Disruption of the Contents of Endogenous Hormones Cause Pollen Development Obstruction and Abortion in Male-Sterile Hybrid Lily Populations
Abstract
:1. Introduction
2. Results
2.1. Observation of the Phenotype and Histological Microstructure of Lily Anthers
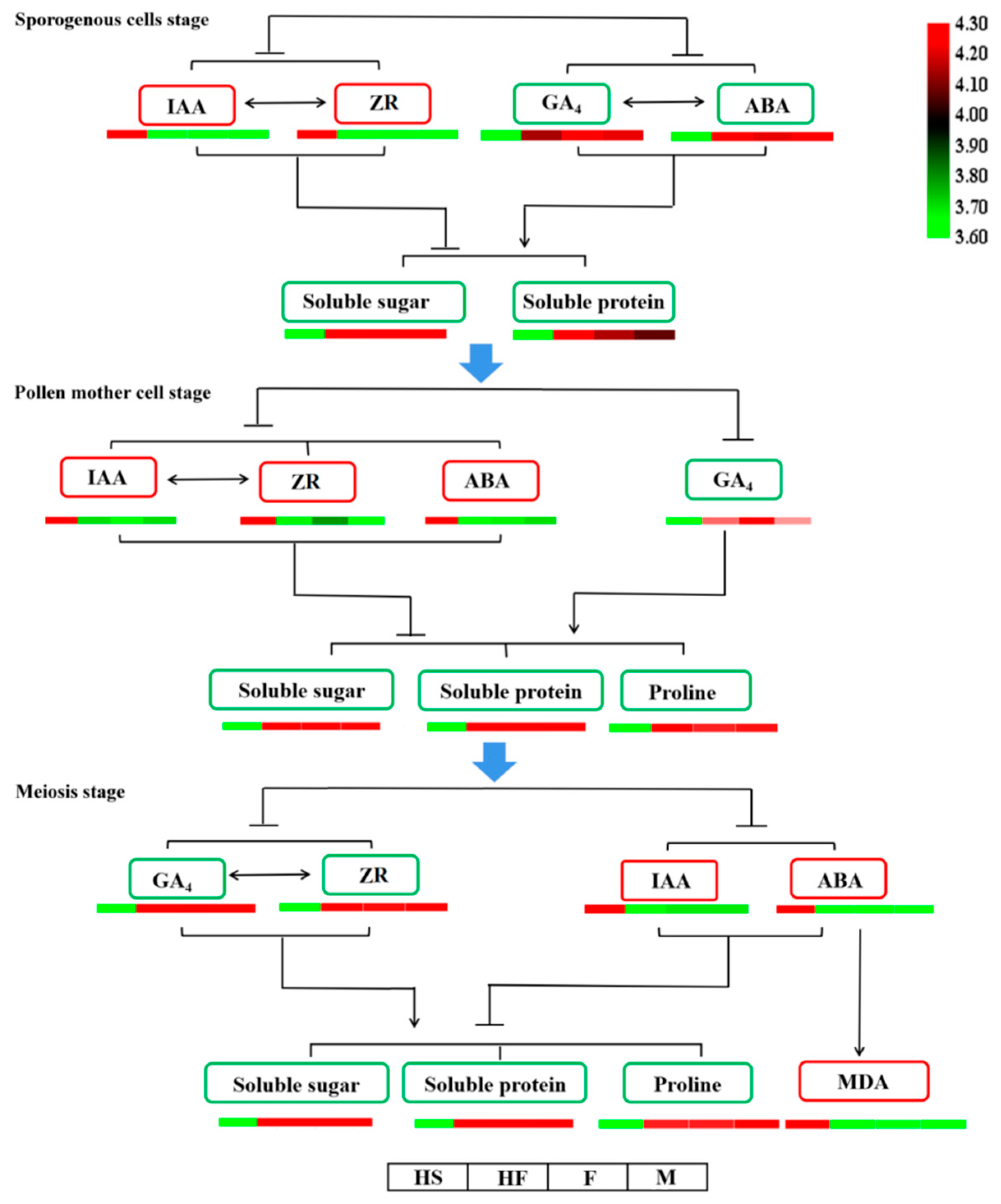
2.2. The Endogenous Hormone Content Changed during Anther Development in Lily
2.3. Endogenous Substance Content Determination
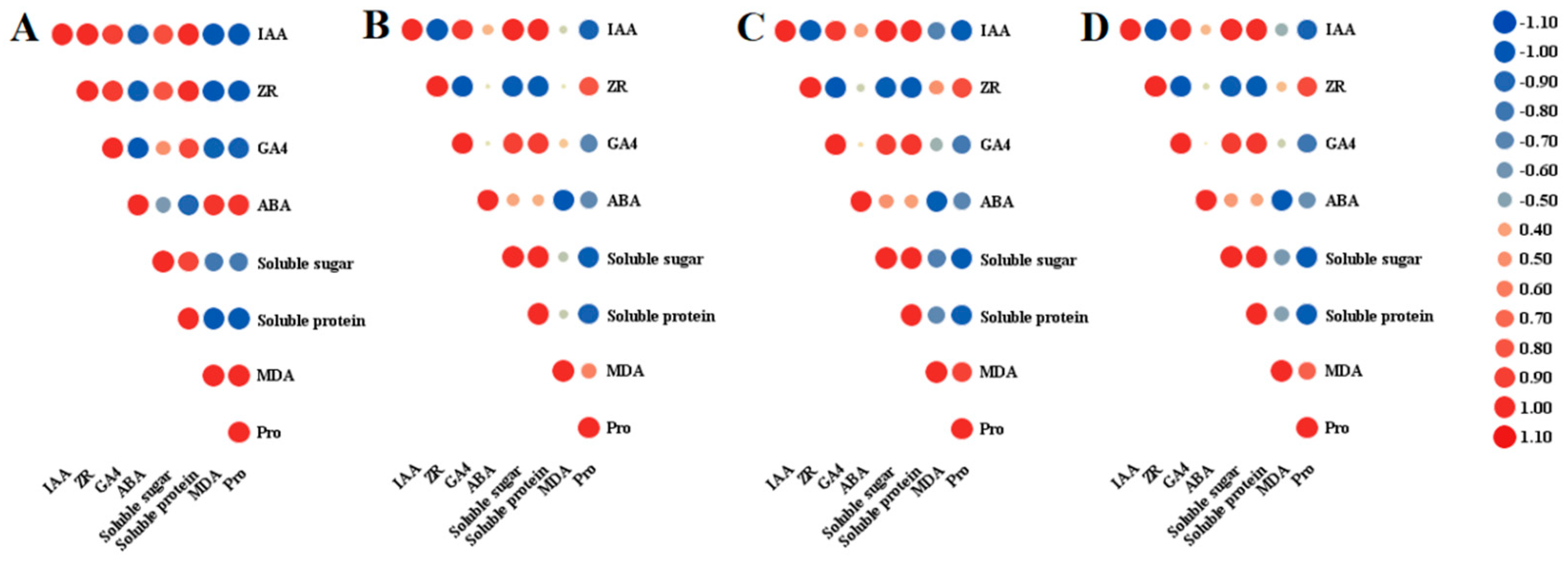
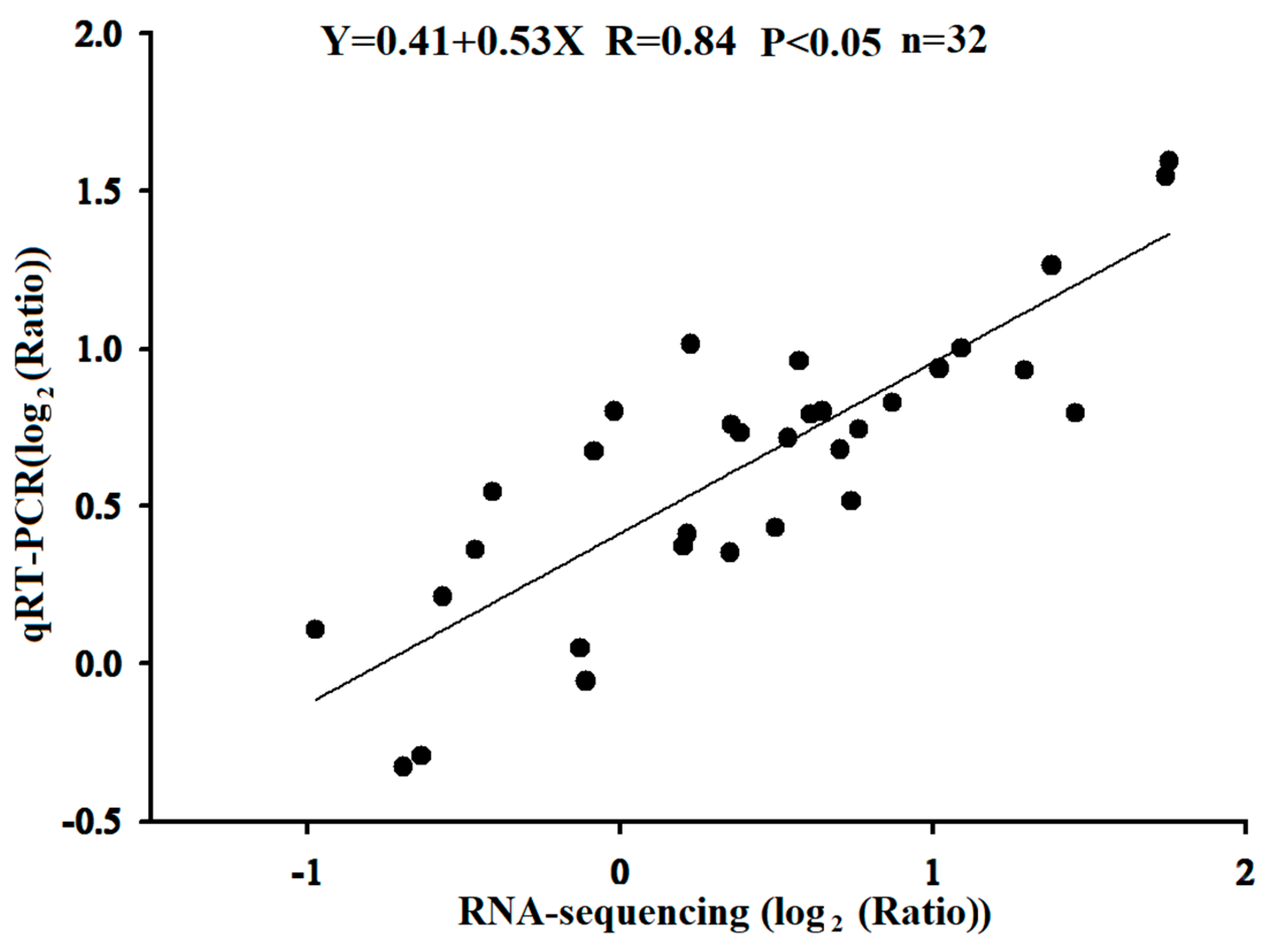
2.4. Relationship between Endogenous Substances and Endogenous Hormones during Anther Development in Lilium
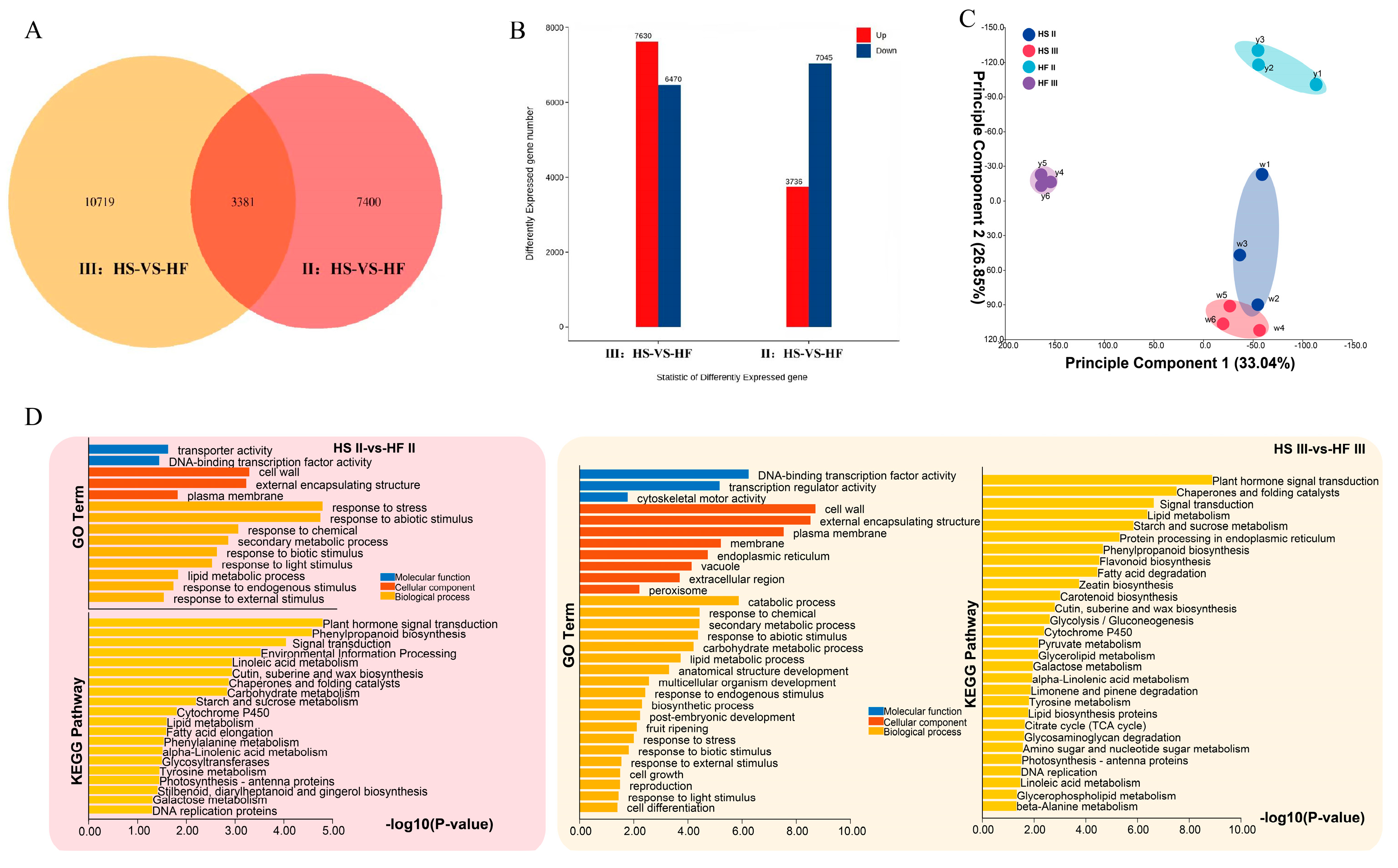
2.5. Screening of Related Genes
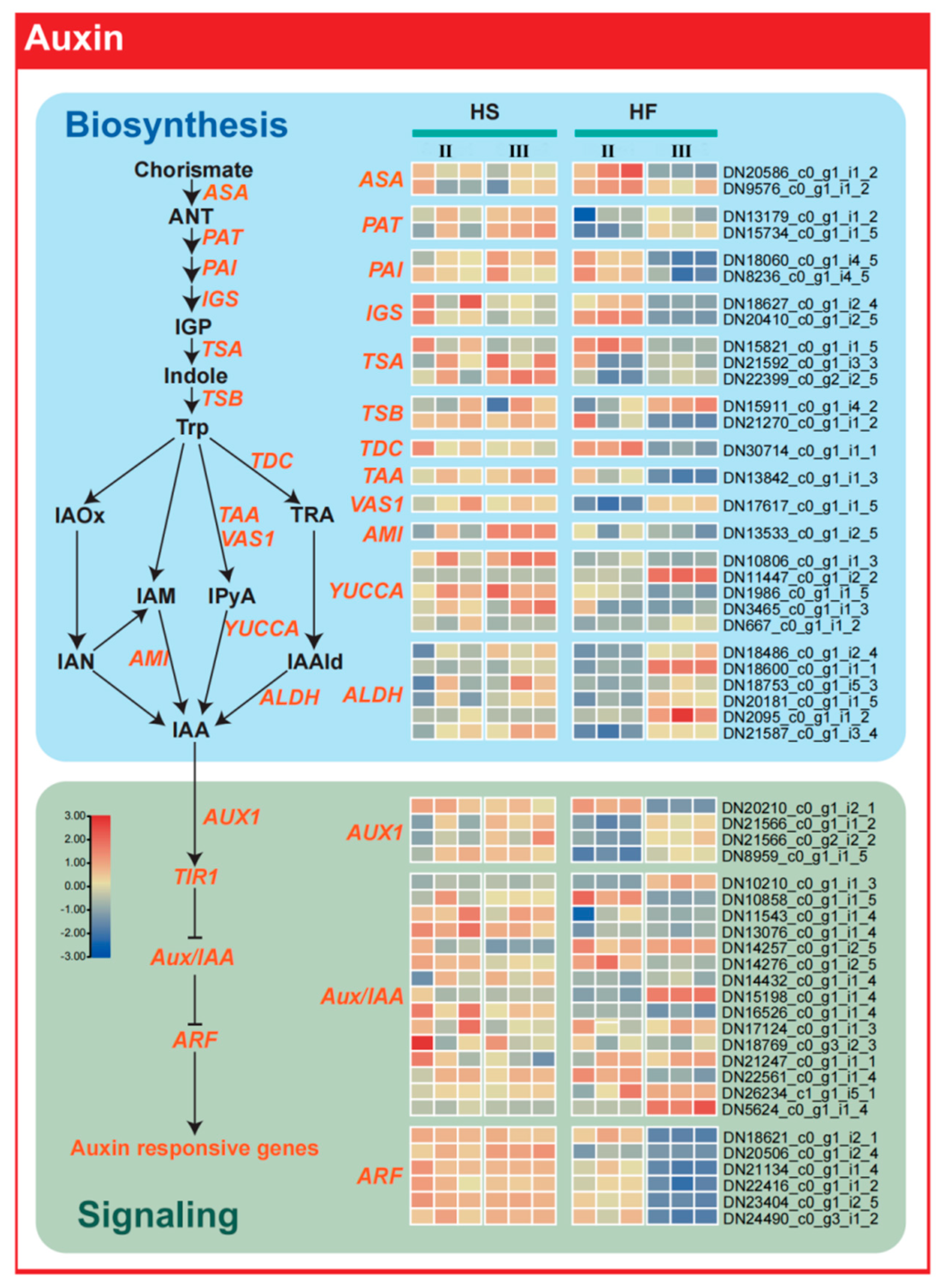
2.6. Auxin Synthesis−Related Genes Regulate Male Sterility
2.7. Abscisic Acid Synthesis−Related Genes Regulate Male Sterility
2.8. Regulation of the Expression of Genes Associated with Male Sterility
3. Discussion
4. Materials and Methods
4.1. Test Materials
4.2. Observation of Lily Anther Morphology
4.3. Examination of the Endogenous Hormone Content
4.4. Determination of the Contents of Endogenous Substances
4.5. Transcriptome Sequencing of Aborted Pollen
4.6. qRT–PCR for Hormone−Related Genes
4.7. Data Processing
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kamenetsky, R.; Okubo, H. Ornamental geophytes from basic science to sustainable production introduction. In Ornamental Geophytes: From Basic Science to Sustainable Production; CRC Press: Boca Raton, FL, USA, 2013; pp. Xv–Xvii. [Google Scholar]
- Tong, Z.; Li, Q.; Yang, Y.; Dai, F.; Gao, J.; Hong, B. Isolation and expression analysis of LoPIP2, a lily (Lilium Oriental Hybrids) aquaporin gene involved in desiccation-induced anther dehiscence. Sci. Hortic. 2013, 164, 316–322. [Google Scholar] [CrossRef]
- Xu, L.F.; Yang, P.P.; Yuan, S.X.; Feng, Y.Y.; Xu, H.; Cao, Y.W.; Ming, J. Transcriptome analysis identifies key candidate genes mediating purple ovary coloration in asiatic hybrid lilies. Int. J. Mol. Sci. 2016, 17, 1881. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Cao, X.; Yi, M.; Wu, J.; He, J. Isolation and characterization of LoAMS gene in anther development of lily (Lilium oriental hybrids). N. Z. J. Crop Hortic. 2020, 48, 257–269. [Google Scholar] [CrossRef]
- He, L.; Liu, X.Y.; Wu, Z.; Teng, N.J. Transcriptome and metabolome analyses provide insights into the stomium degeneration mechanism in lily. Int. J. Mol. Sci. 2021, 22, 12124. [Google Scholar] [CrossRef]
- Dong, T.T.; Wang, L.X.; Wang, R.; Yang, X.; Jia, W.J.; Yi, M.F.; Zhou, X.F.; He, J.N. Transcriptomic analysis reveals candidate genes associated with anther development in Lilium Oriental Hybrid ‘Siberia’. Front. Plant Sci. 2023, 14, 1128911. [Google Scholar] [CrossRef]
- Tong, Z.; Dong, T.; Li, Q.; Wang, R.; He, J.; Hong, B. R2R3-type LoMYB21 affects jasmonate-regulated development and dehiscence of anthers in lily (Lilium oriental hybrids). Hortic. Plant J. 2023. [Google Scholar] [CrossRef]
- Fu, Q.; Cao, Y.; Li, Y.Y. Advanced on studies and spplications of photo-thermo-sensitive male sterility in wheat. J. Triticeae Crops 2010, 30, 576–580. [Google Scholar]
- Cheng, H.; Song, S.; Xiao, L.; Soo, H.M.; Cheng, Z.; Xie, D.; Peng, J. Gibberellin acts through jasmonate to control the expression of MYB21, MYB24, and MYB57 to promote stamen filament growth in Arabidopsis. PLoS Genet. 2009, 5, e1000440. [Google Scholar] [CrossRef]
- Ye, Q.Q.; Zhu, W.J.; Li, L.; Zhang, S.S.; Yin, Y.H.; Ma, H.; Wang, X.L. Brassinosteroids control male fertility by regulating the expression of key genes involved in Arabidopsis anther and pollen development. Proc. Natl. Acad. Sci. USA 2010, 107, 6100–6105. [Google Scholar] [CrossRef]
- Huang, H.; Liu, B.; Liu, L.Y.; Song, S.S. Jasmonate action in plant growth and development. J. Exp. Bot. 2017, 68, 1349–1359. [Google Scholar] [CrossRef]
- Wu, S.W.; Fang, C.W.; Li, Z.W.; Wang, Y.B.; Pan, S.S.; Wu, Y.R.; An, X.L.; Long, Y.; Wan, X.Y. ATP-binding cassette G transporters and their multiple roles especially for male fertility in Arabidopsis, Rice and Maize. Int. J. Mol. Sci. 2022, 23, 9304. [Google Scholar] [CrossRef] [PubMed]
- Li, J.T.; Zhao, Y.; Chu, H.W.; Wang, L.K.; Fu, Y.R.; Liu, P.; Upadhyaya, N.; Chen, C.L.; Mou, T.M.; Feng, Y.Q.; et al. SHOEBOX modulates root meristem size in rice through dose-dependent effects of gibberellins on cell elongation and proliferation. PLoS Genet. 2015, 11, e1005464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.B.; Yuan, S.H.; Liu, Z.H.; Luo, L.Q.; Guo, H.Y.; Li, Y.M.; Bai, J.F.; Zhao, C.P.; Zhang, L.P. Comparative transcriptome analysis reveals hormone signal transduction and sucrose metabolism related genes involved in the regulation of anther dehiscence in photo-thermo-sensitive genic male sterile Wheat. Biomolecules 2022, 12, 1149. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.L.; Hao, M.Y.; Mei, D.S.; Zaman, Q.U.; Sang, S.F.; Wang, H.; Wang, W.X.; Fu, L.; Cheng, H.T.; Hu, Q. Transcriptome and hormone comparison of three cytoplasmic male sterile systems in Brassica napus. Int. J. Mol. Sci. 2018, 19, 4022. [Google Scholar] [CrossRef]
- Cecchetti, V.; Celebrin, D.; Napoli, N.; Ghelli, R.; Brunetti, P.; Costantino, P.; Cardarelli, M. An auxin maximum in the middle layer controls stamen development and pollen maturation in Arabidopsis. New Phytol. 2017, 213, 1194–1207. [Google Scholar] [CrossRef]
- Rezaul, I.M.; Feng, B.H.; Chen, T.T.; Fu, W.M.; Zhang, C.X.; Tao, L.X.; Fu, G.F. Abscisic acid prevents pollen abortion under high-temperature stress by mediating sugar metabolism in rice spikelets. Physiol. Plant. 2019, 165, 644–663. [Google Scholar] [CrossRef]
- Koornneef, M.; Vanderveen, J.H. Induction and analysis of gibberellin sensitive mutants in Arabidopsis-Thaliana (L) Heynh. Theor. Appl. Genet. 1980, 58, 257–263. [Google Scholar] [CrossRef]
- Fan, X.M.; Yuan, D.Y.; Tian, X.M.; Zhu, Z.J.; Liu, M.L.; Cao, H.P. Comprehensive transcriptome analysis of phytohormone biosynthesis and signaling genes in the flowers of Chinese Chinquapin (Castanea henryi). J. Agric. Food Chem. 2017, 65, 10332–10349. [Google Scholar] [CrossRef]
- Miao, J.; Yang, W.; Liu, C.; Li, M.; Qiao, N.; Shen, H. Dynamic changes of physiological and biochemical substances and energy metabolism enzyme activities in bud of welsh onion CMS line. Acta Bot. Boreali-Occident. Sin. 2010, 30, 1142–1148. [Google Scholar]
- Zhu, Y.; Dun, X.L.; Zhou, Z.F.; Xia, S.Q.; Yi, B.; Wen, J.; Shen, J.X.; Ma, C.Z.; Tu, J.X.; Fu, T.D. A separation defect of tapetum cells and microspore mother cells results in male sterility in Brassica napus: The role of abscisic acid in early anther development. Plant Mol. Biol. 2010, 72, 111–123. [Google Scholar] [CrossRef]
- Millar, A.A.; Gubler, F. The Arabidopsis GAMYB-like genes, MYB33 and MYB65, are microRNA-regulated genes that redundantly facilitate anther development. Plant Cell 2005, 17, 705–721. [Google Scholar] [CrossRef]
- Gubler, F.; Kalla, R.; Roberts, J.K.; Jacobsen, J.V. Gibberellin-regulated expression of a Myb gene in barley aleurone cells-evidence for Myb transactivation of a high-Pl alpha-amylase gene promoter. Plant Cell 1995, 7, 1879–1891. [Google Scholar] [CrossRef] [PubMed]
- Biancucci, M.; Mattioli, R.; Forlani, G.; Funck, D.; Costantino, P.; Trovato, M. Role of proline and GABA in sexual reproduction of angiosperms. Front. Plant Sci. 2015, 6, 680. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Biancucci, M.; El Shall, A.; Mosca, L.; Costantino, P.; Funck, D.; Trovato, M. Proline synthesis in developing microspores is required for pollen development and fertility. BMC Plant Biol. 2018, 18, 356. [Google Scholar] [CrossRef]
- Jia, W.; Zheng, J.; Cui, G.; Ma, L.; Duan, Q.; Du, W.; Wang, X. Relationship between Lilium fertility and hormone, endogenous substance content, energy metabolism enzyme activity during anther development. Acta Bot. Boreali-Occident. Sin. 2019, 39, 480–488. [Google Scholar] [CrossRef]
- Higginson, T.; Li, S.F.; Parish, R.W. AtMYB103 regulates tapetum and trichome development in Arabidopsis thaliana. Plant J. 2003, 35, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.B.; Zhu, J.; Gao, J.F.; Wang, C.; Li, H.; Li, H.; Zhang, H.Q.; Zhang, S.; Wang, D.M.; Wang, Q.X.; et al. Transcription factor AtMYB103 is required for anther development by regulating tapetum development, callose dissolution and exine formation in Arabidopsis. Plant J. 2007, 52, 528–538. [Google Scholar] [CrossRef]
- Sawhney, V.K.; Shukla, A. Male-sterility in flowering plants-are plant-growth substances involved. Am. J. Bot. 1994, 81, 1640–1647. [Google Scholar] [CrossRef]
- Xia, T.; Liu, J. Study on the relation between auxin, zeatin and cytoplasmic male sterility in maize (Zea mays L.). Zuo Wu Xue Bao 1994, 20, 26–32. [Google Scholar]
- Wilson, Z.A.; Song, J.; Taylor, B.; Yang, C. The final split: The regulation of anther dehiscence. J. Exp. Bot. 2011, 62, 1633–1649. [Google Scholar] [CrossRef]
- Sanders, P.M.; Bui, A.Q.; Le, B.H.; Goldberg, R.B. Differentiation and degeneration of cells that play a major role in tobacco anther dehiscence. Sex. Plant Reprod. 2005, 17, 219–241. [Google Scholar] [CrossRef]
- Li, H.; Yuan, Z.; Vizcay-Barrena, G.; Yang, C.Y.; Liang, W.Q.; Zong, J.; Wilson, Z.A.; Zhang, D.B. Persistent tapetal cell1 encodes a PHD-finger protein that is required for tapetal cell death and pollen development in Rice. Plant Physiol. 2011, 156, 615–630. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; He, J.; Wu, J.; Gong, B.; Cao, X.; Seng, S.; Wu, Z.; Wu, C.; Liu, C.; Yi, M. Characterization and functional analysis of transcription factor LoMYB80 related to anther development in Lily (Lilium Oriental Hybrids). J. Plant Growth Regul. 2015, 34, 545–557. [Google Scholar] [CrossRef]
- Xia, C.; Zhang, L.C.; Zou, C.; Gu, Y.Q.; Duan, J.L.; Zhao, G.Y.; Wu, J.J.; Liu, Y.; Fang, X.H.; Gao, L.F.; et al. A TRIM insertion in the promoter of Ms2 causes male sterility in wheat. Nat. Commun. 2017, 8, 15407. [Google Scholar] [CrossRef]
- Yang, K.; Chen, Y.; Shi, M.; Converse, R.; Chen, X.; Zhao, B.M.; Zhang, Y.; Lv, J. A novel dominant rice male sterility mutant, OsDMS-1, simultaneously controlled by independent loci on chromosomes 1, 2, and 3. Mol. Breed. 2017, 37, 25. [Google Scholar] [CrossRef]
- Wu, Y.L.; Min, L.; Wu, Z.C.; Yang, L.; Zhu, L.F.; Yang, X.Y.; Yuan, D.J.; Guo, X.P.; Zhang, X.L. Defective pollen wall contributes to male sterility in the male sterile line 1355A of cotton. Sci. Rep. 2015, 5, 9608. [Google Scholar] [CrossRef]
- Polowick, P.L.; Sawhney, V.K. Ultrastructure of the tapetal cell wall in the stamenless-2 mutant of tomato (Lycopersicon esculentum): Correlation between structure and male-sterility. Protoplasma 1995, 189, 249–255. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Z.Y.; Li, C.Y.; Zhang, Y.; Feng, H. Comparative transcriptome analysis of fertile and sterile buds from a genetically male sterile line of Chinese cabbage. Vitr. Cell. Dev. Biol.-Plant 2016, 52, 130–139. [Google Scholar] [CrossRef]
- Hu, Y.J.; Wu, Q.S.; Liu, S.A.; Wei, L.; Chen, X.J.; Yan, Z.X.; Yu, J.H.; Zeng, L.B.; Ding, Y. Study of rice pollen grains by multispectral imaging microscopy. Microsc. Res. Tech. 2005, 68, 335–346. [Google Scholar] [CrossRef]
- Song, X.; Sun, X.; Wang, H.; Liu, Y.; Zhang, J. Biochemical changes in anthers of "Dong A" genetic male sterile lines of cotton. Acta Bot. Boreali.-Occident. Sin. 2004, 24, 243–247. [Google Scholar]
- Liu, J.; Hou, X.; Chen, X.; Zhang, J.; Wang, S.; Pan, B. Studies on biochemical characters in sweet pepper (Capsicum annuum L. )CMS line and its maintainer line. Acta Hortic. Sin. 2006, 33, 629–631. [Google Scholar]
- Podolyan, A.; Luneva, O.; Klimenko, E.; Breygina, M. Oxygen radicals and cytoplasm zoning in growing lily pollen tubes. Plant Reprod. 2021, 34, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.X.; Lv, M.L.; Liang, Y.; Ma, Z.M.; Cao, J.S. Identification of novel and conserved miRNAs involved in pollen development in Brassica campestris ssp. chinensis by high-throughput sequencing and degradome analysis. BMC Genom. 2014, 15, 146. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.F.; Dai, X.H.; Zhao, Y.D. Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Gene Dev. 2006, 20, 1790–1799. [Google Scholar] [CrossRef] [PubMed]
- Cecchetti, V.; Altamura, M.M.; Falasca, G.; Costantino, P.; Cardarelli, M. Auxin regulates Arabidopsis anther dehiscence, pollen maturation, and filament elongation. Plant Cell 2008, 20, 1760–1774. [Google Scholar] [CrossRef]
- Israeli, A.; Reed, J.W.; Ori, N. Genetic dissection of the auxin response network. Nat. Plants 2020, 6, 1082–1090. [Google Scholar] [CrossRef]
- Gomez, J.F.; Talle, B.; Wilson, Z.A. Anther and pollen development: A conserved developmental pathway. J. Integr. Plant Biol. 2015, 57, 876–891. [Google Scholar] [CrossRef]
- Yu, J.; Jiang, M.Y.; Guo, C.K. Crop Pollen development under drought: From the phenotype to the mechanism. Int. J. Mol. Sci. 2019, 20, 1550. [Google Scholar] [CrossRef]
- Ji, X.M.; Dong, B.D.; Shiran, B.; Talbot, M.J.; Edlington, J.E.; Hughes, T.; White, R.G.; Gubler, F.; Dolferus, R. Control of abscisic acid catabolism and abscisic acid homeostasis is important for reproductive stage stress tolerance in Cereals. Plant Physiol. 2011, 156, 647–662. [Google Scholar] [CrossRef]
- Song, S.S.; Qi, T.C.; Huang, H.; Xie, D.X. Regulation of stamen development by coordinated actions of jasmonate, auxin, and gibberellin in Arabidopsis. Mol. Plant 2013, 6, 1065–1073. [Google Scholar] [CrossRef]
- Singh, S.; Sawhney, V.K. Cytokinins in a normal line and the Ogura (Ogu) cytoplasmic aale-sterile line of rapeseed (Brassica-Napus). Plant Sci. 1992, 86, 147–154. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, K.; Fu, J.; Qiao, A. Relationships between cytoplasmic male sterility and endogenous hormone content of pepper bud. J. South China Agric. Univ. 2010, 31, 1–4. [Google Scholar]
- Wang, H.; Wu, Z.d.; Han, Y.; Fang, Z. Relationships between endogenous hormone contents and cytoplasmic male sterility in sugarbeet. Sci. Agric. Sin. 2008, 41, 1134–1141. [Google Scholar]
- Willige, B.C.; Ghosh, S.; Nill, C.; Zourelidou, M.; Dohmann, E.M.N.; Maier, A.; Schwechheimer, C. The DELLA domain of GA INSENSITIVE mediates the interaction with the GA INSENSITIVE DWARF1A gibberellin receptor of Arabidopsis. Plant Cell 2007, 19, 1209–1220. [Google Scholar] [CrossRef]
- Chen, M.L.; Fu, X.M.; Liu, J.Q.; Ye, T.T.; Hou, S.Y.; Huang, Y.Q.; Yuan, B.F.; Wu, Y.; Feng, Y.Q. Highly sensitive and quantitative profiling of acidic phytohormones using derivatization approach coupled with nano-LC-ESI-Q-TOF-MS analysis. J. Chromatogr. B 2012, 905, 67–74. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, F.; Feng, Y.Q. Determination of cytokinins in plant samples by polymer monolith microextraction coupled with hydrophilic interaction chromatography-tandem mass spectrometry. Anal. Methods 2010, 2, 1676–1685. [Google Scholar] [CrossRef]
- Wu, Z.; Liang, J.; Wang, C.; Zhao, X.; Zhong, X.; Cao, X.; Li, G.; He, J.; Yi, M. Overexpression of lily HsfA3s in Arabidopsis confers increased thermotolerance and salt sensitivity via alterations in proline catabolism. J. Exp. Bot. 2018, 69, 2005–2021. [Google Scholar] [CrossRef]
- Nguyen, G.N.; Hailstones, D.L.; Wilkes, M.; Sutton, B.G. Drought-induced oxidative conditions in rice anthers leading to a programmed cell death and pollen abortion. J. Agron. Crop Sci. 2009, 195, 157–164. [Google Scholar] [CrossRef]





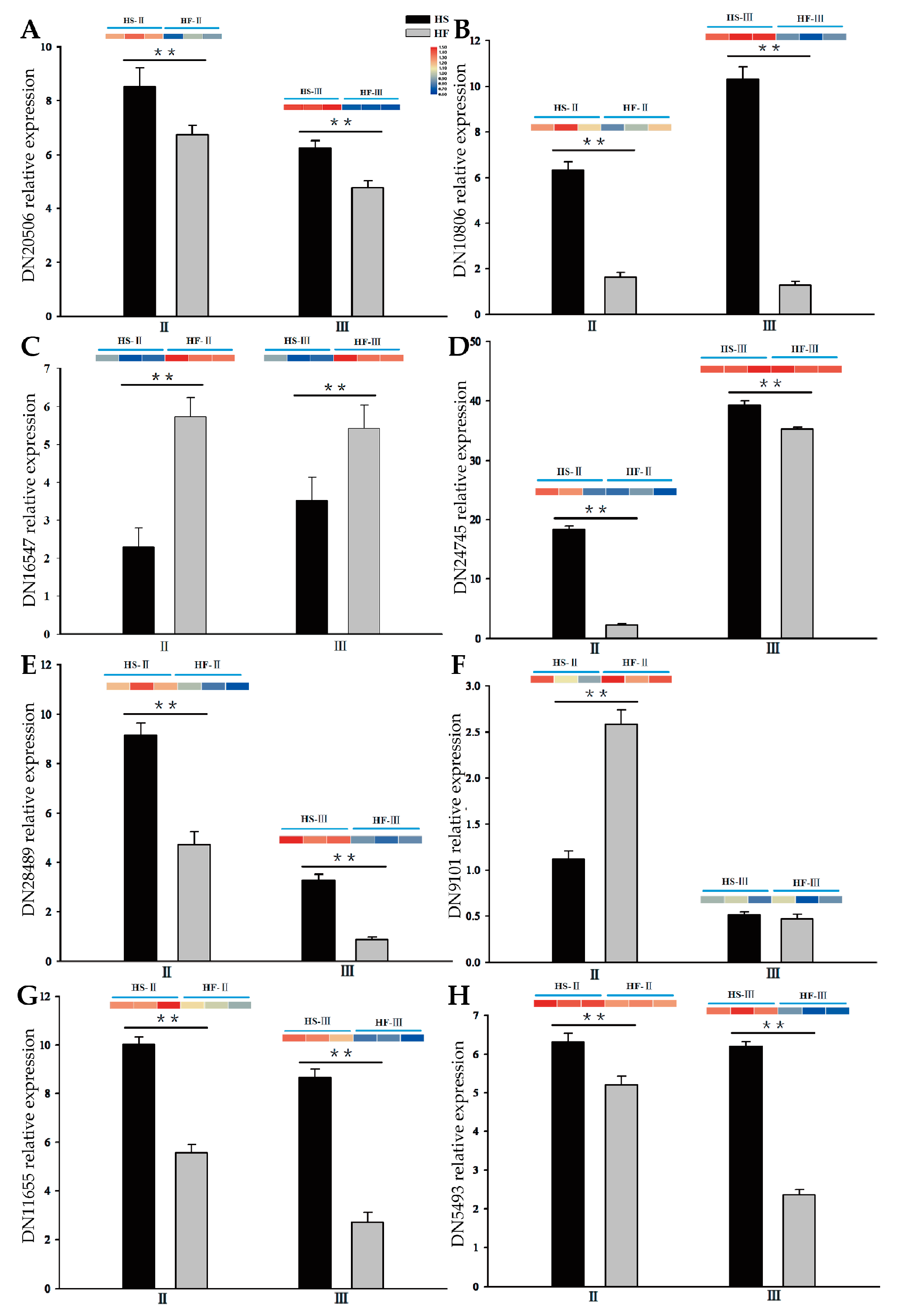
| Gene | KEGG Description | |
|---|---|---|
| IAA | DN20506_c0_g1_i2_4 | Auxin response factor |
| DN10806_c0_g1_i1_3 | Indole−3−pyruvate monooxygenase | |
| Cytokinin | DN24745_c1_g2_i2_2 | Pseudohistidine−containing phosphotransfer protein |
| DN16547_c0_g1_i1_2 | Cytokinin synthase | |
| GA | DN9101_c0_g1_i2_5 | Gibberellin 2−beta−dioxygenase |
| DN28489_c0_g1_i1_1 | Gibberellin receptor | |
| ABA | DN11655_c0_g1_i1_2 | Abscisic acid receptor |
| DN5493_c0_g1_i2_3 | Abscisic−aldehyde oxidase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, W.; Li, X.; Wang, R.; Duan, Q.; He, J.; Gao, J.; Wang, J. Disruption of the Contents of Endogenous Hormones Cause Pollen Development Obstruction and Abortion in Male-Sterile Hybrid Lily Populations. Plants 2023, 12, 3804. https://doi.org/10.3390/plants12223804
Jia W, Li X, Wang R, Duan Q, He J, Gao J, Wang J. Disruption of the Contents of Endogenous Hormones Cause Pollen Development Obstruction and Abortion in Male-Sterile Hybrid Lily Populations. Plants. 2023; 12(22):3804. https://doi.org/10.3390/plants12223804
Chicago/Turabian StyleJia, Wenjie, Xiang Li, Rui Wang, Qing Duan, Junna He, Junping Gao, and Jihua Wang. 2023. "Disruption of the Contents of Endogenous Hormones Cause Pollen Development Obstruction and Abortion in Male-Sterile Hybrid Lily Populations" Plants 12, no. 22: 3804. https://doi.org/10.3390/plants12223804






