Bioactive Compound-Loaded Nanocarriers for Hair Growth Promotion: Current Status and Future Perspectives
Abstract
:1. Introduction
2. Human Hair Development in the Embryo—Molecular Mechanisms
2.1. Anagen
2.2. Catagen
2.3. Telogen
2.4. Exogen
3. Follicle Cycle Mechanisms in Hair
3.1. Anagen
3.2. Catagen
3.3. Telogen
4. Recent Nanotechnology-Based Formulations for Human Hair Growth
5. Biomedical Applications of Nanomaterials
6. Nanoformulations for Hair Follicles (HF)
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamilton, J.B. Patterned loss of hair in man: Types and incidence. Ann. N. Y. Acad. Sci. 1951, 53, 708–728. [Google Scholar] [CrossRef]
- Shankar, D.K.; Chakravarthi, M.; Shilpakar, R. Male androgenetic alopecia: Population-based study in 1005 subjects. Int. J. Trichol. 2009, 1, 131–133. [Google Scholar] [CrossRef]
- Birch, M.; Messenger, J.; Messenger, A. Hair density, hair diameter and the prevalence of female pattern hair loss. Br. J. Dermatol. 2001, 144, 297–304. [Google Scholar] [CrossRef]
- Norwood, O.T.T. Incidence of female androgenetic alopecia (female pattern alopecia). Dermatol. Surg. 2001, 27, 53–54. [Google Scholar]
- Lolli, F.; Pallotti, F.; Rossi, A.; Fortuna, M.C.; Caro, G.; Lenzi, A.; Sansone, A.; Lombardo, F. Androgenetic alopecia: A review. Endocrine 2017, 57, 9–17. [Google Scholar] [CrossRef]
- Mahe, Y.F.; Michelet, J.-F.; Billoni, N.; Jarrousse, F.; Buan, B.; Commo, S.; Saint-Leger, D.; Bernard, B.A. Androgenetic alopecia and microinflammation. Int. J. Dermatol. 2000, 39, 576–584. [Google Scholar] [CrossRef]
- Magro, C.M.; Rossi, A.; Poe, J.; Manhas-Bhutani, S.; Sadick, N. The role of inflammation and immunity in the pathogenesis of andro-genetic alopecia. J. Drugs Dermatol. JDD 2011, 10, 1404–1411. [Google Scholar]
- Martinez-Jacobo, L.; Ancer-Arellano, C.I.; Ortiz-Lopez, R.; Salinas-Santander, M.; Villarreal-Villarreal, C.D.; Ancer-Rodriguez, J.; Camacho-Zamora, B.; Zomosa-Signoret, V.; La Garza, C.E.M.-D.; Ocampo-Candiani, J.; et al. Evaluation of the Expression of Genes Associated with Inflammation and Apoptosis in Androgenetic Alopecia by Targeted RNA-Seq. Ski. Appendage Disord. 2017, 4, 268–273. [Google Scholar] [CrossRef]
- Dey-Rao, R.; Sinha, A. A genomic approach to susceptibility and pathogenesis leads to identifying potential novel therapeutic targets in androgenetic alopecia. Genomics 2017, 109, 165–176. [Google Scholar] [CrossRef]
- Upton, J.H.; Hannen, R.F.; Bahta, A.W.; Farjo, N.; Farjo, B.; Philpott, M.P. Oxidative Stress–Associated Senescence in Dermal Papilla Cells of Men with Androgenetic Alopecia. J. Investig. Dermatol. 2015, 135, 1244–1252. [Google Scholar] [CrossRef]
- Prie, B.; Iosif, L.; Tivig, I.; Stoian, I.; Giurcaneanu, C. Oxidative stress in androgenetic alopecia. J. Med. Life 2016, 9, 79–83. [Google Scholar]
- Hibino, T.; Nishiyama, T. Role of TGF-β2 in the human hair cycle. J. Dermatol. Sci. 2004, 35, 9–18. [Google Scholar] [CrossRef]
- Kwack, M.H.; Ahn, J.S.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Dihydrotestosterone-Inducible IL-6 Inhibits Elongation of Human Hair Shafts by Suppressing Matrix Cell Proliferation and Promotes Regression of Hair Follicles in Mice. J. Investig. Dermatol. 2012, 132, 43–49. [Google Scholar] [CrossRef]
- Titeca, G.; Goudetsidis, L.; Francq, B.; Sampogna, F.; Gieler, U.; Tomas-Aragones, L.; Lien, L.; Jemec, G.B.E.; Misery, L.; Szabo, C.; et al. ‘The psychosocial burden of alopecia areata and androgenetica’: A cross-sectional multicentre study among dermatological out-patients in 13 European countries. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 406–411. [Google Scholar] [CrossRef]
- Goren, A.; Shapiro, J.; Roberts, J.; Desai, N.; Zarrab, Z.; Pietrzak, A.; Lotti, T. Clinical utility and validity of minoxidil response testing in androgenetic alopecia. Dermatol. Ther. 2015, 28, 13–16. [Google Scholar] [CrossRef]
- Lee, S.W.; Juhasz, M.; Mobasher, P.; Ekelem, C.; Mesinkovska, N.A. A systematic review of topical finasteride in the treatment of androgenetic alopecia in men and women. J. Drugs Dermatol. JDD 2018, 17, 457. [Google Scholar]
- Monti, D.; Tampucci, S.; Burgalassi, S.; Chetoni, P.; Lenzi, C.; Pirone, A.; Mailland, F. Topical Formulations Containing Finasteride. Part I: In Vitro Permeation/Penetration Study and In Vivo Pharmacokinetics in Hairless Rat. J. Pharm. Sci. 2014, 103, 2307–2314. [Google Scholar] [CrossRef]
- Olsen, E.A.; Whiting, D.; Bergfeld, W.; Miller, J.; Hordinsky, M.; Wanser, R.; Zhang, P.; Kohut, B. A multicenter, randomized, placebo-controlled, double-blind clinical trial of a novel formulation of 5% minoxidil topical foam versus placebo in the treatment of androgenetic alopecia in men. J. Am. Acad. Dermatol. 2007, 57, 767–774. [Google Scholar] [CrossRef]
- Price, V.H.; Roberts, J.L.; Hordinsky, M.; Olsen, E.A.; Savin, R.; Bergfeld, W.; Fiedler, V.; Lucky, A.; Whiting, D.A.; Pappas, F.; et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J. Am. Acad. Dermatol. 2000, 43, 768–776. [Google Scholar] [CrossRef]
- Whiting, D.A.; Olsen, E.A.; Savin, R.; Halper, L.; Rodgers, A.; Wang, L.; Hustad, C.; Palmisano, J. Efficacy and tolerability of finasteride 1 mg in men aged 41 to 60 years with male pattern hair loss. Eur. J. Dermatol. 2003, 13, 150–160. [Google Scholar]
- Friedman, E.S.; Friedman, P.M.; Cohen, D.E.; Washenik, K. Allergic contact dermatitis to topical minoxidil solution: Etiology and treatment. J. Am. Acad. Dermatol. 2002, 46, 309–312. [Google Scholar]
- Lawson, C.N.; Hollinger, J.; Sethi, S.; Rodney, I.; Sarkar, R.; Dlova, N.; Callender, V.D. Updates in the understanding and treatments of skin & hair disorders in women of color. Int. J. Women’s Dermatol. 2017, 3, S21–S37. [Google Scholar] [CrossRef]
- Khumalo, N.; Jessop, S.; Gumedze, F.; Ehrlich, R. Hairdressing and the prevalence of scalp disease in African adults. Br. J. Dermatol. 2007, 157, 981–988. [Google Scholar] [CrossRef]
- Samrao, A.; Price, V.H.; Zedek, D.; Mirmirani, P. The “Fringe Sign”—A useful clinical finding in traction alopecia of the marginal hair line. Dermatol. Online J. 2011, 17, 11. [Google Scholar]
- Uwakwe, L.; De Souza, B.; Tovar-Garza, A.; McMichael, A. Intralesional Triamcinolone Acetonide in the Treatment of Traction Alopecia. J. Drugs Dermatol. 2020, 19, 128–130. [Google Scholar] [CrossRef]
- Callender, V.D.; McMichael, A.J.; Cohen, G.F. Medical and surgical therapies for alopecias in black women. Dermatol. Ther. 2004, 17, 164–176. [Google Scholar] [CrossRef]
- Khumalo, N.; Ngwanya, R. Traction alopecia: 2% topical minoxidil shows promise. Report of two cases. J. Eur. Acad. Dermatol. Venereol. JEADV 2007, 21, 433–434. [Google Scholar]
- Ogunleye, T.A.; McMichael, A.; Olsen, E.A. Central centrifugal cicatricial alopecia: What has been achieved, current clues for future research. Dermatol. Clin. 2014, 32, 173–181. [Google Scholar]
- Malki, L.; Sarig, O.; Romano, M.-T.; Méchin, M.-C.; Peled, A.; Pavlovsky, M.; Warshauer, E.; Samuelov, L.; Uwakwe, L.; Briskin, V.; et al. Variant PADI3 in Central Centrifugal Cicatricial Alopecia. N. Engl. J. Med. 2019, 380, 833–841. [Google Scholar] [CrossRef]
- Dinh, Q.Q.; Sinclair, R. Female pattern hair loss: Current treatment concepts. Clin. Interv. Aging 2007, 2, 189–199. [Google Scholar]
- Roe, A.L.; McMillan, D.A.; Mahony, C. A Tiered Approach for the Evaluation of the Safety of Botanicals Used as Dietary Supplements: An Industry Strategy. Clin. Pharmacol. Ther. 2018, 104, 446–457. [Google Scholar] [CrossRef]
- Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. The Hair Follicle as a Dynamic Miniorgan. Curr. Biol. 2009, 19, R132–R142. [Google Scholar] [CrossRef]
- Yang, C.-C.; Cotsarelis, G. Review of hair follicle dermal cells. J. Dermatol. Sci. 2010, 57, 2–11. [Google Scholar] [CrossRef]
- Perrimon, N.; Pitsouli, C.; Shilo, B.-Z. Signaling Mechanisms Controlling Cell Fate and Embryonic Patterning. Cold Spring Harb. Perspect. Biol. 2012, 4, a005975. [Google Scholar] [CrossRef]
- Woo, W.-M.; Zhen, H.H.; Oro, A.E. Shh maintains dermal papilla identity and hair morphogenesis via a Noggin—Shh regulatory loop. Genes Dev. 2012, 26, 1235–1246. [Google Scholar]
- Kumari, S.; Goyal, A.; Garg, M.; Antonescu, A.; Sindhu, R.K. Lyotropic Liquid Crystal System for Drug Delivery of Astaxanthin: Physical Characterization and Enhanced Antioxidant Potential. Crystals 2023, 13, 142. [Google Scholar] [CrossRef]
- Rishikaysh, P.; Dev, K.; Diaz, D.; Qureshi, W.M.S.; Filip, S.; Mokry, J. Signaling Involved in Hair Follicle Morphogenesis and Development. Int. J. Mol. Sci. 2014, 15, 1647–1670. [Google Scholar] [CrossRef]
- Oh, J.W.; Kloepper, J.; Langan, E.A.; Kim, Y.; Yeo, J.; Kim, M.J.; Hsi, T.-C.; Rose, C.; Yoon, G.S.; Lee, S.-J.; et al. A Guide to Studying Human Hair Follicle Cycling In Vivo. J. Investig. Dermatol. 2016, 136, 34–44. [Google Scholar] [CrossRef]
- Singh, S.; Sindhu, R.K.; Alsayegh, A.A.; Batiha, G.E.; Alotaibi, S.S.; Albogami, S.M.; Conte-Junior, C.A. Formulation Development and Investigations on Therapeutic Potential of Nanogel from Beta vulgaris L. Extract in Testosterone-Induced Alopecia. BioMed Res. Int. 2023, 2023, 1777631. [Google Scholar] [CrossRef]
- Ohyama, M.; Terunuma, A.; Tock, C.L.; Radonovich, M.F.; Pise-Masison, C.A.; Hopping, S.B.; Brady, J.N.; Udey, M.C.; Vogel, J.C. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J. Clin. Investig. 2005, 116, 249–260. [Google Scholar] [CrossRef]
- Plikus, M.V.; Chuong, C.-M. Complex Hair Cycle Domain Patterns and Regenerative Hair Waves in Living Rodents. J. Investig. Dermatol. 2008, 128, 1071–1080. [Google Scholar] [CrossRef]
- Tobin, D.J. Aging of the hair follicle pigmentation system. Int. J. Trichol. 2009, 1, 83–93. [Google Scholar] [CrossRef]
- Mesler, A.L.; Veniaminova, N.A.; Lull, M.V.; Wong, S.Y. Hair Follicle Terminal Differentiation Is Orchestrated by Distinct Early and Late Matrix Progenitors. Cell Rep. 2017, 19, 809–821. [Google Scholar] [CrossRef]
- Kruglikov, I.L.; Scherer, P.E. Dermal adipocytes and hair cycling: Is spatial heterogeneity a characteristic feature of the dermal adipose tissue depot? Exp. Dermatol. 2016, 25, 258–262. [Google Scholar] [CrossRef]
- Geyfman, M.; Plikus, M.V.; Treffeisen, E.; Andersen, B.; Paus, R. Resting no more: Re-defining telogen, the maintenance stage of the hair growth cycle. Biol. Rev. 2014, 90, 1179–1196. [Google Scholar] [CrossRef]
- Lyle, S.; Elder, D.E.; Christofidou-Solomidou, M.; Liu, Y.; Albelda, S.; Cotsarelis, G. Human Hair Follicle Bulge Cells are Biochemically Distinct and Possess an Epithelial Stem Cell Phenotype. J. Investig. Dermatol. Symp. Proc. 1999, 4, 296–301. [Google Scholar] [CrossRef]
- Mistriotis, P.; Andreadis, S.T. Hair follicle: A novel source of multipotent stem cells for tissue engineering and regenerative medicine. Tissue Eng. Part B Rev. 2013, 19, 265–278. [Google Scholar] [CrossRef]
- Roh, C.; Tao, Q.; Photopoulos, C.; Lyle, S. In Vitro Differences Between Keratinocyte Stem Cells and Transit-Amplifying Cells of the Human Hair Follicle. J. Investig. Dermatol. 2005, 125, 1099–1105. [Google Scholar] [CrossRef]
- Martel, J.; Badri, T. Anatomy, Head, Hair, Follicle; StatPearls Publishing: Treasure Island, FL, USA, 2018; p. 470321. [Google Scholar]
- Hsu, Y.-C.; Pasolli, H.A.; Fuchs, E. Dynamics between Stem Cells, Niche, and Progeny in the Hair Follicle. Cell 2011, 144, 92–105. [Google Scholar] [CrossRef]
- Plikus, M.V. New Activators and Inhibitors in the Hair Cycle Clock: Targeting Stem Cells’ State of Competence. J. Investig. Dermatol. 2012, 132, 1321–1324. [Google Scholar] [CrossRef] [PubMed]
- Burg, D.; Yamamoto, M.; Namekata, M.; Haklani, J.; Koike, K.; Halasz, M. Promotion of anagen, increased hair density and reduction of hair fall in a clinical setting following identification of FGF5-inhibiting compounds via a novel 2-stage process. Clin. Cosmet. Investig. Dermatol. 2017, 10, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.N.; Nguyen, H. Identifying Quiescent Stem Cells in Hair Follicles. Cell. Quiescence Methods Protoc. 2017, 1686, 137–147. [Google Scholar] [CrossRef]
- Higgins, C.A.; Westgate, G.E.; Jahoda, C.A. From Telogen to Exogen: Mechanisms Underlying Formation and Subsequent Loss of the Hair Club Fiber. J. Investig. Dermatol. 2009, 129, 2100–2108. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef]
- Erdoğan, B. Anatomy and Physiology of Hair. In Hair and Scalp Disorders; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Harrison, S.; Sinclair, R. Telogen effluvium. Clin. Exp. Dermatol. Clin. Dermatol. 2002, 27, 389–395. [Google Scholar] [CrossRef]
- Soteriou, D.; Kostic, L.; Sedov, E.; Yosefzon, Y.; Steller, H.; Fuchs, Y. Isolating hair follicle stem cells and epidermal keratinocytes from dorsal mouse skin. J. Vis. Exp. 2016, 110, e53931. [Google Scholar]
- Cotsarelis, G.; Sun, T.-T.; Lavker, R.M. Label-retaining cells reside in the bulge area of pilosebaceous unit: Implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 1990, 61, 1329–1337. [Google Scholar] [CrossRef]
- Lyle, S.; Christofidou-Solomidou, M.; Liu, Y.; Elder, D.E.; Albelda, S.; Cotsarelis, G. The C8/144B monoclonal antibody recognizes cytokeratin 15 and defines the location of human hair follicle stem cells. J. Cell Sci. 1998, 111, 3179–3188. [Google Scholar] [CrossRef]
- Oshima, H.; Rochat, A.; Kedzia, C.; Kobayashi, K.; Barrandon, Y. Morphogenesis and Renewal of Hair Follicles from Adult Multipotent Stem Cells. Cell 2001, 104, 233–245. [Google Scholar] [CrossRef]
- Harries, M.J.; Paus, R. The Pathogenesis of Primary Cicatricial Alopecias. Am. J. Pathol. 2010, 177, 2152–2162. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.; Teh, M.-T.; Mackenzie, I.C.; Waseem, A. Keratin K15 as a Biomarker of Epidermal Stem Cells. Int. J. Mol. Sci. 2013, 14, 19385–19398. [Google Scholar] [CrossRef] [PubMed]
- Rompolas, P.; Greco, V. (Eds.) Stem cell dynamics in the hair follicle niche. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Myung, P.; Ito, M. Dissecting the bulge in hair regeneration. J. Clin. Investig. 2012, 122, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Houschyar, K.S.; Momeni, A.; Pyles, M.N.; Maan, Z.N.; Whittam, A.J.; Siemers, F. Wnt signaling induces epithelial differentiation during cutaneous wound healing. Organogenesis 2015, 11, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. Mutant laboratory mice with abnormalities in hair follicle morphogenesis, cycling, and/or structure: An update. J. Dermatol. Sci. 2013, 69, 6–29. [Google Scholar] [CrossRef] [PubMed]
- Leishman, E.; Howard, J.M.; Garcia, G.E.; Miao, Q.; Ku, A.T.; Dekker, J.D.; Tucker, H.; Nguyen, H. Foxp1 maintains hair follicle stem cell quiescence through regulation of Fgf18. Development 2013, 140, 3809–3818. [Google Scholar] [CrossRef]
- Ohnemus, U.; Uenalan, M.; Conrad, F.; Handjiski, B.; Mecklenburg, L.; Nakamura, M.; Inzunza, J.; Gustafsson, J.-A.; Paus, R. Hair Cycle Control by Estrogens: Catagen Induction via Estrogen Receptor (ER)-α Is Checked by ERβ Signaling. Endocrinology 2005, 146, 1214–1225. [Google Scholar] [CrossRef]
- Narhi, K.; Jarvinen, E.; Birchmeier, W.; Taketo, M.M.; Mikkola, M.L.; Thesleff, I. Sustained epithelial β-catenin activity induces precocious hair development but disrupts hair follicle down-growth and hair shaft formation. Development 2008, 135, 1019–1028. [Google Scholar] [CrossRef]
- Kandyba, E.; Kobielak, K. Wnt7b Is an Important Intrinsic Regulator of Hair Follicle Stem Cell Homeostasis and Hair Follicle Cycling. Stem Cells 2014, 32, 886–901. [Google Scholar] [CrossRef]
- Lien, W.-H.; Polak, L.; Lin, M.; Lay, K.; Zheng, D.; Fuchs, E. In vivo transcriptional governance of hair follicle stem cells by canonical Wnt regulators. Nature 2014, 16, 179–190. [Google Scholar] [CrossRef]
- Sethi, J.K.; Vidal-Puig, A. Wnt signalling and the control of cellular metabolism. Biochem. J. 2010, 427, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kobielak, K.; Stokes, N.; de la Cruz, J.; Polak, L.; Fuchs, E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proc. Natl. Acad. Sci. USA 2007, 104, 10063–10068. [Google Scholar] [CrossRef] [PubMed]
- Tumbar, T.; Guasch, G.; Greco, V.; Blanpain, C.; Lowry, W.E.; Rendl, M.; Fuchs, E. Defining the Epithelial Stem Cell Niche in Skin. Science 2004, 303, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.J.; Liu, Y.; Marles, L.; Yang, Z.; Trempus, C.; Li, S.; Lin, J.; Sawicki, J.A.; Cotsarelis, G. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 2004, 22, 411–417. [Google Scholar] [CrossRef]
- Rajendran, R.L.; Gangadaran, P.; Bak, S.S.; Oh, J.M.; Kalimuthu, S.; Lee, H.W.; Baek, S.H.; Zhu, L.; Sung, Y.K.; Jeong, S.Y.; et al. Extracellular vesicles derived from MSCs activates dermal papilla cell in vitro and promotes hair follicle conversion from telogen to anagen in mice. Sci. Rep. 2017, 7, 15560. [Google Scholar] [CrossRef]
- Blanpain, C.; Fuchs, E. Epidermal Stem Cells of the Skin. Annu. Rev. Cell Dev. Biol. 2006, 22, 339–373. [Google Scholar] [CrossRef]
- Horsley, V.; Aliprantis, A.O.; Polak, L.; Glimcher, L.H.; Fuchs, E. NFATc1 Balances Quiescence and Proliferation of Skin Stem Cells. Cell 2008, 132, 299–310. [Google Scholar] [CrossRef]
- Kandyba, E.; Leung, Y.; Chen, Y.-B.; Widelitz, R.; Chuong, C.-M.; Kobielak, K. Competitive balance of intrabulge BMP/Wnt signaling reveals a robust gene network ruling stem cell homeostasis and cyclic activation. Proc. Natl. Acad. Sci. USA 2013, 110, 1351–1356. [Google Scholar] [CrossRef]
- Samuelov, L.; Sprecher, E.; Tsuruta, D.; Bíró, T.; Kloepper, J.E.; Paus, R. P-Cadherin Regulates Human Hair Growth and Cycling via Canonical Wnt Signaling and Transforming Growth Factor-β2. J. Investig. Dermatol. 2012, 132, 2332–2341. [Google Scholar] [CrossRef]
- Woo, W.-M.; Oro, A.E. SnapShot: Hair follicle stem cells. Cell 2011, 146, 334. [Google Scholar] [CrossRef]
- Greco, V.; Chen, T.; Rendl, M.; Schober, M.; Pasolli, H.A.; Stokes, N.; dela Cruz-Racelis, J.; Fuchs, E. A Two-Step Mechanism for Stem Cell Activation during Hair Regeneration. Cell Stem Cell 2009, 4, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Enshell-Seijffers, D.; Lindon, C.; Kashiwagi, M.; Morgan, B.A. β-catenin Activity in the Dermal Papilla Regulates Morphogenesis and Regeneration of Hair. Dev. Cell 2010, 18, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Che, X.; Zheng, Q.; Wu, A.; Pan, K.; Shao, A.; Wu, Q.; Zhang, J.; Hong, Y. Caspases: A Molecular Switch Node in the Crosstalk between Autophagy and Apoptosis. Int. J. Biol. Sci. 2014, 10, 1072–1083. [Google Scholar] [CrossRef]
- Chuma, M.; Endo-Umeda, K.; Shimba, S.; Yamada, S.; Makishima, M. Hairless Modulates Ligand-Dependent Activation of the Vitamin D Receptor-Retinoid X Receptor Heterodimer. Biol. Pharm. Bull. 2012, 35, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Teichert, A.; Elalieh, H.; Bikle, D. Disruption of the hedgehog signaling pathway contributes to the hair follicle cycling deficiency in Vdr knockout mice. J. Cell Physiol. 2010, 225, 482–489. [Google Scholar] [CrossRef]
- Tampucci, S.; Burgalassi, S.; Chetoni, P.; Lenzi, C.; Pirone, A.; Mailland, F.; Caserini, M.; Monti, D. Topical Formulations Containing Finasteride. Part II: Determination of Finasteride Penetration into Hair Follicles using the Differential Stripping Technique. J. Pharm. Sci. 2014, 103, 2323–2329. [Google Scholar] [CrossRef]
- Higgins, C.A.; Petukhova, L.; Harel, S.; Ho, Y.Y.; Drill, E.; Shapiro, L.; Wajid, M.; Christiano, A.M. FGF5 is a crucial regulator of hair length in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 10648–10653. [Google Scholar] [CrossRef]
- Foitzik, K.; Spexard, T.; Nakamura, M.; Halsner, U.; Paus, R. Towards Dissecting the Pathogenesis of Retinoid-Induced Hair Loss: All-Trans Retinoic Acid Induces Premature Hair Follicle Regression (Catagen) by Upregulation of Transforming Growth Factor-β2 in the Dermal Papilla. J. Investig. Dermatol. 2005, 124, 1119–1126. [Google Scholar] [CrossRef]
- Plikus, M.V.; Mayer, J.A.; de La Cruz, D.; Baker, R.E.; Maini, P.K.; Maxson, R.; Chuong, C.M. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature 2008, 451, 340–344. [Google Scholar] [CrossRef]
- Castellana, D.; Paus, R.; Perez-Moreno, M. Macrophages Contribute to the Cyclic Activation of Adult Hair Follicle Stem Cells. PLoS Biol. 2014, 12, e1002002. [Google Scholar] [CrossRef]
- Kim, M.-J.; Choe, S. BMPs and their clinical potentials. BMB Rep. 2011, 44, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Morgan, B.A. The Dermal Papilla: An Instructive Niche for Epithelial Stem and Progenitor Cells in Development and Regeneration of the Hair Follicle. Cold Spring Harb. Perspect. Med. 2014, 4, a015180. [Google Scholar] [CrossRef] [PubMed]
- Mecklenburg, L.; Tobin, D.J.; Cirlan, M.V.; Craciun, C.; Paus, R. Premature termination of hair follicle morphogenesis and accelerated hair follicle cycling in Iasi congenital atrichia (fzica) mice points to fuzzy as a key element of hair cycle control. Exp. Dermatol. 2005, 14, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Chueh, S.-C.; Lin, S.-J.; Chen, C.-C.; Lei, M.; Wang, L.M.; Widelitz, R.; Hughes, M.W.; Jiang, T.-X.; Chuong, C.M. Therapeutic strategy for hair regeneration: Hair cycle activation, niche environment modulation, wound-induced follicle neogenesis, and stem cell engineering. Expert Opin. Biol. Ther. 2013, 13, 377–391. [Google Scholar] [CrossRef]
- Garza, L.A.; Yang, C.-C.; Zhao, T.; Blatt, H.B.; Lee, M.; He, H.; Stanton, D.C.; Carrasco, L.; Spiegel, J.H.; Tobias, J.W.; et al. Bald scalp in men with androgenetic alopecia retains hair follicle stem cells but lacks CD200-rich and CD34-positive hair follicle progenitor cells. J. Clin. Investig. 2011, 121, 613–622. [Google Scholar] [CrossRef]
- Stenn, K.S.; Cotsarelis, G. Bioengineering the hair follicle: Fringe benefits of stem cell technology. Curr. Opin. Biotechnol. 2005, 16, 493–497. [Google Scholar] [CrossRef]
- Steinberg, M.S.; Takeichi, M. Experimental specification of cell sorting, tissue spreading, and specific spatial patterning by quantitative differences in cadherin expression. Proc. Natl. Acad. Sci. USA 1994, 91, 206–209. [Google Scholar] [CrossRef]
- Reynolds, A.; Jahoda, C. Cultured dermal papilla cells induce follicle formation and hair growth by transdifferentiation of an adult epidermis. Development 1992, 115, 587–593. [Google Scholar] [CrossRef]
- Inoue, K.; Kato, H.; Sato, T.; Osada, A.; Aoi, N.; Suga, H.; Eto, H.; Gonda, K.; Yoshimura, K. Evaluation of Animal Models for the Hair-Inducing Capacity of Cultured Human Dermal Papilla Cells. Cells Tissues Organs 2009, 190, 102–110. [Google Scholar] [CrossRef]
- Osada, A.; Iwabuchi, T.; Kishimoto, J.; Hamazaki, T.S.; Okochi, H. Long-Term Culture of Mouse Vibrissal Dermal Papilla Cells and De Novo Hair Follicle Induction. Tissue Eng. 2007, 13, 975–982. [Google Scholar] [CrossRef]
- Kishimoto, J.; Burgeson, R.E.; Morgan, B.A. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 2000, 14, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Rendl, M.; Polak, L.; Fuchs, E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008, 22, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, K.-E.; Asakawa, K.; Ishibashi, N.; Toki, H.; Ogawa, M.; Hasegawa, T.; Irié, T.; Tachikawa, T.; Sato, A.; Takeda, A.; et al. Fully functional hair follicle regeneration through the rearrangement of stem cells and their niches. Nat. Commun. 2012, 3, 784. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, T.; Yan, L.; Shimizu, A.; Maruo, S.; Fukuda, J. Preparation of hair beads and hair follicle germs for regenerative medicine. Biomaterials 2019, 212, 55–63. [Google Scholar] [CrossRef]
- Pagani, A.; Aitzetmüller, M.M.; Brett, E.A.; König, V.; Wenny, R.; Thor, D.; Radtke, C.; Huemer, G.M.; Machens, H.G.; Duscher, D. Skin rejuvenation through HIF-1α modulation. Plast. Reconstr. Surg. 2018, 141, 600e–607e. [Google Scholar] [CrossRef] [PubMed]
- Rathman-Josserand, M.; Genty, G.; Lecardonnel, J.; Chabane, S.; Cousson, A.; Michelet, J.F.; Bernard, B.A. Human Hair Follicle Stem/Progenitor Cells Express Hypoxia Markers. J. Investig. Dermatol. 2013, 133, 2094–2097. [Google Scholar] [CrossRef]
- Rezvani, H.R.; Ali, N.; Nissen, L.J.; Harfouche, G.; De Verneuil, H.; Taïeb, A.; Mazurier, F. HIF-1α in epidermis: Oxygen sensing, cutaneous angiogenesis, cancer, and non-cancer disorders. J. Investig. Dermatol. 2011, 131, 1793–1805. [Google Scholar] [CrossRef]
- Yum, S.; Jeong, S.; Kim, D.; Lee, S.; Kim, W.; Yoo, J.-W.; Kim, J.A.; Kwon, O.S.; Kim, D.D.; Min, D.S.; et al. Minoxidil induction of VEGF is mediated by inhibition of HIF-prolyl hydrox-ylase. Int. J. Mol. Sci. 2017, 19, 53. [Google Scholar] [CrossRef]
- Duscher, D.; Januszyk, M.; Maan, Z.N.; Whittam, A.J.; Hu, M.S.; Walmsley, G.G.; Dong, Y.; Khong, S.M.; Longaker, M.T.; Gurtner, G.T. Comparison of the hydroxylase inhibitor DMOG and the iron chelator deferoxamine in diabetic and aged wound healing. Plast. Reconstr. Surg. 2017, 139, 695e. [Google Scholar] [CrossRef]
- Hong, W.X.; Hu, M.S.; Esquivel, M.; Liang, G.Y.; Rennert, R.C.; McArdle, A.; Paik, K.J.; Duscher, D.; Gurtner, G.C.; Lorenz, H.P.; et al. The Role of Hypoxia-Inducible Factor in Wound Healing. Adv. Wound Care 2014, 3, 390–399. [Google Scholar] [CrossRef]
- Duscher, D.; Neofytou, E.; Wong, V.W.; Maan, Z.N.; Rennert, R.C.; Inayathullah, M.; Januszyk, M.; Rodrigues, M.; Malkovskiy, A.V.; Whitmore, A.J.; et al. Transdermal deferoxamine prevents pressure-induced diabetic ulcers. Proc. Natl. Acad. Sci. USA 2015, 112, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.H.; Sun, Q.; Ratti, K.; Lee, S.-H.; Zheng, Y.; Takeo, M.; Lee, W.; Rabbani, P.; Plikus, M.V.; Cain, J.E.; et al. Hedgehog stimulates hair follicle neogenesis by creating inductive dermis during murine skin wound healing. Nat. Commun. 2018, 9, 4903. [Google Scholar] [CrossRef] [PubMed]
- Luanpitpong, S.; Nimmannit, U.; Pongrakhananon, V.; Chanvorachote, P. Emblica (Phyllanthus emblica Linn.) fruit extract promotes proliferation in dermal papilla cell of human hair follicle. Res. J. Med. Plant 2011, 5, 95–100. [Google Scholar]
- Shah, C.S.; Qudry, J.S. A Text Book of Pharmacognosy, 11th ed.; Shah Prakashan, B.S., Ed.; Shah Publisher: Ahmadabad, India, 1996; p. 119. [Google Scholar]
- Jammanesh, A.; Arbabi Bidgoli, S.; Ghaffari, S.; Avadi, M.R. Formulation, characterization and toxicity assessment of Ginkgo biloba extract solid lipid nanoparticle in female mice. Nanomed. Res. J. 2021, 6, 28–40. [Google Scholar] [CrossRef]
- Saansoomchai, P.; Limmongkon, A.; Surangkul, D.; Chewonarin, T.; Srikummool, M. Enhanced VEGF expression in hair follicle dermal papilla cells by Centella asiatica Linn. Chiang Mai Univ. J. Nat. Sci. 2018, 17, 25–37. [Google Scholar] [CrossRef]
- Hay, I.; Jamieson, M.; Ormerod, A. The use of aromatherapy as a successful treatment for alopecia areata. J. Eur. Acad. Dermatol. Venereol. 1998, 11, S147. [Google Scholar] [CrossRef]
- Dhanukar, S.A.; Thahe, U.M. Therapeutic Approaches. In Ayurveda Revisited; Popular Prakashan: Bombay, India, 1989; pp. 74–130. [Google Scholar]
- Shimizu, K.; Kondo, R.; Sakai, K.; Shoyama, Y.; Sato, H.; Ueno, T. Steroid 5α-Reductase Inhibitory Activity and Hair Regrowth Effects of an Extract from Boehmeria nipononivea. Biosci. Biotechnol. Biochem. 2000, 64, 875–877. [Google Scholar] [CrossRef]
- Husain, A.; Virman, O.P.; Popli, S.P.; Misra, L.N.; Gupta, M.M.; Srivastava, G.N.; Abraham, Z.; Singh, A.K. Dictionary of Medicinal Plants; Central Institute of Medicinal and Aromatic Plants: Lucknow, Uttar Pradesh, India, 1992; p. 89. [Google Scholar]
- Nandeesh, R.; Kumar, B.S.A.; Lakshman, K.; Khan, S.; Swamy, V.B.N.; Bharathi, T.; Ganapathy, S. Evaluation of Hair Growth Activity of Buxus wallichiana Baill Extract in Rats. Iran. J. Basic Med. Sci. 2009, 11, 236–241. [Google Scholar] [CrossRef]
- Mukerji, B.K. Indian Pharmaceutical Codex; CSIR: Mumbai, India, 1953; pp. 78–79. [Google Scholar]
- Dry, F.W. The coat of the mouse (Mus musculus). J. Genet. 1926, 16, 287–340. [Google Scholar] [CrossRef]
- Shen, L.; Cui, Y. Effects of the leaf of Ginkgo biloba L. extract on blood rheology in animals. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Medica 1998, 23, 622–623. [Google Scholar]
- Butler, H.; Poucher, W.A. Perfumes Cosmetics and Soaps; Chapman and Hall: London, UK, 1993; p. 130. [Google Scholar]
- Liu, W.; Xu, S.; Che, C. Anti-proliferative effect of ginseng saponins on human prostate cancer cell line. Life Sci. 2000, 67, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Suzuki, R.; Koide, C.; Suzuki, T.; Matsuda, H.; Kubo, M. Effect of Leaves of Ginkgo biloba on Hair Regrowth in C3H Strain Mice. YAKUGAKU ZASSHI J. Pharm. Soc. Jpn. 1993, 113, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, M.; Yoo, Y.C.; Matsuzawa, K.; Sato, K.; Saiki, I.; Tonooka, S.; Samukawa, K.; Azuma, I. Inhibitory effect of tumor metastasis in mice by saponins, ginsenoside-Rb2, 20(R)- and 20(S)-ginsenoside-Rg3, of red ginseng. Biol. Pharm. Bull. 1995, 18, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Gupta, S.K.; Prabha, S. Hair growth activity of Nardostachys jatamansi and Cyperus rotundus rhizomes extract on chemotherapy induced alopecia. Int. J. Drug Dis. Herbal. Res. 2011, 1, 52–54. [Google Scholar]
- Pérez-Ornelas, V.; Cabeza, M.; Bratoeff, E.; Heuze, I.; Sánchez, M.; Ramírez, E.; Naranjo-Rodríguez, E. New 5α-reductase inhibitors: In vitro and in vivo effects. Steroids 2005, 70, 217–224. [Google Scholar] [CrossRef]
- Barbareschi, M. The use of minoxidil in the treatment of male and female androgenetic alopecia: A story of more than 30 years. G. Ital. Dermatol. Venereol. 2018, 153, 102–106. [Google Scholar] [CrossRef]
- Mallard, C.; Louis, F.; At, E. Cream Gels Comprising at Least One Retnoid and Benzoyl Peroxde. U.S. Patent No. 8,957,112 B2, 17 February 2015. [Google Scholar]
- Burry, J.S.; Evans, R.L.; Andrew, G. Turner Composition Comprising Azole Fungicide and Water Soluble Metal Salt. EP Patent No. 2421498A1, 24 August 2016. [Google Scholar]
- Tanaka, F. Acidic Composition for External Use and Agent for Accelerating Infiltration of Cosmetic Preparation, Hair-Growing Agent, and Preparation for External Use Each Containing the Composition into Skin or the Like. US Patent No. 20120165291, 1 March 2012. [Google Scholar]
- Bosco, M.; Stucchi, L.; Fabbian, M.; Picotti, F. Use of Glycosaminoglycan Lipoate Esters in the Trichology Field. WO Patent No. 2012080223A1, 2 August 2012. [Google Scholar]
- Gleich, P. Use of a Protease-Containing Hair Growth Reducing Agent. DE Patent No. 102010015120B4, 16 April 2012. [Google Scholar]
- Renshun, G. Composition for Preventing Hair Loss and Stimulating Hair Growth. CN Patent No. 102724959B, 15 October 2013. [Google Scholar]
- Marinkovich, M.P.; Gao, J.; Xu, X.; Rajadas, J. Methods for Modulating Hair Growth Using Truncated Laminin-511. WO Patent No. 2013148377A1, 15 March 2013. [Google Scholar]
- Duranton, A.; Breton, L. Use of Taurine for the Treatment of Alopecia. CA Patent No. 2489308C, 23 June 2013. [Google Scholar]
- Giuliani, G.; Paus, R.; Ramot, Y.; Baroni, S.; Viti, F.; Bellinvia, S. Methods of Treating Hair Related Conditions. WO Patent No. 2014041140A1, 20 March 2014. [Google Scholar]
- Santhanam, U.; Hong, Q.; Yim, S. Dickkopf-1 Expression Modulating Compositions and Uses Thereof. US Patent No. 20140065086, 3 June 2014. [Google Scholar]
- Sizhe, L. Chinese Herbal Medicinal Shampoo and Preparation Method for Same. CN Patent No. 103520048A, 22 January 2014. [Google Scholar]
- Kawano, M. Hair Growth Agent/Hair Tonic. EP Patent No. 2674148A1, 18 December 2013. [Google Scholar]
- Moser, P.; Danoux, L.; Pauly, G. Cosmetic and Pharmaceutical Uses of an Extract of a Plant Belonging to the Genus Buchholzia. US Patent No. 8603545B2, 15 March 2013. [Google Scholar]
- Shimazaki, A.; Shin, Y.; Yasushi, M. Composition for Hair Growth. WO Patent No. 2013180229A1, 15 February 2013. [Google Scholar]
- Duran, G.A. Formulation and Method for Treating Hair Loss. WO Patent No. 2013167927A1, 14 November 2013. [Google Scholar]
- Huchel, U.; Kropf, U.; Welss, T.; Giesen, M.; Bock, A. Advanced Glycation end Products as Active Ingredients. EP Patent No. 2162115B1, 18 December 2013. [Google Scholar]
- Shihong, M.; Shan, K.X.; Weiming, C.Z. Formula and Preparation Method of Natural Plant Anti-Hair-Loss and Anti-Dandruff Shampoo. CN Patent No. 103445997A, 15 March 2013. [Google Scholar]
- Ueno, R.; Habe, T.; Sekida, T. Composition and Method for Promoting Hair Growth. US Patent No. 20140171496A1, 12 August 2014. [Google Scholar]
- Bertrand, M.; Henriat, P. Topical Composition. US Patent No. 20140170246A1, 20 February 2014. [Google Scholar]
- Chung, Y.J.; Kim, M.U. Wnt Family-Derived Peptide and Uses Thereof. EP Patent No. 2740741A1, 19 March 2014. [Google Scholar]
- Bruning, E.; Euen, T.; Gunn, G.K.; Liebel, F.; Tucker-Samaras, S.; VanWyck, D.; Santora, D. Methods and Compositions for Enhancing Hair Quality Using Blackberry Extract. US Patent No. 8962041B2, 14 August 2014. [Google Scholar]
- Kobayashi, T.; Shizuka, U. Moisturizing Agent. WO Patent No. 2015012198A1, 29 January 2015. [Google Scholar]
- Price, V.H.; Menefee, E.; Strauss, P.C. Changes in hair weight and hair count in men with androgenetic alopecia, after application of 5% and 2% topical minoxidil, placebo, or no treatment. J. Am. Acad. Dermatol. 1999, 41, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Yamazaki, M.; Naruto, S.; Asanuma, Y.; Kubo, M. Antiandrogenic and hair growth promoting activities of Lygodii Spora (spore of Lygodium japonicum) I. Active constituents inhibiting testosterone 5 aplhareductase. Biol. Pharm. Bull. 2002, 25, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Singh, V. Phytoconstituents and hair stimulant formulation from Nordostachys jatamansi. In Proceedings of the International Congress on Traditional Asian Medicine, Halle (Saale), Germany, 18–24 August 2002; pp. 18–24. [Google Scholar]
- Saraf, S.; Pathak, A.K.; Dixit, V.K. Hair growth promoting activity of Tridax procumbens. Fitoter 1991, 62, 495–498. [Google Scholar]
- Sharquie, K.E.; Al-Obaidi, H.K. Onion Juice (Allium cepa L.), A New Topical Treatment for Alopecia Areata. J. Dermatol. 2002, 29, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Muradoglu, F.; Oguz, H.I.; Yildiz, K.; Yilmaz, H. Some chemical composition of walnut (Juglans regia L.) selections from Eastern Turkey. Afr. J. Agric. Res. 2010, 5, 2379–2385. [Google Scholar]
- Harada, N.; Okajima, K.; Arai, M.; Kurihara, H.; Nakagata, N. Administration of capsaicin and isoflavone promotes hair growth by increasing insulin-like growth factor-I production in mice and in humans with alopecia. Growth Horm. IGF Res. 2007, 17, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Kamiya, T.; Yokoo, Y.; Hasegawa, A. Procyanidin Oligomers Selectively and Intensively Promote Proliferation of Mouse Hair Epithelial Cells In Vitro and Activate Hair Follicle Growth In Vivo11The authors disclosed conflict of interest. J. Investig. Dermatol. 1999, 112, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Kamimura, A.; Kagoura, M.; Toyoda, M.; Morohashi, M. Investigation of the topical application of procyanidin oligomers from apples to identify their potential use as a hair-growing agent. J. Cosmet. Dermatol. 2005, 4, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S. Green tea and the skin. J. Am. Acad. Dermatol. 2005, 52, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Philpott, M.P.; Kealey, T. Cyclical Changes in Rat Vibrissa Follicles Maintained In Vitro. J. Investig. Dermatol. 2000, 115, 1152–1155. [Google Scholar] [CrossRef]
- Wikramanayake, T.C.; Rodriguez, R.; Choudhary, S.; Mauro, L.M.; Nouri, K.; Schachner, L.A.; Jimenez, J.J. Effects of the Lexington LaserComb on hair regrowth in the C3H/HeJ mouse model of alopecia areata. Lasers Med. Sci. 2012, 27, 431–436. [Google Scholar] [CrossRef]
- Hoffmann, R.; Happle, R. Current understanding of androgenetic alopecia. Part II: Clinical aspects and treatment. Eur. J. Dermatol. 2000, 10, 410–417. [Google Scholar]
- Zarei, M.; Wikramanayake, T.C.; Falto-Aizpurua, L.; Schachner, L.A.; Jimenez, J.J. Low level laser therapy and hair regrowth: An evidence-based review. Lasers Med. Sci. 2016, 31, 363–371. [Google Scholar] [CrossRef]
- Wikramanayake, T.C.; Villasante, A.C.; Mauro, L.M.; Nouri, K.; Schachner, L.A.; Perez, C.I.; Jimenez, J.J. Low-level laser treatment accelerated hair regrowth in a rat model of chemotherapy-induced alopecia (CIA). Lasers Med. Sci. 2012, 28, 701–706. [Google Scholar] [CrossRef]
- Gilhar, A.; Shalaginov, R.; Assy, B.; Serafimovich, S.; Kalish, R.S. Alopecia areata is a T-lymphocyte mediated autoimmune disease: Lesional human T-lymphocytes transfer alopecia areata to human skin grafts on SCID mice. In Journal of Investigative Dermatology Symposium Proceedings; Elsevier: Amsterdam, The Netherlands, 1999; Volume 4, pp. 207–210. [Google Scholar]
- Orasan, M.S.; Roman, I.I.; Coneac, A.; Muresan, A.; Orasan, R.I. Hair loss and regeneration performed on animal models. Clujul Medical 2016, 89, 327. [Google Scholar] [CrossRef] [PubMed]
- Van Neste, D.; de Brouwer, B. Human hair follicle grafts in nude mice: An important in vivo model for investigating the control of hair growthp. In Hair and Its Disorders: Biology, Pathology and Management; Martin Dunitz: London, UK, 2000; pp. 115–122. [Google Scholar]
- Paus, R.; Stenn, K.S.; Link, R.E. The induction of anagen hair growth in telogen mouse skin by cyclosporine A administration. Labor. Investig. J. Tech. Methods Pathol. 1989, 60, 365–369. [Google Scholar]
- Müller-Röver, S.; Foitzik, K.; Paus, R.; Handjiski, B.; van der Veen, C.; Eichmüller, S.; McKay, I.A.; Stenn, K.S. A Comprehensive Guide for the Accurate Classification of Murine Hair Follicles in Distinct Hair Cycle Stages. J. Investig. Dermatol. 2001, 117, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J.E.; Sugawara, K.; Al-Nuaimi, Y.; Gáspár, E.; van Beek, N.; Paus, R. Methods in hair research: How to objectively distinguish between anagen and catagen in human hair follicle organ culture. Exp. Dermatol. 2010, 19, 305–312. [Google Scholar] [CrossRef]
- Gnann, L.A.; Castro, R.F.; Azzalis, L.A.; Feder, D.; Perazzo, F.F.; Pereira, E.C.; Rosa, P.C.P.; Junqueira, V.B.C.; Rocha, K.C.; Machado, C.D.A.; et al. Hematological and hepatic effects of vascular epidermal growth factor (VEGF) used to stimulate hair growth in an animal model. BMC Dermatol. 2013, 13, 15. [Google Scholar] [CrossRef]
- Singh, S.; Shukla, V.K. Current regulations for Herbal Medicines in India. Int. J. Drug Regul. Aff. 2021, 9, 30–34. [Google Scholar] [CrossRef]
- Santos, A.C.; Ferreira, C.; Veiga, F.; Ribeiro, A.J.; Panchal, A.; Lvov, Y.; Agarwal, A. Halloysite clay nanotubes for life sciences applications: From drug encapsulation to bioscaffold. Adv. Colloid Interface Sci. 2018, 257, 58–70. [Google Scholar] [CrossRef]
- Mavridi-Printezi, A.; Guernelli, M.; Menichetti, A.; Montalti, M. Bio-Applications of Multifunctional Melanin Nanoparticles: From Nanomedicine to Nanocosmetics. Nanomaterials 2020, 10, 2276. [Google Scholar] [CrossRef]
- Liu, M.; Jia, Z.; Jia, D.; Zhou, C. Recent advance in research on halloysite nanotubes-polymer nanocomposite. Prog. Polym. Sci. 2014, 39, 1498–1525. [Google Scholar] [CrossRef]
- Abdullayev, E.; Joshi, A.; Wei, W.; Zhao, Y.; Lvov, Y. Enlargement of Halloysite Clay Nanotube Lumen by Selective Etching of Aluminum Oxide. ACS Nano 2012, 6, 7216–7226. [Google Scholar] [CrossRef]
- Santos, A.C.; Panchal, A.; Rahman, N.; Pereira-Silva, M.; Pereira, I.; Veiga, F.; Lvov, Y. Evolution of Hair Treatment and Care: Prospects of Nanotube-Based Formulations. Nanomaterials 2019, 9, 903. [Google Scholar] [CrossRef] [PubMed]
- Asenov, A.; Oliveira, F.A.; Speare, R.; Liesenfeld, O.; Hengge, U.R.; Heukelbach, J. Efficacy of chemical and botanical over-the-counter pediculicides available in Brazil, and off-label treatments, against head lice ex vivo. Int. J. Dermatol. 2010, 49, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Downs, A.M.; Stafford, K.A.; Hunt, L.P.; Ravenscroft, J.C.; Coles, G.C. Widespread insecticide resistance in head lice to the over-the-counter pediculocides in England, and the emergence of carbaryl resistance: Therapeutics. Br. J. Dermatol. 2002, 146, 88–93. [Google Scholar] [CrossRef]
- Tian, L.; Li, X.; Ji, H.; Yu, Q.; Yang, M.; Guo, L.; Huang, L.; Gao, W. Melanin-like nanoparticles: Advances in surface modification and tumour photothermal therapy. J. Nanobiotechnology 2022, 20, 485. [Google Scholar] [CrossRef]
- Sentamilselvi, G.; Janaki, C.; Murugusundram, S. Trichomycoses. Int. J. Trichol. 2009, 1, 100. [Google Scholar] [CrossRef] [PubMed]
- Chanprapaph, K.; Udompanich, S.; Visessiri, Y.; Ngamjanyaporn, P.; Suchonwanit, P. Nonscarring alopecia in systemic lupus erythematosus: A cross-sectional study with trichoscopic, histopathologic, and immunopathologic analyses. J. Am. Acad. Dermatol. 2019, 81, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Macri, L.K.; Kaplan, H.M.; Kohn, J. Nanoparticles and nanofibers for topical drug delivery. J. Control. Release 2016, 240, 77–92. [Google Scholar] [CrossRef]
- Rosen, J.; Landriscina, A.; Friedman, A.J. Nanotechnology-Based Cosmetics for Hair Care. Cosmetics 2015, 2, 211–224. [Google Scholar] [CrossRef]
- Chen, P.; Miao, Y.; Zhang, F.; Huang, J.; Chen, Y.; Fan, Z.; Yang, L.; Wang, J.; Hu, Z. Nanoscale microenvironment engineering based on layer-by-layer self-assembly to regulate hair follicle stem cell fate for regenerative medicine. Theranostics 2020, 10, 11673–11689. [Google Scholar] [CrossRef]
- Enyiğit, T.; Sonvico, F.; Rossi, A.; Tekmen, I.; Santi, P.; Colombo, P.; Nicoli, S.; Özer, Ö. In vivo assessment of clobetasol propionate-loaded lecithin-chitosan nanoparticles for skin delivery. Int. J. Mol. Sci. 2016, 18, 32. [Google Scholar] [CrossRef]
- Matos, B.N.; Reis, T.; Gratieri, T.; Gelfuso, G.M. Chitosan nanoparticles for targeting and sustaining minoxidil sulphate delivery to hair follicles. Int. J. Biol. Macromol. 2015, 75, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Zürcher, S.; Dorcier, A.; Luengo, G.S.; Spencer, N.D. Adsorption and Lubricating Properties of Poly(l-lysine)-graft-poly(ethylene glycol) on Human-Hair Surfaces. ACS Appl. Mater. Interfaces 2009, 1, 1938–1945. [Google Scholar] [CrossRef] [PubMed]
- Leal Cardoso, J.H.; Noronha Coelho de Souza, A.; Militão de Souza, F.; Sa Preire, S.; Pinçon, C. Treatment of Head Louse Infestation with a Novel Mixture Made of Semi-Crystalline Polymers and Plant Extracts: Blind, Randomized, Controlled, Superiority Trial. Cosmetics 2020, 7, 25. [Google Scholar] [CrossRef]
- Wang, W.; Chen, L.; Huang, X.; Shao, A. Preparation and Characterization of Minoxidil Loaded Nanostructured Lipid Carriers. AAPS PharmSciTech 2016, 18, 509–516. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, B.; Bakshi, G.; Katare, O.P. Development of Liposomal Systems of Finasteride for Topical Applications: Design, Characterization, and In Vitro Evaluation. Pharm. Dev. Technol. 2007, 12, 591–601. [Google Scholar] [CrossRef]
- Haveli, S.D.; Walter, P.; Patriarche, G.; Ayache, J.; Castaing, J.; Van Elslande, E.; Tsoucaris, G.; Wang, P.A.; Kagan, H.B. Hair fiber as a nano-reactor in controlled synthesis of fluorescent gold nanoparticles. Nano Lett. 2012, 12, 6212–6217. [Google Scholar] [CrossRef]
- Marimuthu, S.; Rahuman, A.A.; Santhoshkumar, T.; Jayaseelan, C.; Kirthi, A.V.; Bagavan, A.; Kamaraj, C.; Elango, G.; Zahir, A.A.; Rajakumar, G.; et al. Lousicidal activity of synthesized silver nanoparticles using Lawsonia inermis leaf aqueous extract against Pediculus humanus capitis and Bovicola ovis. Parasitol. Res. 2012, 111, 2023–2033. [Google Scholar] [CrossRef]
- Al Mahrooqi, J.H.; Khutoryanskiy, V.V.; Williams, A.C. Thiolated and PEGylated silica nanoparticle delivery to hair follicles. Int. J. Pharm. 2021, 593, 120130. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, M.; Bao, F.; Xing, M.; Wang, E.; Xu, Q.; Huan, Z.; Guo, F.; Chang, J. Multifunctional Zn doped hollow mesoporous silica/polycaprolactoneelectrospun membranes with enhanced hair follicle regeneration and antibacterial activity for wound healing. Nanoscale 2019, 11, 6315–6333. [Google Scholar] [CrossRef]
- Cavallaro, G.; Milioto, S.; Konnova, S.; Fakhrullina, G.; Akhatova, F.; Lazzara, G.; Fakhrullin, R.; Lvov, Y. Halloysite/Keratin Nanocomposite for Human Hair Photoprotection Coating. ACS Appl. Mater. Interfaces 2020, 12, 24348–24362. [Google Scholar] [CrossRef]
- Leerunyakul, K.; Suchonwanit, P. Asian hair: A review of structures, properties, and distinctive disorders. Clin. Cosmet. Investig. Dermatol. 2020, 13, 309. [Google Scholar] [CrossRef]
- Guryanov, I.; Naumenko, E.; Fakhrullin, R. Hair surface engineering: Combining nanoarchitectonics with hair topical and beauty formulations. Appl. Surf. Sci. Adv. 2022, 7, 100188. [Google Scholar] [CrossRef]
- Pucek, A.; Tokarek, B.; Waglewska, E.; Bazylińska, U. Recent Advances in the Structural Design of Photosensitive Agent Formulations Using “Soft” Colloidal Nanocarriers. Pharmaceutics 2020, 12, 587. [Google Scholar] [CrossRef] [PubMed]
- Nazir, H.; Wang, L.; Lian, G.; Zhu, S.; Zhang, Y.; Liu, Y.; Ma, G. Multilayered silicone oil droplets of narrow size distribution: Preparation and improved deposition on hair B. Biointerfaces 2012, 100, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Hu, Z.; Liao, M.; Cai, Y.; Meng, L.; Liu, Z.; Chen, Y.; Liu, Y.; Lu, N. A novel preparation method for silicone oil nanoemulsions and its application for coating hair with silicone. Int. J. Nanomed. 2012, 7, 5719–5724. [Google Scholar] [CrossRef]
- Sonneville-Aubrun, O.; Simonnet, J.T.; L’alloret, F. Nanoemulsions: A new vehicle for skincare products. Adv. Coll. Interface Sci. 2004, 108, 145–149. [Google Scholar] [CrossRef]
- Gavazzoni Dias, M.F. Hair cosmetics: An overview. Int. J. Trichology 2015, 7, 2–15. [Google Scholar] [CrossRef]
- Song, C.; Liu, S. A new healthy sunscreen system for human: Solid lipid nannoparticles as carrier for 3,4,5-trimethoxybenzoylchitin and the improvement by adding Vitamin E. Int. J. Biol. Macromol. 2005, 36, 116–119. [Google Scholar] [CrossRef]
- Nogueira, A.C.S.; Joekes, I. Hair color changes and protein damage caused by ultraviolet radiation. J. Photochem. Photobiol. B Biol. 2004, 74, 109–117. [Google Scholar] [CrossRef]
- Panchal, A.; Fakhrullina, G.; Fakhrullin, R.; Lvov, Y. Self-assembly of clay nanotubes on hair surface for medical and cosmetic formulations. Nanoscale 2018, 10, 18205–18216. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F.; Evtugyn, V.G.; Rozhina, E.; Fakhrullin, R.F. Nanohydrogel Formation within the Halloysite Lumen for Triggered and Sustained Release. ACS Appl. Mater. Interfaces 2018, 10, 8265–8273. [Google Scholar] [CrossRef] [PubMed]
- Tarasova, E.; Naumenko, E.; Rozhina, E.; Akhatova, F.; Fakhrullin, R. Cytocompatibility and uptake of polycations-modified halloysite clay nanotubes. Appl. Clay Sci. 2019, 169, 21–30. [Google Scholar] [CrossRef]
- Guryanov, I.; Naumenko, E.; Akhatova, F.; Lazzara, G.; Cavallaro, G.; Nigamatzyanova, L.; Fakhrullin, R. Selective cytotoxic activity of prodigiosin@ halloysitenanoformulation. Front. Bioeng. Biotechnol. 2020, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Naumenko, E.A.; Guryanov, I.D.; Yendluri, R.; Lvov, Y.M.; Fakhrullin, R.F. Clay nanotube–biopolymer composite scaffolds for tissue engineering. Nanoscale 2016, 8, 7257–7271. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.; Scott, F.H.; Lvov, Y.; Stavitskaya, A.; Akhatova, F.; Konnova, S.; Fakhrullina, G.; Fakhrullin, R. Clay Nanotube Immobilization on Animal Hair for Sustained Anti-Lice Protection. Pharmaceutics 2021, 13, 1477. [Google Scholar] [CrossRef]
- Nanda, A.; Nanda, S.; Nguyen, T.A.; Slimani, Y.; Rajendran, S. (Eds.) Nanocosmetics: Fundamentals, Applications and Toxicity; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Thun, M.J.; Altekruse, S.F.; Namboodiri, M.M.; Calle, E.E.; Myers, D.G.; Heath, C.W., Jr. Hair dye use and risk of fatal cancers in US women. J. Natl. Cancer Inst. 1994, 86, 210–215. [Google Scholar] [CrossRef]
- Baki, G.; Alexander, K.S. Introduction to Cosmetic Formulation and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Lee, H.Y.; Jeong, Y.I.; Choi, K.C. Hair dye-incorporated poly-γ-glutamic acid/glycol chitosan nanoparticles based on ion-complex formation. Int. J. Nanomed. 2011, 6, 2879. [Google Scholar]
- Im, K.M.; Kim, T.-W.; Jeon, J.-R. Metal-Chelation-Assisted Deposition of Polydopamine on Human Hair: A Ready-to-Use Eumelanin-Based Hair Dyeing Methodology. ACS Biomater. Sci. Eng. 2017, 3, 628–636. [Google Scholar] [CrossRef]
- Gao, Z.F.; Wang, X.Y.; Gao, J.B.; Xia, F. Rapid preparation of polydopamine coating as a multifunctional hair dye. RSC Adv. 2019, 9, 20492–20496. [Google Scholar] [CrossRef]
- Trelles, M.A.; Almudever, P.; Alcolea, J.M.; Cortijo, J.; Serrano, G.; Expósito, I.; Royo, J.; Leclère, F.M. Cuttlefish Ink Melanin Encapsulated in Nanolipid Bubbles and Applied Through a Micro-Needling Procedure Easily Stains White Hair Facilitating Photoepilation. J. Drugs Dermatol. 2016, 15, 615–625. [Google Scholar]
- Gourlaouen, L.; Lee, K. Composition and Method of Dyeing Keratin Fibers Comprising Luminescent Semiconductive Nanoparticles. U.S. Patent application US 10/764,436, 16 December 2004. [Google Scholar]
- Luo, C.; Zhou, L.; Chiou, K.; Huang, J. Multifunctional Graphene Hair Dye. Chem 2018, 4, 784–794. [Google Scholar] [CrossRef]
- Gandhi, P.R.; Jayaseelan, C.; Mary, R.R.; Mathivanan, D.; Suseem, S.R. Acaricidal, pediculicidal and larvicidal activity of synthesized ZnO nanoparticles using Momordicacharantia leaf extract against blood feeding parasites. Exp. Parasitol. 2017, 181, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, M.; Clark, L.; Levitt, J. Therapy for Head Lice Based on Life Cycle, Resistance, and Safety Considerations. Pediatrics 2007, 119, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P.; Palombo, M.; Cardillo, A.; Del Ciotto, P.; Morganti, G.; Gazzaniga, G. Anti-dandruff and anti-oily efficacy of hair formulations with a repairing and restructuring activity. The positive influence of the Zn-chitin nanofibrils complexes. J. Appl. Cosmetol. 2012, 30, 149–159. [Google Scholar]
- Lamore, S.D.; Cabello, C.M.; Wondrak, G.T. The topical antimicrobial zinc pyrithione is a heat shock response inducer that causes DNA damage and PARP-dependent energy crisis in human skin cells. Cell Stress Chaperon 2009, 15, 309–322. [Google Scholar] [CrossRef]
- Gao, W.; Thamphiwatana, S.; Angsantikul, P.; Zhang, L. Nanoparticle approaches against bacterial infections. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 532–547. [Google Scholar] [CrossRef]
- Zakharova, O.V.; Godymchuk, A.Y.; Gusev, A.A.; Gulchenko, S.I.; Vasyukova, I.A.; Kuznetsov, D.V. Considerable Variation of Antibacterial Activity of Cu Nanoparticles Suspensions Depending on the Storage Time, Dispersive Medium, and Particle Sizes. BioMed Res. Int. 2015, 2015, 412530. [Google Scholar] [CrossRef]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 2007, 18, 225103. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Rilda, Y.; Damara, D.; Putri, Y.E.; Refinel, R.; Agustien, A.; Pardi, H. Pseudomonas aeruginosa antibacterial textile cotton fiber construction based on ZnO–TiO2 nanorods template. Heliyon 2020, 6, e03710. [Google Scholar] [CrossRef]
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of Nanotechnology in Cosmeceuticals: A Review of Recent Advances. J. Pharm. 2018, 2018, 342020. [Google Scholar] [CrossRef] [PubMed]
- Vickers, N.J. Animal communication: When I’m calling you, will you answer too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Dhawan, G.; Gupta, S.; Kumar, P. Recent Advances in a Polydopamine-Mediated Antimicrobial Adhesion System. Front. Microbiol. 2021, 11, 607099. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-Y.; Huang, C.-J. Functionalization of Polydopamine via the Aza-Michael Reaction for Antimicrobial Interfaces. Langmuir 2016, 32, 5019–5028. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Qu, X.; Tan, H.; Song, J.; Lei, M.; Kim, E.; Payne, G.F.; Liu, C. Role of polydopamine’s redox-activity on its pro-oxidant, radical-scavenging, and antimicrobial activities. Actabiomaterialia 2019, 88, 181–196. [Google Scholar]
- Smith, A.; Perelman, M.; Hinchcliffe, M. Chitosan: A promising safe and immune-enhancing adjuvant for intranasal vaccines. Hum. Vaccines Immunother. 2014, 10, 797–807. [Google Scholar] [CrossRef]
- Azuma, K.; Koizumi, R.; Izawa, H.; Morimoto, M.; Saimoto, H.; Osaki, T.; Ito, N.; Yamashita, M.; Tsuka, T.; Imagawa, T.; et al. Hair growth-promoting activities of chitosan and surface-deacetylated chitin nanofibers. Int. J. Biol. Macromol. 2018, 126, 11–17. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef]
- Kravanja, G.; Primožič, M.; Knez, Ž.; Leitgeb, M. Chitosan-Based (Nano)Materials for Novel Biomedical Applications. Molecules 2019, 24, 1960. [Google Scholar] [CrossRef]
- Pereira, M.N.; Ushirobira, C.Y.; Cunha-Filho, M.S.; Gelfuso, G.M.; Gratieri, T. Nanotechnology advances for hair loss. Ther. Deliv. 2018, 9, 593–603. [Google Scholar] [CrossRef]
- Gupta, A.; Aggarwal, G.; Singla, S.; Arora, R. Transfersomes: A Novel Vesicular Carrier for Enhanced Transdermal Delivery of Sertraline: Development, Characterization, and Performance Evaluation. Sci. Pharm. 2012, 80, 1061–1080. [Google Scholar] [CrossRef] [PubMed]
- Pelikh, O.; Eckert, R.W.; Pinnapireddy, S.R.; Keck, C.M. Hair follicle targeting with curcumin nanocrystals: Influence of the formulation properties on the penetration efficacy. J. Control. Release 2020, 329, 598–613. [Google Scholar] [CrossRef] [PubMed]
- Vidlářová, L.; Romero, G.B.; Hanuš, J.; Štěpánek, F.; Müller, R.H. Nanocrystals for dermal penetration enhancement—Effect of concentration and underlying mechanisms using curcumin as model. Eur. J. Pharm. Biopharm. 2016, 104, 216–225. [Google Scholar] [CrossRef] [PubMed]

| Sl No. | Plant | Formulation | Source | Mechansimof Action | References |
|---|---|---|---|---|---|
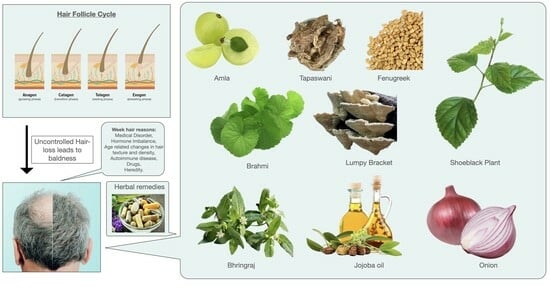
| 1 | Amla or Indian gooseberry | Fruit juice, capsule, and hair oil | Emblica officinalis linn. Family—euphorbiaceae | Powerful inhibitor of 5-alpha reductase | [107] |
| 2 | Brahmi, water hyssop | Capsule, booti, and powder | Bacopa monnieri linn. Family—plantaginaceae | Metal chelation at the initiation level and also as a chain breaker | [108] |
| 3 | Tapaswani | Capsule, powder, and tablet | Nardostachys jatamansi Family—caprifoliaceae | Increases the expression of hair growth factor | [109] |
| 4 | Fenugreek | Hair oil, capsule, and powder | Trigonella foenum graecum Family—fabaceae | Intervening in the anagen-to-catagen and catagen-to-telogen stages of the hair cycle | [110] |
| 5 | Rose mallow, China rose, and shoeblack plant | Paste of fresh or dry flower extract | Hibiscus rosa-sinensis linn. Family—malvaceae | Improve the build-up of keratin and boost the formation of new hair follicles | [111] |
| 6 | Umbrella polypore, lumpy bracket | Capsule, power, and extract | Polyporus umbellatus Family—polyporaceae | Inhibiting catagen entry in the human hair follicle organ | [112] |
| 7 | Rosemary | Oil and tincture | Rosmarinus officinalis linn. Family—lamiaceae | Blockage of DHT precursor, 5-alpha-reductase | [113] |
| 8 | Daisy and aster | Hair oil, tablet | Arnica montana Family—asteraceae | Increases blood circulation in the scalp and hair follicles | [114] |
| 9 | Ramie | Oil | Boehmeria nipononivea Family—urticaceae | Short t telogen, causing premature entry of resting hair follicles | [115] |
| 10 | Himalayan boxwood | Fresh or dry bark extract | Buxus wallichiana baill Family—buxaceae | 5-alpha reductase inhibition | [116] |
| 11 | Maidenhair tree | Fresh or dry fruit and leaf extract | Ginkgo biloba tree linn. Family—ginkgoaceae | Improves circulation in the scalp | [117] |
| 12 | Brahmi | Hair cleanser, oil, powder, fresh leaves | Centella asiatica Family—umbelliferae | Makes the follicles and roots stronger, helping better and stronger hair to grow back | [118] |
| 13 | Bhringraj | Oil, hair tonic | Eclipta alba linn. Family—asteraceae | Increases blood circulation to the scalp and roots | [119] |
| 14 | Coconut | Oil, shampoos, serum | Cocos nucifera linn. Family—palmae | The vitamins and essential fatty acids naturally found in coconut oil nourish the scalp and help to remove sebum build-up from hair follicles. | [120] |
| 15 | Ginseng radix | Powder, capsule, serum | Panax ginseng Family—araliaceae | Prevent apoptosis of hair follicle cells and inhibit 5-α reductase | [121] |
| 16 | Sage oil | Oil, tincture | Salvia officinalis linn. Family—labiatae | Improve blood circulation to the scalp | [122] |
| 17 | Holy basil oil, tulsi | Powder, oil, tincture, leaf and seed extracts | Ocimum sanctum Family—lamiaceae | Increases blood flow and makes the hair root healthy | [123] |
| 18 | Jojoba oil | Oil | Simmondsia chinensis Family—simmondiaceae | Stimulates circulation in the scalp, nourishing and strengthening the hair follicles to grow | [124] |
| 19 | Japanese fern spores | Oil, powder | Climbing greenery Family—lygodiaceae | 5-alpha reductase inhibition | [125] |
| 20 | Ghamra and coatbutton | Oil | Tridax procumbens linn. Family—daisy | Unknown | [126] |
| 21 | Indian subcontinen | Oil | Cuscuta reflexa roxb Family—convolvulaceae | Unknown | [127] |
| 22 | Onion | Oil, shampoo | Allium cepa l Family—liliaceae | Onion juice can provide extra sulfur to support strong and thick hair, thus preventing hair loss and promoting hair growth | [128] |
| 23 | Tuber fleeceflower | Oil, tonic | Polygonium multiflorum thumb Family—polygonaceae | [129] | |
| 24 | Peppers, capsicums | Powder, oil, and extract | Capsicum annum linn. Family—solanaceae | PI3K/AKT pathway | [130] |
| 25 | Northern white cedar | Oil, serum | Thujae occidentalis semen Family—cupressaceae | Enhances circulation to the scalp | [131] |
| 26 | Grape seeds and blueberry | Oil, shampoo, capsule | Proanthocyanidin class of flavonoids Family—sulfotransferase | Catalyze the release of histamine | [132] |
| 27 | Green tea | Oil, shampoo, paste, gel, and serum | Camellia sinensis Family—theaceae | Selectively inhibiting 5-alpha reductase | [133] |
| Sl No. | Patent No | Formulation | Route | Excipient | Mechanism of Action | References |
|---|---|---|---|---|---|---|
| 1 | US8957112 | Cream gel | Dermatology | Dibenzoyl peroxide | Benzoyl peroxide exhibits bactericidal effects against Cutibacterium acnes, a key component of acne vulgaris. | [134] |
| 2 | CN10126973 B | Shampoo | Subcutaneous | Reynoutria multiflora, plastycladus orientalis, Notopterygium forbesii Boiss, dried root of Angelica sinensis, erial parts of Eclipta prostrata L., pericarpium zanthoxyli, Menthae Haplocalycis Herba, Zingiberis Rhizoma Recens | Bactericidal | [135] |
| 3 | EP2421498 A1 | Conditioner | Topical | 6-Benzyladenine, mixtures of the esters of these fatty acids with the polyglycerol mixture, fatty acid esters | Bactericidal | [136] |
| 4 | US20120165291 | Cosmetic preparation | Topical | Azole antifungals and zinc salt of gluconic acid | Promote DNA and RNA production. | [137] |
| 5 | WO2012080223 A1 | Film | Topical | Glycosaminoglycan alkoxy, formic acid, α-lipoic acid | Bactericidal | [138] |
| 6 | US20120165291 A1 | Viscous aqueous solution | Topical | Polysaccharide(Acidic) and water | Increase the contents of VEGF and HGF in the skin tissue of alopecia areata | [139] |
| 7 | DE102010015120 B4 | Cosmetic preparation | Topical | Trypsin, glycosylated, Ca2+, and chymotrypsin | Bactericidal | [140] |
| 8 | CN102724959 B | Oil | Topical | Extract of duchesneae indicae | Elongate the anagen phase and abrogate the effects of androgen | [141] |
| 9 | WO2013148377 A1 | Micro needle device | Topical | Laminin-511 | Laminin-511 promoted hair growth through morphogenic signaling, resulting in Shh and noggin expression | [142] |
| 10 | CA2489308 C | Tablet | Oral | Taurine | Flushes toxins from the scalp, removing excess sebum as well as dead skin cells and DHT | [143] |
| 11 | WO2014041140 A1 | Pharmaceutical salt | Epidermal | 3-(4-aminophenyl)-2-methoxypropanoic acid | Bactericidal | [144] |
| 12 | US20140065086 | Cosmetic preparation | Topical | DICKKOPF-1 | Attenuation of the hair growth process by inhibiting Wnt/β-catenin signaling via the LRP5/6 co-receptor | [145]. |
| 13 | CN103520048 A | Shampoo | Topical | Polyquaternium-10, Chinese herb medication, ammonium lauryl sulfate | Inhibits the 5-alpha reductase enzyme | [146] |
| 14 | EP2674148 A1 | Hair tonic | Topical | Boat orchids (Genus cymbidium) | Restriction of fungal growth | [147] |
| 15 | US8603545 | Cosmetic | Topical | Genus buchholzia | Induces the anagen phase in resting hair follicles | [148] |
| 16 | WO2013180229 A1 | Hair tonic | Topical | Group of alkoxycarbonyls | Stimulates hair growth, is not fully understood | [149] |
| 17 | WO2013167927 A1 | Injectable | Inj. | Biotin, organic silicon, minerals, pentoxyfilline, and hydrochloride salt form of procaine | Enhances keratin production | [150] |
| 18 | EP2162115 B1 | Balm, lotion, emulsion, paste, tablet, cream, foam or spray, particularly in emulsified form | Oral and topical | Advanced glycation end products | Unknown | [151] |
| 19 | CN103445997 A | Shampoo | Topical | Cacumen biotae, root of Polygonum, lycii radicis cortex, Curcuma longa, deionized water, lauryl sodium sulphate (SLS), sodium alkylethersulfate, Alkyl polyglycosides, emulsified silicone, Cocamidopropyl betaine (CAPB), hexadecyl alcohol, stearyl polyoxyethyl hydroxyethyl ammonium chloride, ethylene glycol stearate diester, ammonium chloride, chitin polysaccharide, essence, kathon, acrylamide methyl ammonium oxide, guar gum | Anti-fungal | [152] |
| 20 | US20140171496 A1 | Moisturizer | Topical | Prostaglandin compound | Stimulation of hair follicle stem cells by prostaglandin E2 collagen matrix | [153] |
| 21 | US20140170246 A1 | Foam | Topical | Depilatory agent | Disrupts the disulfide bonds of hair keratin | [154] |
| 22 | EP2740741 A1 | Cosmetics | Topical | Acetyl group, fluorenyl methoxy carbonyl group, formyl group, palmitoyl group, myristyl group, stearyl group, and polyethylene glycol (peg) | Promotes melanin production | [155] |
| 23 | US8962041 B2 | Cosmetics | Topical | Blackberry | Increases blood flow to scalp | [156] |
| 24 | WO2015012198 A1 | Moisturizer | Topical | Kluyveromyces and a polyhydric alcohol | Unknown | [157] |
| Cell Studies for Hair Growth | Composition | Results | References |
|---|---|---|---|
| Minoxidil—topical (5% w/v) | Minoxidil (50 mg/mL) propyleneglycol (500 mg/mL), ethanol (300 mg/mL), water | Three patients who used a 5% minoxidil solution for a year saw some hair regrowth. Two of these three individuals developed barely discernible, tiny, pigmented terminal hairs in scalp locations that had previously had only vellus hairs. Hair regrowth occurred after 4 and 20 weeks, respectively. The third patient exhibited a noticeable restoration of bigger, thicker, more pigmented terminal hair. | [158] |
| Finasteride (0.5% topical solution) | Finasteride | There was an observable increase in hair count (baseline = 876 hairs) with finasteride treatment, measured in a 1-inch diameter circular area of balding vertex scalp. Self-assessment of patients confirmed that there was a decrease in the rate of hair loss with an increase in the growth of hair with finasteridetreatment. | [159] |
| Intralesional triamcinolone acetonide injection | Triamcinolone acetonide | A total of 3 injections of triamcinolone acetonide 2.5 mg/mL were given at an interval of 3 weeks to the patients. The results showed that, after follow-up, more than 50% hair regrowth was observed in 27 (67.5%) patients with intralesional steroids at the end of the treatment. | [160] |
| Spironolactone (systemic) | Spironolactone | Spironolactone at a dose of 200 mg daily was observed to reduce loss of hair by 50–62.9% in a case study of 4 patients. It has also been observed that there is an increase in the total number of anagen hairs. | [161] |
| Dutasteride (oral) | Dutasteride | Dutasteride was seen to increase the mean hair counts by 12.2/cm2 compared with 4.7/cm2 in the placebo group in the treatment of MPHL. | [162] |
| Valproic acid | Valproic acid | Forty male patients with moderate AGA were involved in a study. They received treatment with either VPA (sodium valproate, 8.3%) or placebo spray for 24 weeks. Twenty-seven out of forty patients (n = 15, VPA group; n = 12, placebo group) completed the whole protocol with good compliance. The mean change in total hair count was seen to be significantly increased in the VPA group compared with the placebo group. | [163] |
| Flutamide (topical) | Flutamide | According to a study, flutamide at a dose of 250 mg daily showed an improvement in the growth of hair compared with 5 mg of finasteride daily and 50 mg of cyproterone acetate daily. Flutamide showed a reduction of 21% in Ludwig scores compared with the other two drugs. | [164] |
| Saw Palmetto (topical) | Contains fatty acids (85–90%), carotenoids, lipases, tannins, and sugars, as well as beta-sitosterol, anthranilic acid, capric acid, caproic acid, caprylic acid, carotene, ferulic acid, linoleic acid, myristic acid, lauric acid, oleic acid, palmitic acid, 1-monolaurin, and 1-mono-myristin | In a 2012 study involving 100 males taking 320 milligrams (mg) of saw palmetto each day over 2 years, it was seen that 38% of those who took saw palmetto had improvements in their hair loss. | [165] |
| Ketoconazole (topical) | Ketoconazole | 2% Ketoconazole shampoo on MPHL has been observed to increase hair density along with increasing the size and proportion of anagen follicles. | [166] |
| Green tea (topical) | Antioxidants such as polyphenols and flavonoids that contain catechins and their derivatives, epicatechin (EC), epigallocatechin gallate (EGCG), epigallo catechins, epicatechin gallate, linoleic and linolenic acids, and vitamins | [167] | |
| Pumpkin seed | Polyunsaturated fatty acids of 80% palmitic acid, myristic acid, stearic acid, oleic acid, and linoleic acid, as well as vitamin E, α-tocopherols, γ-tocopherols, carotenoid, phytoestrogens, and phytosterols | [168] | |
| Rosemary oil (topical) | Contains esters (2.6%) largely as borneol, cineoles, and several terpenes, chiefly a-pinene, camphene, 1% and 2% volatile oil containing 0.8% and 6% esters, and 8% and 20% alcohols, respectively | [169] | |
| Grapeseed oil (topical) | Anthocyanins, flavan-3-ols (example: catechins), vitamin-E (α-tocopherol), petiole, linoleic acid, flavonoids (resveratrol, quercetin and catechin, and polyphenols (flavonoids, phenolic acids, phenolic alcohols, stilbenes, and lignans), and trimer gallate, unsaturated fatty acids, and phytosterols | [170] | |
| Licorice (topical) | Glycyrrhetinic acids rich in flavonoids such as liquiritin, isoliquiritin, neoisoliquiritin, liquiritigenin, glycerin, glyzaglabrin, and licoisofavines. | [171] | |
| Tinfal Plus Serum (topical) | Minoxidil 5% + Aminexil 1.5% | [172] | |
| Keraglo Eva (topical) | Biotin (10 Mg), folic acid (300 Mcg), selenium (40 Mcg) (173) | [173] | |
| Organ model studies | |||
| Human hair follicle (HF) unit | The anagen–catagen transition in organ-cultured, severed human scalp HFs differs from the transformation in vivo in a number of ways, including the absence of the bulge, isthmus area, sebaceous gland, and tissue interactions with the dermis and subcutis. As a result, it has long been unclear to what degree in vivo morphological criteria may be transferred to HF organ culture settings. This article ma an effort to address these technical issues. | [174] | |
| Rat vibrissae follicle unit | The histopathology of the proximal follicle bulb revealed that vibrissa follicles extracted from 12-day-old rats were in the anagen stage of their hair development cycle. The extended dermal papilla (DP) was located inside the follicular bulb and was surrounded by highly basophilic epithelial matrix cells, which displayed typical patterns of lineage-restricted differentiation, giving rise to the keratinized hair fiber and inner root sheath. | [175] | |
| Full-thickness human scalp skin | |||
| Animal Models | |||
| Anagen phase induction models | The anagen–catagen transition in severed human scalp organ culture HFs differs from this metamorphosis in vivo in a number of ways, including the absence of the bulge, isthmus area, sebaceous gland, and tissue interactions with the dermis and subcutis. As a result, it has long been unclear to what degree in vivo morphological criteria may be transferred to HF organ culture settings. This article made an effort to address these technical issues. | [176] | |
| Androgen effect modulation models | |||
| Mesocricetus auratus (golden hamster) | This model was used to assess macroscopic and microscopic evaluation (hair diameter analysis) as an animal model for hair regrowth. | [177] | |
| C3 H mouse model | Even though the increase in density of hair of the animal and the wave pattern hair cycle development provided drawbacks, they were the most extensively reported for hair growth promotion. After just two weeks of treatment, laser therapy administered to C3 H mice for 20 s daily, three times per week, caused a substantially longer development phase, with the majority of the follicles from the examined region in the anagen hair growth phase. | [178] | |
| Progenitor cell population in mice | These cells are comparable to human cells. These mature cells were tested on immunodeficient mice animal models, and the findings demonstrated the creation of new hair follicles and enhanced hair regrowth. | [179] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.; Mohapatra, H.; Arora, K.; Babbar, R.; Arora, R.; Arora, P.; Kumar, P.; Algın Yapar, E.; Rani, K.; Meenu, M.; et al. Bioactive Compound-Loaded Nanocarriers for Hair Growth Promotion: Current Status and Future Perspectives. Plants 2023, 12, 3739. https://doi.org/10.3390/plants12213739
Sharma A, Mohapatra H, Arora K, Babbar R, Arora R, Arora P, Kumar P, Algın Yapar E, Rani K, Meenu M, et al. Bioactive Compound-Loaded Nanocarriers for Hair Growth Promotion: Current Status and Future Perspectives. Plants. 2023; 12(21):3739. https://doi.org/10.3390/plants12213739
Chicago/Turabian StyleSharma, Arvind, Harapriya Mohapatra, Kanika Arora, Ritchu Babbar, Rashmi Arora, Poonam Arora, Pradeep Kumar, Evren Algın Yapar, Kailash Rani, Maninder Meenu, and et al. 2023. "Bioactive Compound-Loaded Nanocarriers for Hair Growth Promotion: Current Status and Future Perspectives" Plants 12, no. 21: 3739. https://doi.org/10.3390/plants12213739