Potential of Biochar as a Peat Substitute in Growth Media for Lavandula angustifolia, Salvia rosmarinus and Fragaria × ananassa
Abstract
:1. Introduction
2. Results
2.1. Biochars Chemistry
2.2. Salvia rosmarinus
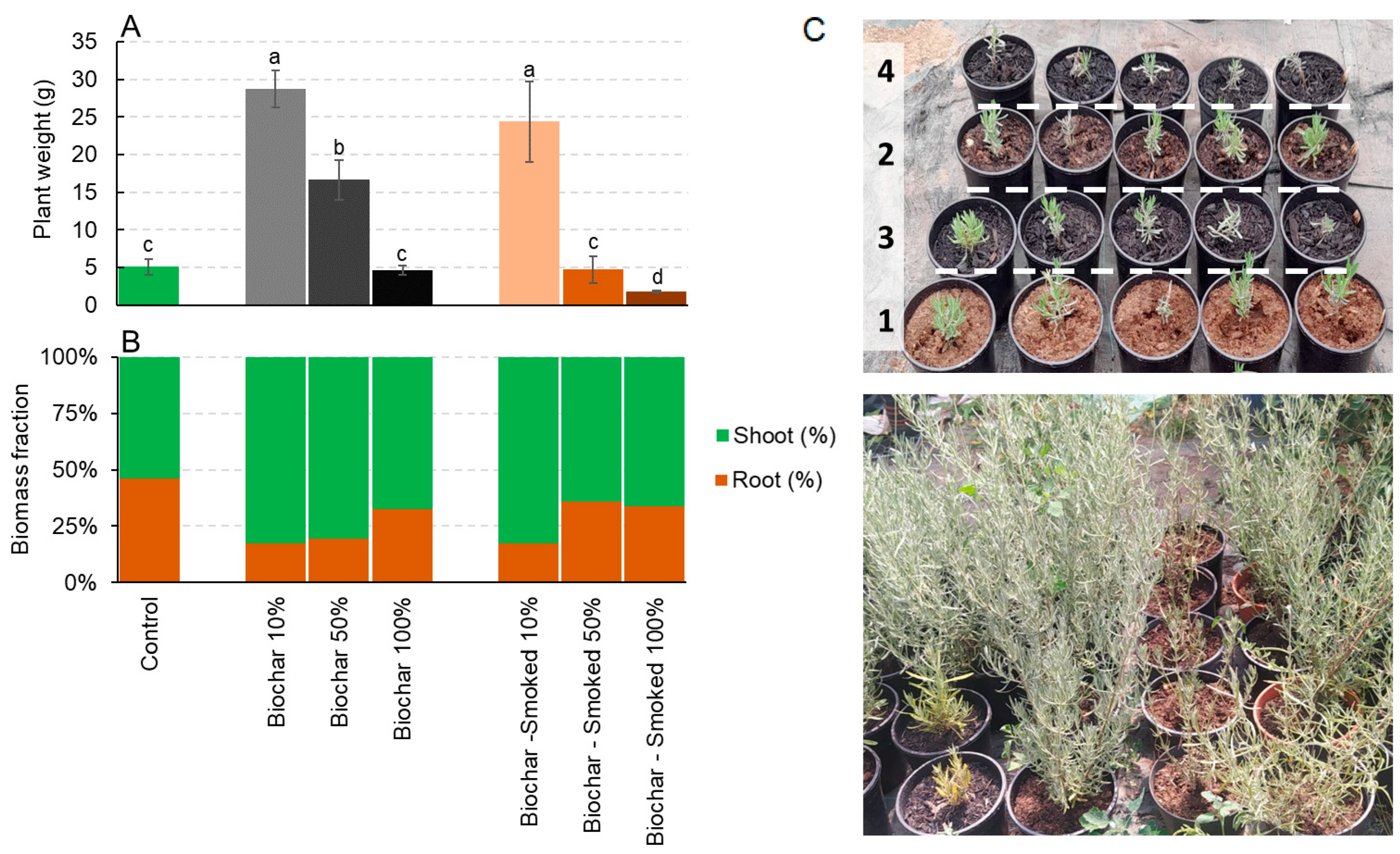
2.3. Lavandula angustifolia
2.4. Fragaria × ananassa
3. Discussion
4. Materials and Methods
4.1. Study Site
4.2. Experimental Design
4.3. Biochar and Peat Chemical Analyses
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lourenco, M.; Fitchett, J.M.; Woodborne, S. Peat definitions: A critical review. Prog. Phys. Geogr. Earth Environ. 2023, 47, 506–520. [Google Scholar] [CrossRef]
- Humpenöder, F.; Karstens, K.; Lotze-Campen, H.; Leifeld, J.; Menichetti, L.; Barthelmes, A.; Popp, A. Peatland protection and restoration are key for climate change mitigation. Environ. Res. Lett. 2020, 15, 104093. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X.; Tian, Y.; Gong, X. Composted Green Waste as a Substitute for Peat in Growth Media: Effects on Growth and Nutrition of Calathea insignis. PLoS ONE 2013, 8, e78121. [Google Scholar] [CrossRef]
- Fascella, G. Growing substrates alternative to peat for ornamental plants. In Soilless Culture-Use of Substrates for the Production of Quality Horticultural Crops; InTech: London, UK, 2015. [Google Scholar]
- Méndez, A.; Paz-Ferreiro, J.; Gil, E. The effect of paper sludge and biochar addition on brown peat and coir based growing media properties. Sci. Hortic. 2015, 193, 225–230. [Google Scholar] [CrossRef]
- Biochar (IBI). Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil|International Biochar Initiative; International Biochar Initiative: Canandaigua, NY, USA, 2012; 47p. [Google Scholar]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science and Technology; Routledge: New York, NY, USA, 2012. [Google Scholar]
- Oleszczuk, P.; Rycaj, M.; Lehmann, J.; Cornelissen, G. Influence of activated carbon and biochar on phytotoxicity of air-dried sewage sludges to Lepidium sativum. Ecotoxicol. Environ. Saf. 2012, 80, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, G.; Ippolito, F.; Cesarano, G.; Vinale, F.; Lombardi, N.; Crasto, A.; Woo, S.L.; Scala, F. Biochar chemistry defined by 13C-CPMAS NMR explains opposite effects on soilborne microbes and crop plants. Appl. Soil Ecol. 2018, 124, 351–361. [Google Scholar] [CrossRef]
- Hagemann, N.; Joseph, S.; Schmidt, H.-P.; Kammann, C.I.; Harter, J.; Borch, T.; Young, R.B.; Varga, K.; Taherymoosavi, S.; Elliott, K.W.; et al. Organic coating on biochar explains its nutrient retention and stimulation of soil fertility. Nat. Commun. 2017, 8, 1089. [Google Scholar] [CrossRef]
- Kammann, C.I.; Schmidt, H.P.; Messerschmidt, N.; Linsel, S.; Steffens, D.; Muller, C.; Koyro, H.W.; Conte, P.; Stephen, J. Plant growth improvement mediated by nitrate capture in co-composted biochar. Sci. Rep. 2015, 5, 11080. [Google Scholar] [CrossRef]
- Manikandan, A.; Subramanian, K.S. Urea intercalated biochar—A slow release fertilizer production and characterisation. Indian J. Sci. Technol. 2013, 6, 5579–5584. [Google Scholar] [CrossRef]
- Shi, W.; Ju, Y.Y.; Bian, R.J.; Li, L.Q.; Joseph, S.; Mitchell, D.R.G.; Munroe, P.; Taherymoosavi, S.; Pan, G.X. Biochar bound urea boosts plant growth and reduces nitrogen leaching. Sci. Total Environ. 2020, 701, 13442. [Google Scholar] [CrossRef]
- Bonanomi, G.; Zotti, M.; Abd-ElGawad, A.M.; Iacomino, G.; Nappi, A.; Grauso, L.; Idbella, M. Plant-growth promotion by biochar-organic amendments mixtures explained by selective chemicals adsorption of inhibitory compounds. J. Environ. Chem. Eng. 2023, 11, 109009. [Google Scholar] [CrossRef]
- Kammann, C.; Glaser, B.; Schmidt, H.P. Combining biochar and organic amendments. In Biochar in European Soils and Agriculture: Science and Practice; Shackley, S., Ruysschaert, G., Zwart, K., Glaser, B., Eds.; Routledge: New York, NY, USA, 2016; pp. 136–164. [Google Scholar]
- Bonanomi, G.; Incerti, G.; Barile, E.; Capodilupo, M.; Antignani, V.; Mingo, A.; Lanzotti, V.; Scala, F.; Mazzoleni, S. Phytotoxicity, not nitrogen immobilization, explains plant litter inhibitory effects: Evidence from solid-state13C NMR spectroscopy. New Phytol. 2011, 191, 1018–1030. [Google Scholar] [CrossRef]
- Light, M.; van Staden, J.; Bornman, C. The potential of smoke in seed technology. S. Afr. J. Bot. 2004, 70, 97–101. [Google Scholar] [CrossRef]
- Chiwocha, S.D.; Dixon, K.W.; Flematti, G.R.; Ghisalberti, E.L.; Merritt, D.J.; Nelson, D.C.; Riseborough, J.-A.M.; Smith, S.M.; Stevens, J. Karrikins: A new family of plant growth regulators in smoke. Plant Sci. 2009, 177, 252–256. [Google Scholar] [CrossRef]
- Yildizli, G.; Coral, G.; Ayaz, F. Anti-bacterial, anti-fungal, and anti-inflammatory activities of wood vinegar: A potential remedy for major plant diseases and inflammatory reactions. Biomass Convers. Biorefin. 2022, 1–10. [Google Scholar] [CrossRef]
- Yatagai, M.; Nishimoto, M.; Hori, K.; Ohira, T.; Shibata, A. Termiticidal activity of wood vinegar, its components and their homologues. J. Wood Sci. 2002, 48, 338–342. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Xing, Z.J.; Duan, Z.K.; Li, M.; Wang, Y. Effects of steam activation on the pore structure and surface chemistry of activated carbon derived from bamboo waste. Appl. Surf. Sci. 2014, 315, 279–286. [Google Scholar] [CrossRef]
- Dispenza, V.; De Pasquale, C.; Fascella, G.; Mammano, M.M.; Alonzo, G. Use of biochar as peat substitute for growing substrates of Euphorbia × lomi potted plants. Span. J. Agric. Res. 2017, 14, e0908. [Google Scholar] [CrossRef]
- Álvarez, J.M.; Pasian, C.; Lal, R.; López, R.; Díaz, M.J.; Fernández, M. Morpho-physiological plant quality when biochar and vermicompost are used as growing media replacement in urban horticulture. Urban For. Urban Green. 2018, 34, 175–180. [Google Scholar] [CrossRef]
- Margenot, A.J.; Griffin, D.E.; Alves, B.S.; Rippner, D.A.; Li, C.; Parikh, S.J. Substitution of peat moss with softwood biochar for soil-free marigold growth. Ind. Crops Prod. 2018, 112, 160–169. [Google Scholar] [CrossRef]
- Dunn, C.; Freeman, C. Peatlands: Our greatest source of carbon credits? Carbon Manag. 2011, 2, 289–301. [Google Scholar] [CrossRef]
- Bonanomi, G.; Jesu, G.; Zotti, M.; Idbella, M.; D’Errico, G.; Laudonia, S.; Vinale, F.; Abd-ElGawad, A. Biochar-derived smoke-water exerts biological effects on nematodes, insects, and higher plants but not fungi. Sci. Total Environ. 2021, 750, 142307. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Defining hormesis. Hum. Exp. Toxicol. 2002, 21, 91–97. [Google Scholar] [CrossRef]
- Flematti, G.R.; Ghisalberti, E.L.; Dixon, K.W.; Trengove, R.D. A compound from smoke that promotes seed germination. Science 2004, 305, 977. [Google Scholar] [CrossRef]
- Fascella, G.; Mammano, M.M.; D’Angiolillo, F.; Pannico, A.; Rouphael, Y. Coniferous wood biochar as substrate component of two containerized Lavender species: Effects on morpho-physiological traits and nutrients partitioning. Sci. Hortic. 2020, 267, 109356. [Google Scholar] [CrossRef]
- De Tender, C.A.; Debode, J.; Vandecasteele, B.; D’Hose, T.; Cremelie, P.; Haegeman, A.; Ruttink, T.; Dawyndt, P.; Maes, M. Biological, physicochemical and plant health responses in lettuce and strawberry in soil or peat amended with biochar. Appl. Soil Ecol. 2016, 107, 1–12. [Google Scholar] [CrossRef]
- Graber, E.R.; Frenkel, O.; Jaiswal, A.K.; Elad, Y. How may biochar influence severity of diseases caused by soilborne pathogens? Carbon Manag. 2014, 5, 169–183. [Google Scholar] [CrossRef]
- Thies, J.E.; Rillig, M.C. Characteristics of biochar: Biological properties. In Biochar for Environmental Management: Science and Technology; Earthscan: London, UK, 2009; Chapter 6; pp. 85–105. [Google Scholar]
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Rillig, M.C. Mycorrhizal responses to biochar in soil–concepts and mechanisms. Plant Soil 2017, 300, 9–20. [Google Scholar] [CrossRef]
- Masiello, C.A.; Chen, Y.; Gao, X.; Liu, S.; Cheng, H.-Y.; Bennett, M.R.; Rudgers, J.A.; Wagner, D.S.; Zygourakis, K.; Silberg, J.J. Biochar and microbial signaling: Production conditions determine effects on microbial communication. Environ. Sci. Technol. 2013, 47, 11496–11503. [Google Scholar] [CrossRef]
- Kolton, M.; Harel, Y.M.; Pasternak, Z.; Graber, E.R.; Elad, Y.; Cytryn, E. Impact of biochar application to soil on the root-associated bacterial community structure of fully developed greenhouse pepper plants. Appl. Environ. Microb. 2011, 77, 4924–4930. [Google Scholar] [CrossRef]
- Idbella, M.; De Filippis, F.; Zotti, M.; Sequino, G.; Abd-ElGawad, A.M.; Fechtali, T.; Mazzoleni, S.; Bonanomi, G. Specific microbiome signatures under the canopy of Mediterranean shrubs. Appl. Soil Ecol. 2022, 173, 104407. [Google Scholar] [CrossRef]
- Brewin, N.J. Root nodules (legume–rhizobium symbiosis). In eLS (Encyclopedia of Life Sciences); John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- DeLuca, T.H.; Gundale, M.J.; MacKenzie, M.D.; Jones, D.L. Biochar effects on soil nutrient transformations. Biochar Environ. Manag. Sci. Technol. Implement. 2015, 2, 421–454. [Google Scholar]
- Buss, W.; Graham, M.C.; Shepherd, J.G.; Mašek, O. Risks and benefits of marginal biomass-derived biochars for plant growth. Sci. Total Environ. 2016, 569–570, 496–506. [Google Scholar] [CrossRef]
- UNI EN 13037; Soil Improvers And Growing Media. Determination of pH. UNI Ente Italiano di Normazione: Milano, Italy, 2012.
- UNI EN 13041; Soil Improvers And Growing Media. Determination of Physical Properties. Dry Bulk Density, Air Volume, Water Volume, Shrinkage Value and Total Pore Space. UNI Ente Italiano di Normazione: Milano, Italy, 2012.
- UNI EN 13038; Soil Improvers and Growing Media. Determination of Electrical Conductivity. UNI Ente Italiano di Normazione: Milano, Italy, 2012.
- UNI EN 14775; Solid Biofuels—Determination of Ash Content. UNI Ente Italiano di Normazione: Milano, Italy, 2010.
- UNI EN 13654-2; Soil IMPROVERS and Cultivation Substrates—Determination of Nitrogen—Dumas Method. UNI Ente Italiano di Normazione: Milano, Italy, 2001.
- UNI EN 13650; Soil Improvers and Growing Media—Extraction of Aqua Regia Soluble Elements. UNI Ente Italiano di Normazione: Milano, Italy, 2002.
- UNI EN 15428; Soil Improvers and Growing Media—Determination of Particle Size Distribution. UNI Ente Italiano di Normazione: Milano, Italy, 2008.
- Violante, P. Metodi di Analisi Chimica del Suolo. [Methods of Soil Chemical Analyses]; Franco Angeli: Milan, Italy, 2000. [Google Scholar]



| Parameters | Unit | Biochar | Smoked Biochar | Peat |
|---|---|---|---|---|
| Density | g/L | 438 | 457 | |
| pH | pH unit | 7.9 | 7.5 | 5.70 |
| Electric conductibility | mS/m | 29 | 49 | 42.38 |
| Humidity | % m/m | 59.2 | 60.3 | |
| Total limestone (CaCO3) | % s.s. | 7.1 | 5.2 | |
| Total carbon | % s.s. | 55.7 | 70.7 | 44.50 |
| Ashes 550 °C | 26.51 | 19.04 | ||
| Molar ratio H:C | 0.29 | 0.29 | ||
| Total nitrogen | % s.s. | 0.49 | 0.64 | 1.09 |
| Total potassium | % s.s. | 0.92 | 0.76 | |
| Total calcium | % s.s. | 2.95 | 2.38 | |
| Total magnesium | % s.s. | 0.43 | 0.31 | |
| Total sodium | mg/kg s.s. | 1258.00 | 884.24 | |
| Water retention | % m/m | 74.85 | 73.53 | |
| Particle size fraction < 5.00 mm | % m/m s.s | 87 | 77 | |
| Particle size fraction < 2.00 mm | % m/m s.s | 47 | 44 | |
| Particle size fraction < 0.50 mm | % m/m s.s | 25 | 24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacomino, G.; Cozzolino, A.; Idbella, M.; Amoroso, G.; Bertoli, T.; Bonanomi, G.; Motti, R. Potential of Biochar as a Peat Substitute in Growth Media for Lavandula angustifolia, Salvia rosmarinus and Fragaria × ananassa. Plants 2023, 12, 3689. https://doi.org/10.3390/plants12213689
Iacomino G, Cozzolino A, Idbella M, Amoroso G, Bertoli T, Bonanomi G, Motti R. Potential of Biochar as a Peat Substitute in Growth Media for Lavandula angustifolia, Salvia rosmarinus and Fragaria × ananassa. Plants. 2023; 12(21):3689. https://doi.org/10.3390/plants12213689
Chicago/Turabian StyleIacomino, Giuseppina, Alessia Cozzolino, Mohamed Idbella, Giandomenico Amoroso, Tomaso Bertoli, Giuliano Bonanomi, and Riccardo Motti. 2023. "Potential of Biochar as a Peat Substitute in Growth Media for Lavandula angustifolia, Salvia rosmarinus and Fragaria × ananassa" Plants 12, no. 21: 3689. https://doi.org/10.3390/plants12213689








