Decoding the Multifaceted Potential of Artemisia monosperma: Comprehensive Insights into Allelopathy, Antimicrobial Activity, and Phytochemical Profile for Sustainable Agriculture
Abstract
:1. Introduction
2. Results
2.1. Allelopathic Effect of A. monosperma against Selected Weeds and Crops
2.1.1. Chenopodium murale
2.1.2. Amaranthus viridis
2.1.3. Solanum lycopersicum
2.1.4. Cucumis sativus
2.2. Antimicrobial Potential of A. monosperma
2.2.1. Antifungal Effect
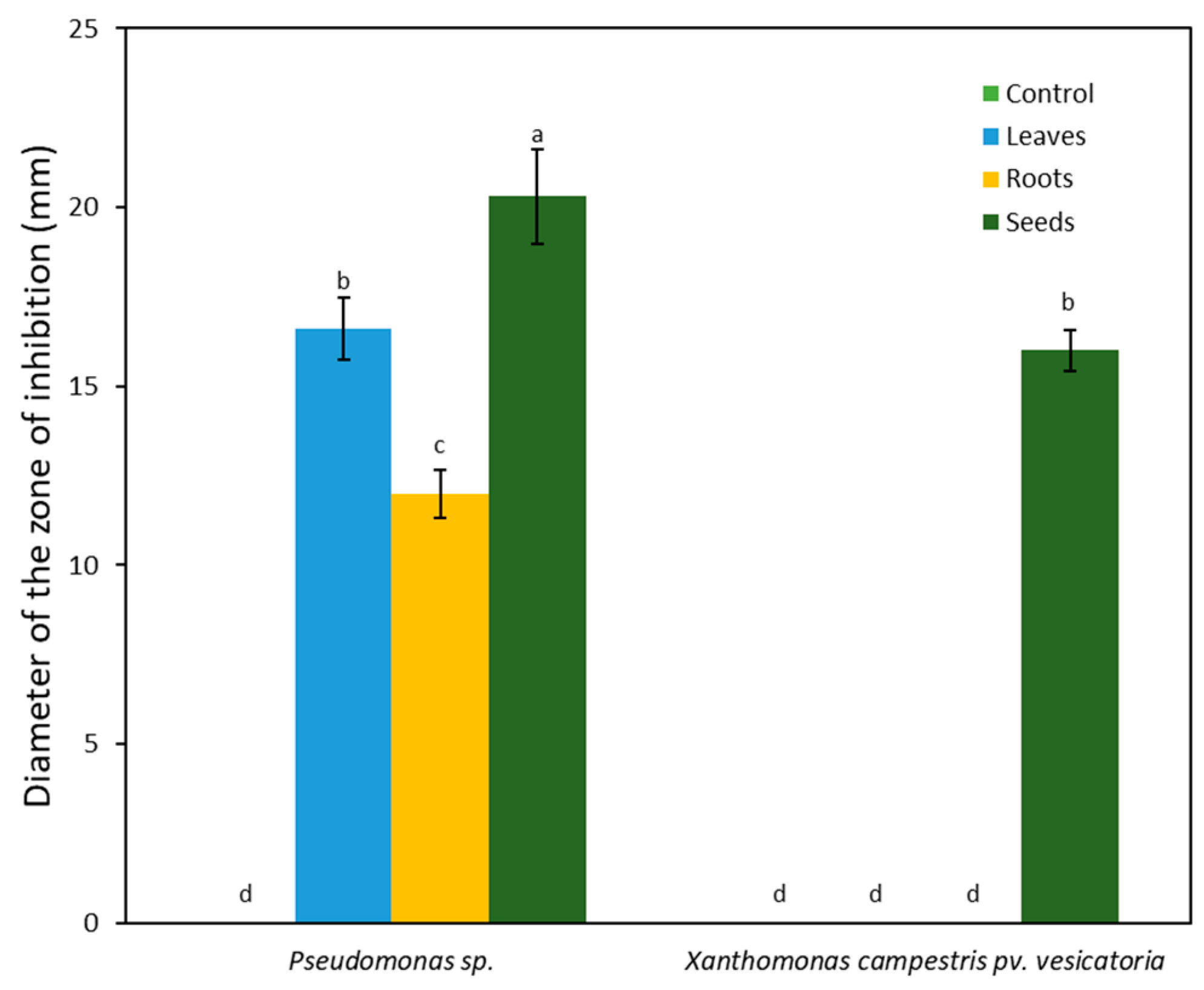
2.2.2. Antibacterial Activity
2.3. Chemical Profiling of A. monosperma
2.3.1. Quantification of Phenolic Acids by HPLC
2.3.2. Phytochemical Screening Using GC-MS
3. Discussion
4. Materials and Methods
4.1. Collection of Plant Materials
4.2. Preparation of Aqueous Extracts
4.3. Petri-Dish Bioassay of Seed Germination
4.4. Growth Inhibition by Aqueous Extracts
4.5. Antimicrobial Potential of Artemisia Monosperma
4.5.1. Antifungal Effects
4.5.2. Antibacterial Effects
4.6. Phytochemical Profiling via GC-MS
4.7. Phenolic Acids Quantification via HPLC
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rice, E.L. Allelopathy—An Overview. In Chemically Mediated Interactions between Plants and Other Organisms; Cooper-Driver, G.A., Swain, T., Conn, E.E., Eds.; Springer US: Boston, MA, USA, 1985; pp. 81–105. [Google Scholar]
- Einhellig, F.A. Allelopathy: Current Status and Future Goals. In Allelopathy; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1994; Volume 582, pp. 1–24. [Google Scholar]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Griepentrog, H.W.; Dedousis, A.P. Mechanical Weed Control. In Soil Engineering; Dedousis, A.P., Bartzanas, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 171–179. [Google Scholar]
- Singh, H.P.; Batish, D.R.; Kohli, R.K. Allelopathic Interactions and Allelochemicals: New Possibilities for Sustainable Weed Management. Crit. Rev. Plant Sci. 2003, 22, 239–311. [Google Scholar] [CrossRef]
- Gianessi, L.P. The increasing importance of herbicides in worldwide crop production. Pest Manag. Sci. 2013, 69, 1099–1105. [Google Scholar] [CrossRef]
- Carballido, J.; Rodríguez-Lizana, A.; Agüera, J.; Pérez-Ruiz, M. Field sprayer for inter and intra-row weed control: Performance and labor savings. Span. J. Agric. Res. 2013, 11, 642–651. [Google Scholar] [CrossRef]
- Cattani, D.; Cesconetto, P.A.; Tavares, M.K.; Parisotto, E.B.; De Oliveira, P.A.; Rieg, C.E.H.; Leite, M.C.; Prediger, R.D.S.; Wendt, N.C.; Razzera, G.; et al. Developmental exposure to glyphosate-based herbicide and depressive-like behavior in adult offspring: Implication of glutamate excitotoxicity and oxidative stress. Toxicology 2017, 387, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Arno, M.; Costanzo, M.; Malatesta, M.; Séralini, G.-E.; Antoniou, M.N. Transcriptome profile analysis reflects rat liver and kidney damage following chronic ultra-low dose Roundup exposure. Environ. Health 2015, 14, 70. [Google Scholar] [CrossRef]
- Grube, A.; Donaldson, D.; Kiely, T.; Wu, L. Pesticides Industry Sales and Usage; US EPA: Washington, DC, USA, 2011; p. 20460. [Google Scholar]
- Alsharekh, A.; El-Sheikh, M.A.; Alatar, A.A.; Abdel-Salam, E.M. Natural Control of Weed Invasions in Hyper-Arid Arable Farms: Allelopathic Potential Effect of Conocarpus erectus against Common Weeds and Vegetables. Agronomy 2022, 12, 703. [Google Scholar] [CrossRef]
- Hussain, M.I.; El-Sheikh, M.A.; Reigosa, M.J. Allelopathic Potential of Aqueous Extract from Acacia melanoxylon R. Br. on Lactuca sativa. Plants 2020, 9, 1228. [Google Scholar] [CrossRef]
- Al-Samarai, G.F.; Mahdi, W.M.; Al-Hilali, B.M. Reducing environmental pollution by chemical herbicides using natural plant derivatives–Allelopathy effect. Ann. Agric. Environ. Med. 2018, 25, 449–452. [Google Scholar] [CrossRef]
- Weston, L.A.; Duke, S.O. Weed and Crop Allelopathy. Crit. Rev. Plant Sci. 2003, 22, 367–389. [Google Scholar] [CrossRef]
- Bachheti, A.; Sharma, A.; Bachheti, R.K.; Husen, A.; Pandey, D.P. Plant Allelochemicals and Their Various Applications. In Co-Evolution of Secondary Metabolites; Merillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–25. [Google Scholar]
- Mallik, A.U. Allelopathy: Advances, Challenges and Opportunities. In Allelopathy in Sustainable Agriculture and Forestry; Zeng, R.S., Mallik, A.U., Luo, S.M., Eds.; Springer New York: New York, NY, USA, 2008; pp. 25–38. [Google Scholar]
- Bhadoria, P.B.S. Allelopathy: A Natural Way towards Weed Management. J. Exp. Agric. Int. 2010, 1, 7–20. [Google Scholar] [CrossRef]
- Duke, S.O.; Rimando, A.M.; Baerson, S.R.; Scheffler, B.E.; Ota, E.; Belz, R.G. Strategies for the Use of Natural Products for Weed Management. J. Pestic. Sci. 2002, 27, 298–306. [Google Scholar] [CrossRef]
- Motmainna, M.; Juraimi, A.S.; Uddin, M.K.; Asib, N.B.; Islam, A.K.M.M.; Ahmad-Hamdani, M.S.; Hasan, M. Phytochemical Constituents and Allelopathic Potential of Parthenium hysterophorus L. in Comparison to Commercial Herbicides to Control Weeds. Plants 2021, 10, 1445. [Google Scholar] [CrossRef]
- Afifi, H.S.; Al Marzooqi, H.M.; Tabbaa, M.J.; Arran, A.A. Phytochemicals of Conocarpus spp. as a Natural and Safe Source of Phenolic Compounds and Antioxidants. Molecules 2021, 26, 1069. [Google Scholar] [CrossRef]
- Weston, L.A. Utilization of Allelopathy for Weed Management in Agroecosystems. Agron. J. 1996, 88, 860–866. [Google Scholar] [CrossRef]
- Weston, L.A. History and Current Trends in the Use of Allelopathy for Weed Management. HortTechnology 2005, 15, 529–534. [Google Scholar] [CrossRef]
- Hijazi, A.M.; Salhab, A.S. Effects of Artemisia monosperma ethanolic leaves extract on implantation, mid-term abortion and parturition of pregnant rats. J. Ethnopharmacol. 2010, 128, 446–451. [Google Scholar] [CrossRef]
- El Zalabani, S.M.; Tadros, S.H.; El Sayed, A.M.; Daboub, A.A.; Sleem, A.A. Chemical Profile and Biological Activities of Essential oil of Aerial parts of Artemisia monosperma Del. Growing in Libya. Pharmacogn. J. 2017, 9, 578–586. [Google Scholar] [CrossRef]
- Algandaby, M.M.; El-Darier, S.M. Management of the noxious weed; Medicago polymorpha L. via allelopathy of some medicinal plants from Taif region, Saudi Arabia. Saudi J. Biol. Sci. 2018, 25, 1339–1347. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z. Research Progress on the use of Plant Allelopathy in Agriculture and the Physiological and Ecological Mechanisms of Allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef] [PubMed]
- Elmetwally, I.; Shehata, S.; Abdelgawad, K.; Elkhawaga, F. Utilization of Phenolic Compounds Extracted from Agro-Industrial Wastes as Natural Herbicides. Egypt. J. Chem. 2022, 65, 265–274. [Google Scholar] [CrossRef]
- Li, Z.-H.; Wang, Q.; Ruan, X.; Pan, C.-D.; Jiang, D.-A. Phenolics and Plant Allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef] [PubMed]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; Foletto-Felipe, M.d.P.; Abrahão, J.; et al. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Alwathnani, H.A. Antibacterial Activity of Aqueous Extracts of Artemisia Species Against Some Pathogenic Bacteria. Biosci. Biotechnol. Res. Asia 2017, 14, 621–624. [Google Scholar] [CrossRef]
- Zayyat, M.; Khedr, A.-H.; Amer, N. Phytochemical and antimicrobial activity of two Egyptian xerophytes: Artemisia jaudica L. and Artemisia monosperma Delile. Afr. J. Biol. Sci. 2018, 14, 127–140. [Google Scholar] [CrossRef]
- Barkosky, R.R.; Einhellig, F.A.; Butler, J.L. Caffeic Acid-Induced Changes in Plant–Water Relationships and Photosynthesis in Leafy Spurge Euphorbia esula. J. Chem. Ecol. 2000, 26, 2095–2109. [Google Scholar] [CrossRef]
- Macias, F.A.; Galindo, J.C.G.; Molinillo, J.M.G. Allelopathy: Chemistry and Mode of Action of Allelochemicals, 1st ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Chou, C.-H.; Leu, L.-L. Allelopathic substances and interactions of Delonix regia (Boj) Raf. J. Chem. Ecol. 1992, 18, 2285–2303. [Google Scholar] [CrossRef]
- Haramoto, E.R.; Gallandt, E.R. Brassica cover cropping: II. Effects on growth and interference of green bean (Phaseolus vulgaris) and redroot pigweed (Amaranthus retroflexus). Weed Sci. 2005, 53, 702–708. [Google Scholar] [CrossRef]
- Burgos, N.R.; Talbert, R.E. Differential activity of allelochemicals from Secale cereale in seedling bioassays. Weed Sci. 2000, 48, 302–310. [Google Scholar] [CrossRef]
- Hijazi, K.; Abdel-Gaber, A.; Younes, G.; Habchi, R. Comparative study of the effect of an acidic anion on the mild steel corrosion inhibition using Rhus coriaria plant extract and its quercetin component. Port. Electrochim. Acta 2021, 39, 237–252. [Google Scholar] [CrossRef]
- Heatley, N.G. A method for the assay of penicillin. Biochem. J. 1944, 38, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Nasr, F.A.; Noman, O.M.; Alqahtani, A.S.; Qamar, W.; Ahamad, S.R.; Al-Mishari, A.A.; Alyhya, N.; Farooq, M. Phytochemical constituents and anticancer activities of Tarchonanthus camphoratus essential oils grown in Saudi Arabia. Saudi Pharm. J. 2020, 28, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Li, C.; Di, H.; Liao, Y.; Liu, H. A Universal HPLC Method for the Determination of Phenolic Acids in Compound Herbal Medicines. J. Agric. Food Chem. 2005, 53, 6624–6629. [Google Scholar] [CrossRef] [PubMed]


| Plant Parts | Concentration (%) | Seed Germination | Plant Growth | ||
|---|---|---|---|---|---|
| Shoot Length (cm) | Root Length (cm) | Shoot Length (cm) | Root Length (cm) | ||
| Leaves * | 0 | 5.26 ± 0.17 a | 3.19 ± 0.32 a | 5.40 ± 0.21 a | 2.85 ± 0.41 a |
| 25 | 2.65 ± 0.08 bc | 1.10 ± 0.07 ce | 3.92 ± 0.19 b | 1.83 ± 0.25 ab | |
| 50 | 2.17 ± 0.05 cd | 0.82 ± 0.13 ce | 4.00 ± 0.16 b | 1.45 ± 0.14 b | |
| 75 | 1.61 ± 0.18 de | 0.24 ± 0.03 e | 4.03 ± 0.21 b | 1.55 ± 0.14 b | |
| 100 | 1.00 ± 0.17 ef | 0.23 ± 0.04 e | 3.70 ± 0.45 b | 1.31 ± 0.16 b | |
| Roots | 0 | 5.26 ± 0.17 a | 3.19 ± 0.31 a | 5.40 ± 0.21 a | 2.85 ± 0.41 a |
| 25 | 3.20 ± 0.11 b | 3.07 ± 0.24 a | 4.41 ± 0.27 ab | 1.87 ± 0.12 ab | |
| 50 | 3.21 ± 0.14 b | 2.38 ± 0.21 ab | 4.2 ± 0.14 b | 2.35 ± 0.17 ab | |
| 75 | 3.15 ± 0.12 b | 1.50 ± 0.18 bc | 4.23 ± 0.21 ab | 1.48 ± 0.21 b | |
| 100 | 2.79 ± 0.15 bc | 1.19 ± 0.14 cd | 3.80 ± 0.19 b | 1.28 ± 0.14 b | |
| Seeds | 0 | 5.26 ± 0.17 a | 3.19 ± 0.34 a | 5.40 ± 0.21 a | 2.85 ± 0.41 a |
| 25 | 2.61 ± 0.15 bc | 0.98 ± 0.11 ce | 4.55 ± 0.14 ab | 1.72 ± 0.09 b | |
| 50 | 2.34 ± 0.08 c | 0.67 ± 0.06 ce | 4.71 ± 0.12 ab | 1.81 ± 0.14 ab | |
| 75 | 1.66 ± 0.14 de | 0.34 ± 0.06 de | 4.57 ± 0.11 ab | 1.86 ± 0.09 ab | |
| 100 | 0.85 ± 0.09 f | 0.33 ± 0.05 de | 3.93 ± 0.47 b | 1.34 ± 0.16 b | |
| f-values | Part | 85.27 | 67.38 | 3.81 | 0.70 |
| Concentration | 323.08 | 100.15 | 17.71 | 18.86 | |
| Part × Concentration | 9.91 | 6.11 | 0.46 | 1.02 | |
| p-values | Part | 0.00 | 0.00 | 0.02 | 0.50 |
| Concentration | 0.00 | 0.00 | 0.00 | 0.00 | |
| Part × Concentration | 0.00 | 0.00 | 0.88 | 0.43 | |
| Plant Parts | Concentration (%) | Seed Germination | Plant Growth | ||
|---|---|---|---|---|---|
| Shoot Length (cm) | Root Length (cm) | Shoot Length (cm) | Root Length (cm) | ||
| Leaves * | 0 | 2.26 ± 0.07 ab | 1.48 ± 0.07 a | 4.05 ± 0.17 a | 1.90 ± 0.10 a |
| 25 | 1.91 ± 0.15 ab | 0.81 ± 0.06 bc | 3.03 ± 0.23 ab | 0.75 ± 0.10 bd | |
| 50 | 1.73 ± 0.13 bc | 0.55 ± 0.06 ce | 2.4 ± 0.44 b | 0.54 ± 0.12 d | |
| 75 | 0.64 ± 0.17 ef | 0.33 ± 0.09 ef | 2.98 ± 0.37 ab | 0.66 ± 0.12 cd | |
| 100 | 0.21 ± 0.11 f | 0.09 ± 0.05 f | 3.40 ± 0.44 ab | 0.75 ± 0.10 bd | |
| Roots | 0 | 2.26 ± 0.07 ab | 1.48 ± 0.07 a | 4.05 ± 0.17 a | 1.9 ± 0.10 a |
| 25 | 2.35 ± 0.08 a | 1.69 ± 0.04 a | 3.69 ± 0.28 ab | 0.79 ± 0.17 bd | |
| 50 | 1.88 ± 0.07 ab | 1.06 ± 0.06 b | 3.45 ± 0.27 ab | 0.79 ± 0.18 bd | |
| 75 | 1.28 ± 0.16 cd | 0.80 ± 0.10 bc | 3.39 ± 0.31 ab | 0.52 ± 0.15 d | |
| 100 | 1.05 ± 0.24 de | 0.72 ± 0.68 | 2.86 ± 0.53 ab | 0.55 ± 0.15 d | |
| Seeds | 0 | 2.26 ± 0.07 ab | 1.48 ± 0.07 a | 4.05 ± 0.17 a | 1.9 ± 0.10 a |
| 25 | 1.15 ± 0.08 de | 0.97 ± 0.07 b | 3.83 ± 0.23 ab | 1.28 ± 0.10 b | |
| 50 | 0.73 ± 0.08 df | 0.51 ± 0.05 ce | 3.84 ± 0.30 ab | 1.23 ± 0.08 bc | |
| 75 | 0.61 ± 0.04 ef | 0.35 ± 0.03 def | 3.22 ± 0.26 ab | 1.18 ± 0.08 bc | |
| 100 | 0.41 ± 0.05 f | 0.27 ± 0.04 ef | 3.13 ± 0.364 ab | 1.00 ± 0.15 bd | |
| f-values | Part | 48.39 | 61.76 | 2.54 | 17.60 |
| Concentration | 104.21 | 111.20 | 4.24 | 44.99 | |
| Part × Concentration | 7.61 | 5.15 | 1.46 | 1.79 | |
| p-values | Part | 0.00 | 0.00 | 0.08 | 0.00 |
| Concentration | 0.00 | 0.00 | 0.00 | 0.00 | |
| Part × Concentration | 0.00 | 0.00 | 0.18 | 0.08 | |
| Plant Parts | Concentration (%) | Seed Germination | Plant Growth | ||
|---|---|---|---|---|---|
| Shoot Length (cm) | Root Length (cm) | Shoot Length (cm) | Root Length (cm) | ||
| Leaves * | 0 | 6.94 ± 0.26 a | 6.10 ± 0.32 a | 9.43 ± 0.23 ab | 3.24 ± 0.24 ab |
| 25 | 4.78 ± 0.17 b | 1.16 ± 0.16 dg | 8.4 ± 0.40 bc | 2.99 ± 0.24 ab | |
| 50 | 3.57 ± 0.22 bc | 1.16 ± 0.18 dg | 7.52 ± 0.48 cd | 2.59 ± 0.19 ab | |
| 75 | 3.17 ± 0.19 cd | 0.91 ± 0.11 efg | 7.82 ± 0.57 bd | 2.63 ± 0.24 ab | |
| 100 | 1.47 ± 0.21 e | 0.28 ± 0.05 g | 6.24 ± 0.54 d | 2.95 ± 0.27 ab | |
| Roots | 0 | 6.94 ± 0.26 a | 6.1 ± 0.32 a | 9.43 ± 0.23 ab | 3.24 ± 0.24 ab |
| 25 | 7.45 ± 0.30 a | 5.31 ± 0.38 ab | 9.19 ± 0.24 abc | 2.63 ± 0.19 ab | |
| 50 | 7.41 ± 0.35 a | 4.78 ± 0.31 b | 9.16 ± 0.39 abc | 2.94 ± 0.23 ab | |
| 75 | 7.40 ± 0.27 a | 4.13 ± 0.44 b | 9.06 ± 0.37 abc | 2.40 ± 0.36 ab | |
| 100 | 6.84 ± 0.36 a | 2.63 ± 0.31 c | 10.51 ± 0.27 a | 2.78 ± 0.21 ab | |
| Seeds | 0 | 6.94 ± 0.26 a | 6.1 ± 0.32 a | 9.43 ± 0.23 ab | 3.24 ± 0.24 ab |
| 25 | 3.87 ± 0.44 bc | 2.04 ± 0.17 cdf | 8.11 ± 0.38 bd | 2.40 ± 0.52 ab | |
| 50 | 1.93 ± 0.11 de | 2.22 ± 0.32 cde | 8.19 ± 0.23 bc | 3.26 ± 0.94 ab | |
| 75 | 1.92 ± 0.16 de | 2.43 ± 0.15 cd | 9.41 ± 0.48 ab | 3.15 ± 0.67 ab | |
| 100 | 1.58 ± 0.26 e | 0.88 ± 0.18 fg | 7.31 ± 0.48 cd | 2.64 ± 0.75 ab | |
| f-values | Part | 308.14 | 127.36 | 21.59 | 0.43 |
| Concentration | 81.43 | 132.26 | 5.79 | 2.66 | |
| Part × Concentration | 23.86 | 10.16 | 5.75 | 1.49 | |
| p-values | Part | 0.00 | 0.00 | 0.00 | 0.65 |
| Concentration | 0.00 | 0.00 | 0.00 | 0.04 | |
| Part × Concentration | 0.00 | 0.00 | 0.00 | 0.16 | |
| Plant Parts | Concentration (%) | Seed Germination | Plant Growth | ||
|---|---|---|---|---|---|
| Shoot Length (cm) | Root Length (cm) | Shoot Length (cm) | Root Length (cm) | ||
| Leaves * | 0 | 7.09 ± 0.26 ac | 10.22 ± 0.77 bc | 12.51 ± 0.37 bcd | 11.20 ± 0.47 ab |
| 25 | 1.94 ± 0.63 ef | 1.35 ± 0.47 d | 11.95 ± 0.84 cd | 10.15 ± 0.72 ab | |
| 50 | 0.27 ± 0.23 f | 0.19 ± 0.17 d | 12.70 ± 0.42 bcd | 12.75 ± 0.98 a | |
| 75 | 0.22 ± 0.20 f | 0.15 ± 0.13 d | 10.86 ± 0.61 d | 12.00 ± 1.08 a | |
| 100 | 0.03 ± 0.019 f | 0.02 ± 0.01 d | 11.04 ± 0.46 d | 11.24 ± 0.95 ab | |
| Roots | 0 | 7.09 ± 0.26 ac | 10.22 ± 0.77 bc | 12.51 ± 0.37 bcd | 11.20 ± 0.47 ab |
| 25 | 8.50 ± 0.96 a | 11.56 ± 1.66 ac | 15.70 ± 0.198 a | 9.70 ± 0.72 ab | |
| 50 | 8.69 ± 1.08 a | 12.68 ± 1.20 ab | 14.55 ± 0.39 ab | 8.00 ± 1.28 b | |
| 75 | 0.13 ± 0.03 f | 0.08 ± 0.02 d | 11.58 ± 0.67 d | 7.96 ± 0.67 b | |
| 100 | 0.09 ± 0.03 f | 0.05 ± 0.01 d | 14.13 ± 0.99 ac | 11.27 ± 0.59 ab | |
| Seeds | 0 | 7.09 ± 0.26 ac | 10.22 ± 0.77 bc | 12.51 ± 0.37 bcd | 11.20 ± 0.47 ab |
| 25 | 7.91 ± 0.291 ab | 15.17 ± 0.96 a | 12.58 ± 0.32 bcd | 10.40 ± 0.48 ab | |
| 50 | 5.94 ± 0.46 bc | 14.01 ± 0.87 ab | 12.82 ± 0.36 bcd | 10.55 ± 0.46 ab | |
| 75 | 4.88 ± 0.72 cd | 8.08 ± 1.46 c | 12.47 ± 0.35 bcd | 10.94 ± 0.67 ab | |
| 100 | 2.99 ± 0.47 de | 3.47 ± 0.69 d | 12.19 ± 0.33 bcd | 10.39 ± 0.40 ab | |
| f-values | Part | 80.35 | 109.01 | 17.10 | 7.75 |
| Concentration | 84.47 | 74.88 | 6.12 | 1.19 | |
| Part × Concentration | 21.04 | 20.52 | 3.47 | 2.78 | |
| p-values | Part | 0.00 | 0.00 | 0.00 | 0.00 |
| Concentration | 0.00 | 0.00 | 0.00 | 0.32 | |
| Part × Concentration | 0.00 | 0.00 | 0.00 | 0.01 | |
| Phenolic Acid Compounds (ppm) | Leaves | Roots | Seeds |
|---|---|---|---|
| Gallic acid * | 18.60 ± 0.20 c | 12.82 ± 0.40 d | 17.60 ± 0.70 c |
| Caffeic acid | 116.30± 0.30 a | 10.59 ± 0.29 e | 10.56 ± 0.43 e |
| Ferulic acid | 9.06 ± 0.16 ef | 7.56 ± 0.29 f | 63.10 ± 0.58 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sheikh, M.A.; Alsharekh, A.; Alatar, A.A.; Rizwana, H. Decoding the Multifaceted Potential of Artemisia monosperma: Comprehensive Insights into Allelopathy, Antimicrobial Activity, and Phytochemical Profile for Sustainable Agriculture. Plants 2023, 12, 3695. https://doi.org/10.3390/plants12213695
El-Sheikh MA, Alsharekh A, Alatar AA, Rizwana H. Decoding the Multifaceted Potential of Artemisia monosperma: Comprehensive Insights into Allelopathy, Antimicrobial Activity, and Phytochemical Profile for Sustainable Agriculture. Plants. 2023; 12(21):3695. https://doi.org/10.3390/plants12213695
Chicago/Turabian StyleEl-Sheikh, Mohamed A., Anfal Alsharekh, Abdulrahman A. Alatar, and Humaira Rizwana. 2023. "Decoding the Multifaceted Potential of Artemisia monosperma: Comprehensive Insights into Allelopathy, Antimicrobial Activity, and Phytochemical Profile for Sustainable Agriculture" Plants 12, no. 21: 3695. https://doi.org/10.3390/plants12213695






