Multifarious Characterization and Efficacy of Three Phosphate-Solubilizing Aspergillus Species as Biostimulants in Improving Root Induction of Cassava and Sugarcane Stem Cuttings
Abstract
:1. Introduction
2. Results
2.1. Characterization of Plant Growth Promotion Properties
2.1.1. Determination of IAA Production
2.1.2. Determination of Siderophore Production
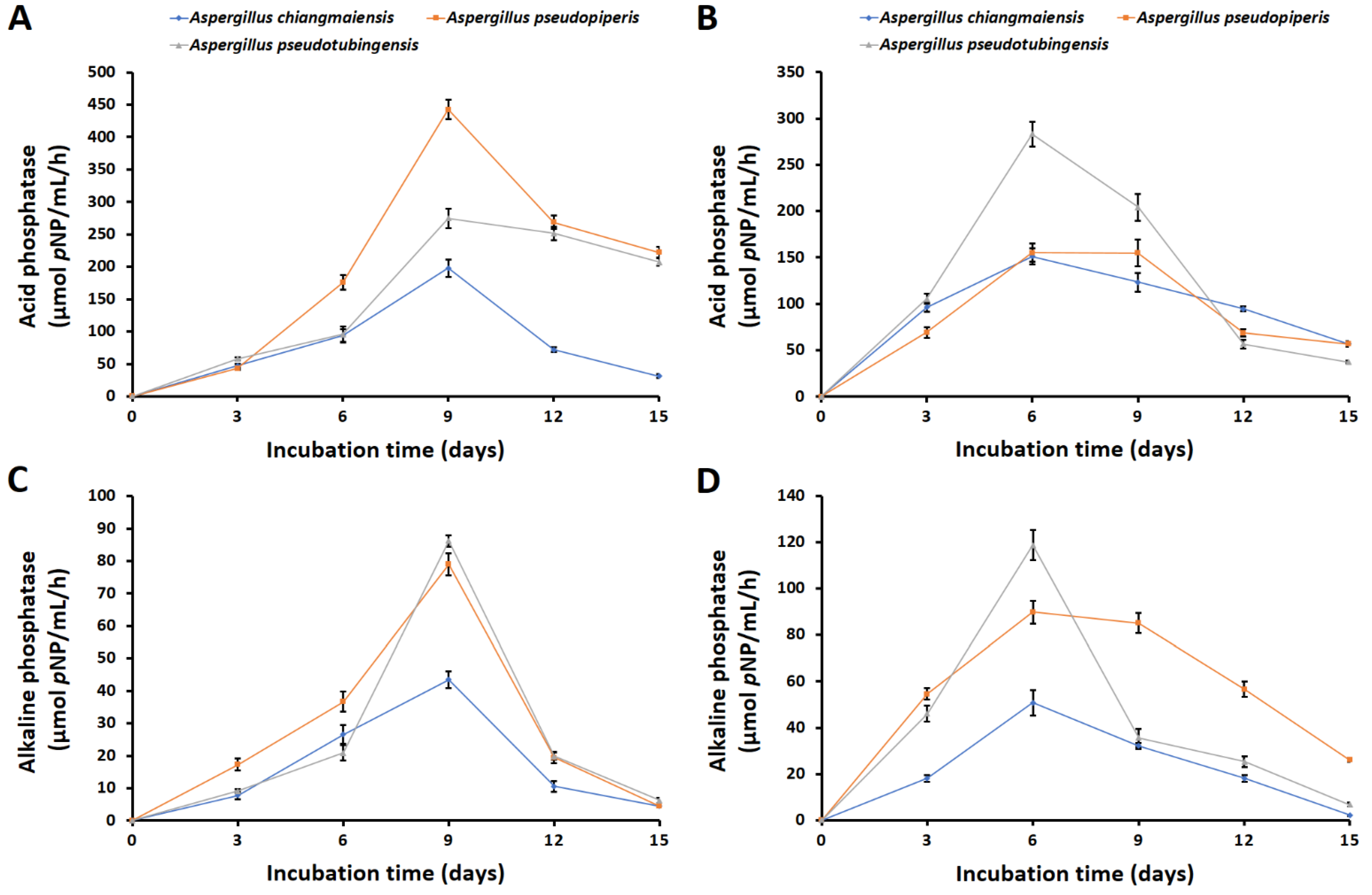
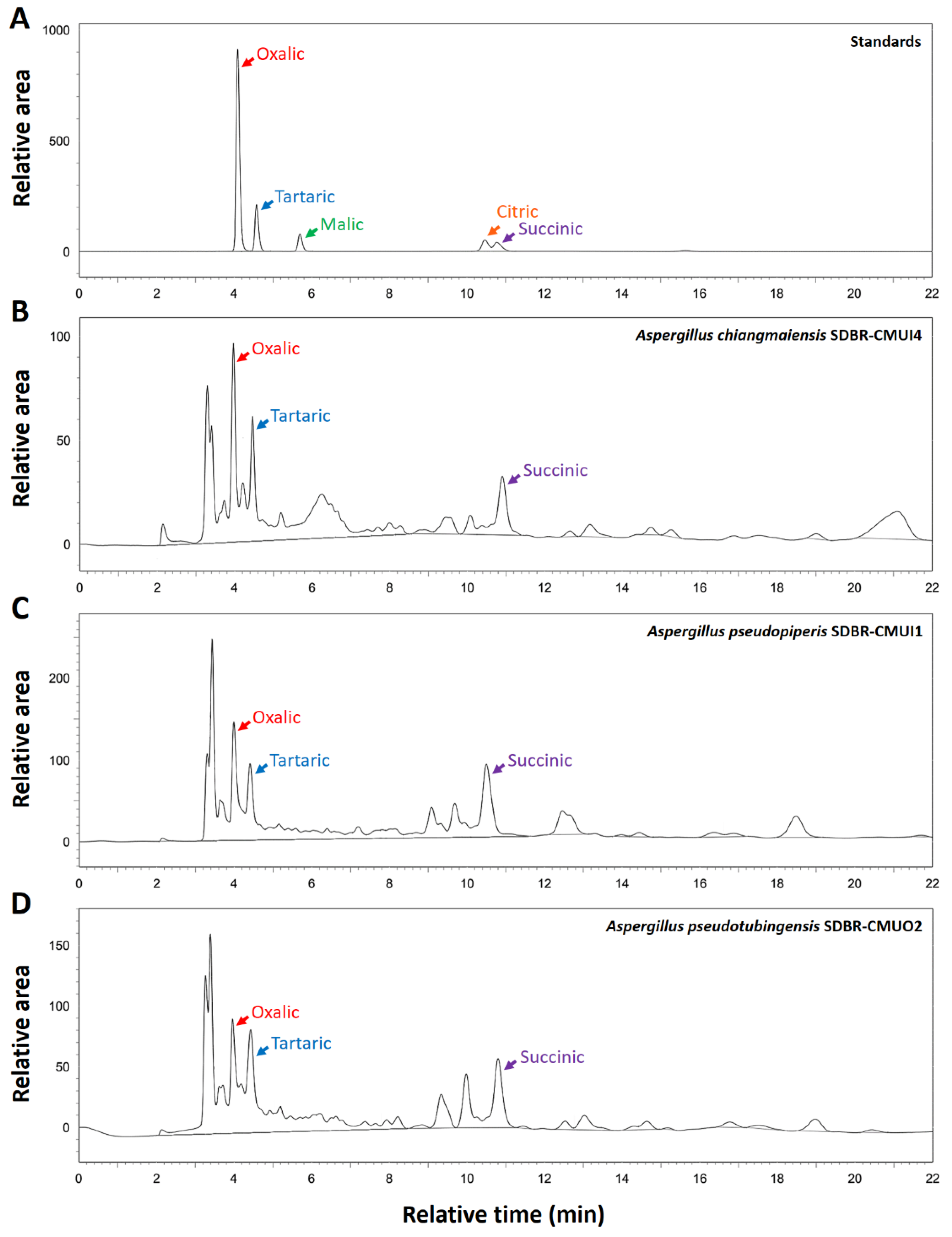
2.1.3. Solubilization of Phosphate Minerals, Phosphatase Activities, and Organic Acid Production
2.2. Drought, pH, Temperature, and Salinity Tolerances
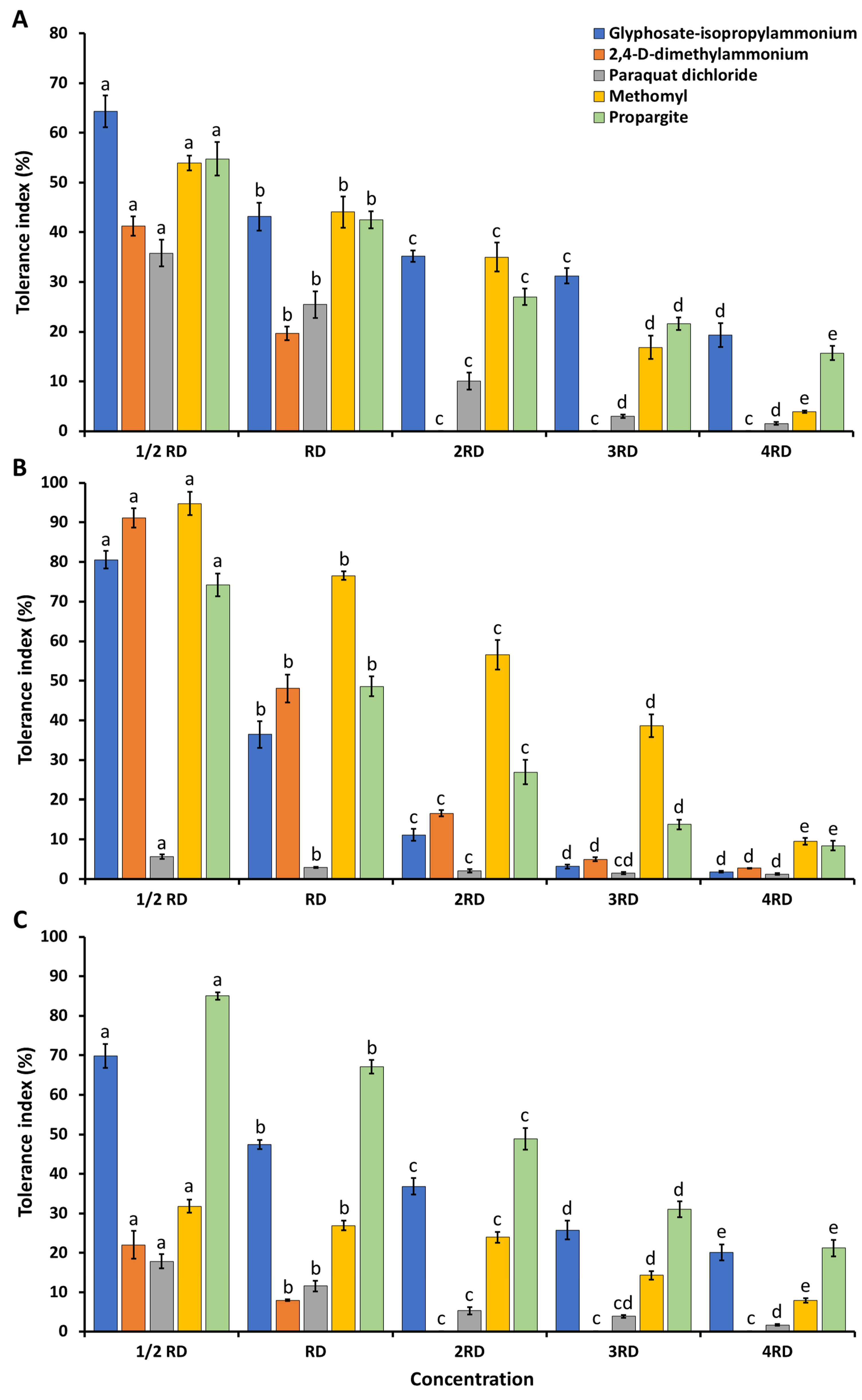
2.3. Agrochemical Tolerance
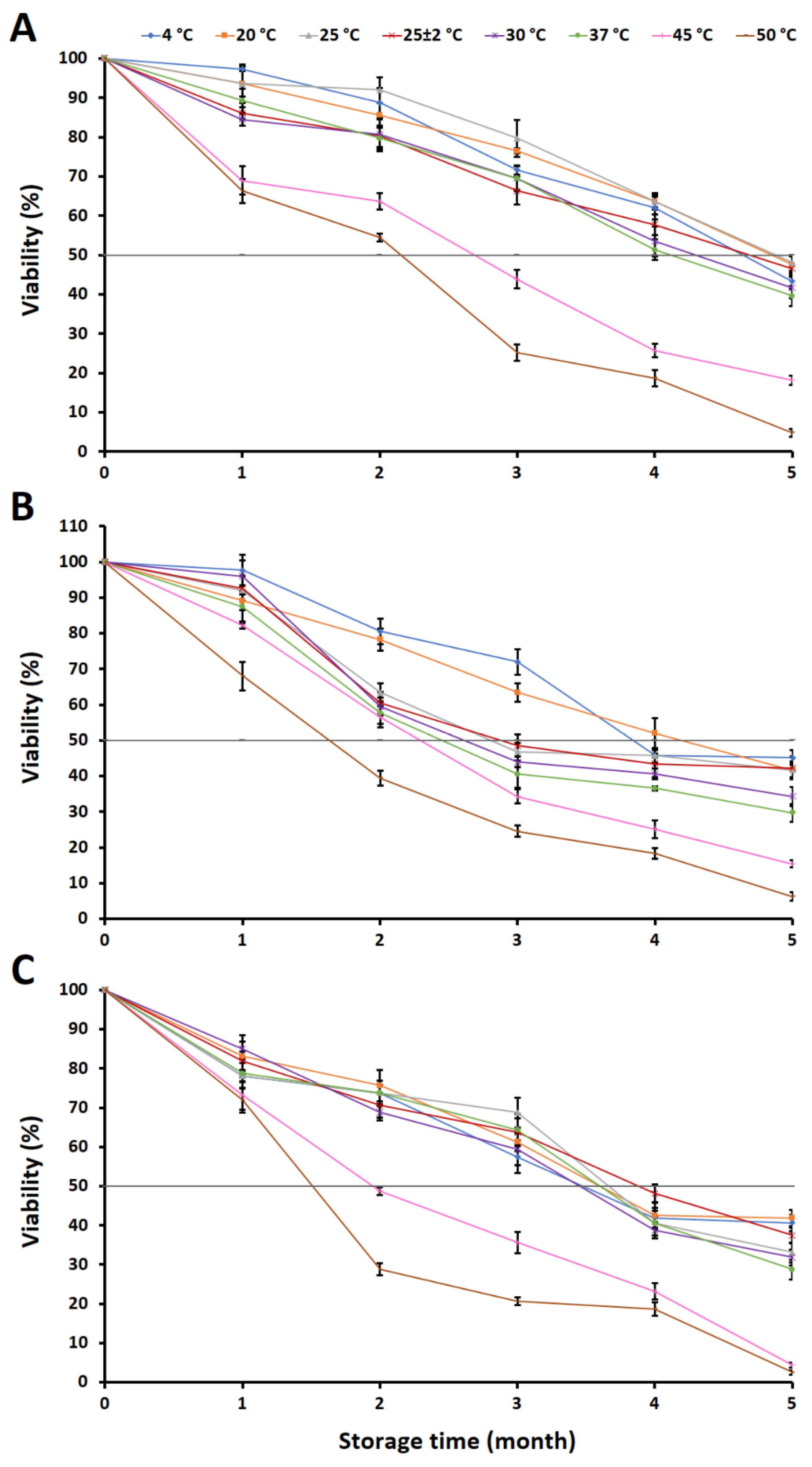
2.4. Evaluation of Fungal Viability in a Granular Inoculum
2.5. Root Induction of Cassava and Sugarcane Stem Cuttings under Greenhouse Conditions
2.5.1. Cassava Stem Cuttings
2.5.2. Sugarcane Stem Cuttings
3. Discussion
4. Materials and Methods
4.1. Fungal Strains
4.2. Characterization of Plant Growth Promotion Properties
4.2.1. Determination of IAA Production
4.2.2. Determination of Siderophore Production
4.2.3. Solubilization of Phosphate Minerals, Phosphatase Activities, and Organic Acid Production
4.3. Drought, pH, Temperature, and Salinity Tolerances
4.3.1. Drought Tolerance
4.3.2. pH Tolerance
4.3.3. Temperature Tolerance
4.3.4. Salinity Tolerance
4.4. Agrochemical Tolerance
4.5. Evaluation of Fungal Viability in a Granular Inoculum
4.5.1. Preparation of Granular Inoculum
4.5.2. Evaluation of Fungal Viability
4.6. Root Inductions of Cassava and Sugarcane Stem Cuttings under Greenhouse Conditions
4.6.1. Measurement of Plant Growth
4.6.2. Determination of Chlorophyll and Cellular Inorganic Phosphate Contents in Plants
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, S.; Kumar, S.; Mohapatra, T. Interaction between macro- and micro-nutrients in plants. Front. Plant Sci. 2021, 12, 665583. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Ahemad, M.; Ahemad, M.; Wani, P.A. Plant growth promotion by phosphate solubilizing fungi–current perspective. Arch. Agron. Soil Sci. 2010, 56, 73–98. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2, 587. [Google Scholar] [CrossRef]
- Jain, R.; Saxena, J.; Sharma, V. Differential effects of immobilized and free forms of phosphate-solubilizing fungal strains on the growth and phosphorus uptake of mung bean plants. Ann. Microbiol. 2014, 64, 1523–1534. [Google Scholar] [CrossRef]
- Gyaneshwar, P.; Naresh, K.G.; Parekh, L.J.; Poole, P.S. Role of soil microorganisms in improving P nutrition of plants. Plant Soil 2002, 245, 83–93. [Google Scholar] [CrossRef]
- Mendoza-Arroyo, G.E.; Chan-Bacab, M.J.; Aguila-Ramírez, R.N.; Ortega-Morales, B.O.; Solís, R.E.C.; Chab-Ruiz, A.O.; Cob-Rivera, K.I.; Dzib-Castillo, B.; Tun-Che, R.E.; Camacho-Chab, J.C. Inorganic phosphate solubilization by a novel isolated bacterial strain Enterobacter sp. ITCB-09 and its application potential as biofertilizer. Agriculture 2020, 10, 383. [Google Scholar] [CrossRef]
- Mengel, K.; Kirkby, E.A.; Kosegarten, H.; Appel, T. Phosphorus. In Principles of Plant Nutrition; Mengel, K., Kirkby, E.A., Kosegarten, H., Appel, T., Eds.; Springer: Dordrecht, The Netherlands, 2001; pp. 453–479. [Google Scholar] [CrossRef]
- Zhou, K.; Binkley, D.; Doxtader, K.G. A new method for estimating gross phosphorus mineralization and immobilization rates in soils. Plant Soil 1992, 147, 243–250. [Google Scholar] [CrossRef]
- Bechtaoui, N.; Rabiu, M.K.; Raklami, A.; Oufdou, K.; Hafidi, M.; Jemo, M. Phosphate-dependent regulation of growth and stresses management in plants. Front. Plant Sci. 2021, 12, 679916. [Google Scholar] [CrossRef]
- Rengel, Z.; Marschner, P. Nutrient availability and management in the rhizosphere: Exploiting genotypic differences. New Phytol. 2005, 168, 305–312. [Google Scholar] [CrossRef]
- Hasnain, M.; Chen, J.; Ahmed, N.; Memon, S.; Wang, L.; Wang, Y.; Wang, P. The effects of fertilizer type and application time on soil properties, plant traits, yield and quality of tomato. Sustainability 2020, 12, 9065. [Google Scholar] [CrossRef]
- Chang, C.H.; Yang, S.S. Thermo-tolerant phosphate-solubilizing microbes for multi-functional biofertilizer preparation. Bioresour. Technol. 2009, 100, 1648–1658. [Google Scholar] [CrossRef] [PubMed]
- Padmavathi, T. Optimization of phosphate solubilization by Aspergillus niger using plackett-burman and response surface methodology. J. Soil Sci. Plant Nutr. 2015, 15, 781–793. [Google Scholar] [CrossRef]
- Kalayu, G. Phosphate solubilizing microorganisms: Promising approach as biofertilizers. Int. J. Agron. 2019, 2019, 4917256. [Google Scholar] [CrossRef]
- Shrivastava, M.; Srivastava, P.C.; D’Souza, S.F. Phosphate-solubilizing microbes: Diversity and phosphates solubilization mechanism. In Rhizospheric Microbes in Soil; Meena, V., Ed.; Springer: Singapore, 2018; pp. 137–165. [Google Scholar] [CrossRef]
- Kucey, R.M.N. Phosphate solubilizing bacteria and fungi in various cultivated and virgin Alberta soils. Can. J. Soil Sci. 1983, 63, 671–678. [Google Scholar] [CrossRef]
- Kaul, S.; Sharma, S.; Apra; Dhar, M.K. Phosphate-solubilising fungi and their potential role in sustainable agriculture. In Biofertilizers for Sustainable Agriculture and Environment; Soil Biology; Giri, B., Prasad, R., Wu, Q.S., Varma, A., Eds.; Springer: Cham, Switzerland, 2019; Volume 55, pp. 371–393. [Google Scholar] [CrossRef]
- Mendes, G.O.; Freitas, A.L.M.; Pereira, O.L.; Silva, I.R.; Vassilev, B.; Costa, M.D. Mechanism of phosphate solubilization by fungal isolates when exposed to different P sources. Ann. Microbiol. 2014, 64, 239–249. [Google Scholar] [CrossRef]
- Pawar, V.C.; Thaker, V.S. Acid phosphatase and invertase activities of Aspergillus niger. Mycoscience 2009, 50, 323–330. [Google Scholar] [CrossRef]
- Singh, H.; Reddy, M.S. Effect of inoculation with phosphate solubilizing fungus on growth and nutrient uptake of wheat and maize plants fertilized with rock phosphate in alkaline soils. Eur. J. Soil Biol. 2011, 47, 30–34. [Google Scholar] [CrossRef]
- Mayadunna, N.; Karunarathna, S.C.; Asad, S.; Stephenson, S.L.; Elgorban, A.M.; Al-Rejaie, S.; Kumla, J.; Yapa, N.; Suwannarach, N. Isolation of phosphate-solubilizing microorganisms and the formulation of biofertilizer for sustainable processing of phosphate rock. Life 2023, 13, 782. [Google Scholar] [CrossRef]
- Mittal, V.; Singh, O.; Nayyar, H.; Kaur, J.; Tewari, R. Stimulatory effect of phosphate-solubilizing fungal strains (Aspergillus awamori and Penicillium citrinum) on the yield of chickpea (Cicer arietinum L. cv. GPF2). Soil Biol. Biochem. 2008, 40, 718–727. [Google Scholar] [CrossRef]
- Khuna, S.; Suwannarach, N.; Kumla, J.; Frisvad, J.C.; Matsui, K.; Nuangmek, W.; Lumyong, S. Growth enhancement of Arabidopsis (Arabidopsis thaliana) and onion (Allium cepa) with inoculation of three newly identified mineral-solubilizing fungi in the genus Aspergillus section Nigri. Front. Microbiol. 2021, 12, 705896. [Google Scholar] [CrossRef]
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S.C. Phosphate-solubilizing microorganisms: Mechanism and their role in phosphate solubilization and uptake. J. Soil Sci. Plant Nutr. 2021, 21, 49–68. [Google Scholar] [CrossRef]
- Jain, R.; Saxena, J.; Sharma, V. Effect of phosphate-solubilizing fungi Aspergillus awamori S29 on mungbean (Vigna radiata cv. RMG 492) growth. Folia Microbiol. 2012, 57, 533–541. [Google Scholar] [CrossRef]
- Patel, S.; Panchal, B.; Karmakar, N.; Katagi, R.B.; Jha, S. Solubilization of rock phosphate by two Rhizopus species isolated from coastal areas of South Gujarat and its effect on chickpea. Ecol. Environ. Conserv. 2015, 21, 223–231. [Google Scholar]
- Kaur, G.; Reddy, M.S. Improvement of crop yield by phosphate-solubilizing Aspergillus species in organic farming. Arch. Agron. Soil Sci. 2016, 63, 24–34. [Google Scholar] [CrossRef]
- Saxena, J.; Saini, A.; Ravi, I.; Chandra, S.; Garg, V. Consortium of phosphate-solubilizing bacteria and fungi for promotion of growth and yield of chickpea (Cicer arietinum). J. Crop. Improv. 2015, 29, 353–369. [Google Scholar] [CrossRef]
- Saxena, J.; Rawat, J.; Sanwal, P. Enhancement of growth and yield of Glycine max plants with inoculation of phosphate solubilizing fungus Aspergillus niger K7 and biochar amendment in soil. Commun. Soil Sci. Plant Anal. 2016, 47, 2334–2347. [Google Scholar] [CrossRef]
- Saxena, J.; Saini, A.; Kushwaha, K.; Ariño, A. Synergistic effect of plant growth promoting bacterium Pseudomonas fluorescens and phosphate solubilizing fungus Aspergillus awamori for growth enhancement of chickpea. Indian J. Biochem. Biophys. 2016, 53, 135–143. [Google Scholar]
- Zhao, L.; Liu, Q.; Zhang, Y.; Cui, Q.; Liang, Y. Effect of acid phosphatase produced by Trichoderma asperellum Q1 on growth of Arabidopsis under salt stress. J. Integr. Agric. 2017, 16, 1341–1346. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, W.; Liu, D.; Lu, C.; Zhang, D.; Wu, H.; Dong, D.; Meng, L. Identification and evaluation of Aspergillus tubingensis as a potential biocontrol agent against grey mould on tomato. J. Gen. Plant Pathol. 2018, 84, 148–159. [Google Scholar] [CrossRef]
- Naziya, B.; Murali, M.; Amruthesh, K.N. Plant growth-promoting fungi (PGPF) Instigate plant growth and induce disease resistance in Capsicum annuum L. upon infection with Colletotrichum capsici (Syd.) Butler & Bisby. Biomolecules 2019, 10, 41. [Google Scholar] [CrossRef]
- Aloni, R.; Aloni, E.; Langhans, M.; Ullrich, C.I. Role of cytokinin and auxin in shaping root architecture: Regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann. Bot. 2006, 97, 883–893. [Google Scholar] [CrossRef]
- Fu, J.; Wang, S. Insights into auxin signaling in plant–pathogen interactions. Front. Plant Sci. 2011, 2, 74. [Google Scholar] [CrossRef]
- Tian, H.; De Smet, I.; Ding, Z. Shaping a root system: Regulating lateral versus primary root growth. Trends Plant Sci. 2014, 19, 426–431. [Google Scholar] [CrossRef]
- Keswani, C.; Singh, S.P.; Cueto, L.; García-Estrada, C.; Mezaache-Aichour, S.; Glare, T.R.; Borriss, R.; Singh, S.P.; Blázquez, M.A.; Sansinenea, S. Auxins of microbial origin and their use in agriculture. Appl. Microbiol. Biotechnol. 2020, 104, 8549–8565. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J. Auxin and plant-microbe interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a001438. [Google Scholar] [CrossRef]
- Yadav, J.; Verma, J.P.; Tiwari, K.N. Plant growth promoting activities of fungi and their effect on chickpea plant growth. Asian J. Biol. Sci. 2011, 4, 291–299. [Google Scholar] [CrossRef]
- Gaind, S. Phosphate dissolving fungi: Mechanism and application in alleviation of salt stress in wheat. Microbiol. Res. 2016, 193, 94–102. [Google Scholar] [CrossRef]
- Amrutha, G.; Savalgi, V.P.; Jagadeesh, K.S.; Hebsur, N.S. Isolation screening and selection of phosphate solubilizing fungi from maize rhizosphere. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 988–998. [Google Scholar] [CrossRef]
- Srinivasan, R.; Alagawadi, A.R.; Yandigeri, M.S.; Meena, K.K.; Saxena, A.K. Characterization of phosphate-solubilizing microorganisms from salt-affected soils of India and their effect on growth of sorghum plants [Sorghum bicolor (L.) Moench]. Ann. Microbiol. 2012, 62, 93–105. [Google Scholar] [CrossRef]
- Bader, A.N.; Salerno, G.L.; Covacevich, F.; Consolo, V.F. Native Trichoderma harzianum strains from Argentina produce indole-3-acetic acid and phosphorus solubilization, promote growth and control wilt disease on tomato (Solanum lycopersicum L.). J. King Saud Univ. Sci. 2020, 32, 867–873. [Google Scholar] [CrossRef]
- Imran, M.; Abulreesh, H.H.; Monjed, M.K.; Elbanna, K.; Samreen; Ahmad, I. Multifarious functional traits of free-living rhizospheric fungi, with special reference to Aspergillus spp. isolated from North Indian soil, and their inoculation effect on plant growth. Ann. Microbiol. 2021, 71, 31. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Yan, J.; Zhang, Y.; Wang, H.; Zhang, J.; Ahmed, T.; Li, B. Effect of plant-growth-promoting fungi on eggplant (Solanum melongena L.) in new reclamation land. Agriculture 2021, 11, 1036. [Google Scholar] [CrossRef]
- Nafaa, M.; Rizk, S.M.; Aly, T.A.-G.A.; Rashed, M.A.-S.; Abd El-Moneim, D.; Ben Bacha, A.; Alonazi, M.; Magdy, M. Screening and identification of the rhizosphere fungal communities associated with land reclamation in Egypt. Agriculture 2023, 13, 215. [Google Scholar] [CrossRef]
- Jain, R.; Saxena, J.; Sharma, V. Solubilization of inorganic phosphates by Aspergillus awamori S19 isolated from rhizosphere soil of a semi-arid region. Ann. Microbiol. 2012, 62, 725–735. [Google Scholar] [CrossRef]
- Patel, D.; Patel, A.; Patel, M.; Goswami, D. Talaromyces pinophilus strain M13: A portrayal of novel groundbreaking fungal strain for phytointensification. Environ. Sci. Pollut. Res. 2021, 28, 8758–8769. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Zhai, L.; Zhang, J.; Ren, T.; Fan, B.; Liu, H. Preparation and utilization of phosphate biofertilizers using agricultural waste. J. Integr. Agric. 2015, 14, 158–167. [Google Scholar] [CrossRef]
- Kannahi, M.; Senbagam, N. Studies on siderophore production by microbial isolates obtained from rhizosphere soil and its antibacterial activity. J. Chem. Pharm. Res. 2014, 6, 1142–1145. [Google Scholar]
- Saha, M.; Sarkar, S.; Sarkar, B.; Sharma, B.K.; Bhattacharjee, S.; Tribedi, P. Microbial siderophores and their potential applications: A review. Environ. Sci. Pollut. Res. 2016, 23, 3984–3999. [Google Scholar] [CrossRef]
- Crowley, D.E. Microbial siderophores in the plant rhizosphere. In Iron Nutrition in Plants and Rhizospheric Microorganisms; Barton, L.L., Abadia, J., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 169–198. [Google Scholar] [CrossRef]
- Ahmed, E.; Holmström, S.J.M. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef]
- Babu, A.G.; Shim, J.; Bang, K.; Shea, P.J.; Oh, B. Trichoderma virens PDR-28: A heavy metal-tolerant and plant growth promoting fungus for remediation and bioenergy crop production on mine tailing soil. J. Environ. Manag. 2014, 132, 129–134. [Google Scholar] [CrossRef]
- Babu, A.G.; Kim, S.W.; Yadav, D.R.; Hyum, U.; Adhikari, M.; Lee, Y.S. Penicillium menonorum: A novel fungus to promote growth and nutrient management in cucumber plants. Mycobiology 2015, 43, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Dutta, S. Plant growth promoting activities of a fungal strain Penicillium commune MCC 1720 and it’s effect on growth of black gram. Pharm. Innov. J. 2019, 8, 121–127. [Google Scholar]
- Moreno-Salazar, R.; Sánchez-García, I.; Chan-Cupul, W.; Ruiz-Sánchez, E.; Hernández-Ortega, H.A.; Pineda-Lucatero, J.; Figueroa-Chávez, D. Plant growth, foliar nutritional content and fruit yield of Capsicum chinense biofertilized with Purpureocillium lilacinum under greenhouse conditions. Sci. Hortic. 2020, 261, 108950. [Google Scholar] [CrossRef]
- Xie, Y.; Han, S.; Li, X.; Amombo, E.; Fu, J. Ameliorates of salt stress on bermudagrass by the fungus Aspergillus aculeatus. Mol. Plant-Microbe Interac. 2017, 30, 245–254. [Google Scholar] [CrossRef]
- Muthuraja, R.; Muthukumar, T. Isolation and characterization of potassium solubilizing Aspergillus species isolated from saxum habitats and their effect on maize growth in different soil types. Geomicrobiol. J. 2021, 38, 672–685. [Google Scholar] [CrossRef]
- Hervieux, V.; Yaganza, E.S.; Arul, J.; Tweddell, R.J. Effect of organic and inorganic salts on the development of Helminthosporium solani, the causal agent of potato silver scurf. Plant Dis. 2002, 86, 1014–1018. [Google Scholar] [CrossRef]
- Arriagada, C.A.; Herrera, M.A.; Borie, F.; Ocampo, J.A. Contribution of arbuscular mycorrhizal and saprobe fungi to the aluminum resistance of Eucalyptus globulus. Water Air Soil Pollut. 2007, 182, 383–394. [Google Scholar] [CrossRef]
- Kolaei, E.A.; Cenatus, C.; Tweddell, R.J.; Avis, T.J. Antifungal activity of aluminium-containing salts against the development of carrot cavity spot and potato dry rot. Ann. Appl. Biol. 2013, 136, 311–317. [Google Scholar] [CrossRef]
- Jones, D.J.; Oburger, E. Solubilization of phosphorus by soil microorganisms. In Phosphorus in Action; Bünemann, E.K., Oberson, A., Frossard, E., Eds.; Springer: Heidelberg/Berlin, Germany, 2011; pp. 169–198. [Google Scholar] [CrossRef]
- Mapelli, F.; Marasco, R.; Balloi, A.; Rolli, E.; Cappitelli, F.; Daffonchio, D.; Borin, S. Mineral-microbe interactions: Biotechnological potential of bioweathering. J. Biotechnol. 2012, 157, 473–481. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- Illmer, P.; Barbato, A.; Schinner, F. Solubilization of hardly soluble AlPO4 with P-solubilizing microorganisms. Soil Biol. Biochem. 1995, 27, 265–270. [Google Scholar] [CrossRef]
- Behera, B.C.; Singdevsachan, S.K.; Mishra, R.R.; Dutta, S.K.; Thatoi, H.N. Diversity, mechanism and biotechnology of phosphate solubilising microorganism in mangrove—A review. Biocatal. Agric. Biotechnol. 2014, 3, 97–110. [Google Scholar] [CrossRef]
- Jamshidi, R.; Jalili, B.; Bahmanyar, M.A.; Salek-Gilani, S. Isolation and identification of a phosphate solubilising fungus from soil of a phosphate mine in Chaluse, Iran. Mycology 2016, 7, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Vera-Morales, M.; López Medina, S.E.; Naranjo-Morán, J.; Quevedo, A.; Ratti, M.F. Nematophagous fungi: A review of their phosphorus solubilization potential. Microorganisms 2023, 11, 137. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, C.; Sui, J.; Liu, Z.; Li, Q.; Ji, C.; Song, X.; Hu, Y.; Wang, C.; Sa, R.; et al. Isolation and characterization of phosphofungi, and screening of their plant growth-promoting activities. AMB Express 2018, 8, 63. [Google Scholar] [CrossRef]
- Escobar Diaz, P.A.; Gil, O.J.A.; Barbosa, C.H.; Desoignies, N.; Rigobelo, E.C. Aspergillus spp. and Bacillus spp. as growth promoters in cotton plants under greenhouse conditions. Front. Sustain. Food Syst. 2021, 5, 709267. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y. Effects of phosphate solubilization and phytohormone production of Trichoderma asperellum Q1 on promoting cucumber growth under salt stress. J. Integr. Agric. 2015, 14, 1588–1597. [Google Scholar] [CrossRef]
- Jain, R.; Garg, V.; Saxena, J. Effect of an organophosphate pesticide, monocrotophos, on phosphate-solubilizing efficiency of soil fungal isolates. Appl. Biochem. Biotechnol. 2015, 175, 813–824. [Google Scholar] [CrossRef]
- Della Mónica, I.F.; Godoy, M.S.; Godeas, A.M.; Scervino, J.M. Fungal extracellular phosphatases: Their role in P cycling under different pH and P sources availability. J. Appl. Microbiol. 2017, 124, 155–165. [Google Scholar] [CrossRef]
- Qiao, H.; Sun, X.R.; Wu, X.Q.; Li, G.E.; Wang, Z.; Li, D.W. The phosphate-solubilizing ability of Penicillium guanacastense and its effects on the growth of Pinus massoniana in phosphate-limiting conditions. Biol. Open 2019, 8, bio046797. [Google Scholar] [CrossRef]
- Akintokun, A.K.; Akande, G.A.; Akintokun, P.O.; Popoola, T.O.S.; Babalola, A.O. Solubilization of insoluble phosphate by organic acid-producing fungi isolated from Nigerian soil. Int. J. Soil Sci. 2007, 2, 301–307. [Google Scholar] [CrossRef]
- Kanse, O.S.; Whitelaw-Weckert, M.; Kadam, T.A.; Bhosale, H. Phosphate solubilization by stress-tolerant soil fungus Talaromyces funiculosus SLS8 isolated from the Neem rhizosphere. Ann. Microbiol. 2015, 65, 85–93. [Google Scholar] [CrossRef]
- Bakri, M.M. Tri-calcium and zinc phosphates solubilization by Aspergillus niger and its relation to organic acids production. BioNanoScience 2019, 9, 238–244. [Google Scholar] [CrossRef]
- Li, X.; Sun, X.; Wang, G.; Amombo, E.; Zhou, X.; Du, Z.; Zhang, Y.; Xie, Y.; Fu, J. Inoculation with Aspergillus aculeatus alters the performance of perennial ryegrass under phosphorus deficiency. J. Am. Soc. Hortic. Sci. 2019, 144, 182–192. [Google Scholar] [CrossRef]
- Acevedo, E.; Galindo-Castañeda, T.; Prada, F.; Navia, M.; Romero, H.M. Phosphate solubilizing microorganisms associated with the rhizosphere of oil palm (Elaeis guineensis Jacq.) in Colombia. Appl. Soil Ecol. 2014, 80, 26–33. [Google Scholar] [CrossRef]
- Xiao, C.; Fang, Y.; Chi, R. Phosphate solubilization in vitro by isolated Aspergillus niger and Aspergillus carbonarius. Res. Chem. Intermed. 2015, 41, 2867–2878. [Google Scholar] [CrossRef]
- Li, Z.; Bai, T.; Dai, L.; Wang, F.; Tao, J.; Meng, S.; Hu, Y.; Wang, S.; Hu, S. A study of organic acid production in contrasts between two phosphate solubilizing fungi: Penicillium oxalicum and Aspergillus niger. Sci. Rep. 2016, 6, 25313. [Google Scholar] [CrossRef]
- Qin, W.; Liu, C.; Jiang, W.; Xue, Y.; Wang, G.; Liu, S. A coumarin analogue NFA from endophytic Aspergillus fumigatus improves drought resistance in rice as an antioxidant. BMC Microbiol. 2019, 19, 50. [Google Scholar] [CrossRef]
- Li, X.; Zhao, C.; Zhang, T.; Wang, G.; Amombo, E.; Xie, Y.; Fu, J. Exogenous Aspergillus aculeatus enhances drought and heat tolerance of perennial ryegrass. Front. Microbiol. 2021, 12, 593722. [Google Scholar] [CrossRef]
- Kaur, R.; Saxena, S. Penicillium citrinum, a drought-tolerant endophytic fungus isolated from wheat (Triticum aestivum L.) leaves with plant growth-promoting abilities. Curr. Microbiol. 2023, 80, 184. [Google Scholar] [CrossRef]
- Muthuraja, R.; Muthukumar, T.; Natthapol, C. Drought tolerance of Aspergillus violaceofuscus and Bacillus licheniformis and their influence on tomato growth and potassium uptake in mica amended tropical soils under water-limiting conditions. Front. Plant Sci. 2023, 14, 1114288. [Google Scholar] [CrossRef] [PubMed]
- Ou, T.; Zhang, M.; Gao, H.; Wang, F.; Xu, W.; Liu, X.; Wang, L.; Wang, R.; Xie, J. Study on the potential for stimulating mulberry growth and drought tolerance of plant growth-promoting fungi. Int. J. Mol. Sci. 2023, 24, 4090. [Google Scholar] [CrossRef] [PubMed]
- Rinu, K.; Pandey, A. Temperature-dependent phosphate solubilization by cold- and pH-tolerant species of Aspergillus isolated from Himalayan soil. Mycoscience 2010, 51, 263–271. [Google Scholar] [CrossRef]
- Xiao, C.; Chi, R.; Li, X.; Xia, M.; Xia, Z. Biosolubilization of rock phosphate by three stress-tolerant fungal strains. Appl. Biochem. Biotechnol. 2011, 165, 719–727. [Google Scholar] [CrossRef]
- Passamani, F.R.F.; Hernandes, T.; Lopes, N.A.; Bastos, S.C.; Santiago, W.D.; Cardoso, M.G.; Batista, L.R. Effect of temperature, water activity, and pH on growth and production of ochratoxin A by Aspergillus niger and Aspergillus carbonarius from Brazilian grapes. J. Food Prot. 2014, 77, 1947–1952. [Google Scholar] [CrossRef] [PubMed]
- Kurniati, E.; Arfarita, N.; Imai, T. Potential use of Aspergillus flavus strain KRP1 in utilization of mercury contaminant. Procedia Environ. Sci. 2014, 20, 254–260. [Google Scholar] [CrossRef]
- Al Tamie, M.S.S. Effect of salinity on the fungal occurance in Al-Shega area at Al-Qassim, Saudi Arabia. Res. J. Microbiol. 2014, 9, 287–295. [Google Scholar] [CrossRef]
- Vassilev, N.; Eichler-Löbermann, B.; Vassileva, M. Stress-tolerant P-solubilizing microorganisms. Appl. Microbiol. Biotechnol. 2012, 95, 851–859. [Google Scholar] [CrossRef]
- Viscardi, S.; Ventorino, V.; Duran, P.; Maggio, A.; De Pascale, S.; Mora, M.L.; Pepe, O. Assessment of plant growth promoting activities and abiotic stress tolerance of Azotobacter chroococcum strains for a potential use in sustainable agriculture. J. Soil Sci. Plant Nutr. 2016, 16, 848–863. [Google Scholar] [CrossRef]
- Chaiya, L.; Kumla, J.; Suwannarach, N.; Kiatsiriroat, T.; Lumyong, S. Isolation, characterization, and efficacy of actinobacteria associated with arbuscular mycorrhizal spores in promoting plant growth of chili (Capsicum flutescens L.). Microorganisms 2021, 9, 1274. [Google Scholar] [CrossRef]
- Fan, D.; Smith, D.L. Characterization of selected plant growth-promoting rhizobacteria and their non-host growth promotion effects. Microbiol. Spectr. 2021, 9, e00279-21. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xiao, L.; Li, F.; Xiao, M.; Lin, D.; Long, X.; Wu, Z. Microbial degradation of pesticide residues and an emphasis on the degradation of cypermethrin and 3-phenoxy benzoic acid: A review. Molecules 2018, 23, 2313. [Google Scholar] [CrossRef]
- Spagnoletti, F.N.; Chiocchio, V.M. Tolerance of dark septate endophytic fungi (DSE) to agrochemicals in vitro Tolerancia de hongos endofíticos septados oscuros a agroquímicos in vitro. Rev. Argent. Microbiol. 2020, 52, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Suwannarach, N.; Kumla, J.; Matsui, K.; Lumyong, S. Characterization and efficacy of Muscodor cinnamomi in promoting plant growth and controlling Rhizoctonia root rot in tomatoes. Biol. Control 2015, 90, 25–33. [Google Scholar] [CrossRef]
- Karaoglu, S.A.; Bozdeveci, A.; Pehlivan, N. Characterization of local Trichoderma spp. as potential bio-control agents, screening of in vitro antagonistic activities and fungicide tolerance. J. Biol. Chem. 2018, 46, 247–261. [Google Scholar] [CrossRef]
- Nuangmek, W.; Aiduang, W.; Kumla, J.; Lumyong, S.; Suwannarach, N. Evaluation of a newly identified endophytic fungus, Trichoderma phayaoense for plant growth promotion and biological control of gummy stem blight and wilt of muskmelon. Front. Microbiol. 2021, 12, e634772. [Google Scholar] [CrossRef]
- Eman, A.; Abdel-Megeed, A.; Suliman, A.M.A.; Sadik, M.W.; Sholkamy, E.N. Biodegradation of glyphosate by fungal strains isolated from herbicides polluted-soils in Riyadh area. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 359–381. [Google Scholar] [CrossRef]
- Mohiddin, F.A.; Khan, M.R. Tolerance of fungal and bacterial biocontrol agents to six pesticides commonly used in the control of soil borne plant pathogens. Afr. J. Agric. Res. 2013, 8, 5272–5275. [Google Scholar]
- Andy, I.E.; Edu, G.S.; Bassey, I.U.; Markson, A.A.; Umana, E.I.; Udo, S.E. Biodegradation of paraquat. J. Biopestic. Environ. 2015, 1, 80–85. [Google Scholar]
- Wongputtisin, P.; Supo, C.; Suwannarach, N.; Honda, Y.; Nakazawa, T.; Kumla, J.; Lumyong, S.; Khanongnuch, C. Filamentous fungi with high paraquat-degrading activity isolated from contaminated agricultural soils in northern Thailand. Lett. Appl. Microbiol. 2020, 72, 467–475. [Google Scholar] [CrossRef]
- Spinelli, V.; Ceci, A.; Dal Bosco, C.; Gentili, A.; Persiani, A.M. Glyphosate-eating fungi: Study on fungal saprotrophic strains’ ability to tolerate and utilise glyphosate as a nutritional source and on the ability of Purpureocillium lilacinum to degrade it. Microorganisms 2021, 9, 2179. [Google Scholar] [CrossRef] [PubMed]
- Benito, N.; Magnoli, K.; Carranza, C.S.; Aluffi, M.E.; Magnoli, C.E.; Barberis, C.L. Influence of a glyphosate-based herbicide on growth parameters and aflatoxin B1 production by Aspergillus section Flavi on maize grains. Rev. Argent. Microbiol. 2021, 53, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Carranza, C.S.; Barberis, C.L.; Aluffi, M.E.; Benito, N.; Magnoli, C.E. Native mycota in agricultural soils exposed to pesticides and Aspergillus oryzae tolerance to chlorpyrifos in microcosms assays. Curr. Res. Environ. Appl. Mycol. 2017, 7, 236–248. [Google Scholar] [CrossRef]
- Kumar, S.; Thakur, M.; Rani, A. Trichoderma: Mass production, formulation, quality control, delivery and its scope in commercialization in India for the management of plant diseases. Afr. J. Agric. Res. 2014, 9, 3838–3852. [Google Scholar] [CrossRef]
- Raymond, N.S.; Stöver, D.M.; Jensen, L.S.; Håkansson, S. Survival and phosphate solubilisation activity of desiccated formulations of Penicillium bilaiae and Aspergillus niger influenced by water activity. J. Microbiol. Methods 2018, 150, 39–46. [Google Scholar] [CrossRef]
- Friesen, T.; Hill, G.; Pugsley, T.; Holloway, G.; Zimmerman, D. Experimental determination of viability loss of Penicillium bilaiae conidia during convective air drying. Appl. Microbiol. Biotechnol. 2005, 68, 397–404. [Google Scholar] [CrossRef]
- Friesen, T.J.; Holloway, G.; Hill, G.A.; Pugsley, T.S. Effect of conditions and protectants on the survival of Penicillium bilaiae during storage. Biocontrol Sci. Technol. 2006, 16, 89–98. [Google Scholar] [CrossRef]
- Pindi, P.K.; Satyanarayana, S.D.V. Liquid microbial consortium—A potential tool for sustainable soil health. J. Biofertil. Biopestic. 2012, 3, 124. [Google Scholar] [CrossRef]
- Phiromtan, M.; Mala, T.; Srinives, P. Effect of various carriers and storage temperatures on survival of Azotobacter vinelandii NDD-CK-1 in powder inoculant. Mod. Appl. Sci. 2013, 7, 81–89. [Google Scholar] [CrossRef]
- Raimi, A.; Roopnarain, A.; Adeleke, R. Biofertilizer production in Africa: Current status, factors impeding adoption and strategies for success. Sci. Afr. 2021, 11, e00694. [Google Scholar] [CrossRef]
- Daigle, D.J.; Cotty, P.J. Formulating atoxigenic Aspergillus flavus for field release. Biocontrol Sci. Technol. 1995, 5, 175–184. [Google Scholar] [CrossRef]
- Locatelli, G.O.; dos Santos, G.F.; Botelho, P.S.; Finkler, C.L.L.; Bueno, L.A. Development of Trichoderma sp. formulations in encapsulated granules (CG) and evaluation of conidia shelf-life. Biol. Control 2018, 117, 21–29. [Google Scholar] [CrossRef]
- Mulatu, A.; Alemu, T.; Megersa, N.; Vetukuri, R.R. Optimization of culture conditions and production of bio-fungicides from Trichoderma species under solid-state fermentation using mathematical modeling. Microorganisms 2021, 9, 1675. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, M.O. Microbial inoculation of seed for improved crop performance: Issues and opportunities. Appl. Microbiol. Biotechnol. 2016, 100, 5729–5746. [Google Scholar] [CrossRef]
- Alori, E.T.; Babalola, O.O. Microbial inoculants for improving crop quality and human health in Africa. Front. Microbiol. 2018, 9, 2213. [Google Scholar] [CrossRef]
- Brar, S.K.; Sarma, S.J.; Chaaboun, E. Shelf-life of biofertilizers: An accord between formulations and genetics. J. Biofertil. Biopestici. 2012, 3, e109. [Google Scholar] [CrossRef]
- Melin, P.; Schnürer, J.; Håkansson, S. Formulation and stabilisation of the biocontrol yeast Pichia anomala. Antonie Van Leeuwenhoek 2011, 99, 107–112. [Google Scholar] [CrossRef]
- Parnell, J.J.; Berka, R.; Young, H.A.; Sturino, J.M.; Kang, Y.; Barnhart, D.; DiLeo, M.V. From the lab to the farm: An industrial perspective of plant beneficial microorganisms. Front. Plant Sci. 2016, 7, 1110. [Google Scholar] [CrossRef]
- Accinelli, C.; Saccà, M.L.; Abbas, H.K.; Zablotowicz, R.M.; Wilkinson, J.R. Use of a granular bioplastic formulation for carrying conidia of a non-aflatoxigenic strain of Aspergillus flavus. Bioresour. Technol. 2009, 100, 3997–4004. [Google Scholar] [CrossRef]
- Jain, R.; Gupta, A.; Sharma, V.; Naik, S.; Saxena, J.; Kumar, V.; Prasad, R. Immobilization-based bio-formulation of Aspergillus awamori S29 and evaluation of its shelf life and re-usability in the soil–plant experiment. Curr. Microbiol. 2022, 79, 163. [Google Scholar] [CrossRef]
- Mendes, G.O.; Galvez, A.; Vassileva, M.; Vassilev, N. Fermentation liquid containing microbially solubilized P significantly improved plant growth and P uptake in both soil and soilless experiments. Appl. Soil Ecol. 2017, 117–118, 208–211. [Google Scholar] [CrossRef]
- Tariq, M.R.; Shaheen, F.; Mustafa, S.; Ali, S.; Fatima, A.; Shafiq, M.; Safdar, W.; Sheas, M.N.; Hameed, A.; Nasir, M.A. Phosphate solubilizing microorganisms isolated from medicinal plants improve growth of mint. PeerJ 2022, 10, e13782. [Google Scholar] [CrossRef] [PubMed]
- Lubna; Asaf, S.; Hamayun, M.; Gul, H.; Lee, I.J.; Hussain, A. Aspergillus niger CSR3 regulates plant endogenous hormones and secondary metabolites by producing gibberellins and indoleacetic acid. J. Plant Interact. 2018, 13, 100–111. [Google Scholar] [CrossRef]
- Abdel-Motaal, F.; Kamel, N.; El-Zayat, S.; Abou-Ellail, M. Early blight suppression and plant growth promotion potential of the endophyte Aspergillus flavus in tomato plant. Ann. Agric. Sci. 2020, 65, 117–123. [Google Scholar] [CrossRef]
- Peng, Q.; Xiao, Y.; Zhang, S.; Zhou, C.; Xie, A.; Li, Z.; Tan, A.; Zhou, L.; Xie, Y.; Zhao, J.; et al. Mutation breeding of Aspergillus niger by atmospheric room temperature plasma to enhance phosphorus solubilization ability. PeerJ 2022, 10, e13076. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.C.; Kuo, Y.L.; Chao, C.C.; Chao, W.L. Solubilization of inorganic phosphates and plant growth promotion by Aspergillus niger. Biol. Fertil. Soils 2007, 43, 575–584. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Petropoulos, S.A. Biostimulants application: A low input cropping management tool for sustainable farming of vegetables. Biomolecules 2021, 11, 698. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Tzortzakis, N.; Petropoulos, S.A. Sustainable agriculture systems in vegetable production using chitin and chitosan as plant biostimulants. Biomolecules 2021, 11, 819. [Google Scholar] [CrossRef]
- Tsavkelova, E.A.; Cherdyntseva, T.A.; Botina, S.G.; Netrusov, A.I. Bacteria associated with orchid roots and microbial production of auxin. Microbiol. Res. 2007, 162, 69–76. [Google Scholar] [CrossRef]
- Kumla, J.; Suwannarach, N.; Bussaban, B.; Matsui, K.; Lumyong, S. Indole-3-acetic acid production, solubilization of insoluble metal minerals and metal tolerance of some sclerodermatoid fungi collected from northern Thailand. Ann. Microbiol. 2014, 64, 707–720. [Google Scholar] [CrossRef]
- Schwyn, B.; Neiland, J. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Lim, Y.; Lee, S.E.; Yang, N.W.; Rhee, J.H. CAS agar diffusion assay for the measurement of siderophores in biological fluids. J. Microbiol. Methods 2001, 44, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Fomina, M.A.; Alexander, I.J.; Colpaert, J.V.; Gadd, G.M. Solubilization of toxic metal minerals and metal tolerance of mycorrhizal fungi. Soil Biol. Biochem. 2005, 37, 851–866. [Google Scholar] [CrossRef]
- Elias, F.; Muleta, D.; Woyessa, D. Effects of phosphate solubilizing fungi on growth and yield of haricot bean (Phaseolus vulgaris L.) plants. J. Agric. Sci. 2016, 8, 204–218. [Google Scholar] [CrossRef]
- Fiske, C.; Subbarow, Y. The colorimetric determination of phosphorus. J. Biol. Chem. 1925, 66, 375–400. [Google Scholar] [CrossRef]
- Holman, W.I.M. A new technique for the determination of phosphorus by the molybdenum blue method. Biochem. J. 1943, 37, 256–259. [Google Scholar] [CrossRef]
- Gaind, S.; Singh, S. Production, purification and characterization of neutral phytase from thermotolerant Aspergillus flavus ITCC 6720. Int. Biodeterior. Biodegrad. 2015, 99, 15–22. [Google Scholar] [CrossRef]
- Adhikari, P.; Pandey, A. Phosphate solubilization potential of endophytic fungi isolated from Taxus wallichiana Zucc. roots. Rhizosphere 2019, 9, 2–9. [Google Scholar] [CrossRef]
- Hallsworth, J.E.; Nomura, Y.; Iwahara, M. Ethanol-induced water stress and fungal growth. J. Ferment. Bioeng. 1998, 86, 451–456. [Google Scholar] [CrossRef]
- Tresner, H.D.; Hayes, J.A. Sodium chloride tolerance of terrestrial fungi. Appl. Microbiol. 1971, 22, 210–213. [Google Scholar] [CrossRef]
- Sriram, S.; Roopa, K.P.; Savitha, M.J. Extended shelf-life of liquid fermentation derived talc formulations of Trichoderma harzianum with the addition of glycerol in the production medium. Crop. Prot. 2011, 30, 1334–1339. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Liang, Y.; Urano, D.; Liao, K.L.; Hedrick, T.L.; Gao, Y.; Jones, A.M. A nondestructive method to estimate the chlorophyll content of Arabidopsis seedlings. Plant Methods 2017, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N. Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol. 1966, 8, 115–118. [Google Scholar] [CrossRef]
- Wang, L.; Dong, J.; Gao, Z.; Liu, D. The Arabidopsis gene HYPERSENSITIVE TO PHOSPHATE STARVATION 3 encodes ETHYLENE OVERPRODUCTION 1. Plant Cell Physiol. 2012, 53, 1093–1105. [Google Scholar] [CrossRef]





| Plant Growth Promotion Properties | A. chiangmaiensis SDBR-CMUI4 | A. pseudopiperis SDBR-CMUI1 | A. pseudotubingensis SDBR-CMUO2 |
|---|---|---|---|
| IAA production (µg/mL) | − | 33.37 | − |
| Siderophore production | + | + | + |
| Drought tolerance (aw) | 0.837–0.998 | 0.859–0.998 | 0.837–0.998 |
| pH tolerance | 4.0–9.0 | 4.0–9.0 | 4.0–9.0 |
| Temperature tolerance (°C) | 4–40 | 4–40 | 4–40 |
| Salinity tolerance (% NaCl) | Up to 17% | Up to 16% | Up to 17% |
| Treatment Number | Treatment Details |
|---|---|
| T1 | Soil (control) |
| T2 | Soil (3 kg) + Ca3(PO4)2 (1.5 g) |
| T3 | Soil (3 kg) + inoculum of Aspergillus chiangmaiensis SDBR-CMUI4 (3 g) |
| T4 | Soil (3 kg) + inoculum of Aspergillus pseudopiperis SDBR-CMUI1 (3 g) |
| T5 | Soil (3 kg) + inoculum of Aspergillus pseudotubingensis SDBR-CMUO2 (3 g) |
| T6 | Soil (3 kg) + Ca3(PO4)2 (1.5 g) + inoculum of A. chiangmaiensis SDBR-CMUI4 (3 g) |
| T7 | Soil (3 kg) + Ca3(PO4)2 (1.5 g) + inoculum of A. pseudopiperis SDBR-CMUI1 (3 g) |
| T8 | Soil (3 kg) + Ca3(PO4)2 (1.5 g) + inoculum of A. pseudotubingensis SDBR-CMUO2 (3 g) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khuna, S.; Kumla, J.; Srinuanpan, S.; Lumyong, S.; Suwannarach, N. Multifarious Characterization and Efficacy of Three Phosphate-Solubilizing Aspergillus Species as Biostimulants in Improving Root Induction of Cassava and Sugarcane Stem Cuttings. Plants 2023, 12, 3630. https://doi.org/10.3390/plants12203630
Khuna S, Kumla J, Srinuanpan S, Lumyong S, Suwannarach N. Multifarious Characterization and Efficacy of Three Phosphate-Solubilizing Aspergillus Species as Biostimulants in Improving Root Induction of Cassava and Sugarcane Stem Cuttings. Plants. 2023; 12(20):3630. https://doi.org/10.3390/plants12203630
Chicago/Turabian StyleKhuna, Surapong, Jaturong Kumla, Sirasit Srinuanpan, Saisamorn Lumyong, and Nakarin Suwannarach. 2023. "Multifarious Characterization and Efficacy of Three Phosphate-Solubilizing Aspergillus Species as Biostimulants in Improving Root Induction of Cassava and Sugarcane Stem Cuttings" Plants 12, no. 20: 3630. https://doi.org/10.3390/plants12203630








