Spatial and Temporal Characteristics of Phytoplankton Communities in Drinking Water Source Reservoirs in Shenzhen, China
Abstract
:1. Introduction
2. Results
2.1. Phytoplankton Community Composition
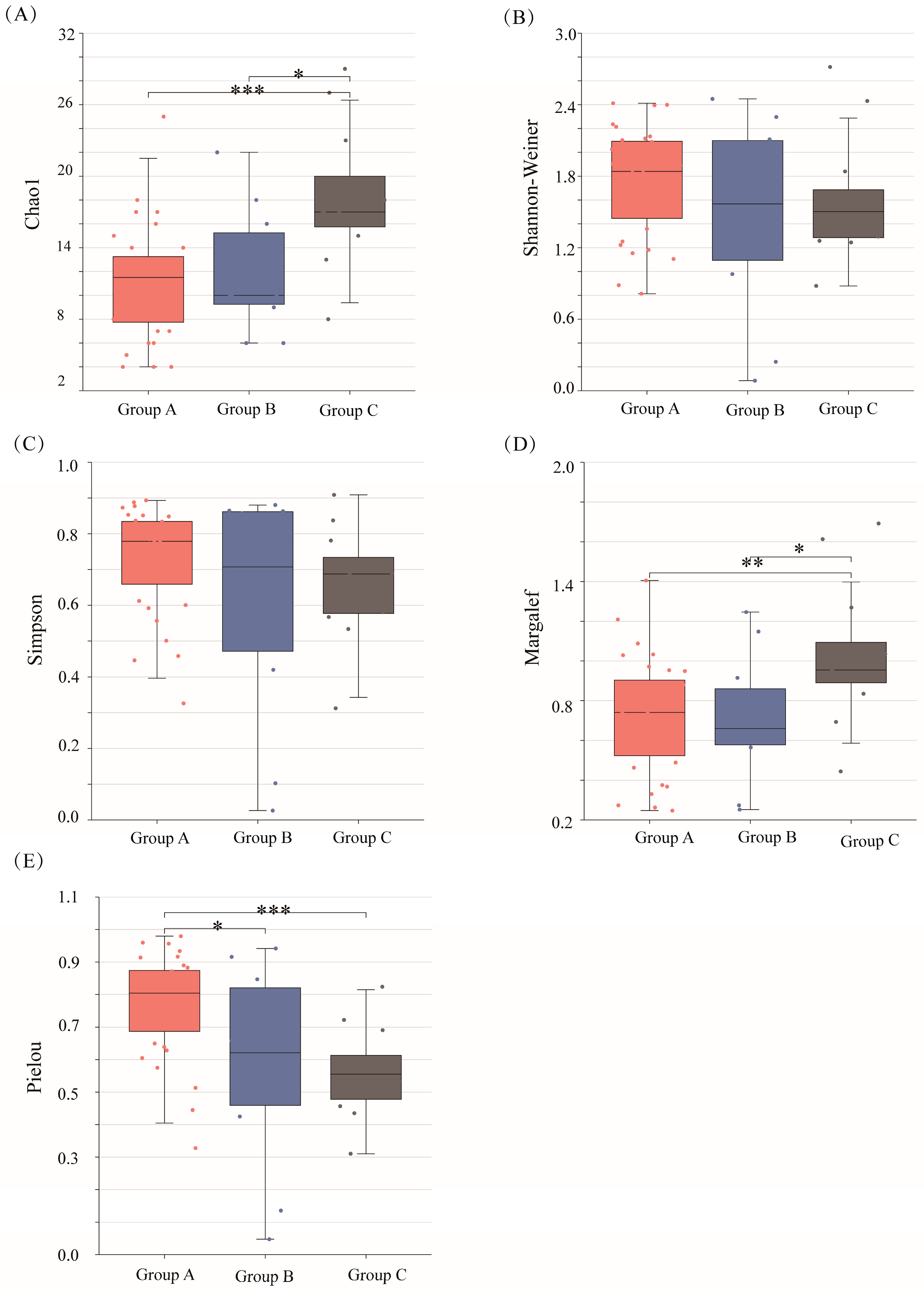
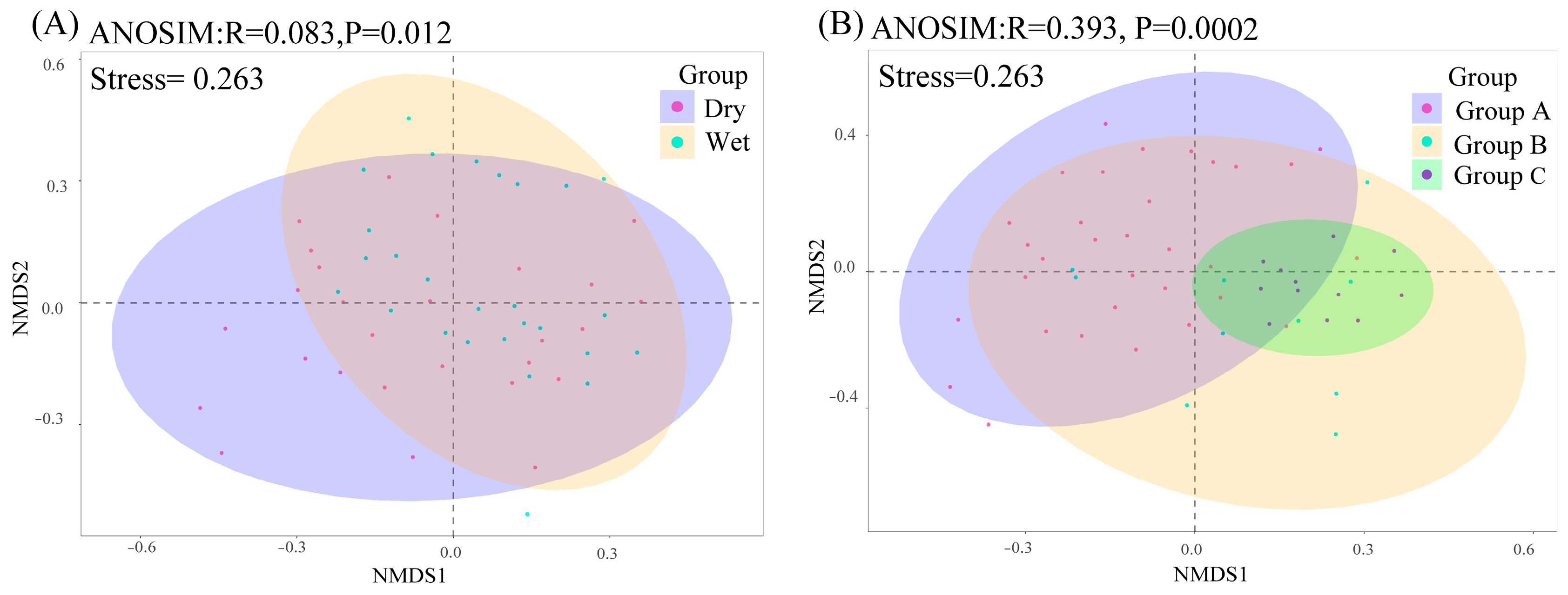
2.2. Phytoplankton Community Alpha and Beta Diversities
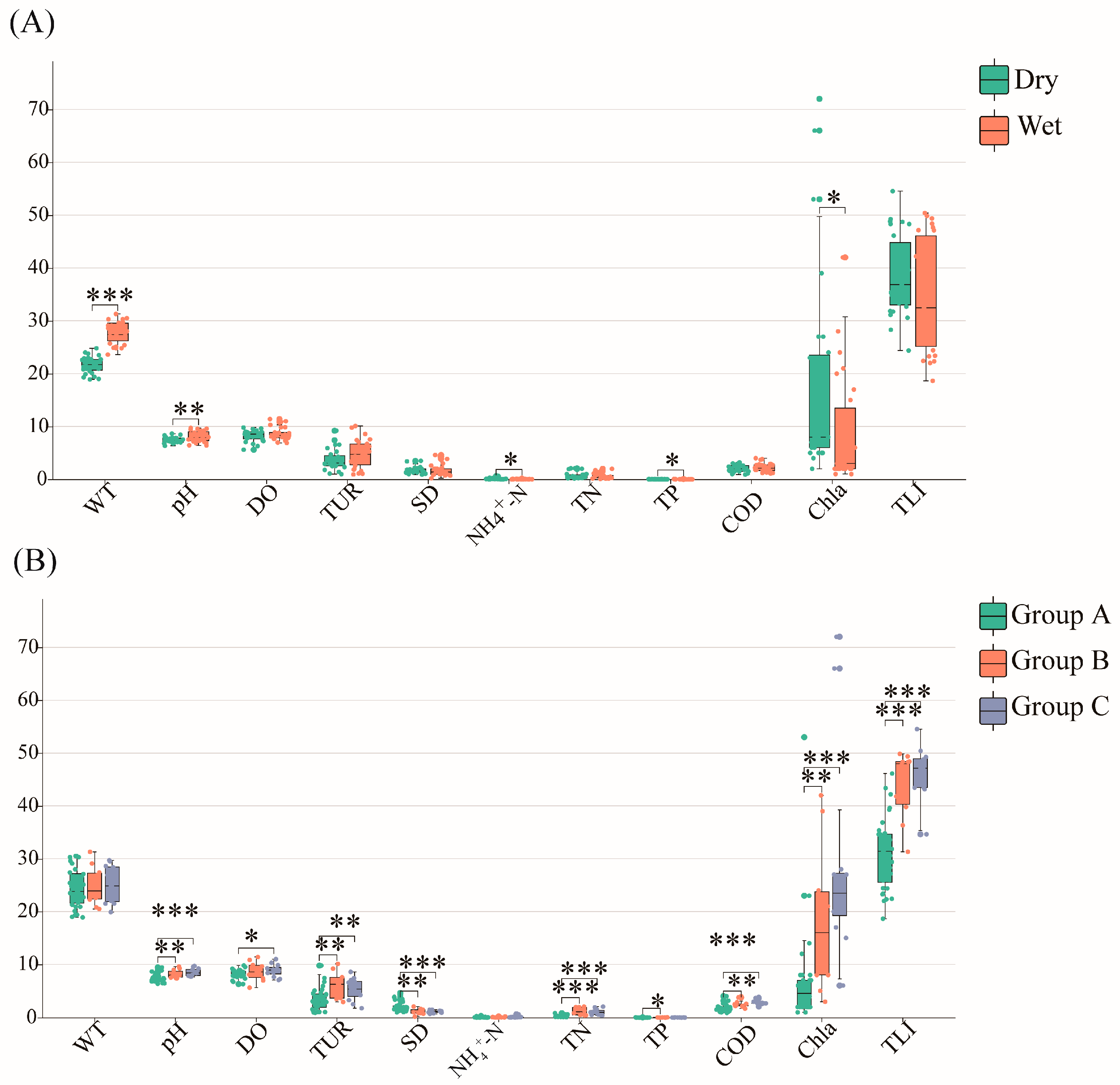
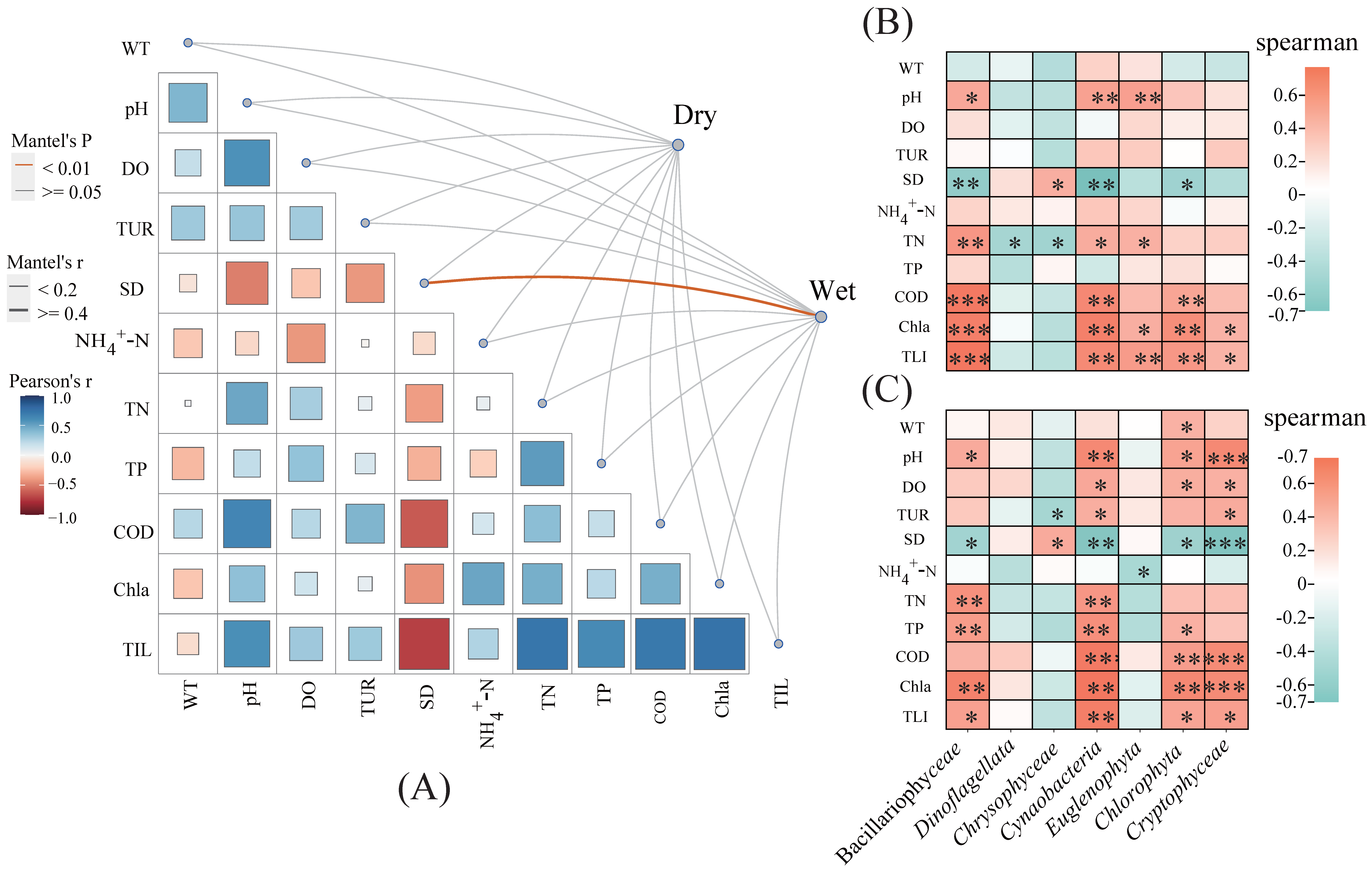
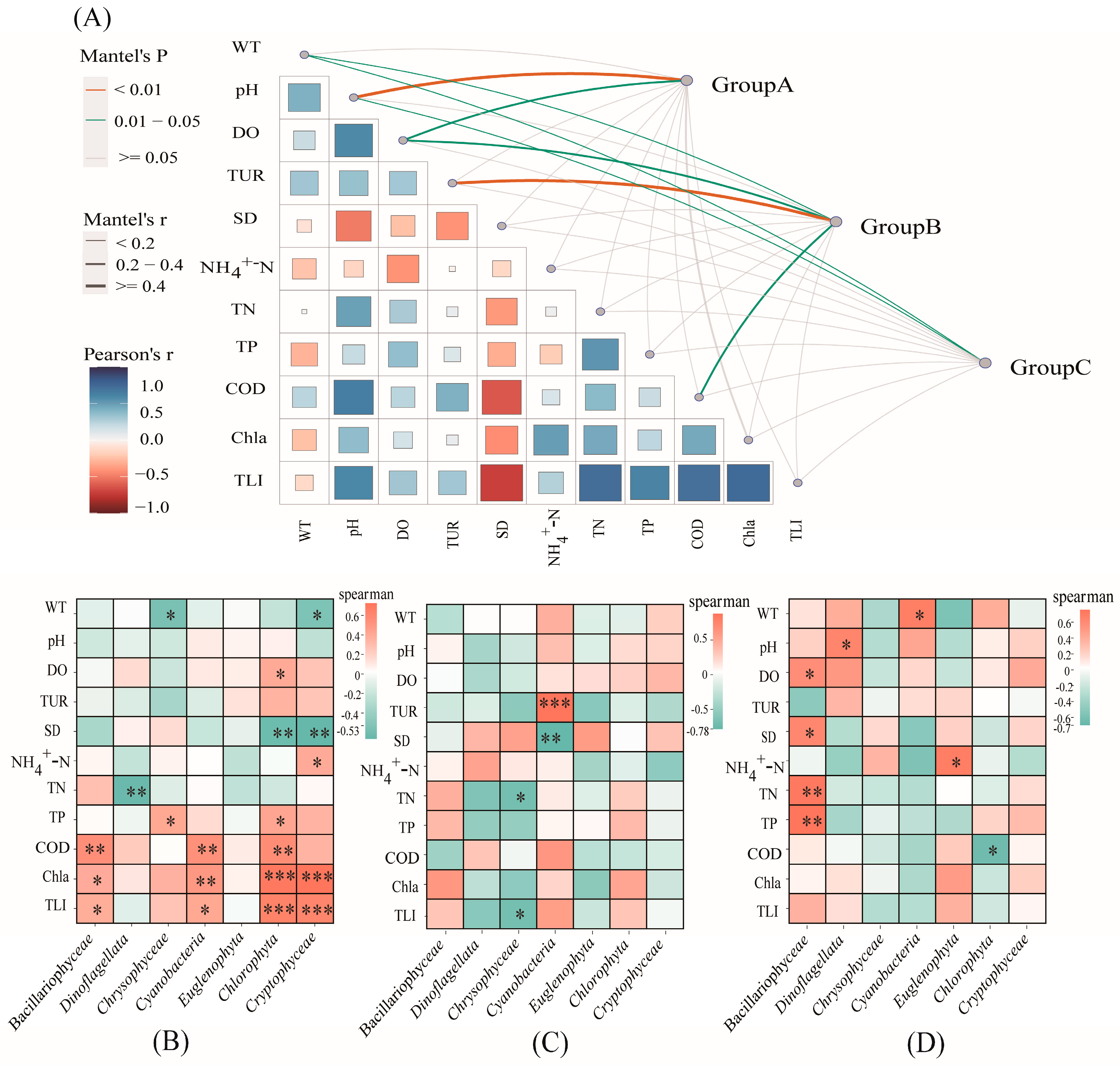
2.3. Relationships between Phytoplankton Communities and Environmental Factors
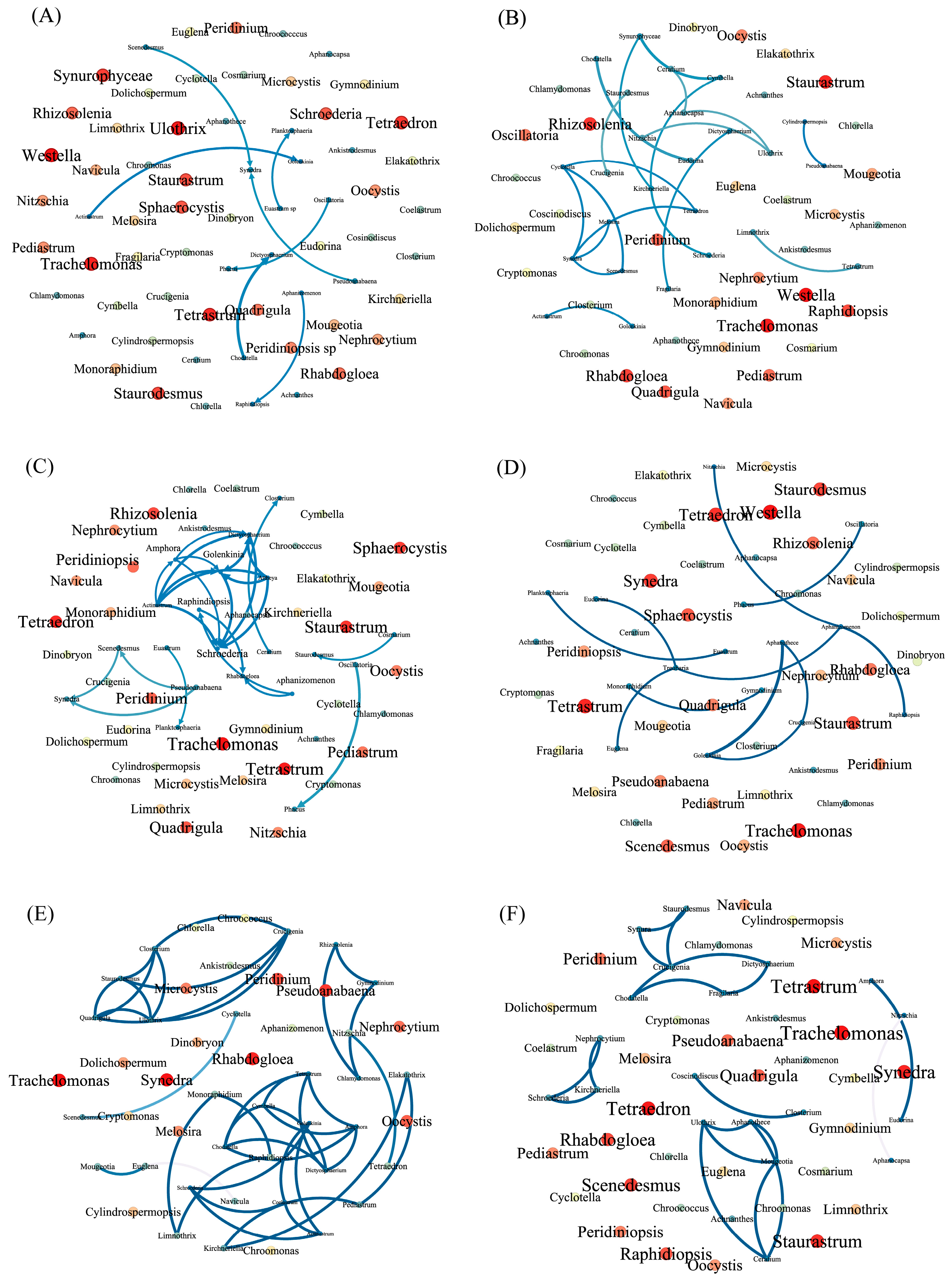
2.4. Co-Occurrence Patterns of Phytoplankton Communities
3. Discussion
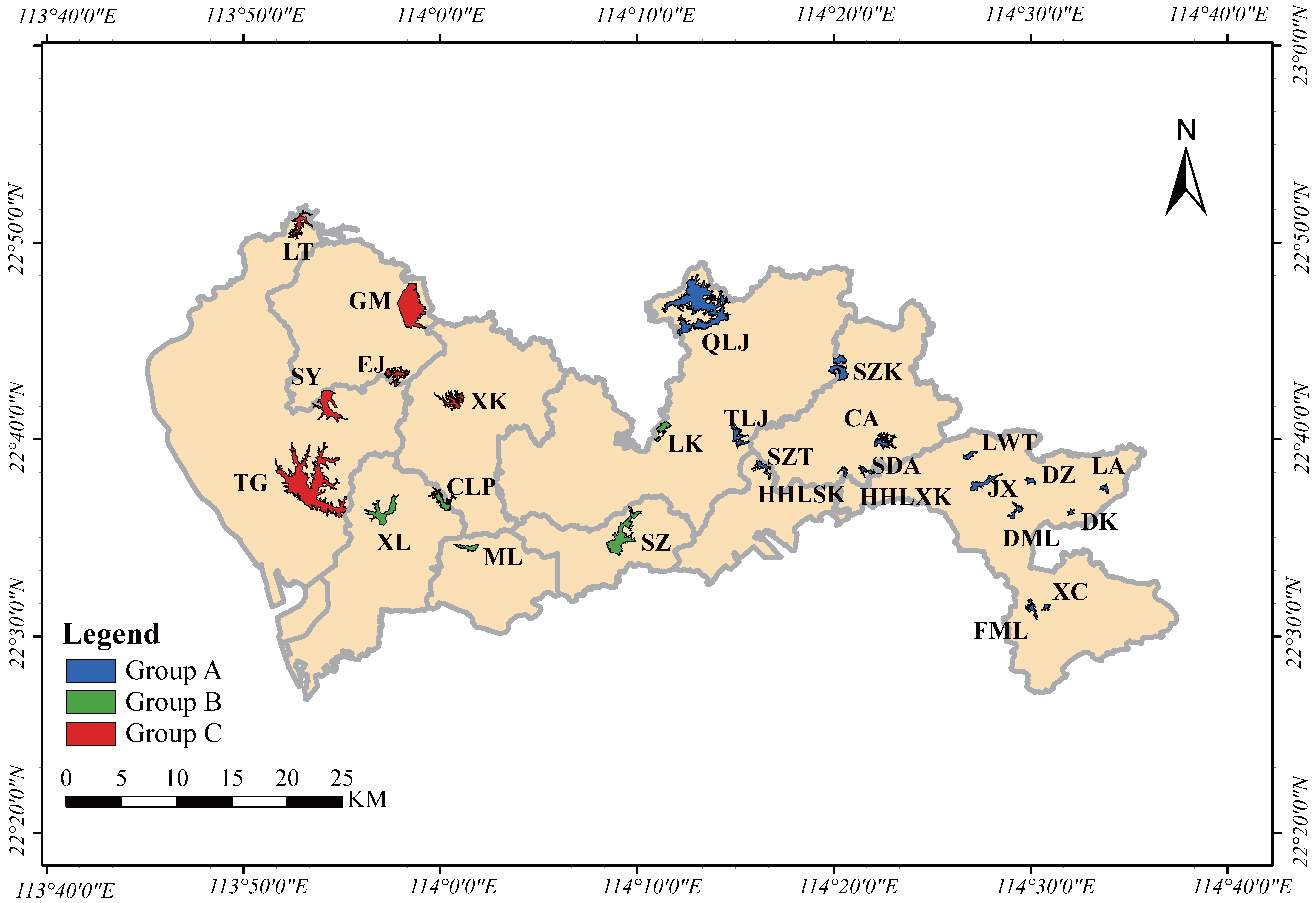
3.1. Spatiotemporal Characteristics of Phytoplankton Communities
3.2. Relationships between Phytoplankton Members
3.3. Environmental Factors Affecting Phytoplankton Community Structure
4. Materials and Methods
4.1. Phytoplankton Community Alpha and Beta Diversities
4.2. Quantitative Identification of Phytoplankton Samples
4.3. Measurement of Environmental Factors
4.4. Phytoplankton Community Alpha and Beta Diversities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, T.L.; Li, X.; Rijnaarts, H.; Grotenhuis, T.; Ma, W.X.; Sun, X.; Xu, J.L. Effects of storm runoff on the thermal regime and water quality of a deep, stratified reservoir in a temperate monsoon zone, in Northwest China. Sci. Total Environ. 2014, 485, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.M.; Chen, S.N.; Huang, T.L.; Li, B.Q.; Li, N.; Liu, K.W.; Zong, R.R.; Miao, Y.T.; Huang, X. Community Compositions of Phytoplankton and Eukaryotes during the Mixing Periods of a Drinking Water Reservoir: Dynamics and Interactions. Int. J. Environ. Res. Public Health 2020, 17, 1128. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Yang, J.; Liu, L.M. Temporal pattern prevails over spatial variability in phytoplankton communities from a subtropical water supply reservoir. Oceanol. Hydrobiol. Stud. 2013, 42, 420–430. [Google Scholar] [CrossRef]
- Zhao, C.S.; Shao, N.F.; Yang, S.T.; Ren, H.; Ge, Y.R.; Zhang, Z.S.; Feng, P.; Liu, W.L. Quantitative assessment of the effects of human activities on phytoplankton communities in lakes and reservoirs. Sci. Total Environ. 2019, 665, 213–225. [Google Scholar] [CrossRef]
- Gao, J.S.; Zhu, J.; Wang, M.W.; Dong, W.Y. Dominance and growth factors of Pseudanabaena sp. in drinking water source reservoirs, Southern China. Sustainability 2018, 10, 3936. [Google Scholar] [CrossRef]
- Naeem, S.; Hahn, D.R.; Schuurman, G. Producer-decomposer co-dependency influences biodiversity effects. Nature 2000, 403, 762–764. [Google Scholar] [CrossRef]
- Niu, Y.; Shen, H.; Chen, J.; Xie, P.; Yang, X.; Tao, M.; Ma, Z.M.; Qi, M. Phytoplankton community succession shaping bacterioplankton community composition in Lake Taihu, China. Water Res. 2011, 45, 4169–4182. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, P.; Li, X.D.; Yang, S.X.; Chao, X.; Liu, H.Q.; Ba, S. Distribution patterns and community assembly processes of eukaryotic microorganisms along an altitudinal gradient in the middle reaches of the Yarlung Zangbo River. Water Res. 2023, 239, 10. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Yang, J.; Liu, L.M.; Yu, X.Q.; Yu, Z.; Chiang, P.C. Temperature and nutrients are significant drivers of seasonal shift in phytoplankton community from a drinking water reservoir, subtropical China. Environ. Sci. Pollut. Res. 2014, 21, 5917–5928. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Qian, X.; Han, B.P.; Luo, L.C.; Hamilton, D.P. Effects of local climate and hydrological conditions on the thermal regime of a reservoir at Tropic of Cancer, in southern China. Water Res. 2012, 46, 2591–2604. [Google Scholar] [CrossRef]
- Duan, H.T.; Tao, M.; Loiselle, S.A.; Zhao, W.; Cao, Z.G.; Ma, R.H.; Tang, X.X. MODIS observations of cyanobacterial risks in a eutrophic lake: Implications for long-term safety evaluation in drinking-water source. Water Res. 2017, 122, 455–470. [Google Scholar] [CrossRef]
- Wang, Z.H.; Lin, Q.Q.; Hu, R.; Fan, C.L.; Han, B.P. Pollution by Blue green Algae(Cyanobacteria)in Reservoirs of Guangdong Province and Water Quality Evaluation. J. Trop. Subtrop. Bot. 2004, 12, 117–123. [Google Scholar]
- Lei, L.M.; Peng, L.; Huang, X.H.; Han, B.P. Occurrence and dominance of Cylindrospermopsis raciborskii and dissolved cylindrospermopsin in urban reservoirs used for drinking water supply, South China. Environ. Monit. Assess. 2014, 270, 12. [Google Scholar] [CrossRef]
- Hu, R.; Lin, Q.Q.; Wang, Z.H.; Han, B.P. Phytoplankton Composition and Distribution in Typical Reservoirs of Guangdong Province. Acta Ecol. Sin. 2002, 22, 1939–1944. [Google Scholar]
- Lin, Q.Q.; Han, B.P. Seasonal Fluctuation of Cyanobacteria Population in Tropical Through-flowing Reservoirs. J. Trop. Subtrop. Bot. 2007, 15, 152–159. [Google Scholar]
- Liu, X. Eutrophication Assessment and Modelling of Water Quality: A Case Study in Tiegang Reservoir of Shenzhen; Sichuan Normal University: Chengdu, China, 2016. [Google Scholar]
- Jia, N.N.; Wang, Y.L.; Guan, Y.Y.; Chen, Y.X.; Li, R.H.; Yu, G.L. Occurrence of Raphidiopsis raciborskii blooms in cool waters: Synergistic effects of nitrogen availability and ecotypes with adaptation to low temperature. Environ. Pollut. 2020, 270, 116070. [Google Scholar] [CrossRef]
- Yang, Y.M.; Yu, G.L.; Chen, Y.X.; Jia, N.N.; Li, R.H. Four decades of progress in cylindrospermopsin research: The ins and outs of a potent cyanotoxin. J. Hazard. Mater. 2021, 406, 124653. [Google Scholar] [CrossRef] [PubMed]
- China, Shenzhen. Overview of Each District (New Administrative District). 2023. Available online: http://www.sz.gov.cn/cn/zjsz/gqgl/ (accessed on 15 August 2023).
- Huang, C.C.; Wang, X.L.; Yang, H.; Li, Y.M.; Wang, Y.H.; Chen, X.; Xu, L.J. Satellite data regarding the eutrophication response to human activities in the plateau lake Dianchi in China from 1974 to 2009. Sci. Total Environ. 2014, 485–486, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Bai, X.T.; Dong, A.; Li, C.M.; Lu, H. A Study on the Correlation between the Spatial and Temporal Distribution of Phytoplankton Community and Environmental Factors in Changchun Section of the Yitong River. Pol. J. Environ. Stud. 2022, 31, 3955–3969. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.H.; Yang, J.W.; Liu, A.C.H.; Lu, H.P.; Gong, G.C.; Shiah, F.K.; Hsieh, C.H. Deterministic Assembly Processes Strengthen the Effects of beta-Diversity on Community Biomass of Marine Bacterioplankton. Msystems 2023, 8, e0097022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.G.; Zhang, J.; Liu, Y.; Shi, P.; Wei, G.H. Co-occurrence patterns of soybean rhizosphere microbiome at a continental scale. Soil Biol. Biochem. 2018, 118, 178–186. [Google Scholar] [CrossRef]
- Schmidt, K.C.; Jackrel, S.L.; Smith, D.J.; Dick, G.J.; Denef, V.J. Genotype and host microbiome alter competitive interactions between Microcystis aeruginosa and Chlorella sorokiniana. Harmful Algae 2020, 99, 101939. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, X.J.; Lu, J.P.; Liu, Q.; Nan, F.R.; Liu, X.D.; Xie, S.L.; Feng, J. Seasonal co-occurrence patterns of bacteria and eukaryotic phytoplankton and the ecological response in urban aquatic ecosystem. J. Oceanol. Limnol. 2022, 40, 1508–1529. [Google Scholar] [CrossRef]
- Xu, H.Z.; Liu, W.; Zhang, S.S.; Wei, J.L.; Li, Y.Z.; Pei, H.Y. Cyanobacterial bloom intensities determine planktonic eukaryote community structure and stability. Sci. Total Environ. 2022, 838, 156637. [Google Scholar] [CrossRef]
- Escalas, A.; Catherine, A.; Maloufi, S.; Cellamare, M.; Hamlaoui, S.; Yepremian, C.; Louvard, C.; Troussellier, M.; Bernard, C. Drivers and ecological consequences of dominance in periurban phytoplankton communities using networks approaches. Water Res. 2019, 163, 114893. [Google Scholar] [CrossRef]
- Zhao, C.S.; Shao, N.F.; Yang, S.T.; Ren, H.; Ge, Y.R.; Feng, P.; Dong, B.E.; Zhao, Y. Predicting cyanobacteria bloom occurrence in lakes and reservoirs before blooms occur. Sci. Total Environ. 2019, 670, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Peng, Y.Q.; Zou, X.N.; Zhang, T.T.; Wu, C.; Qiao, Y.M.; Yang, H.Y. Phytoplankton Community Structure and Trophic Status Evaluation of Large Reservoir in Southern China. Environ. Sci. Technol. 2021, 44, 186–194. [Google Scholar]
- Hu, J.; Hu, X.; Mi, W.J.; Ju, Z.C.; Wang, H.R.; Dong, F.Y. Spatial Variation of Phytoplankton Community Structure and Its Relationship with Environmental Factors in the Sandy River in Summer. Ecol. Environ. Sci. 2016, 25, 9. [Google Scholar]
- Nunes, P.; Roland, F.; Amado, A.M.; Resende, N.D.; Cardoso, S.J. Responses of Phytoplanktonic Chlorophyll-a Composition to Inorganic Turbidity Caused by Mine Tailings. Front. Environ. Sci. 2022, 9, 605838. [Google Scholar] [CrossRef]
- Yuan, Y.X.; Jiang, M.; Liu, X.T.; Yu, H.X.; Otte, M.L.; Ma, C.X.; Her, Y.G. Environmental variables influencing phytoplankton communities in hydrologically connected aquatic habitats in the Lake Xingkai basin. Ecol. Indic. 2018, 91, 1–12. [Google Scholar] [CrossRef]
- Xiao, L.J.; Xie, J.; Tan, L.; Lei, L.M.; Peng, L.; Wang, Z.H.; Naselli-Flores, L. Iron enrichment from hypoxic hypolimnion supports the blooming of Raphidiopsis raciborskii in a tropical reservoir. Water Res. 2022, 219, 118562. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.Z.; Gao, W.L.; Li, Y.Y.; Wang, W.P.; Wang, H.T.; Liu, H.; Fan, P.P.; Fohrer, N.; Wu, N.C. Dissolved Oxygen and Water Temperature Drive Vertical Spatiotemporal Variation of Phytoplankton Community: Evidence from the Largest Diversion Water Source Area. Int. J. Environ. Res. Public Health 2023, 20, 4307. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.Y.; Soranno, P.A.; Wagner, T. The role of phosphorus and nitrogen on chlorophyll a: Evidence from hundreds of lakes. Water Res. 2020, 185, 116236. [Google Scholar] [CrossRef] [PubMed]
- Amorim, C.A.; Moura, A.D. Ecological impacts of freshwater algal blooms on water quality, plankton biodiversity, structure, and ecosystem functioning. Sci. Total Environ. 2021, 758, 143605. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.J.; Wei, Y.X. The Freshwater Algae of China, Systematics, Taxonomy and Ecology; Science Press: Beijing, China, 2006. [Google Scholar]


| Reservoir | Dry Season | Wet Season |
|---|---|---|
| CA | Microcystis sp. (0.35), Cylindrospermopsis sp. (0.22), Chlorella sp. (0.22) | Cyclotella sp. (0.36), Eudorina sp. (0.21) |
| DK | Pseudoanabaena sp. (0.59), Chlorella sp. (0.19) | Microcystis sp. (0.25) |
| DML | Cryptomonas sp. (0.38), Staurastrum sp. (0.19) | Staurodesmus sp. (0.19), Staurastrum sp. (0.17) |
| DZ | Ankistrodesmus sp. (0.57) | Scenedesmus sp. (0.33), Cyclotella sp. (0.25), Cryptomonas sp. (0.25), Cylindrospermopsis sp. (0.17) |
| FML | Coelastrum sp. (0.35), Westella sp. (0.3) | Coelastrum sp. (0.69) |
| HHLSK | Microcystis sp. (0.625) | Oscillatoria sp. (0.82) |
| HHLXK | Scenedesmus sp. (0.31), Chlorella sp. (0.23), Dolichospermum sp. (0.15) | Cyclotella sp. (0.45) |
| JX | Rhizosolenia sp. (0.28), Chlorella sp. (0.17) | Dolichospermum sp. (0.44) |
| LA | Nephrocytium sp. (0.33) | Coelastrum sp. (0.38), Cylindrospermopsis sp. (0.19) |
| LWT | Crucigenia sp. (0.17) | Nephrocytium sp. (0.43) |
| QLJ | Rhizosolenia sp. (0.19) | Limnothrix sp. (0.26), Euastrum sp. (0.26) |
| SDA | Oscillatoria sp. (0.43), Coelastrum sp. (0.35), Coelastrum sp. (0.35) | Cyclotella sp. (0.64), Melosira sp. (0.16) |
| SZK | Pseudoanabaena sp. (0.5), Cylindrospermopsis sp. (0.17) | Pseudoanabaena sp. (0.35), Microcystis sp. (0.24), Limnothrix sp. (0.29) |
| SZT | Chlorella sp. (0.21), Dinobryon sp. (0.21) | Aphanocapsa sp. (0.54) |
| TLJ | Elakatothrix sp. (0.27), Ankistrodesmus sp. (0.2), Chlorella sp. (0.2) | Cyclotella sp. (0.72) |
| XC | Chlorella sp. (0.43) | Coelastrum sp. (0.72) |
| CLP | Microcystis sp. (0.95) | Microcystis sp. (0.99) |
| LK | Cryptomonas sp. (0.22), Crucigenia sp. (0.20) | Pseudoanabaena sp. (0.5), Limnothrix sp. (0.20) |
| ML | Peridinium sp. (0.21), Scenedesmus sp. (0.18) | Nephrocytium sp. (0.59) |
| SZ | Aphanizomenon sp. (0.21), Mougeotia sp. (0.21) | Cyclotella sp. (0.29) |
| XL | Melosira sp. (0.75) | Dolichospermum sp. (0.22), Pseudoanabaena sp. (0.21) |
| EJ | Pseudoanabaena sp. (0.47) | Pseudoanabaena sp. (0.62) |
| GM | Pseudoanabaena sp. (0.83) | Pseudoanabaena sp. (0.49), Limnothrix sp. (0.21) |
| LT | Cryptomonas sp. (0.22), Crucigenia sp. (0.20) | Limnothrix sp. (0.41), Pseudoanabaena sp. (0.34) |
| SY | Pseudoanabaena sp. (0.34) | Pseudoanabaena sp. (0.30), Aphanizomenon sp. (0.27), Microcystis sp. (0.21) |
| TG | Cylindrospermopsis sp. (0.17), Dolichospermum sp. (0.17) | Pseudoanabaena sp. (0.51), Cylindrospermopsis sp. (0.37) |
| XK | Melosira sp. (0.45), Dolichospermum sp. (0.21), Cyclotella sp. (0.16) | Pseudoanabaena sp. (0.64) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Q.; Jin, X.; Feng, J.; Wu, S.; Wu, J.; Liu, Y.; Xie, Z.; Li, Z.; Chen, C. Spatial and Temporal Characteristics of Phytoplankton Communities in Drinking Water Source Reservoirs in Shenzhen, China. Plants 2023, 12, 3933. https://doi.org/10.3390/plants12233933
Liang Q, Jin X, Feng J, Wu S, Wu J, Liu Y, Xie Z, Li Z, Chen C. Spatial and Temporal Characteristics of Phytoplankton Communities in Drinking Water Source Reservoirs in Shenzhen, China. Plants. 2023; 12(23):3933. https://doi.org/10.3390/plants12233933
Chicago/Turabian StyleLiang, Qiting, Xingliang Jin, Jie Feng, Shenhao Wu, Jiajia Wu, Ying Liu, Zixin Xie, Zhi Li, and Chunxing Chen. 2023. "Spatial and Temporal Characteristics of Phytoplankton Communities in Drinking Water Source Reservoirs in Shenzhen, China" Plants 12, no. 23: 3933. https://doi.org/10.3390/plants12233933




