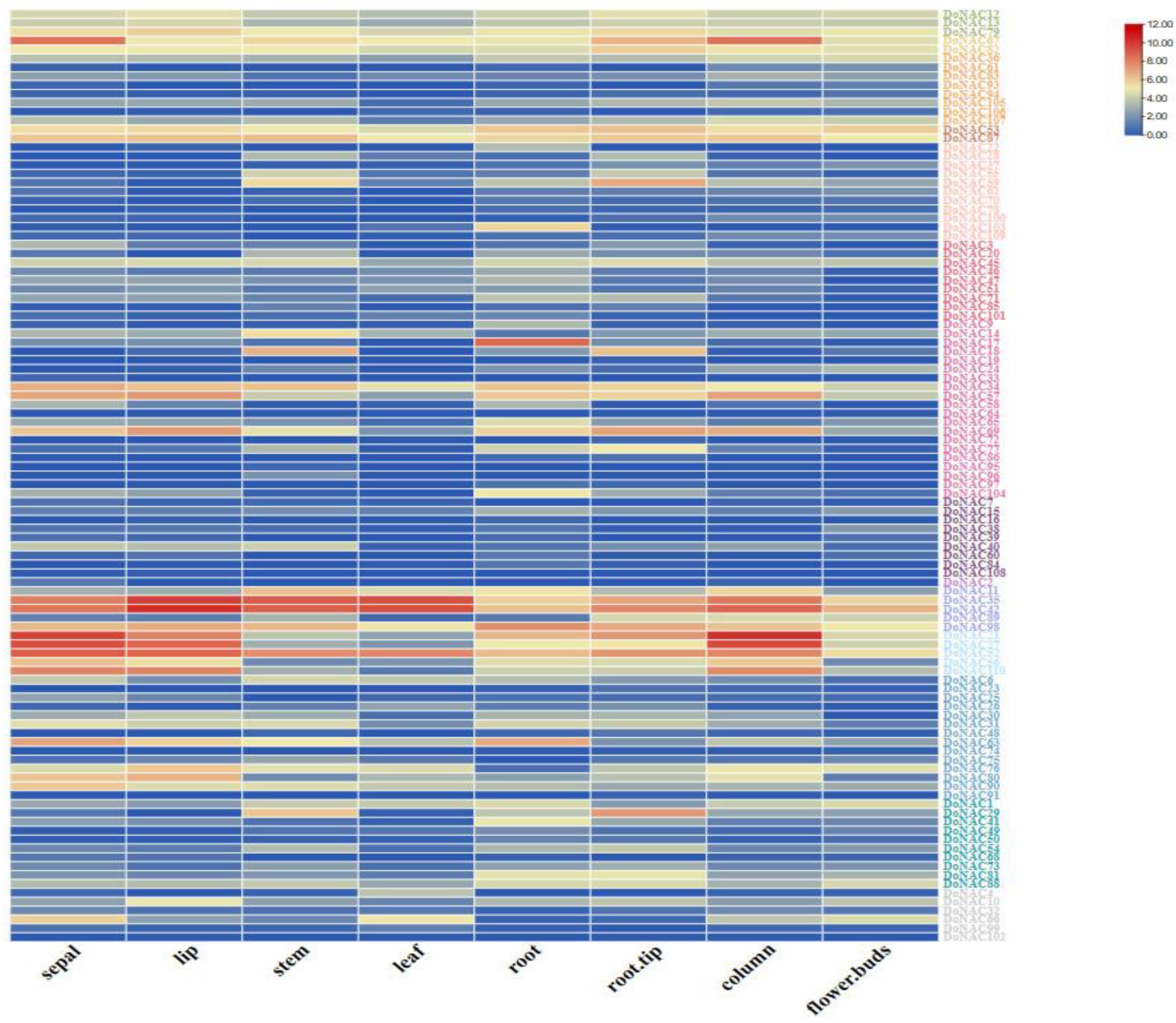
2.4. Expression Profile of DoNACs in Different Tissues
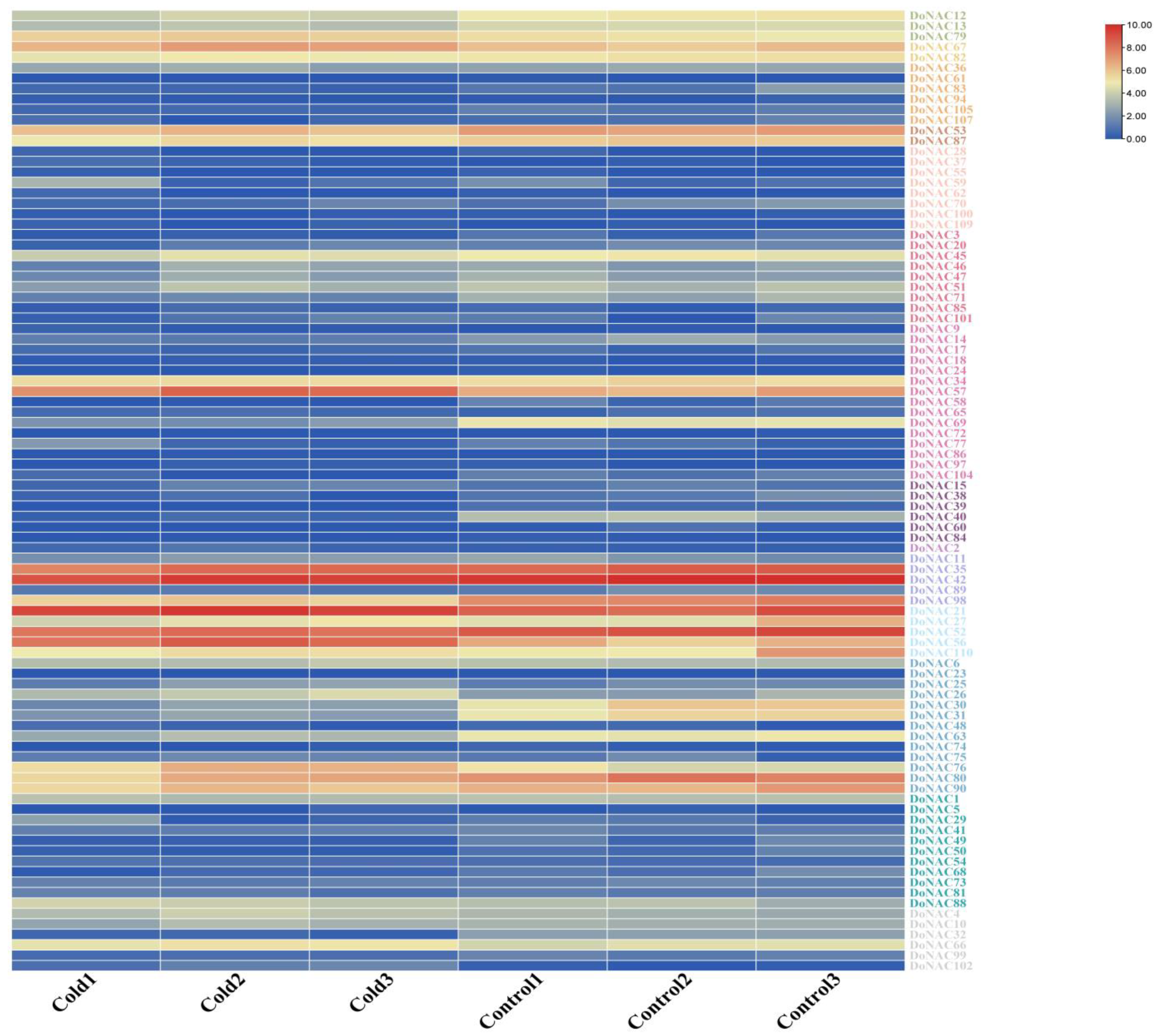
2.5. Expression Analysis of DoNACs under Cold Stress
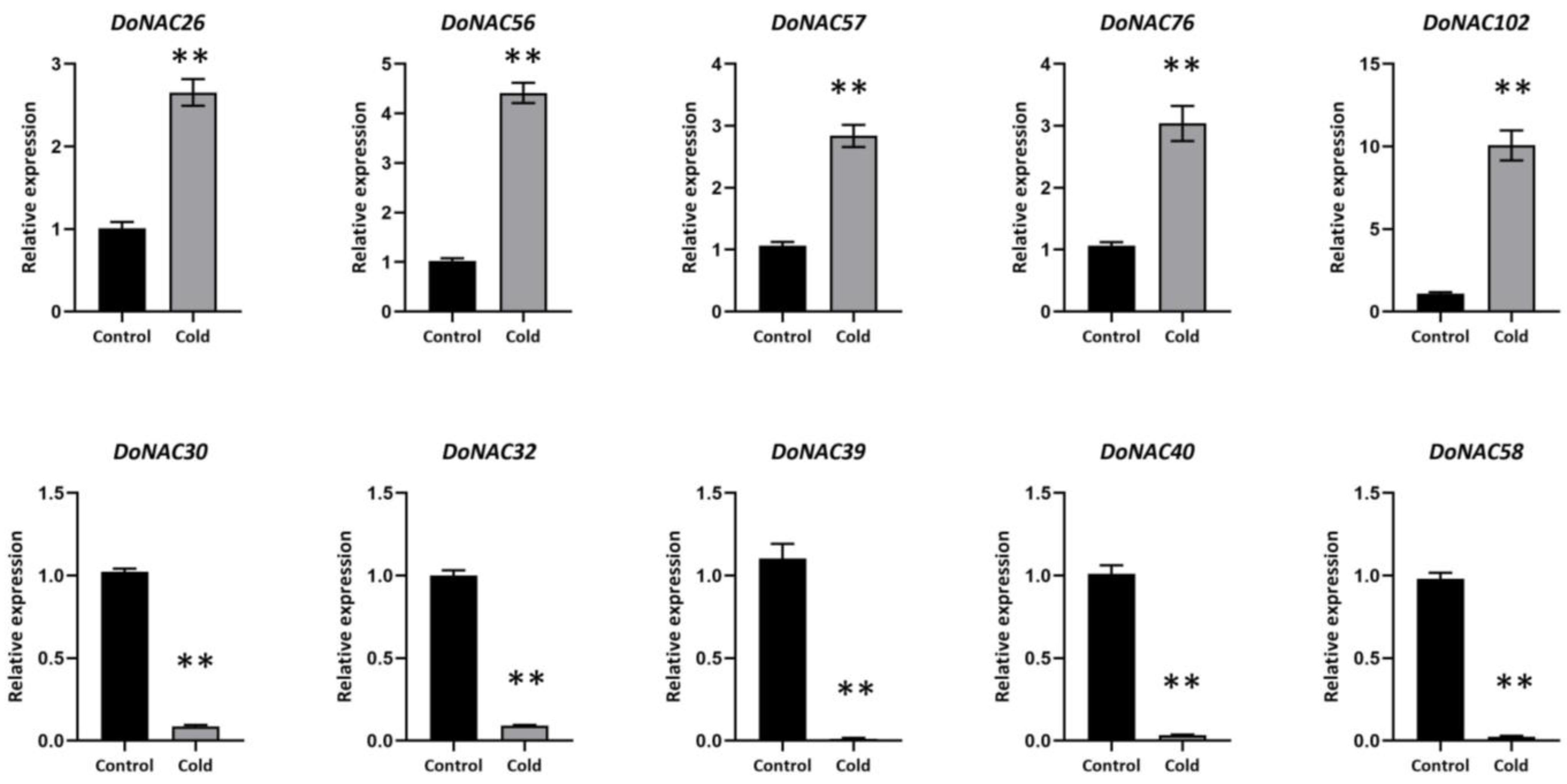
2.6. Validation of the Cold-Responsive DoNACs by qRT-PCR Analysis and Subcellular Localization
3. Discussion
4. Materials and Methods
4.1. Genome-Wide Identification of NAC Family in D. officinale
4.2. Phylogenetic Relationship, Conserved Motif and Cis-Element Analysis
4.3. Expression Analysis in Diverse Tissues and under Cold Stress
4.4. qPCR Validation of the Cold-Responsive DoNACs
4.5. Subcellular Localization of DoNAC-GFP Fusion Proteins
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ooka, H.; Satoh, K.; Doi, K.; Nagata, T.; Otomo, Y.; Murakami, K.; Matsubara, K.; Osato, N.; Kawai, J.; Carninci, P.; et al. Comprehensive analysis of NAC family genes in Oryza sativa and Arabidopsis thaliana. DNA Res. 2003, 10, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.N.; Ernst, H.A.; Leggio, L.L.; Skriver, K. NAC transcription factors: Structurally distinct, functionally diverse. Trends Plant Sci. 2005, 10, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, B.; Rizwan, H.; Sun, K.; Zeng, J.; Shi, M.; Guo, T.; Chen, F. Genome-wide identification and comprehensive analyses of NAC transcription factor gene family and expression analysis under Fusarium kyushuense and drought stress conditions in Passiflora edulis. Front. Plant Sci. 2022, 13, 972734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jin, J.P.; Tang, L.; Zhao, Y.; Gu, X.C.; Gao, G.; Luo, J.C. PlantTFDB 2.0: Update and improvement of the comprehensive plant transcription factor database. Nucleic Acids Res. 2011, 39, D1114–D1117. [Google Scholar] [CrossRef]
- Souer, E.; van Houwelingen, A.; Kloos, D.; Mol, J.; Koes, R. The no apical meristem gene of Petunia is required for pattern formation in embryos and flowers and is expressed at meristem and primordia boundaries. Cell 1996, 85, 159–170. [Google Scholar] [CrossRef]
- Aida, M.; Ishida, T.; Fukaki, H.; Fujisawa, H.; Tasaka, M. Genes involved in organ separation in Arabidopsis: An analysis of the cup-shaped cotyledon mutant. Plant Cell 1997, 9, 841–857. [Google Scholar] [CrossRef]
- Dang, X.; Zhang, B.; Li, C.; Nagawa, S. FvNST1b NAC protein induces secondary cell wall formation in strawberry. Int. J. Mol. Sci. 2022, 23, 13212. [Google Scholar] [CrossRef]
- Christianson, J.A.; Dennis, E.S.; Llewellyn, D.J.; Wilson, I.W. ATAF NAC transcription factors: Regulators of plant stress signaling. Plant Signal. Behav. 2010, 5, 428–432. [Google Scholar] [CrossRef]
- Zhu, G.; Chen, G.; Zhu, J.; Zhu, Y.; Lu, X.; Li, X.; Hu, Y.; Yan, Y. Molecular characterization and expression profiling of NAC transcription factors in Brachypodium distachyon L. PLoS ONE 2015, 10, e0139794. [Google Scholar] [CrossRef]
- Zhu, T.; Nevo, E.; Sun, D.; Peng, J. Phylogenetic analyses unravel the evolutionary history of NAC proteins in plants. Evolution 2012, 66, 1833–1848. [Google Scholar] [CrossRef]
- Kikuchi, K.; Ueguchi-Tanaka, M.; Yoshida, K.; Nagato, Y.; Matsusoka, M.; Hirano, H.Y. Molecular analysis of the NAC gene family in rice. Mol. Gen. Genet. 2000, 262, 1047–1051. [Google Scholar] [CrossRef]
- Puranik, S.; Sahu, P.P.; Srivastava, P.S.; Prasad, M. NAC proteins: Regulation and role in stress tolerance. Trends Plant Sci. 2012, 17, 369–381. [Google Scholar] [CrossRef]
- Diao, P.; Chen, C.; Zhang, Y.; Meng, Q.; Lv, W.; Ma, N. The role of NAC transcription factor in plant cold response. Plant Signal. Behav. 2020, 15, 1785668. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Koyama, H.; Bhati, K.K.; Alok, A. The biotechnological importance of the plant-specific NAC transcription factor family in crop improvement. J. Plant Res. 2021, 134, 475–495. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wang, S.; Zhang, B.; Shang-Guan, K.; Shi, Y.Y.; Zhang, D.; Liu, X.; Wu, K.; Xu, Z.; Fu, X.; et al. A gibberellin-mediated DELLA-NAC signaling cascade regulates cellulose synthesis in rice. Plant Cell 2015, 27, 1681–1696. [Google Scholar] [CrossRef]
- Mao, C.; Ding, W.; Wu, Y.; Yu, J.; He, X.W.; Shou, H.X.; Wu, P. Overexpression of a NAC-domain protein promotes shoot branching in rice. New Phytol. 2007, 176, 288–298. [Google Scholar] [CrossRef]
- Huysmans, M.; Buono, R.A.; Skorzinski, N.; Radio, M.C.; De Winter, F.; Parizot, B.; Mertens, J.; Karimi, M.; Fendrych, M.; Nowack, M.K. NAC transcription factors ANAC087 and ANAC046 control distinct aspects of programmed cell death in the Arabidopsis columella and lateral root cap. Plant Cell 2018, 30, 2197–2213. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.F.; Gan, S.S. AtNAP, a NAC family transcription factor, has an important role in leaf senescence. Plant J. 2006, 46, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Sablowski, R.W.; Meyerowitz, E.M. A homolog of NO APICAL MERISTEM is an immediate target of the floral homeotic genes APETALA3/PISTILLATA. Cell 1998, 92, 93–103. [Google Scholar] [CrossRef]
- Jensen, M.K.; Skriver, K. NAC transcription factor gene regulatory and protein-protein interaction networks in plant stress responses and senescence. Iubmb Life 2014, 66, 156–166. [Google Scholar] [CrossRef]
- Hu, H.; You, J.; Fang, Y.; Zhu, X.; Qi, Z.; Xiong, L. Characterization of transcription factor gene SNAC2 conferring cold and salt tolerance in rice. Plant Mol. Biol. 2008, 67, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Zhang, H.; Huang, L.; Li, D.; Song, F. Overexpression of a stress-responsive NAC transcription factor gene ONAC022 improves drought and salt tolerance in rice. Front. Plant Sci. 2016, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Thirumalaikumar, V.P.; Devkar, V.; Mehterov, N.; Ali, S.; Ozgur, R.; Turkan, I.; Mueller-Roeber, B.; Balazadeh, S. NAC transcription factor JUNGBRUNNEN 1 enhances drought tolerance in tomato. Plant Biotechnol. J. 2018, 16, 354–366. [Google Scholar] [CrossRef]
- De Clercq, I.; Vermeirssen, V.; Van Aken, O.; Vandepoele, K.; Murcha, M.W.; Law, S.R.; Inzé, A.; Ng, S.; Ivanova, A.; Rombaut, D.; et al. The membrane-bound NAC transcription factor ANAC013 functions in mitochondrial retrograde regulation of the oxidative stress response in Arabidopsis. Plant Cell 2013, 25, 3472–3490. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.; Ivanova, A.; Duncan, O.; Law, S.R.; Van Aken, O.; De Clercq, I.; Wang, I.; Carrie, C.; Xu, L.; Kmiec, B.; et al. A membrane-bound NAC transcription factor, ANAC017, mediates mitochondrial retrograde signaling in Arabidopsis. Plant Cell 2013, 25, 3450–3471. [Google Scholar] [CrossRef]
- Shan, W.; Kuang, J.F.; Lu, W.J.; Chen, J.Y. Banana fruit NAC transcription factor MaNAC1 is a direct target of MaICE1 and involved in cold stress through interacting with MaCBF1. Plant Cell Environ. 2014, 37, 2116–2127. [Google Scholar] [CrossRef]
- Shiriga, K.; Sharma, R.; Kumar, K.; Yadav, S.K.; Hossain, F.; Thirunavukkarasu, N. Genome-wide identification and expression pattern of drought-responsive members of the NAC family in maize. Meta Gene 2014, 2, 407–417. [Google Scholar] [CrossRef]
- Le, D.T.; Nishiyama, R.; Watanabe, Y.; Mochida, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S.P. Genome-wide survey and expression analysis of the plant-specific NAC transcription factor family in soybean during development and dehydration stress. DNA Res. 2011, 18, 263–276. [Google Scholar] [CrossRef]
- Rui, Z.; Pan, W.; Zhao, Q.; Hu, H.; Li, X.; Xing, L.; Jia, H.; She, K.; Nie, X. Genome-wide identification, evolution and expression analysis of NAC gene family under salt stress in wild emmer wheat (Triticum dicoccoides. L). Int. J. Biol. Macromol. 2023, 230, 123376. [Google Scholar] [CrossRef]
- Liu, X.H.; Lyu, Y.S.; Yang, W.; Yang, Z.T.; Lu, S.J.; Liu, J.X. A membrane-associated NAC transcription factor OsNTL3 is involved in thermotolerance in rice. Plant Biotechnol. J. 2020, 18, 1317–1329. [Google Scholar] [CrossRef]
- Ng, T.B.; Liu, J.; Wong, J.H.; Ye, X.; Wing, S.S.C.; Tong, Y.; Zhang, K.Y. Review of research on Dendrobium, a prized folk medicine. Appl. Microbiol. Biotechnol. 2012, 93, 1795–1803. [Google Scholar] [CrossRef]
- Wang, Y.H. Traditional uses, chemical constituents, pharmacological activities, and toxicological effects of Dendrobium leaves: A review. J. Ethnopharmacol. 2021, 270, 113851. [Google Scholar] [CrossRef]
- Yang, Q.; Xiang, W.; Li, Z.; Nian, Y.; Fu, X.; Zhou, G.; Li, L.; Zhang, J.; Huang, G.; Han, X.; et al. Genome-wide characterization and expression analysis of HD-ZIP gene family in Dendrobium officinale. Front. Genet. 2022, 13, 797014. [Google Scholar] [CrossRef]
- Mao, X.; Zhang, H.; Qian, X.; Li, A.; Zhao, G.; Jing, R. TaNAC2, a NAC-type wheat transcription factor conferring enhanced multiple abiotic stress tolerances in Arabidopsis. J. Exp. Bot. 2012, 63, 2933–2946. [Google Scholar] [CrossRef]
- Wang, N.; Tang, C.; Fan, X.; He, M.; Gan, P.; Zhang, S.; Hu, Z.; Wang, X.; Yan, T.; Shu, W.; et al. Inactivation of a wheat protein kinase gene confers broad-spectrum resistance to rust fungi. Cell 2022, 185, 2961–2974. [Google Scholar] [CrossRef]
- Guo, L.; Qi, J.; Du, D.; Liu, Y.; Jiang, X. Current advances of Dendrobium officinale polysaccharides in dermatology: A literature review. Pharm. Biol. 2020, 58, 664–673. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, C.; Wang, N.; Xu, Y.; Tang, G.; Xu, L.; Feng, Y. Bioactivities and mechanism of actions of Dendrobium officinale: A comprehensive review. Oxidative Med. Cell Longev. 2022, 2022, 6293355. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, Y.; Adejobi, O.I.; Wang, Y.; Liu, A. Research advances in multi-omics on the traditional Chinese herb Dendrobium officinale. Front. Plant Sci. 2022, 11, 808228. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Dong, W.; Teixeira da Silva, J.A.; He, C.; Si, C.; Duan, J. Ectopic expression of DoFLS1 from Dendrobium officinale enhances flavonol accumulation and abiotic stress tolerance in Arabidopsis thaliana. Protoplasma 2021, 258, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, S.; Wu, X.; Li, J.; Xu, C.; Huang, M.; Wang, H.; Liu, H.; Zhao, Z. Identification of the NAC transcription factor family during early seed development in Akebia trifoliata (Thunb.) Koidz. Plants 2023, 12, 1518. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Li, K.Q.; Xu, X.Y.; Zhang, H.P.; Chen, H.X.; Chen, Y.H.; Hao, J.; Wang, Y.; Huang, X.S.; Zhang, S.L. A novel NAC transcription factor, PbeNAC1, of Pyrus betulifolia confers cold and drought tolerance via interacting with PbeDREBs and activating the expression of stress-responsive genes. Front. Plant Sci. 2017, 8, 1049. [Google Scholar] [CrossRef]
- Nakashima, K.; Yamaguchi-Shinozaki, K. ABA signaling in stress-response and seed development. Plant Cell Rep. 2013, 32, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]




| Gene ID | Gene Name | Protein Size (AA) | pI | Molecular Weight (kDa) | Subcellular |
|---|---|---|---|---|---|
| Dendrobium_GLEAN_10145674 | DoNAC1 | 403 | 5.83 | 45.14922 | Nuclear |
| Dendrobium_GLEAN_10132016 | DoNAC2 | 214 | 8.96 | 24.05615 | Nuclear |
| Dendrobium_GLEAN_10127752 | DoNAC3 | 201 | 10.28 | 23.17154 | Mitochondrial |
| Dendrobium_GLEAN_10125186 | DoNAC4 | 341 | 6.88 | 39.36333 | Nuclear |
| Dendrobium_GLEAN_10125242 | DoNAC5 | 105 | 5 | 11.45904 | Cytoplasmic |
| Dendrobium_GLEAN_10121576 | DoNAC6 | 338 | 7.11 | 38.55624 | Nuclear |
| Dendrobium_GLEAN_10119429 | DoNAC7 | 313 | 6.55 | 36.06974 | Nuclear |
| Dendrobium_GLEAN_10119030 | DoNAC8 | 125 | 9.54 | 14.32841 | Mitochondrial |
| Dendrobium_GLEAN_10116337 | DoNAC9 | 280 | 5.81 | 32.51057 | Cytoplasmic |
| Dendrobium_GLEAN_10115867 | DoNAC10 | 347 | 4.33 | 39.12014 | Nuclear |
| Dendrobium_GLEAN_10114825 | DoNAC11 | 195 | 5.28 | 22.19483 | Cytoplasmic |
| Dendrobium_GLEAN_10113712 | DoNAC12 | 709 | 4.63 | 78.28001 | Nuclear |
| Dendrobium_GLEAN_10113713 | DoNAC13 | 1174 | 4.78 | 129.50276 | Nuclear |
| Dendrobium_GLEAN_10110893 | DoNAC14 | 297 | 9.1 | 34.35212 | Nuclear |
| Dendrobium_GLEAN_10110543 | DoNAC15 | 398 | 6.68 | 45.2573 | Nuclear |
| Dendrobium_GLEAN_10110544 | DoNAC16 | 119 | 6.29 | 14.24564 | Cytoplasmic |
| Dendrobium_GLEAN_10110681 | DoNAC17 | 341 | 6.32 | 38.85693 | Nuclear |
| Dendrobium_GLEAN_10109120 | DoNAC18 | 251 | 9.36 | 28.12291 | Nuclear |
| Dendrobium_GLEAN_10106022 | DoNAC19 | 117 | 9.68 | 13.13935 | Extracellular |
| Dendrobium_GLEAN_10104194 | DoNAC20 | 282 | 9.3 | 32.25563 | Nuclear |
| Dendrobium_GLEAN_10100250 | DoNAC21 | 300 | 5.37 | 34.18254 | Nuclear |
| Dendrobium_GLEAN_10098876 | DoNAC22 | 174 | 9 | 20.14462 | Nuclear |
| Dendrobium_GLEAN_10096163 | DoNAC23 | 641 | 8.75 | 72.99217 | Nuclear |
| Dendrobium_GLEAN_10091520 | DoNAC24 | 284 | 8.7 | 31.92515 | Chloroplast |
| Dendrobium_GLEAN_10089166 | DoNAC25 | 316 | 9.49 | 35.73608 | Nuclear |
| Dendrobium_GLEAN_10089170 | DoNAC26 | 318 | 5.75 | 36.07187 | Cytoplasmic |
| Dendrobium_GLEAN_10088322 | DoNAC27 | 130 | 4.62 | 15.0337 | Nuclear |
| Dendrobium_GLEAN_10087230 | DoNAC28 | 162 | 9.18 | 18.98751 | Mitochondrial |
| Dendrobium_GLEAN_10083779 | DoNAC29 | 293 | 8.37 | 32.75773 | Nuclear |
| Dendrobium_GLEAN_10080940 | DoNAC30 | 262 | 5.23 | 30.17884 | Nuclear |
| Dendrobium_GLEAN_10080942 | DoNAC31 | 262 | 5.32 | 30.16295 | Cytoplasmic |
| Dendrobium_GLEAN_10079456 | DoNAC32 | 285 | 5.95 | 32.94021 | Nuclear |
| Dendrobium_GLEAN_10078554 | DoNAC33 | 101 | 4.78 | 11.51279 | Cytoplasmic |
| Dendrobium_GLEAN_10078555 | DoNAC34 | 359 | 6.05 | 40.56424 | Cytoplasmic |
| Dendrobium_GLEAN_10077726 | DoNAC35 | 160 | 9.74 | 18.50435 | Mitochondrial |
| Dendrobium_GLEAN_10073743 | DoNAC36 | 661 | 6 | 75.60385 | Nuclear |
| Dendrobium_GLEAN_10072577 | DoNAC37 | 151 | 8.76 | 17.89539 | Nuclear |
| Dendrobium_GLEAN_10069509 | DoNAC38 | 208 | 8.57 | 24.32624 | Cytoplasmic |
| Dendrobium_GLEAN_10069510 | DoNAC39 | 267 | 5.08 | 31.15486 | Nuclear |
| Dendrobium_GLEAN_10068907 | DoNAC40 | 308 | 6.49 | 35.85838 | Nuclear |
| Dendrobium_GLEAN_10066548 | DoNAC41 | 304 | 7.69 | 34.92676 | Nuclear |
| Dendrobium_GLEAN_10065340 | DoNAC42 | 221 | 9.17 | 25.23475 | Nuclear |
| Dendrobium_GLEAN_10061197 | DoNAC43 | 128 | 8.28 | 14.26358 | Cytoplasmic |
| Dendrobium_GLEAN_10061198 | DoNAC44 | 220 | 8.59 | 24.31788 | Extracellular |
| Dendrobium_GLEAN_10061199 | DoNAC45 | 112 | 4.69 | 12.52021 | Extracellular |
| Dendrobium_GLEAN_10060620 | DoNAC46 | 251 | 8.78 | 28.10788 | Nuclear |
| Dendrobium_GLEAN_10060621 | DoNAC47 | 97 | 4.52 | 11.14347 | Nuclear |
| Dendrobium_GLEAN_10058619 | DoNAC48 | 278 | 8.77 | 31.99242 | Cytoplasmic |
| Dendrobium_GLEAN_10053684 | DoNAC49 | 313 | 8.92 | 35.57001 | Cytoplasmic |
| Dendrobium_GLEAN_10053685 | DoNAC50 | 187 | 8.48 | 20.87264 | Nuclear |
| Dendrobium_GLEAN_10052939 | DoNAC51 | 251 | 8.95 | 28.01581 | Nuclear |
| Dendrobium_GLEAN_10052503 | DoNAC52 | 311 | 9 | 34.53148 | Nuclear |
| Dendrobium_GLEAN_10052536 | DoNAC53 | 546 | 4.51 | 61.31671 | Nuclear |
| Dendrobium_GLEAN_10051831 | DoNAC54 | 430 | 6.2 | 47.6221 | Nuclear |
| Dendrobium_GLEAN_10049252 | DoNAC55 | 319 | 9.14 | 35.37876 | Nuclear |
| Dendrobium_GLEAN_10049133 | DoNAC56 | 630 | 8.22 | 71.57787 | Cytoplasmic |
| Dendrobium_GLEAN_10048805 | DoNAC57 | 378 | 8.38 | 41.8827 | Nuclear |
| Dendrobium_GLEAN_10048260 | DoNAC58 | 321 | 5.32 | 36.82507 | Nuclear |
| Dendrobium_GLEAN_10046985 | DoNAC59 | 160 | 9.32 | 18.69425 | Nuclear |
| Dendrobium_GLEAN_10046111 | DoNAC60 | 262 | 6.94 | 29.87379 | Extracellular |
| Dendrobium_GLEAN_10045817 | DoNAC61 | 236 | 4.94 | 27.23745 | Nuclear |
| Dendrobium_GLEAN_10043622 | DoNAC62 | 352 | 6.76 | 41.10419 | Nuclear |
| Dendrobium_GLEAN_10042843 | DoNAC63 | 345 | 7.6 | 39.17742 | Nuclear |
| Dendrobium_GLEAN_10042799 | DoNAC64 | 277 | 8.24 | 30.96703 | Nuclear |
| Dendrobium_GLEAN_10042684 | DoNAC65 | 369 | 6.01 | 41.49966 | Nuclear |
| Dendrobium_GLEAN_10042333 | DoNAC66 | 334 | 8.56 | 38.10982 | Nuclear |
| Dendrobium_GLEAN_10042836 | DoNAC67 | 437 | 6.26 | 50.02975 | Nuclear |
| Dendrobium_GLEAN_10042279 | DoNAC68 | 222 | 8.69 | 24.99947 | Nuclear |
| Dendrobium_GLEAN_10042421 | DoNAC69 | 157 | 9.64 | 18.32222 | Cytoplasmic |
| Dendrobium_GLEAN_10041251 | DoNAC70 | 286 | 7.63 | 33.8024 | Nuclear |
| Dendrobium_GLEAN_10040136 | DoNAC71 | 245 | 5.73 | 27.80819 | Cytoplasmic |
| Dendrobium_GLEAN_10039845 | DoNAC72 | 283 | 6.67 | 32.33944 | Nuclear |
| Dendrobium_GLEAN_10039511 | DoNAC73 | 409 | 6.26 | 45.40578 | Nuclear |
| Dendrobium_GLEAN_10037990 | DoNAC74 | 319 | 5.63 | 36.12287 | Cytoplasmic |
| Dendrobium_GLEAN_10036377 | DoNAC75 | 116 | 6.28 | 13.35206 | Nuclear |
| Dendrobium_GLEAN_10036378 | DoNAC76 | 335 | 8.37 | 36.74328 | Nuclear |
| Dendrobium_GLEAN_10036159 | DoNAC77 | 326 | 8.48 | 36.90069 | Nuclear |
| Dendrobium_GLEAN_10034487 | DoNAC78 | 146 | 7.76 | 17.00228 | Cytoplasmic |
| Dendrobium_GLEAN_10032744 | DoNAC79 | 309 | 8.62 | 35.55115 | Cytoplasmic |
| Dendrobium_GLEAN_10032445 | DoNAC80 | 379 | 9.07 | 43.11576 | Nuclear |
| Dendrobium_GLEAN_10030109 | DoNAC81 | 291 | 9.05 | 32.6348 | Nuclear |
| PEQU_23244-D2 | DoNAC82 | 345 | 6.09 | 39.06895 | Nuclear |
| Dendrobium_GLEAN_10030254 | DoNAC83 | 342 | 5.79 | 38.68952 | Nuclear |
| Dendrobium_GLEAN_10027918 | DoNAC84 | 534 | 9.16 | 60.5709 | Nuclear |
| Dendrobium_GLEAN_10027115 | DoNAC85 | 555 | 8.97 | 64.00039 | Plasma Membrane |
| Dendrobium_GLEAN_10026921 | DoNAC86 | 283 | 6.67 | 32.33944 | Nuclear |
| Dendrobium_GLEAN_10023733 | DoNAC87 | 593 | 5.02 | 66.27489 | Nuclear |
| Dendrobium_GLEAN_10022335 | DoNAC88 | 371 | 5.27 | 41.05842 | Cytoplasmic |
| Dendrobium_GLEAN_10018741 | DoNAC89 | 198 | 6.16 | 22.51354 | Nuclear |
| Dendrobium_GLEAN_10016232 | DoNAC90 | 330 | 6.8 | 37.84653 | Nuclear |
| Dendrobium_GLEAN_10016023 | DoNAC91 | 298 | 6.08 | 33.85566 | Nuclear |
| Dendrobium_GLEAN_10015903 | DoNAC92 | 199 | 5.81 | 22.27767 | Nuclear |
| Dendrobium_GLEAN_10014831 | DoNAC93 | 130 | 4.19 | 14.78027 | Nuclear |
| Dendrobium_GLEAN_10014832 | DoNAC94 | 561 | 5.74 | 64.18911 | Nuclear |
| Dendrobium_GLEAN_10014534 | DoNAC95 | 340 | 5.96 | 38.01689 | Nuclear |
| Dendrobium_GLEAN_10014535 | DoNAC96 | 183 | 5.89 | 20.71126 | Cytoplasmic |
| Dendrobium_GLEAN_10014195 | DoNAC97 | 109 | 5.35 | 12.24524 | Cytoplasmic |
| Dendrobium_GLEAN_10013786 | DoNAC98 | 177 | 9.6 | 19.70194 | Chloroplast |
| Dendrobium_GLEAN_10010728 | DoNAC99 | 313 | 7.8 | 35.8342 | Nuclear |
| Dendrobium_GLEAN_10010634 | DoNAC100 | 148 | 8.49 | 17.43163 | Nuclear |
| Dendrobium_GLEAN_10010200 | DoNAC101 | 103 | 9.94 | 12.06804 | Mitochondrial |
| Dendrobium_GLEAN_10008910 | DoNAC102 | 321 | 6.06 | 36.54962 | Nuclear |
| Dendrobium_GLEAN_10008576 | DoNAC103 | 200 | 9.05 | 23.83835 | Plasma Membrane |
| Dendrobium_GLEAN_10007482 | DoNAC104 | 311 | 5.68 | 35.97639 | Nuclear |
| Dendrobium_GLEAN_10006697 | DoNAC105 | 178 | 9.43 | 21.16647 | Cytoplasmic |
| Dendrobium_GLEAN_10005041 | DoNAC106 | 230 | 9.61 | 26.40513 | Nuclear |
| Dendrobium_GLEAN_10003671 | DoNAC107 | 144 | 9.46 | 17.19184 | Cytoplasmic |
| Dendrobium_GLEAN_10003465 | DoNAC108 | 275 | 8.66 | 31.21719 | Nuclear |
| Dendrobium_GLEAN_10003618 | DoNAC109 | 148 | 8.39 | 17.4567 | Nuclear |
| Dendrobium_GLEAN_10000578 | DoNAC110 | 131 | 7.87 | 14.95097 | Nuclear |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Li, Z.; Wang, X.; Jiang, C.; Liu, F.; Nian, Y.; Fu, X.; Zhou, G.; Liu, L.; Wang, H. Genome-Wide Identification and Characterization of the NAC Gene Family and Its Involvement in Cold Response in Dendrobium officinale. Plants 2023, 12, 3626. https://doi.org/10.3390/plants12203626
Yang Q, Li Z, Wang X, Jiang C, Liu F, Nian Y, Fu X, Zhou G, Liu L, Wang H. Genome-Wide Identification and Characterization of the NAC Gene Family and Its Involvement in Cold Response in Dendrobium officinale. Plants. 2023; 12(20):3626. https://doi.org/10.3390/plants12203626
Chicago/Turabian StyleYang, Qianyu, Zhihui Li, Xiao Wang, Chunqian Jiang, Feihong Liu, Yuxin Nian, Xiaoyun Fu, Guangzhu Zhou, Lei Liu, and Hui Wang. 2023. "Genome-Wide Identification and Characterization of the NAC Gene Family and Its Involvement in Cold Response in Dendrobium officinale" Plants 12, no. 20: 3626. https://doi.org/10.3390/plants12203626
APA StyleYang, Q., Li, Z., Wang, X., Jiang, C., Liu, F., Nian, Y., Fu, X., Zhou, G., Liu, L., & Wang, H. (2023). Genome-Wide Identification and Characterization of the NAC Gene Family and Its Involvement in Cold Response in Dendrobium officinale. Plants, 12(20), 3626. https://doi.org/10.3390/plants12203626





