Integrated Assessment of Pb(II) and Cu(II) Metal Ion Phytotoxicity on Medicago sativa L., Triticum aestivum L., and Zea mays L. Plants: Insights into Germination Inhibition, Seedling Development, and Ecosystem Health
Abstract
:1. Introduction
1.1. Understanding Heavy Metals in the Environment: Justifying the Need for Investigating Their Impact
1.2. Impact of Heavy Metals on Cereal Crop Growth and Quality
1.3. Heavy Metal Impacts on Seed Germination for Cereals and Plants Used as Feed Sources for Livestock
2. Results and Discussion
2.1. Effects of Lead (Pb(II)) and Copper (Cu(II)) Metal Ions on Seed Germination and Seedling Development of Alfalfa (Medicago sativa L.) in a Soilless Growing Environment
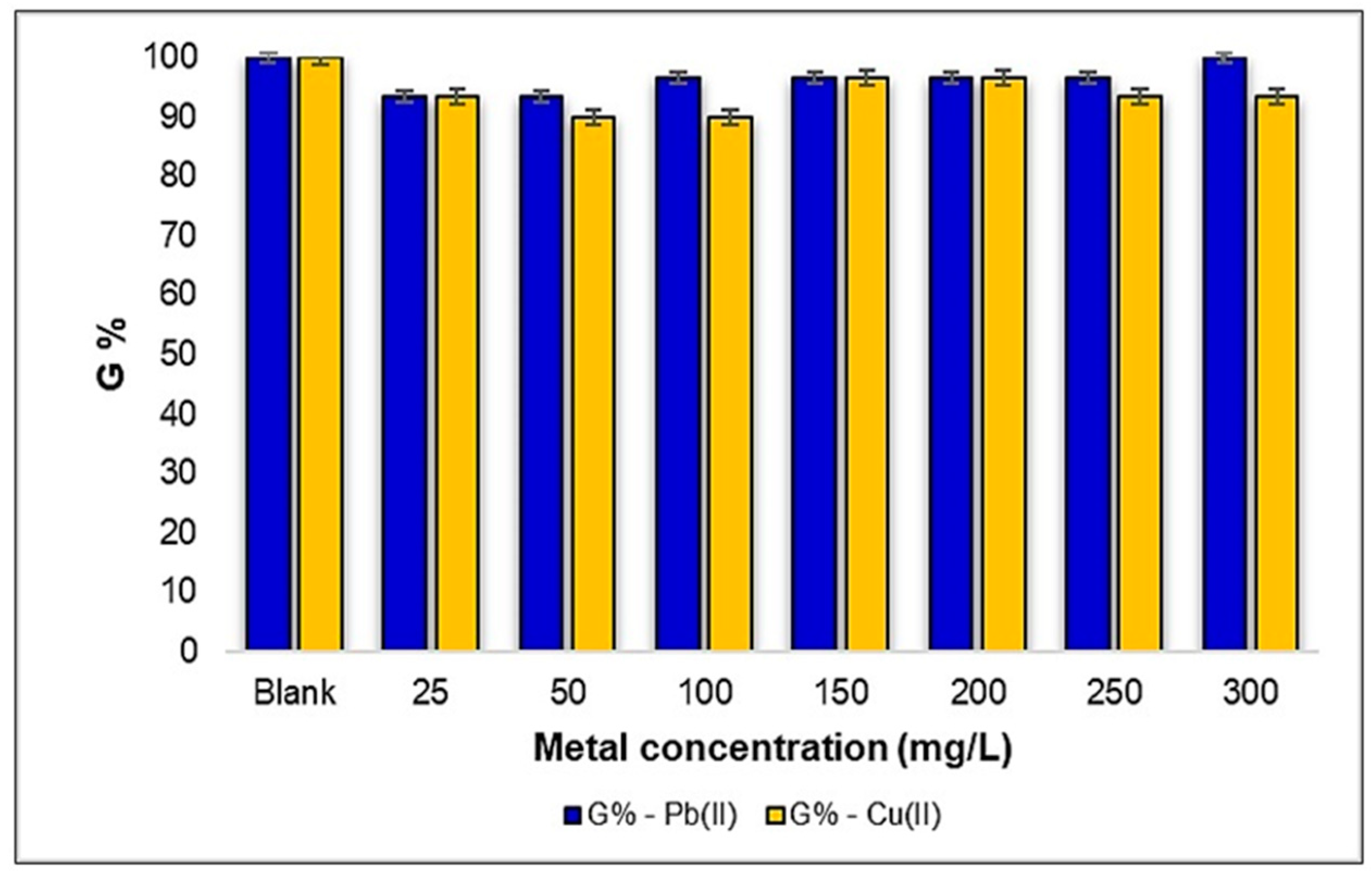
2.1.1. Influence of Pb(II) and Cu(II) Metal Ions on Medicago sativa L. Seeds Degree of Germination (G%)
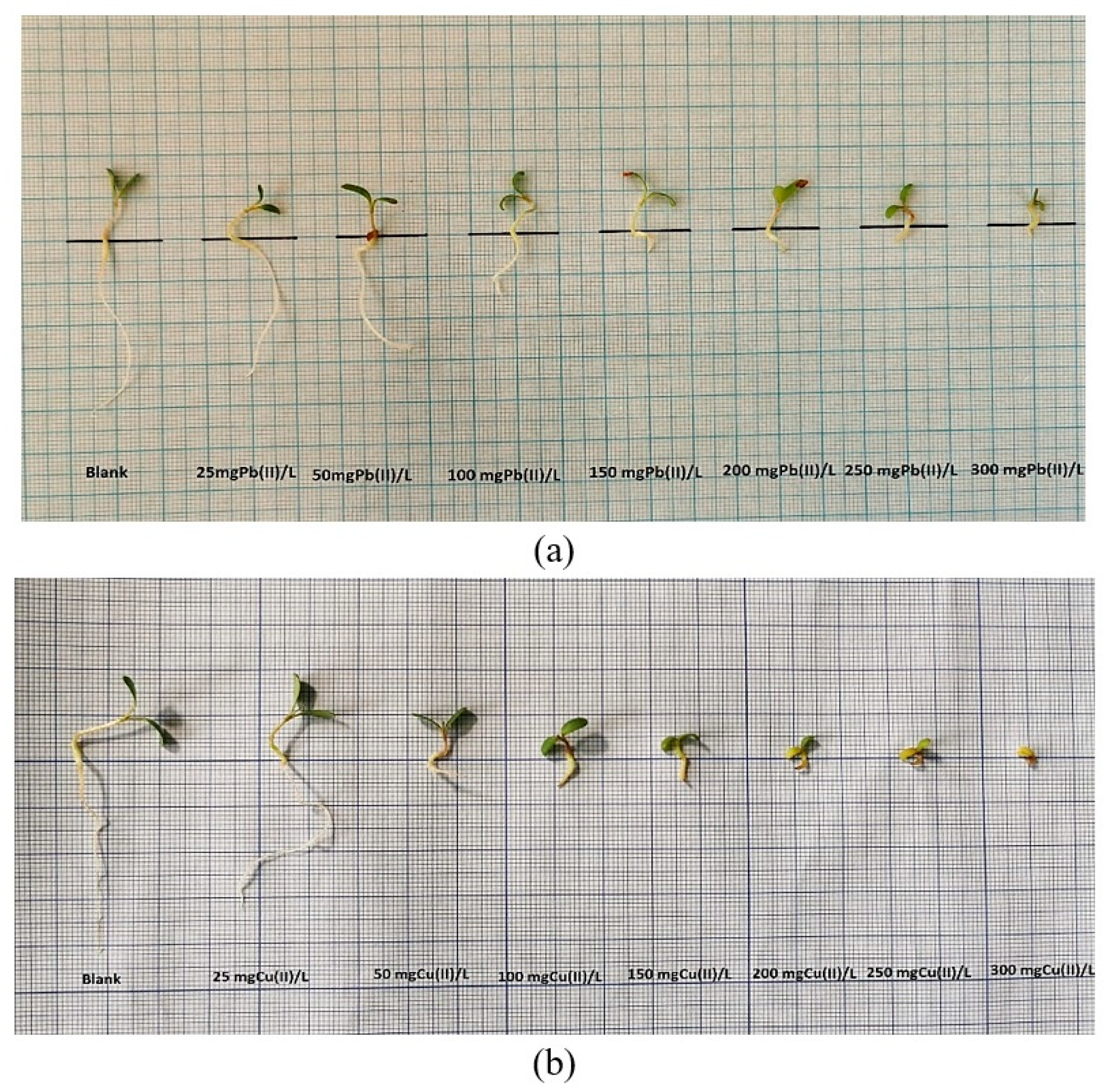
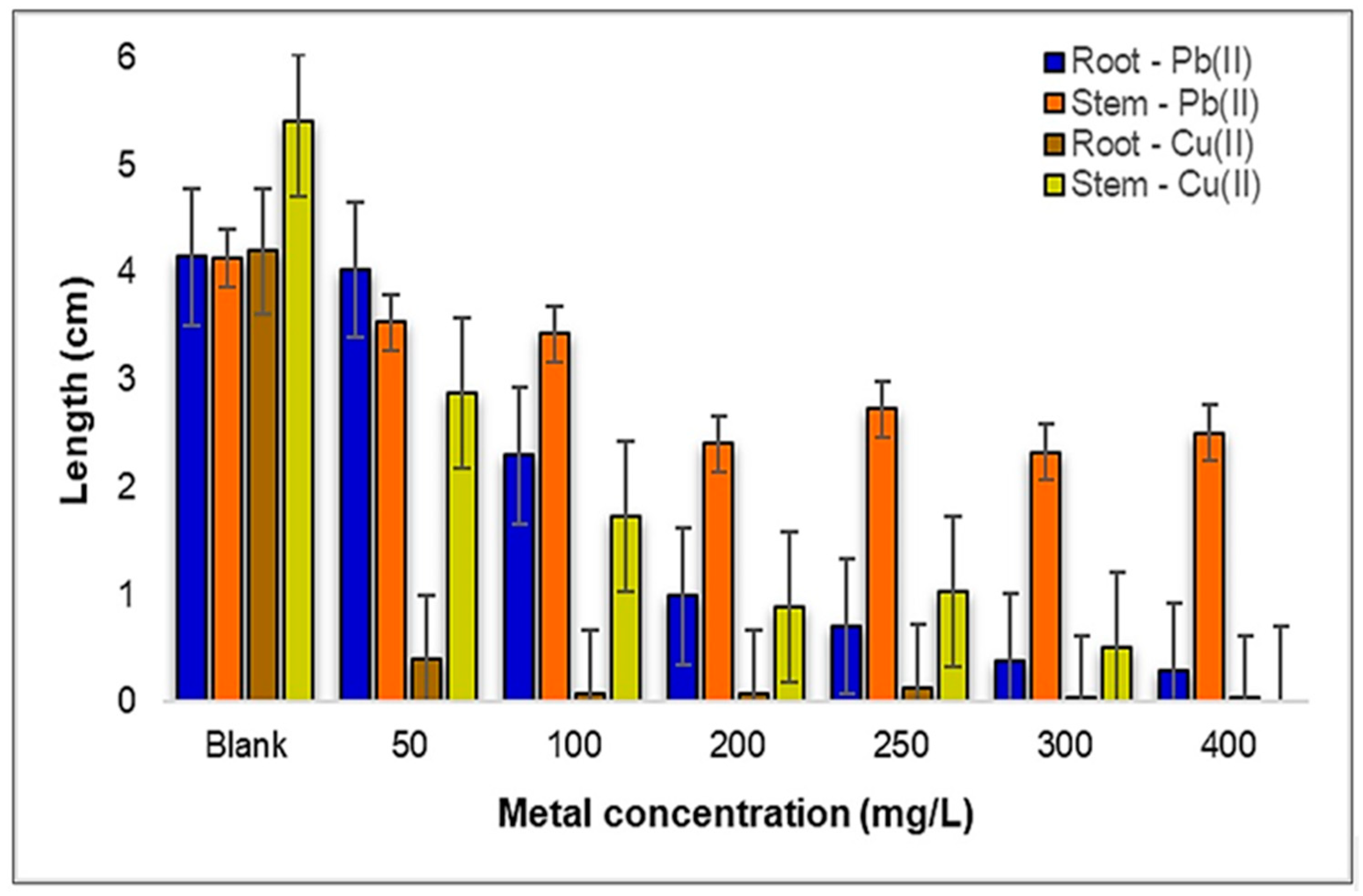
2.1.2. Influence of Pb(II) and Cu(II) on the Growth and Development of Medicago sativa L.
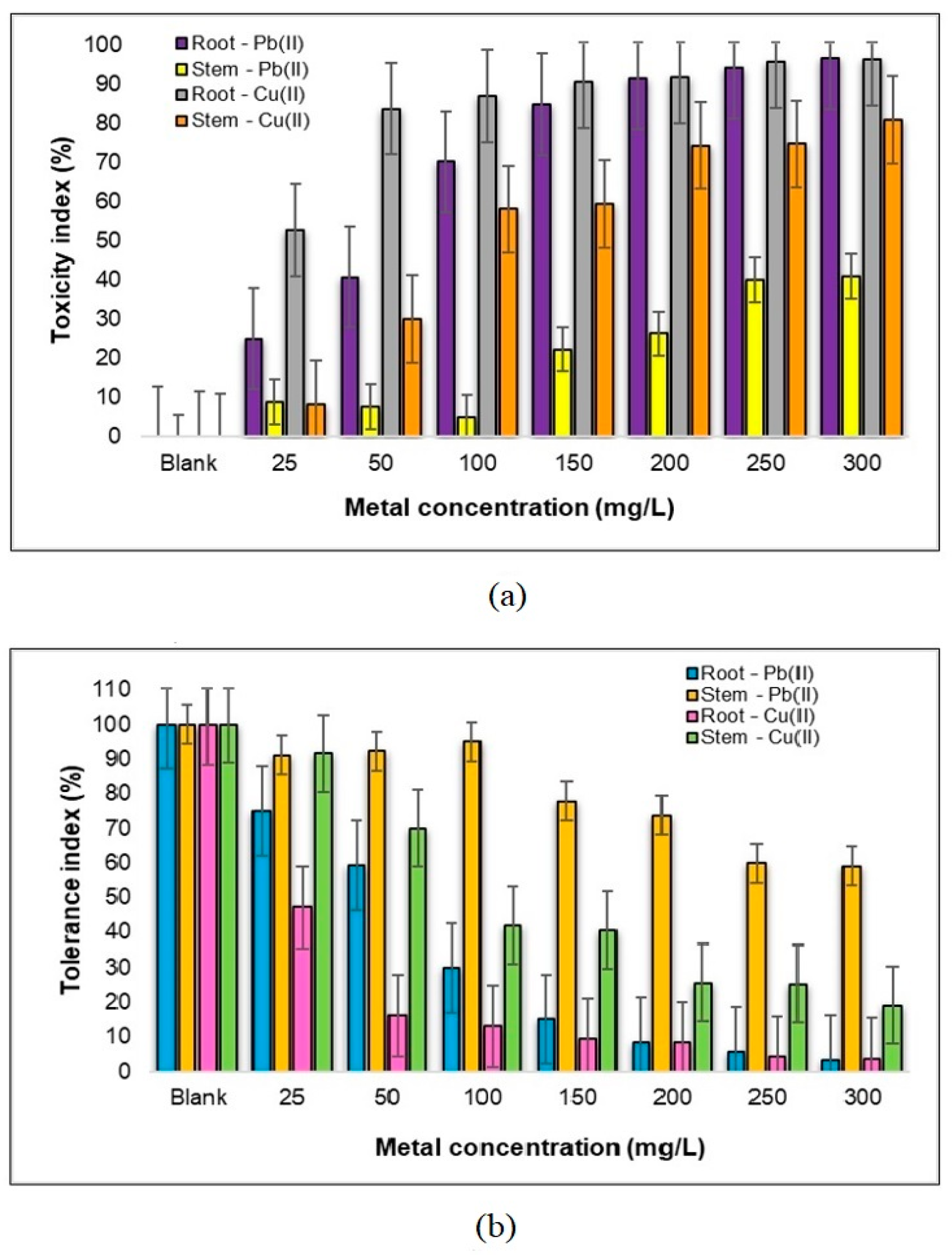
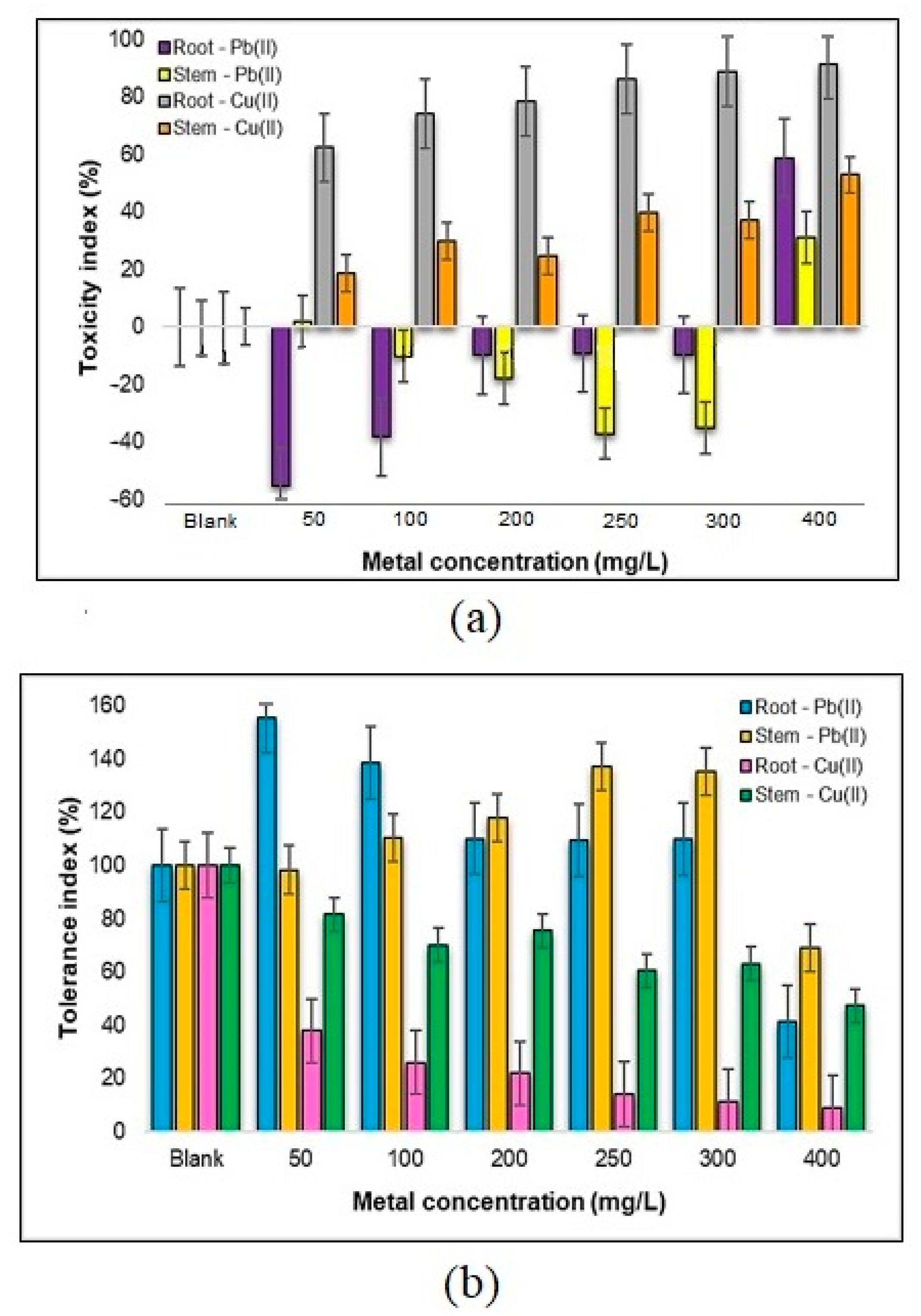
2.1.3. Evaluating Toxicity Levels and Metal Tolerance in the Roots and Stems of Medicago sativa L. Towards Pb(II) and Cu(II)
2.2. The Effects of Pb(II) and Cu(II) Metal Ions on the Growth and Development of Wheat (Triticum aestivum L.) in a Soilless Growing Environment
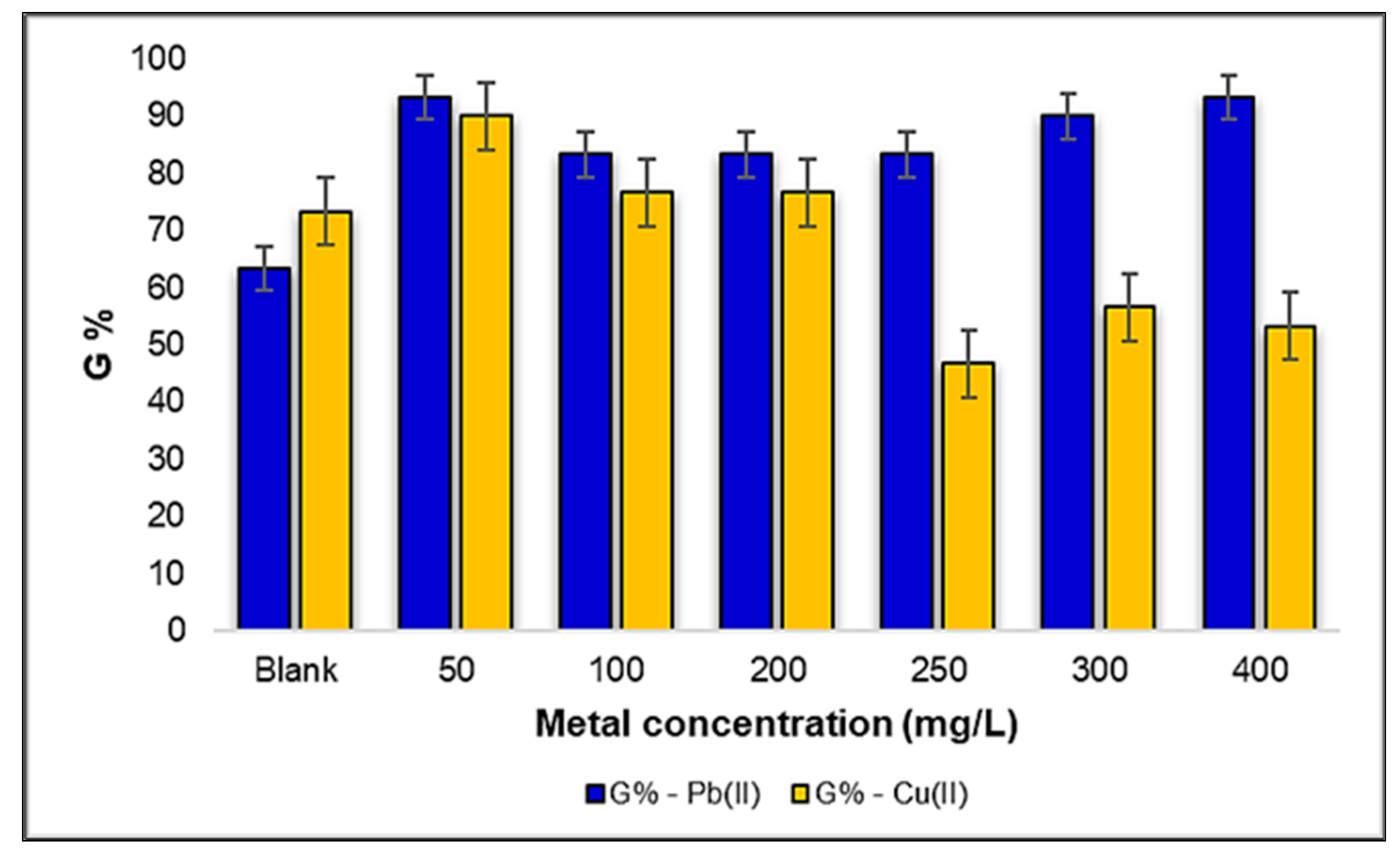
2.2.1. Influence of Pb(II) and Cu(II) Metal Ions on the Germination Percentage (G%) of Triticum aestivum L. Seeds
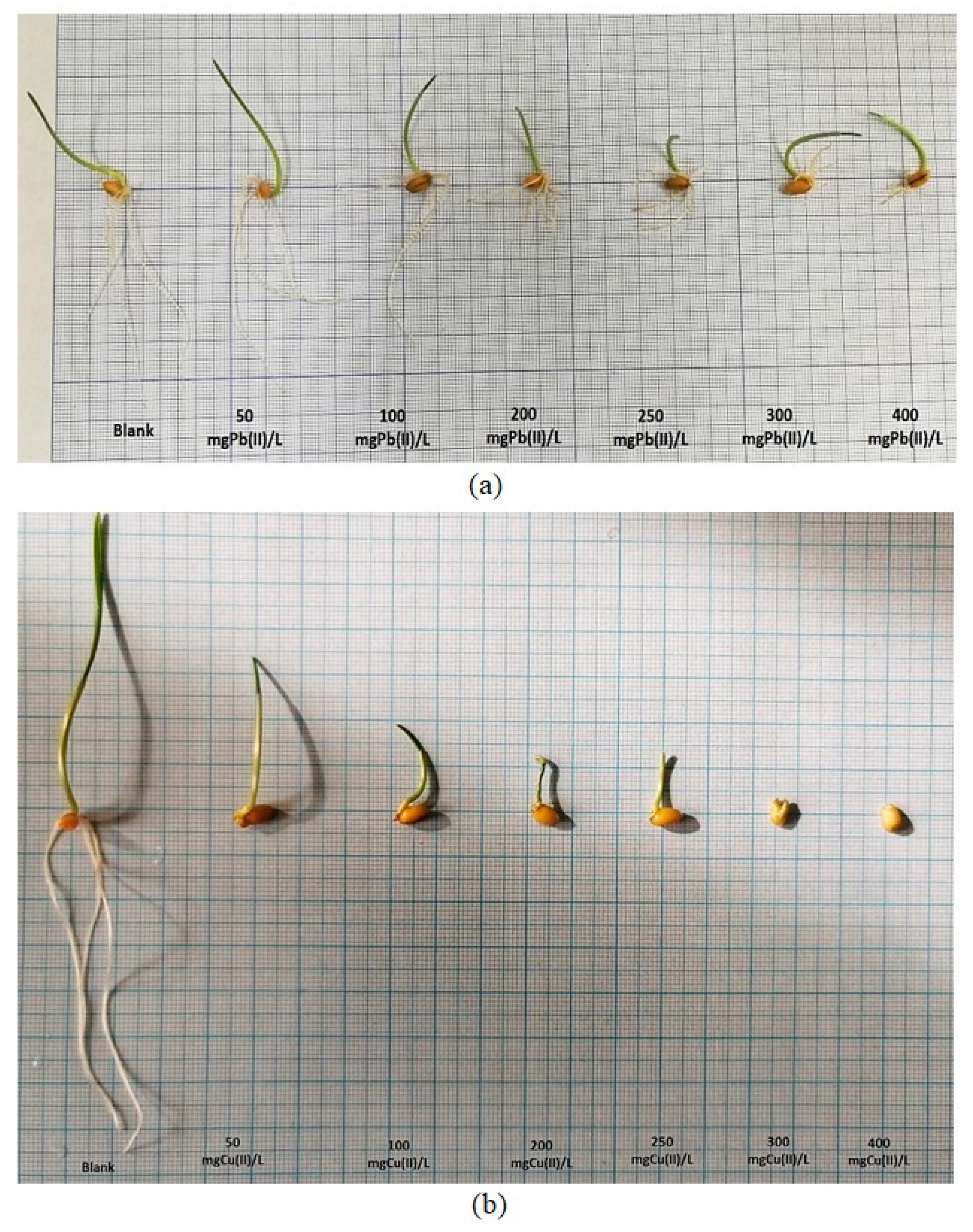
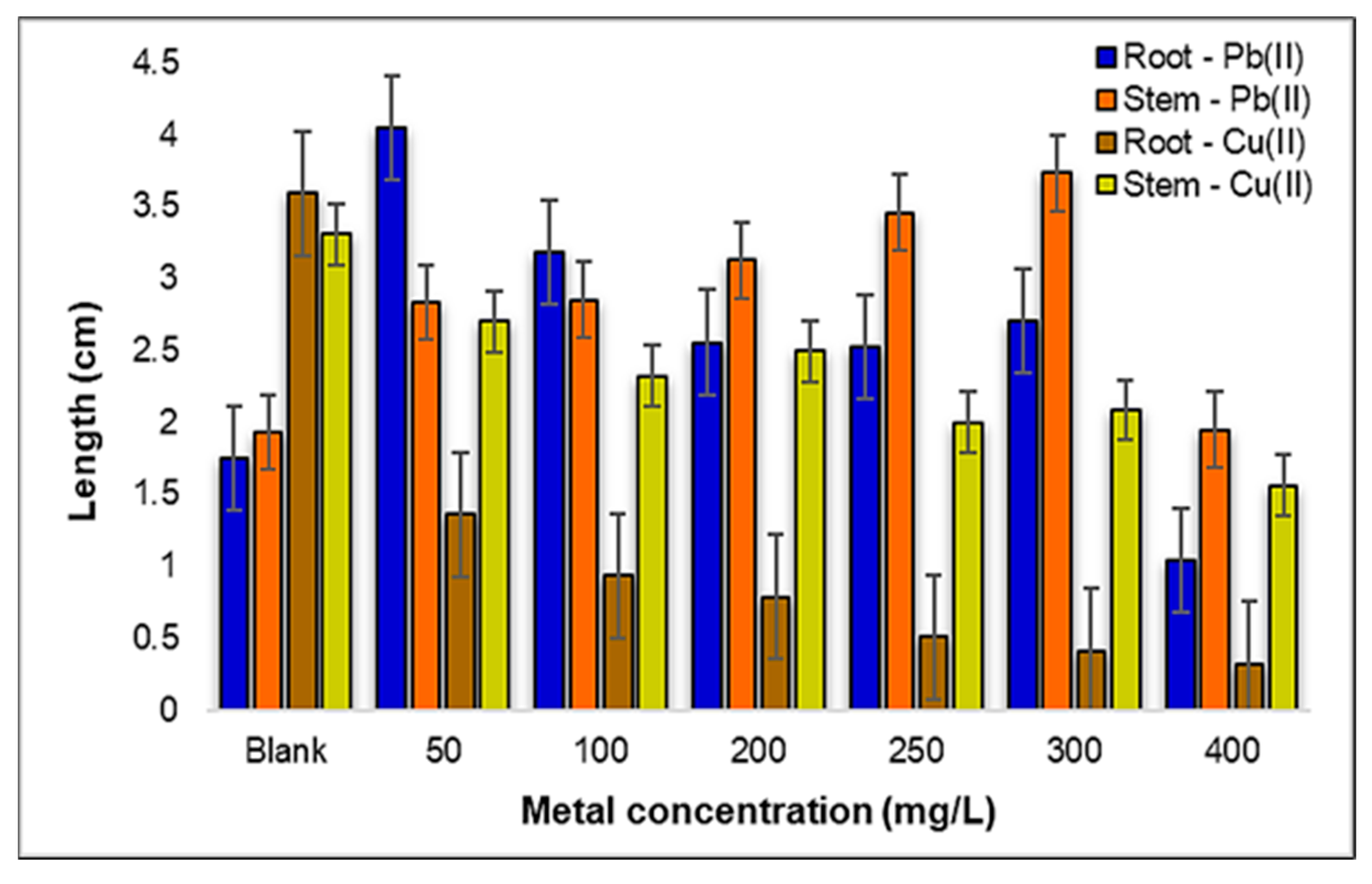
2.2.2. Influence of Pb(II) and Cu(II) Metal Ions on the Growth and Development of Triticum aestivum L.
2.2.3. Toxicity Levels and Tolerance of the Root and Stem of Triticum aestivum L. to Pb(II) and Cu(II) Metals
2.2.4. Influence of Pb(II) and Cu(II) on the Total Biomass of Triticum aestivum L.
2.3. Effects of Pb(II) and Cu(II) on the Development of Corn (Zea mays L.) in a Soilless Growing Environment
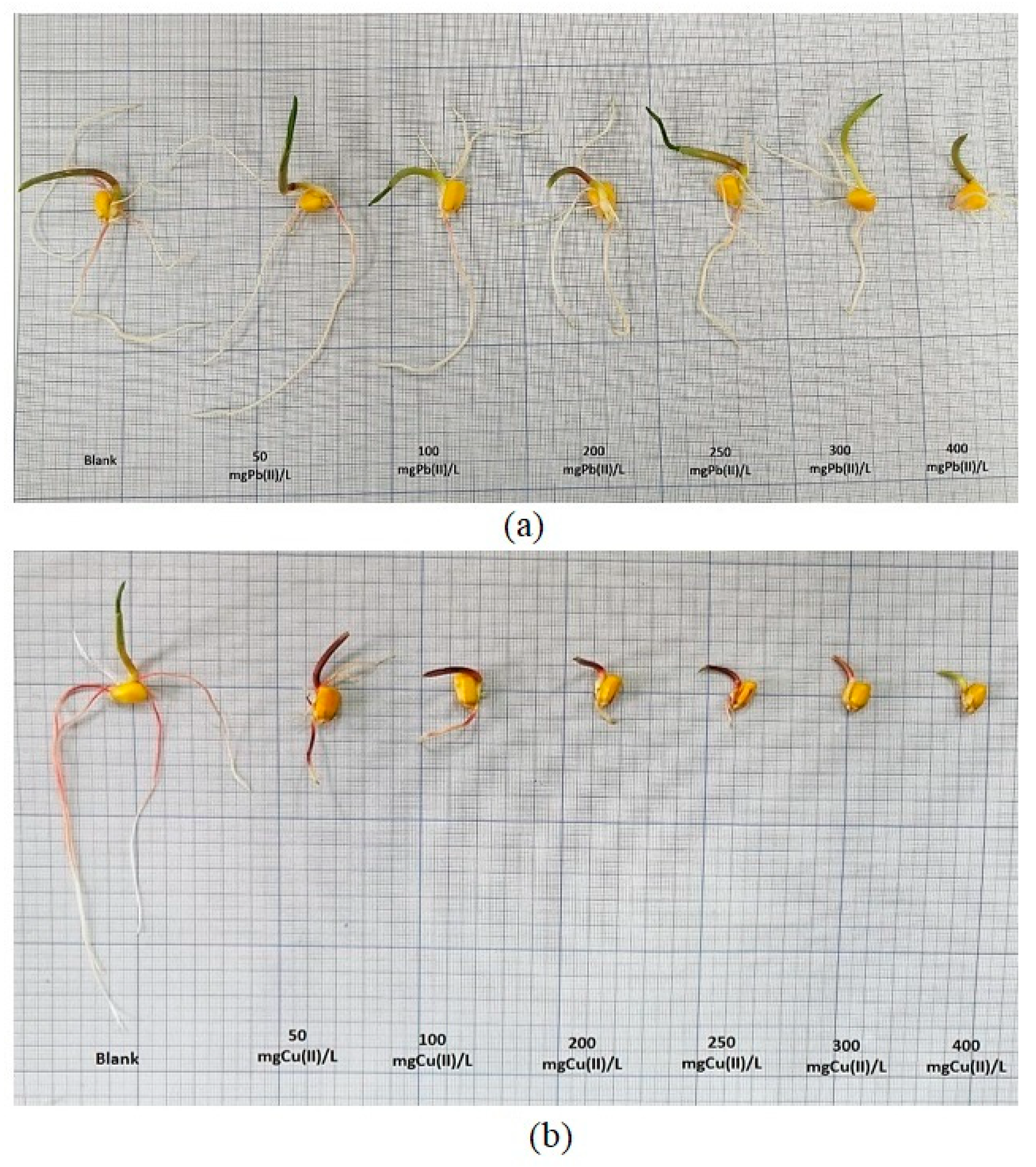
2.3.1. Influence of Pb(II) and Cu(II) on the Degree of Germination of Zea mays L. Seeds (G%)
2.3.2. Influence of Pb(II) and Cu(II) on the Growth and Development of Zea mays L.
2.3.3. The Degree of Toxicity and the Tolerance of the Root and Stem of Zea mays L. to the Pb(II) and Cu(II)
2.3.4. Influence of Pb(II) and Cu(II) on the Total Biomass of Zea mays L.
2.4. Significance of Results
3. Materials and Methods
3.1. Framework for the Application of Phytotoxicity Studies
- Identifying and ranking the phytotoxicity potential of samples;
- Defining the actual degree of phytotoxicity;
- Pinpointing the chemical components within a complex waste mixture contributing to phytotoxicity (referred to as phytotoxicity evaluation or TIE—phytotoxicity identification evaluation).
3.2. Indicators of Heavy Metals Phytotoxicity in Plants
3.2.1. The Degree of Germination
- G is the degree of germination (%);
- Sgerm is the number of germinated seeds in the sample to be analyzed (the sample containing heavy metal solutions of different concentrations);
- Mgerm is the number of germinated seeds in the control sample.
3.2.2. Relative Growth of Plant Root and Stem Lengths
3.2.3. Growth Inhibition Index of Plant Components (El%)
3.2.4. Tolerance Index of Plant Components (Er%)
3.3. Biological Material
3.4. Working Method
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metals toxicity and the environment. Mol. Clin. Environ. Toxicol. 2014, 101, 133–164. [Google Scholar]
- Gavrilescu, M. Water, soil, and plants interactions in a threatened environment. Water 2021, 13, 2746. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.-H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [PubMed]
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy metal contamination in agricultural soil: Environmental pollutants affecting crop health. Agronomy 2023, 13, 1521. [Google Scholar] [CrossRef]
- Sobariu, D.L.; Fertu, D.I.; Diaconu, M.; Pavel, L.V.; Hlihor, R.-M.; Dragoi, E.N.; Curteanu, S.; Lenz, M.; Corvini, P.; Gavrilescu, M. Rhizobacteria and plant symbiosis in heavy metal uptake and its implications for soil bioremediation. New Biotechnol. 2017, 39, 125–134. [Google Scholar]
- AbdElgawad, H.; Zinta, G.; Hamed, B.A.; Selim, S.; Beemster, G.; Hozzein, W.N.; Wadaan, M.A.M.; Asard, H.; Abuelsoud, W. Maize roots and shoots show distinct profiles of oxidative stress and antioxidant defense under heavy metal toxicity. Environ. Pollut. 2020, 258, 113705. [Google Scholar] [CrossRef]
- Schiopu, M.; Gavrilescu, M. Municipal solid waste landfilling and treatment of resulting liquid effluents. Environ. Eng. Manag. J. 2010, 9, 993–1019. [Google Scholar]
- Vasilachi, I.-C.; Asiminicesei, D.M.; Fertu, D.I.; Gavrilescu, M. Occurrence and fate of emerging pollutants in water environment and options for their removal. Water 2021, 13, 181. [Google Scholar] [CrossRef]
- Vouk, D.; Nakic, D.; Bubalo, A.; Bolanca, T. Environmental aspects in selecting optimum variant of sewage sludge management. Environ. Eng. Manag. J. 2022, 21, 443–456. [Google Scholar]
- Masindi, V.; Muedi, K.L. Environmental Contamination by Heavy Metals; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Pintilie, S.; Brinza, L.; Betianu, C.; Pavel, L.V.; Ungureanu, F.; Gavrilescu, M. Modelling and simulation of heavy metals transport in water and sediments. Environ. Eng. Manag. J. 2007, 6, 153–161. [Google Scholar]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Tehrani, G.F.; Rubinos, D.A.; Kelm, U.; Ghadimi, S. Environmental and human health risks of potentially harmful elements in mining-impacted soils: A case study of the Angouran Zn–Pb Mine, Iran. J. Environ. Manag. 2023, 334, 117470. [Google Scholar] [CrossRef]
- Vasseghian, Y.; Almomani, F.; Dragoi, E.-N. Health risk assessment induced by trace toxic metals in tap drinking water: Condorcet principle development. Chemosphere 2022, 286, 131821. [Google Scholar] [CrossRef] [PubMed]
- Pavel, V.L.; Diaconu, M.; Bulgariu, D.; Statescu, F.; Gavrilescu, M. Evaluation of heavy metals toxicity on two microbial strains isolated from soil: Azotobacter sp. and Pichia sp. Environ. Eng. Manag. J. 2012, 11, 165–168. [Google Scholar]
- Baby, R.; Saifullah, B.; Hussein, M.Z. Carbon nanomaterials for the treatment of heavy metal-contaminated water and environmental remediation. Nanoscale Res. Lett. 2019, 14, 341. [Google Scholar] [CrossRef]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M.R. Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar]
- Diaconu, M.; Pavel, V.L.; Hlihor, R.M.; Rosca, M.; Fertu, D.I.; Lenz, M.; Corvini, P.X.; Gavrilescu, M. Characterization of heavy metal toxicity in some plants and microorganisms—A preliminary approach for environmental bioremediation. New Biotechnol. 2020, 56, 130–139. [Google Scholar]
- Vareda, J.P.; Artur, A.J.M.; Durãesa, L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar]
- Nivetha, N.; Srivarshine, B.; Sowmya, B.; Rajendiran, M.; Saravanan, P.; Rajeshkannan, P.; Rajasimman, M.; Pham, T.H.T.; Shanmugam, V.K.; Dragoi, E.N.A. Comprehensive review on bio-stimulation and bio-enhancement towards remediation of heavy metals degeneration. Chemosphere 2023, 312, 137099. [Google Scholar] [CrossRef]
- Rehman, A.U.; Nazir, S.; Irshad, S.; Kamran, T.; Rehman, U.K.; Islam, R.U.; Wahab, Z. Toxicity of heavy metals in plants and animals and their uptake by magnetic iron oxide nanoparticles. J. Mol. Liq. 2021, 321, 114455. [Google Scholar] [CrossRef]
- Gavrilescu, M. Removal of heavy metals from the environment by biosorption. Eng. Life Sci. 2004, 4, 219–232. [Google Scholar] [CrossRef]
- Hlihor, R.M.; Figueiredo, H.; Tavares, T.; Gavrilescu, M. Biosorption potential of dead and living Arthrobacter viscosus biomass in the removal of Cr(VI): Batch and column studies. Process Saf. Environ. Prot. 2017, 108, 44–56. [Google Scholar] [CrossRef]
- Odobasic, A. Determination and Speciation of Trace Heavy Metals in Natural Water by DPASV. In Water Quality Monitoring and Assessment; Voudouris, K., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Cuypers, A.N.N.; Vangronsveld, J.; Clijsters, H. Peroxidases in roots and primary leaves of Phaseolus vulgaris copper and zinc phytotoxicity: A comparison. J. Plant Physiol. 2002, 159, 869–876. [Google Scholar] [CrossRef]
- Achim, C.; Bercu, M.; Bratu, A.; Dumitraș, D. The Impact of Heavy Metals on Plants (Impactul Metalelor Grele Asupra Plantelor). Stiință și Tehnică. 2016. Available online: https://stiintasitehnica.com/impactul-metalelor-grele-asupra-plantelor/ (accessed on 12 August 2023).
- World Bank. World—Cereal Production (Metric Tons). 2021. Available online: https://tradingeconomics.com/world/cereal-production-metric-tons-wb-data.html (accessed on 15 August 2023).
- OECD-FAO. Agricultural Outlook 2022–2031. 2022. Available online: https://www.fao.org/3/CC0308EN/Cereals.pdf (accessed on 12 August 2023).
- FAO. Cereal Supply and Demand Brief. 2023. Available online: https://www.fao.org/worldfoodsituation/csdb/en/ (accessed on 18 July 2023).
- Statista. Cereals—Worldwide. 2023. Available online: https://fr.statista.com/outlook/io/agriculture/cereals/worldwide (accessed on 11 September 2023).
- Talmhaiocta, A.R.; Mara, B.A. Annual Review and Outlook for Agriculture, Food and the Marine; Department’s Annual Review and Outlook for Agriculture, Food and the Marine; Economics and Planning Division: Dublin, Ireland, 2022. [Google Scholar]
- OECD/FAO. OECD-FAO Agricultural Outlook 2018–2027. 2018. Available online: https://www.agri-outlook.org/commodities/Agricultural-Outlook-2018-Cereals.pdf (accessed on 25 August 2023).
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global maize production, consumption and trade: Trends and R&D implications. Food Sec. 2022, 14, 1295–1319. [Google Scholar]
- FAO. Food Outlook. Biannual Report on Global Food Markets. 2023. Available online: https://www.fao.org/3/cc3020en/cc3020en.pdf (accessed on 11 September 2023).
- OECD-FAO. OECD—FAO Agricultural Outlook 2013–2022. 2013. Available online: https://www.oecd.org/berlin/OECD-FAO%20Highlights_FINAL_with_Covers%20(3).pdf (accessed on 12 September 2023).
- EUROSTAT. Agricultural Production—Crops. 2022. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Agricultural_production_-_crops (accessed on 28 August 2023).
- Zakaria, Z.; Zulkafflee, N.S.; Redzuan, N.A.M.; Selamat, J.; Ismail, M.R.; Praveena, S.M.; Tóth, G.; Razis, A.F.A. Understanding potential heavy metal contamination, absorption, translocation and accumulation in rice and human health risks. Plants 2021, 10, 1070. [Google Scholar] [CrossRef]
- Afonne, O.J.; Ifediba, E.C. Heavy metals risks in plant foods—Need to step up precautionary measures. Curr. Opin. Toxicol. 2020, 22, 1–6. [Google Scholar]
- Proshad, R.; Idris, A.M. Evaluation of heavy metals contamination in cereals, vegetables and fruits with probabilistic health hazard in a highly polluted megacity. Environ. Sci. Pollut. Res. Int. 2023, 30, 79525–79550. [Google Scholar]
- Witkowska, D.; Słowik, J.; Chilicka, K. Heavy metals and human health: Possible exposure pathways and the competition for protein binding sites. Molecules 2021, 26, 6060. [Google Scholar] [CrossRef]
- Rubio-Armendáriz, C.; Paz, S.; Gutiérrez, A.J.; González-Weller, D.; Revert, C.; Hardisson, A. Human exposure to toxic metals (Al, Cd, Cr, Ni, Pb, Sr) from the consumption of cereals in Canary Islands. Foods 2021, 10, 1158. [Google Scholar] [CrossRef]
- Sharma, A.; Nagpal, A.K. Contamination of vegetables with heavy metals across the globe: Hampering food security goal. J. Food Sci. Technol. 2020, 57, 391–403. [Google Scholar] [CrossRef]
- Hejna, M.; Gottardo, D.; Baldi, A.; Dell’Orto, V.; Cheli, F.; Zaninelli, M.; Rossi, L. Review: Nutritional ecology of heavy metals. Animal 2018, 12, 2156–2170. [Google Scholar] [CrossRef] [PubMed]
- Rezaeian, M.; Moghadam, M.T.; Kiaei, M.M.; Zadeh, H.M. The effect of heavy metals on the nutritional value of Alfalfa: Comparison of nutrients and heavy metals of Alfalfa (Medicago sativa) in industrial and non-industrial areas. Toxicol. Res. 2020, 36, 183–193. [Google Scholar] [CrossRef]
- Sotiriou, V.; Michas, G.; Xiong, L.; Drosos, M.; Vlachostergios, D.; Papadaki, M.; Mihalakakou, G.; Kargiotidou, A.; Tziouvalekas, M.; Salachas, G.; et al. Effects of heavy metal ions on white clover (Trifolium repens L.) growth in Cd, Pb and Zn contaminated soils using zeolite. J. Soil Sci. Environ. 2023, 2, 4. [Google Scholar] [CrossRef]
- Reed, R.C.; Bradford, K.J.; Khanday, I. Seed germination and vigor: Ensuring crop sustainability in a changing climate. Heredity 2022, 128, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Sanjosé, I.; Navarro-Roldán, F.; Infante-Izquierdo, M.D.; Martínez-Sagarra, G.; Devesa, J.A.; Polo, A.; Ramírez-Acosta, S.; Sánchez-Gullón, E.; Jiménez-Nieva, F.J.; Muñoz-Rodríguez, A.F. Accumulation and effect of heavy metals on the germination and growth of Salsola vermiculata L. seedlings. Diversity 2021, 13, 539. [Google Scholar] [CrossRef]
- Kranner, I.; Colville, L. Metals and seeds: Biochemical and molecular implications and their significance for seed germination. Environ. Exp. Bot. 2011, 23, 93–105. [Google Scholar] [CrossRef]
- Mansoor, S.; Ali, A.; Kour, N.; Bornhorst, J.; AlHarbi, K.; Rinklebe, J.; Abd El Moneim, D.; Ahmad, P.; Chung, Y.S. Heavy metal induced oxidative stress mitigation and ROS scavenging in plants. Plants 2023, 12, 3003. [Google Scholar] [CrossRef]
- Dutta, S.; Mitra, M.; Agarwal, P.; Mahapatra, K.; De, S.; Sett, U.; Roy, S. Oxidative and genotoxic damages in plants in response to heavy metal stress and maintenance of genome stability. Plant Signal. Behav. 2018, 13, e1460048. [Google Scholar] [CrossRef]
- Sethy, S.K.; Ghosh, S. Effect of heavy metals on germination of seeds. J. Nat. Sci. Biol. Med. 2013, 4, 272–275. [Google Scholar]
- Wiszniewska, A. Priming strategies for benefiting plant performance under toxic trace metal exposure. Plants 2021, 10, 623. [Google Scholar] [CrossRef]
- Miller, E.C.; Perron, G.G.; Collins, C.D. Plant-driven changes in soil microbial communities influence seed germination through negative feedbacks. Ecol. Evol. 2019, 9, 9298–9311. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Zhang, H. Niche-processes induced differences in plant growth, carbon balance, stress resistance, and regeneration affect community assembly over succession. PLoS ONE 2020, 15, e0229443. [Google Scholar] [CrossRef] [PubMed]
- Moise, J.A.; Han, S.; Gudynaite-Savitch, L.; Johnson, D.A.; Miki, B.L.A. Seed coats: Structure, development, composition, and biotechnology. In Vitro Cell. Dev. Biol. Plant 2005, 41, 620–644. [Google Scholar] [CrossRef]
- Seregin, I.V.; Kozhevnikova, A.D. Distribution of cadmium, lead, nickel, and strontium in imbibing maize caryopses. Russ. J. Plant Physiol. 2005, 52, 565–569. [Google Scholar] [CrossRef]
- Diaz-Baez, M.C.; Dutka, B.J. Frameworks for the application of toxicity data. In Environmental Toxicity Testing; Thompson, C.K., Wadhia, K., Loibner, A.P., Eds.; CRC Press-Blackwell Publishing: Boca Raton, FL, USA, 2005; pp. 94–130. [Google Scholar]
- Ranal, M.A.; Santana, D.G. How and why to measure the germination process? Rev. Bras. Botânica 2006, 29, 1–11. [Google Scholar] [CrossRef]
- Herranz, J.M.; Ferrandis, P.; Martínez-Sánchez, J.J. Influence of heat on seed germination of seven Mediterranean Leguminosae species. Plant Ecol. 1998, 136, 95–103. [Google Scholar] [CrossRef]
- Kramer-Walter, K.R.; Bellingham, P.J.; Millar, T.R.; Smissen, R.D.; Richardson, S.J.; Laughlin, D.C. Root traits are multidimensional: Specific root length is independent from root tissue density and the plant economic spectrum. J. Ecol. 2016, 104, 1299–1310. [Google Scholar] [CrossRef]
- Sampietro, D.A.; Vattuone, M.A.; Isla, M.I. Plant growth inhibitors isolated from sugarcane (Saccharum officinarum) straw. J. Plant Physiol. 2006, 163, 837–846. [Google Scholar] [CrossRef]
- Nadgórska-Socha, A.; Kandziora-Ciupa, M.; Ciepał, R.; Barczyk, G. Robinia pseudoacacia and Melandrium album in trace elements biomonitoring and air pollution tolerance index study. Int. J. Environ. Sci. Technol. 2016, 13, 1741–1752. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Phytoremediation potential of maize (Zea mays L.). A review. Afr. J. Agric. 2010, 6, 275–287. [Google Scholar]







| Plant Species | Zea mays L., Triticum aestivum L., Medicago sativa L. |
| Incubation temperature | 24 ± 2 °C. |
| Photo-periodicity | 12 h of light/12 h of darkness. |
| Type of test vessel | Petri dishes with a diameter of 10 cm. |
| Number of seeds/plate | 10. |
| The number of replicates | 3 replicates for each concentration to be tested. |
| Duration of the test | 5–7 days. |
| Compounds containing Pb(II) and Cu(II) used to prepare aqueous solutions | PbCl2 and CuCl2:
|
| Biological measurements | The degree of germination, the length of the stems, the length of the roots. |
| Reported parameters |
|
| Equipment used |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasilachi-Mitoseru, I.-C.; Stoleru, V.; Gavrilescu, M. Integrated Assessment of Pb(II) and Cu(II) Metal Ion Phytotoxicity on Medicago sativa L., Triticum aestivum L., and Zea mays L. Plants: Insights into Germination Inhibition, Seedling Development, and Ecosystem Health. Plants 2023, 12, 3754. https://doi.org/10.3390/plants12213754
Vasilachi-Mitoseru I-C, Stoleru V, Gavrilescu M. Integrated Assessment of Pb(II) and Cu(II) Metal Ion Phytotoxicity on Medicago sativa L., Triticum aestivum L., and Zea mays L. Plants: Insights into Germination Inhibition, Seedling Development, and Ecosystem Health. Plants. 2023; 12(21):3754. https://doi.org/10.3390/plants12213754
Chicago/Turabian StyleVasilachi-Mitoseru, Ionela-Catalina, Vasile Stoleru, and Maria Gavrilescu. 2023. "Integrated Assessment of Pb(II) and Cu(II) Metal Ion Phytotoxicity on Medicago sativa L., Triticum aestivum L., and Zea mays L. Plants: Insights into Germination Inhibition, Seedling Development, and Ecosystem Health" Plants 12, no. 21: 3754. https://doi.org/10.3390/plants12213754






