Melatonin-Mediated Enhancement of Photosynthetic Capacity and Photoprotection Improves Salt Tolerance in Wheat
Abstract
:1. Introduction
2. Results
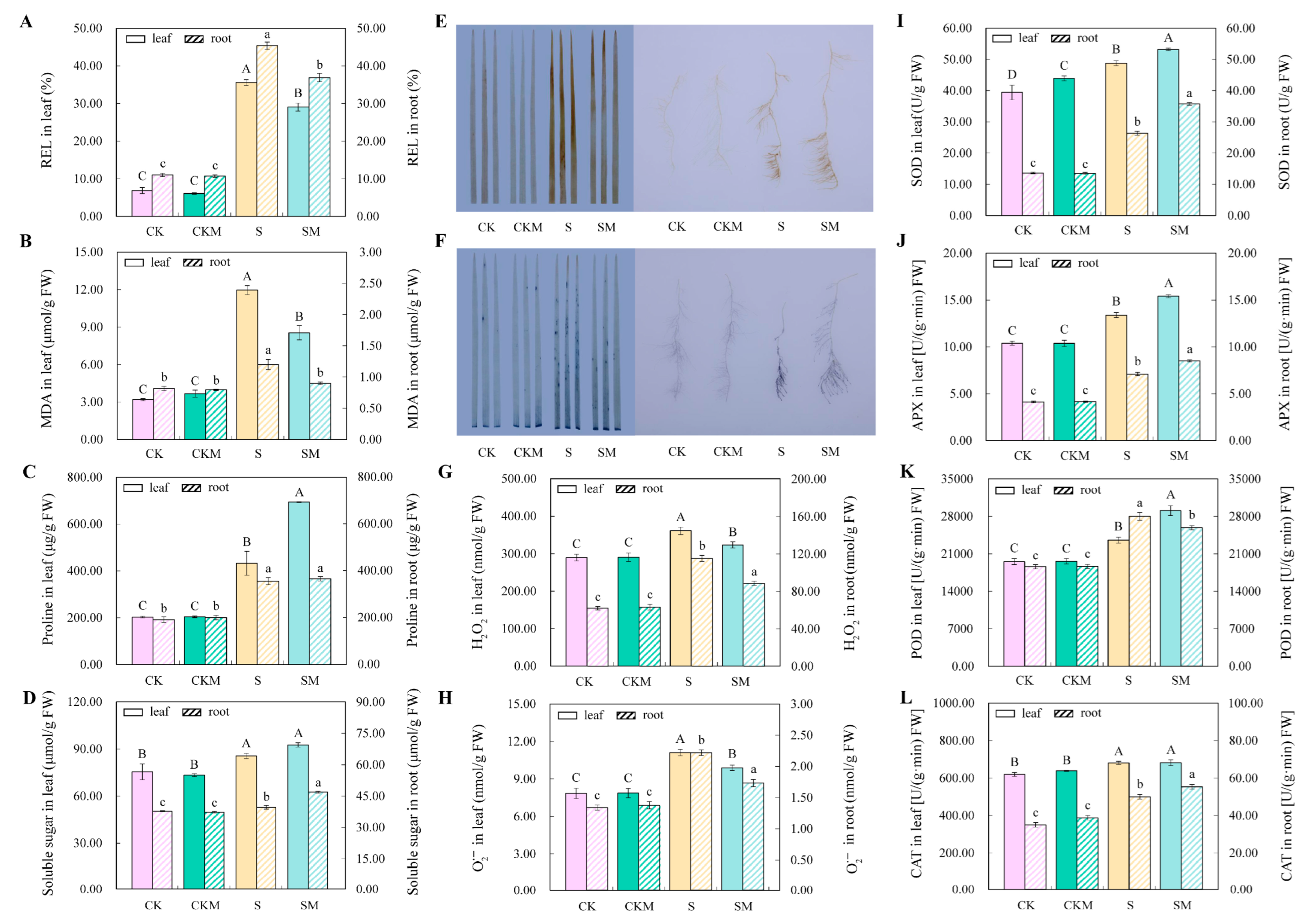
2.1. Phenotypes of Wheat Seedlings in Different Treatments under Salt Stress
2.2. Measuring the Antioxidant and Osmoregulatory Abilities of MT
2.3. Photosynthetic Parameters and Chlorophyll Content of Wheat Seedlings under Different Treatments
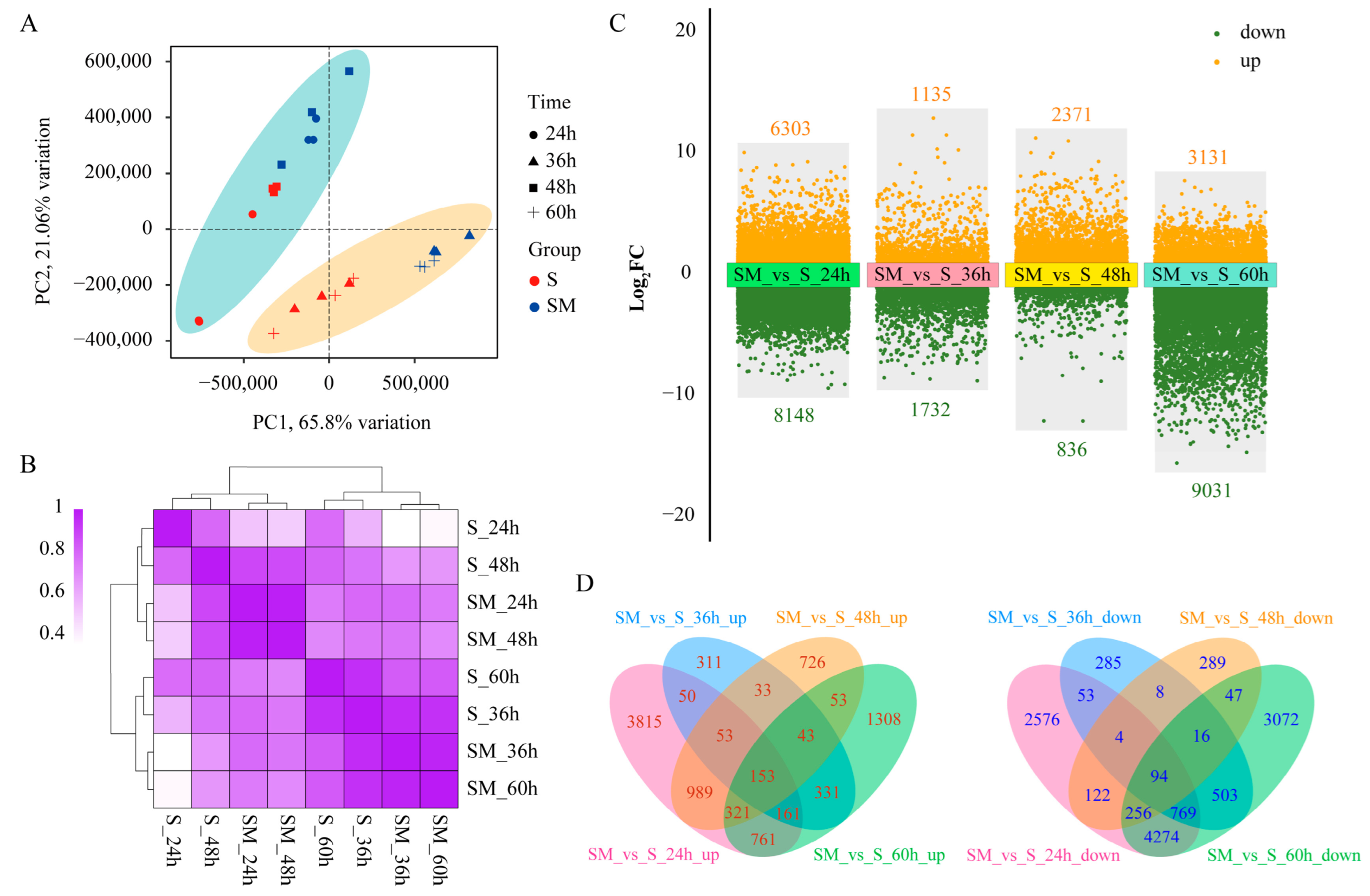
2.4. Comprehensive Transcriptome Analysis of S and SM at Different Times
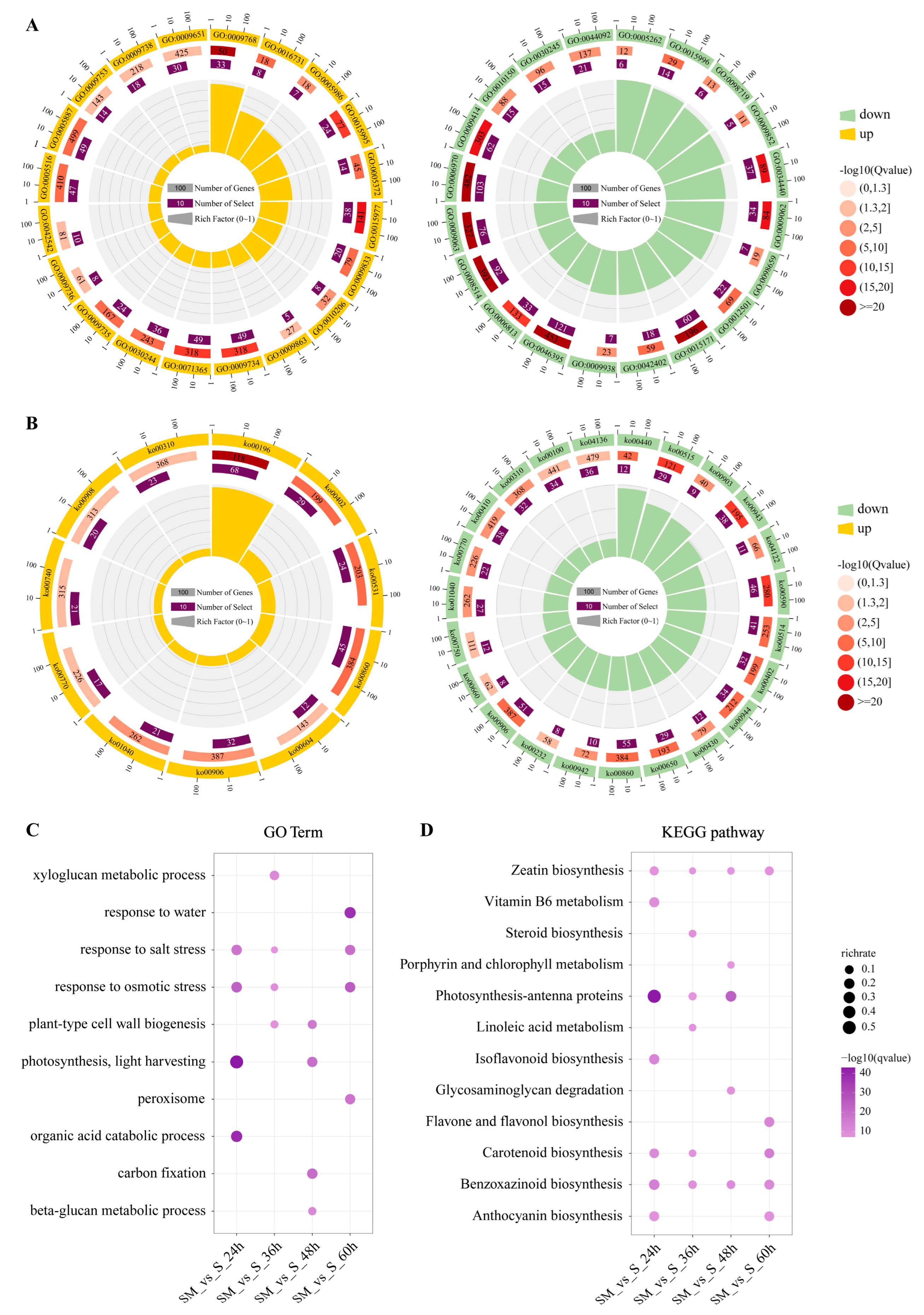
2.5. Functional and Pathway Annotation of DEGs
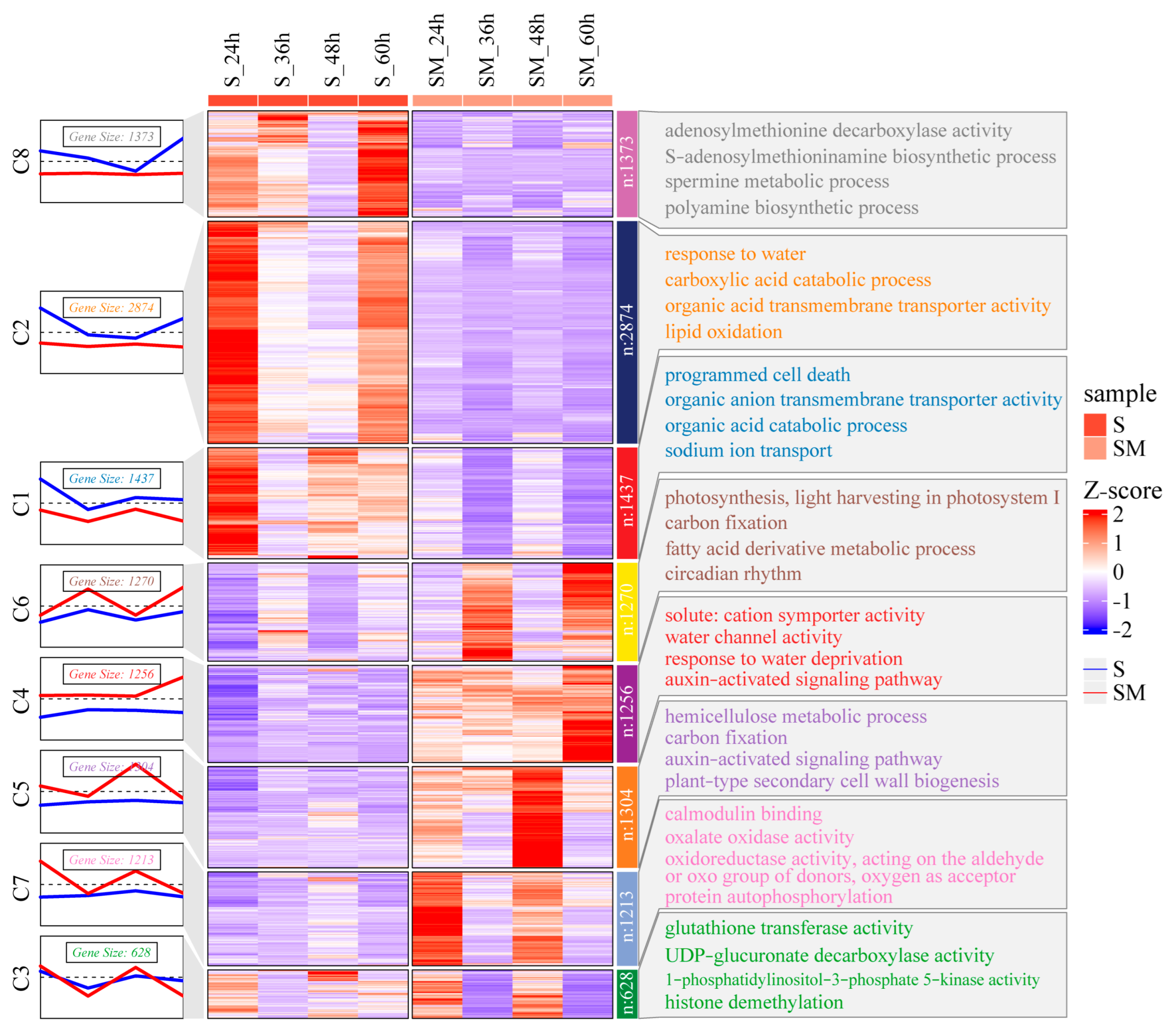
2.6. Expression Pattern Clustering of the DEGs
2.7. Transcription Factor and Co-Expression Network Analysis in S and SM Treatments
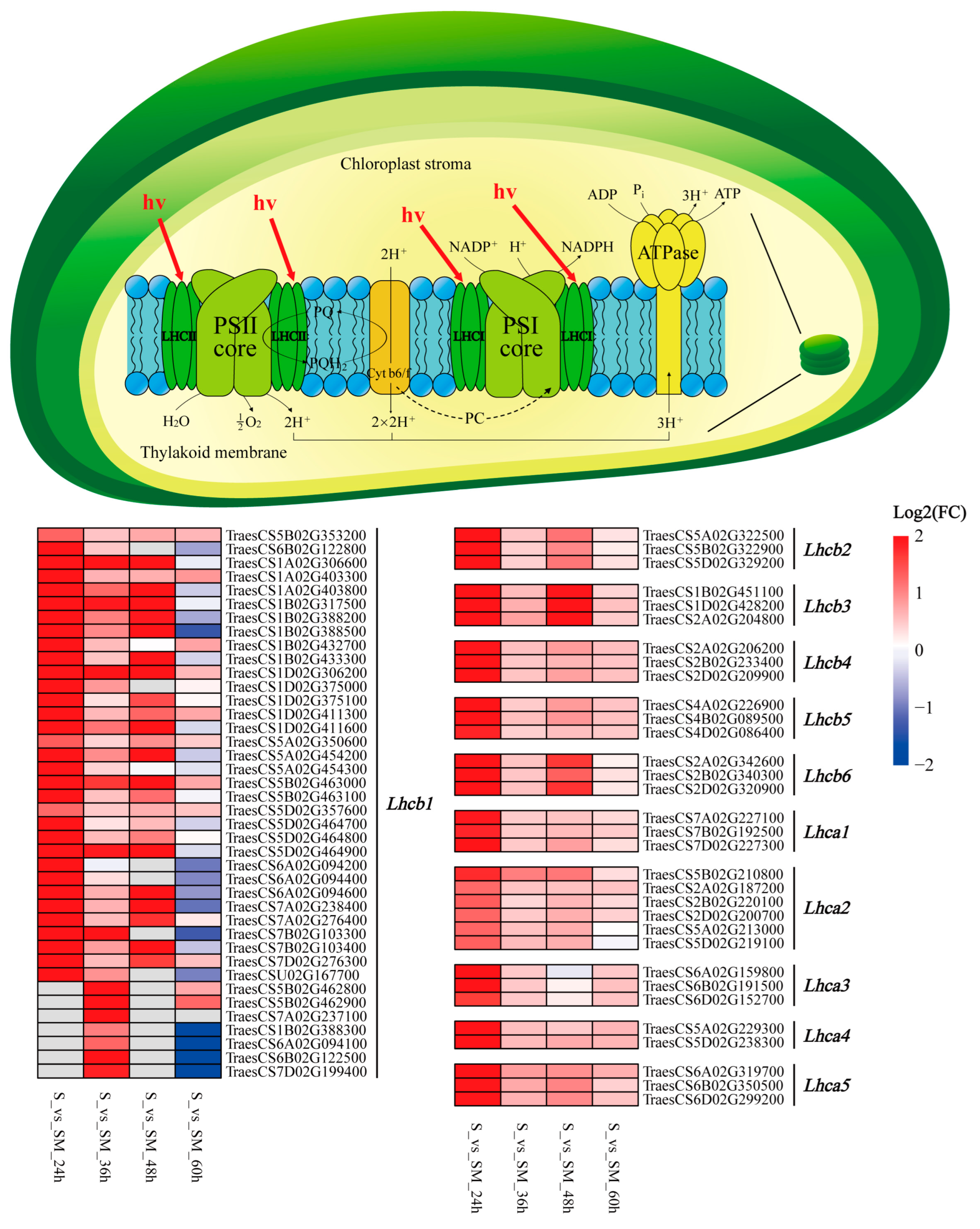
2.8. Effect of Exogenous MT on LHC
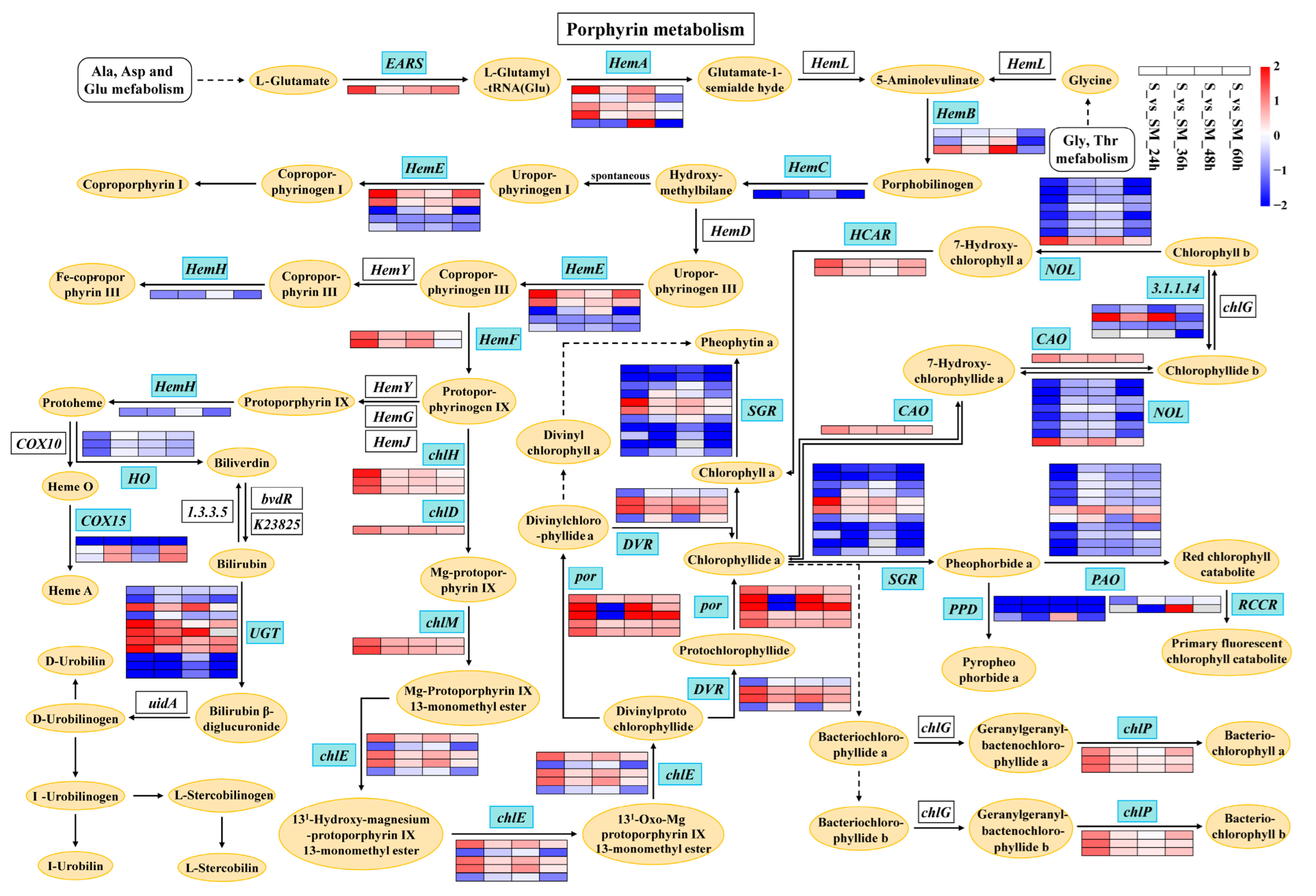
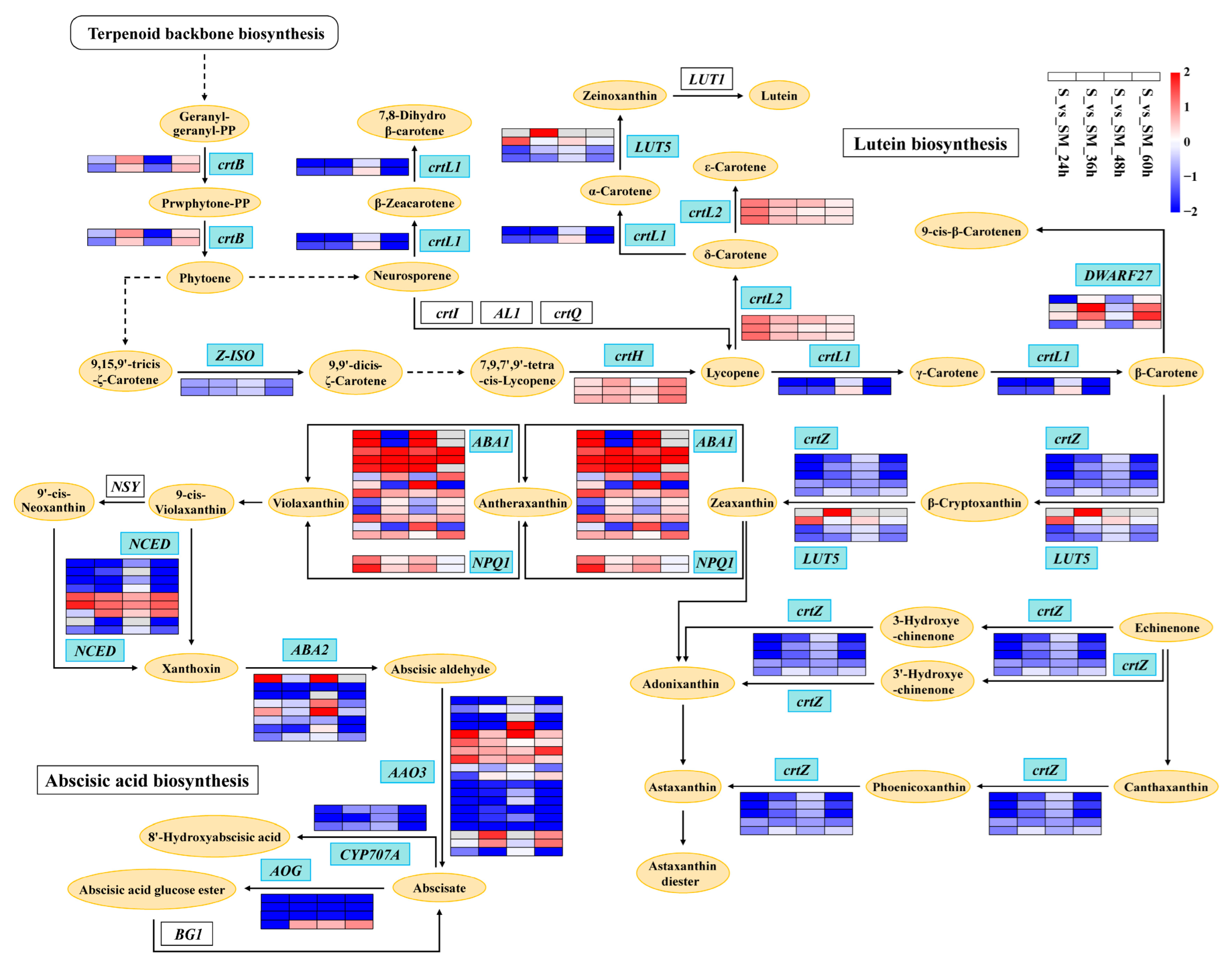
2.9. Effect of Exogenous MT on Photosynthetic Pigments
3. Discussion
3.1. Exogenous MT Alleviates the Growth Inhibition of Wheat Seedlings under Salt Stress
3.2. Exogenous MT Alleviates the Photosynthetic Inhibition Affected by Salt Stress
3.3. Exogenous MT Enhances the Light Harvesting of Photosynthesis
3.4. Exogenous MT Affects the Synthesis of Chlorophylls and Carotenoids
4. Materials and Methods
4.1. Materials and Treatments
4.2. Morphological Observation
4.3. Measurement of Malondialdehyde Content and Relative Electrolyte Leakage Rate (REL)
4.4. Measurement of Proline and Soluble Sugars Contents
4.5. Measurement of Reactive Oxygen Species and Antioxidant Enzyme Activities
4.6. Measurement of Gas Exchange Parameters and Chlorophyll Content
4.7. RNA-Seq Analysis and Quantitative Real-Time PCR Assays
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, J.-K. Plant Salt Tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lu, B.; Liu, L.; Duan, W.; Jiang, D.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Li, C.; et al. Melatonin Promotes Seed Germination under Salt Stress by Regulating ABA and GA3 in Cotton (Gossypium hirsutum L.). Plant Physiol. Biochem. 2021, 162, 506–516. [Google Scholar] [CrossRef]
- Wang, M.; Qi, Z.; Pei, W.; Cheng, Y.; Mao, K.; Ma, F. The Apple Argonaute Gene MdAGO1 Modulates Salt Tolerance. Environ. Exp. Bot. 2023, 207, 105202. [Google Scholar] [CrossRef]
- Wang, M.; Xia, G. The landscape of molecular mechanisms for salt tolerance in wheat. Crop. J. 2018, 6, 42–47. [Google Scholar] [CrossRef]
- Ju, F.; Pang, J.; Sun, L.; Gu, J.; Wang, Z.; Wu, X.; Ali, S.; Wang, Y.; Zhao, W.; Wang, S.; et al. Integrative Transcriptomic, Metabolomic and Physiological Analyses Revealed the Physiological and Molecular Mechanisms by Which Potassium Regulates the Salt Tolerance of Cotton (Gossypium hirsutum L.) Roots. Ind. Crops Prod. 2023, 193, 116177. [Google Scholar] [CrossRef]
- Gao, Y.; Lu, Y.; Wu, M.; Liang, E.; Li, Y.; Zhang, D.; Yin, Z.; Ren, X.; Dai, Y.; Deng, D.; et al. Ability to Remove Na+ and Retain K+ Correlates with Salt Tolerance in Two Maize Inbred Lines Seedlings. Front. Plant Sci. 2016, 7, 1716. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lv, P.; Yan, D.; Zhang, Z.; Xu, X.; Wang, T.; Wang, Y.; Peng, Z.; Yu, C.; Gao, Y.; et al. Exogenous Melatonin Improves Seed Germination of Wheat (Triticum aestivum L.) under Salt Stress. Int. J. Mol. Sci. 2022, 23, 8436. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Gao, X.; Lan, J.; Fu, B. Comparative Physiological and Transcriptome Analysis Reveal the Molecular Mechanism of Melatonin in Regulating Salt Tolerance in Alfalfa (Medicago sativa L.). Front. Plant Sci. 2022, 13, 919177. [Google Scholar] [CrossRef]
- Shahzad, B.; Rehman, A.; Tanveer, M.; Wang, L.; Park, S.K.; Ali, A. Salt Stress in Brassica: Effects, Tolerance Mechanisms, and Management. J. Plant Growth Regul. 2022, 41, 781–795. [Google Scholar] [CrossRef]
- Ismail, A.M.; Horie, T. Genomics, Physiology, and Molecular Breeding Approaches for Improving Salt Tolerance. Annu. Rev. Plant Biol. 2017, 68, 405–434. [Google Scholar] [CrossRef]
- Chen, M.; Yang, Z.; Liu, J.; Zhu, T.; Wei, X.; Fan, H.; Wang, B. Adaptation Mechanism of Salt Excluders under Saline Conditions and Its Applications. Int. J. Mol. Sci. 2018, 19, 3668. [Google Scholar] [CrossRef]
- Park, H.-S.; Kazerooni, E.A.; Kang, S.-M.; Al-Sadi, A.M.; Lee, I.-J. Melatonin Enhances the Tolerance and Recovery Mechanisms in Brassica juncea (L.) Czern. Under Saline Conditions. Front. Plant Sci. 2021, 12, 593717. [Google Scholar] [CrossRef] [PubMed]
- Bazihizina, N.; Colmer, T.D.; Barrett-Lennard, E.G. Response to Non-Uniform Salinity in the Root Zone of the Halophyte Atriplex Nummularia: Growth, Photosynthesis, Water Relations and Tissue Ion Concentrations. Ann. Bot. 2009, 104, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant Salt-Tolerance Mechanism: A Review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Wang, Y.; Yan, Y.; Liu, Y.; Wang, J.; Chen, S. A Review on Plant Responses to Salt Stress and Their Mechanisms of Salt Resistance. Horticulturae 2021, 7, 132. [Google Scholar] [CrossRef]
- Hernández, J.A.; Ferrer, M.A.; Jiménez, A.; Barceló, A.R.; Sevilla, F. Antioxidant Systems and O2 .−/H2O2 Production in the Apoplast of Pea Leaves. Its Relation with Salt-Induced Necrotic Lesions in Minor Veins. Plant Physiol. 2001, 127, 817–831. [Google Scholar] [CrossRef]
- Zhao, G.; Zhao, Y.; Yu, X.; Kiprotich, F.; Han, H.; Guan, R.; Wang, R.; Shen, W. Nitric Oxide Is Required for Melatonin-Enhanced Tolerance against Salinity Stress in Rapeseed (Brassica napus L.) Seedlings. Int. J. Mol. Sci. 2018, 19, 1912. [Google Scholar] [CrossRef]
- Zhu, J.-K. Salt and Drought Stress Signal Transduction in Plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef]
- Kurt-Celebi, A.; Colak, N.; Torun, H.; Dosedělová, V.; Tarkowski, P.; Ayaz, F.A. Exogenous Melatonin Ameliorates Ionizing Radiation-Induced Damage by Modulating Growth, Osmotic Adjustment and Photosynthetic Capacity in Wheat Seedlings. Plant Physiol. Biochem. 2022, 187, 67–76. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.-K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant Hormone Regulation of Abiotic Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; Wei, Z.; Liang, D.; Liu, C.; Yin, L.; Jia, D.; Fu, M.; Ma, F. The Mitigation Effects of Exogenous Melatonin on Salinity-Induced Stress in Malus Hupehensis. J. Pineal Res. 2012, 53, 298–306. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt Tolerance and Salinity Effects on Plants: A Review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Shi, H.; Ishitani, M.; Kim, C.; Zhu, J.-K. The Arabidopsis Thaliana Salt Tolerance Gene SOS1 Encodes a Putative Na+/H+ Antiporter. Proc. Natl. Acad. Sci. USA 2000, 97, 6896–6901. [Google Scholar] [CrossRef] [PubMed]
- Quan, R.; Lin, H.; Mendoza, I.; Zhang, Y.; Cao, W.; Yang, Y.; Shang, M.; Chen, S.; Pardo, J.M.; Guo, Y. SCABP8/CBL10, a Putative Calcium Sensor, Interacts with the Protein Kinase SOS2 to Protect Arabidopsis Shoots from Salt Stress. Plant Cell 2007, 19, 1415–1431. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhu, J.-K. Regulation of Expression of the Vacuolar Na+/H+ Antiporter Gene AtNHX1 by Salt Stress and Abscisic Acid. Plant Mol. Biol. 2002, 50, 543–550. [Google Scholar] [CrossRef]
- Blumwald, E. Sodium Transport and Salt Tolerance in Plants. Curr. Opin. Cell Biol. 2000, 12, 431–434. [Google Scholar] [CrossRef]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic Adjustment and Energy Limitations to Plant Growth in Saline Soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef]
- Hazman, M.; Hause, B.; Eiche, E.; Nick, P.; Riemann, M. Increased Tolerance to Salt Stress in OPDA-Deficient Rice ALLENE OXIDE CYCLASE Mutants Is Linked to an Increased ROS-Scavenging Activity. J. Exp. Bot. 2015, 66, 3339–3352. [Google Scholar] [CrossRef]
- Yu, R.; Zuo, T.; Diao, P.; Fu, J.; Fan, Y.; Wang, Y.; Zhao, Q.; Ma, X.; Lu, W.; Li, A.; et al. Melatonin Enhances Seed Germination and Seedling Growth of Medicago Sativa Under Salinity via a Putative Melatonin Receptor MsPMTR1. Front. Plant Sci. 2021, 12, 702875. [Google Scholar] [CrossRef]
- Calvo, J.R.; González-Yanes, C.; Maldonado, M.D. The Role of Melatonin in the Cells of the Innate Immunity: A Review. J. Pineal Res. 2013, 55, 103–120. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin as a Natural Ally against Oxidative Stress: A Physicochemical Examination. J. Pineal Res. 2011, 51, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kolář, J.; Macháčková, I. Melatonin in Higher Plants: Occurrence and Possible Functions. J. Pineal Res. 2005, 39, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of Melatonin in Plants and Its Effects on Plasma Melatonin Levels and Binding to Melatonin Receptors in Vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in Edible Plants Identified by Radioimmunoassay and by High Performance Liquid Chromatography-Mass Spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef]
- Manchester, L.C.; Tan, D.X.; Reiter, R.J.; Park, W.; Monis, K.; Qi, W. High Levels of Melatonin in the Seeds of Edible Plants: Possible Function in Germ Tissue Protection. Life Sci. 2000, 67, 3023–3029. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. The Physiological Function of Melatonin in Plants. Plant Signal Behav. 2006, 1, 89–95. [Google Scholar] [CrossRef]
- Yan, F.; Wei, H.; Ding, Y.; Li, W.; Chen, L.; Ding, C.; Tang, S.; Jiang, Y.; Liu, Z.; Li, G. Melatonin Enhances Na+/K+ Homeostasis in Rice Seedlings under Salt Stress through Increasing the Root H+-Pump Activity and Na+/K+ Transporters Sensitivity to ROS/RNS. Environ. Exp. Bot. 2021, 182, 104328. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, B.; Zhang, H.-J.; Weeda, S.; Yang, C.; Yang, Z.-C.; Ren, S.; Guo, Y.-D. Melatonin Promotes Water-Stress Tolerance, Lateral Root Formation, and Seed Germination in Cucumber (Cucumis sativus L.). J. Pineal Res. 2013, 54, 15–23. [Google Scholar] [CrossRef]
- Liang, C.; Zheng, G.; Li, W.; Wang, Y.; Hu, B.; Wang, H.; Wu, H.; Qian, Y.; Zhu, X.-G.; Tan, D.-X.; et al. Melatonin Delays Leaf Senescence and Enhances Salt Stress Tolerance in Rice. J. Pineal Res. 2015, 59, 91–101. [Google Scholar] [CrossRef]
- Kołodziejczyk, I.; Kaźmierczak, A.; Posmyk, M.M. Melatonin Application Modifies Antioxidant Defense and Induces Endoreplication in Maize Seeds Exposed to Chilling Stress. Int. J. Mol. Sci. 2021, 22, 8628. [Google Scholar] [CrossRef]
- Ni, J.; Wang, Q.; Shah, F.A.; Liu, W.; Wang, D.; Huang, S.; Fu, S.; Wu, L. Exogenous Melatonin Confers Cadmium Tolerance by Counterbalancing the Hydrogen Peroxide Homeostasis in Wheat Seedlings. Molecules 2018, 23, 799. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.-H.; Huang, B.; Ding, C.-B.; Zhang, Z.-W.; Chen, Y.-E.; Hu, C.; Zhou, L.-J.; Huang, Y.; Liao, J.-Q.; Yuan, S.; et al. Effects of Melatonin on Anti-Oxidative Systems and Photosystem II in Cold-Stressed Rice Seedlings. Front. Plant Sci. 2017, 8, 785. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-E.; Mao, J.-J.; Sun, L.-Q.; Huang, B.; Ding, C.-B.; Gu, Y.; Liao, J.-Q.; Hu, C.; Zhang, Z.-W.; Yuan, S.; et al. Exogenous Melatonin Enhances Salt Stress Tolerance in Maize Seedlings by Improving Antioxidant and Photosynthetic Capacity. Physiol. Plant 2018, 164, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.S.; Guo, S.; Baloch, A.R.; Sun, J.; Shu, S.; Wang, Y.; Ahammed, G.J.; Kabir, K.; Roy, R. Melatonin Alleviates Nickel Phytotoxicity by Improving Photosynthesis, Secondary Metabolism and Oxidative Stress Tolerance in Tomato Seedlings. Ecotoxicol. Environ. Saf. 2020, 197, 110593. [Google Scholar] [CrossRef]
- Tan, D.-X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One Molecule, Many Derivatives: A Never-Ending Interaction of Melatonin with Reactive Oxygen and Nitrogen Species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, X.; Zhang, D.; Liu, H.; Guan, K. Effects of Drought Stress on the Seed Germination and Early Seedling Growth of the Endemic Desert Plant Eremosparton Songoricum (Fabaceae). EXCLI J. 2013, 12, 89–101. [Google Scholar] [PubMed]
- Huang, B.; Chen, Y.-E.; Zhao, Y.-Q.; Ding, C.-B.; Liao, J.-Q.; Hu, C.; Zhou, L.-J.; Zhang, Z.-W.; Yuan, S.; Yuan, M. Exogenous Melatonin Alleviates Oxidative Damages and Protects Photosystem II in Maize Seedlings Under Drought Stress. Front Plant Sci. 2019, 10, 677. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, Y.; Liu, Z.; Jin, W.; Sun, Y. Effects of Melatonin on Seedling Growth, Mineral Nutrition, and Nitrogen Metabolism in Cucumber under Nitrate Stress. J. Pineal Res. 2017, 62, e12403. [Google Scholar] [CrossRef]
- Park, S.; Back, K. Melatonin Promotes Seminal Root Elongation and Root Growth in Transgenic Rice after Germination. J. Pineal Res. 2012, 53, 385–389. [Google Scholar] [CrossRef]
- Javeed, H.M.R.; Ali, M.; Skalicky, M.; Nawaz, F.; Qamar, R.; Rehman, A.U.; Faheem, M.; Mubeen, M.; Iqbal, M.M.; Rahman, M.H.U.; et al. Lipoic Acid Combined with Melatonin Mitigates Oxidative Stress and Promotes Root Formation and Growth in Salt-Stressed Canola Seedlings (Brassica napus L.). Molecules 2021, 26, 3147. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Ye, J.; Wang, B.; Ren, J.; Yin, L.; Deng, X.; Wang, S. Melatonin Mitigates Salt Stress in Wheat Seedlings by Modulating Polyamine Metabolism. Front Plant Sci. 2018, 9, 914. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Feng, Z.; Bai, Q.; He, J.; Wang, Y. Melatonin-Mediated Regulation of Growth and Antioxidant Capacity in Salt-Tolerant Naked Oat under Salt Stress. Int. J. Mol. Sci. 2019, 20, 1176. [Google Scholar] [CrossRef] [PubMed]
- Yeo, A. Molecular Biology of Salt Tolerance in the Context of Whole-Plant Physiology. J. Exp. Bot. 1998, 49, 915–929. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of Enzymatic and Nonenzymatic Antioxidants in Plants during Abiotic Stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive Oxygen Species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tan, D.-X.; Jiang, D.; Liu, F. Melatonin Enhances Cold Tolerance in Drought-Primed Wild-Type and Abscisic Acid-Deficient Mutant Barley. J. Pineal Res. 2016, 61, 328–339. [Google Scholar] [CrossRef]
- Flexas, J.; Carriquí, M. Photosynthesis and Photosynthetic Efficiencies along the Terrestrial Plant’s Phylogeny: Lessons for Improving Crop Photosynthesis. Plant J. 2020, 101, 964–978. [Google Scholar] [CrossRef]
- Hichem, H.; El Naceur, A.; Mounir, D. Effects of Salt Stress on Photosynthesis, PSII Photochemistry and Thermal Energy Dissipation in Leaves of Two Corn (Zea mays L.) Varieties. Photosynthetica 2009, 47, 517–526. [Google Scholar] [CrossRef]
- das Neves, J.P.C.; Ferreira, L.F.P.; Vaz, M.M.; Gazarini, L.C. Gas Exchange in the Salt Marsh Species Atriplex Portulacoides L. and Limoniastrum monopetalum L. in Southern Portugal. Acta Physiol Plant 2008, 30, 91–97. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, W.; Yang, Q.-Y.; Zhang, S.-B.; Hu, H. Effect of Growth Temperature on the Electron Flow for Photorespiration in Leaves of Tobacco Grown in the Field. Physiol. Plant. 2013, 149, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Agarwal, R.M. Salinity Stress Induced Alterations in Antioxidant Metabolism and Nitrogen Assimilation in Wheat (Triticum aestivum L) as Influenced by Potassium Supplementation. Plant Physiol. Biochem. 2017, 115, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Ahanger, M.A.; Zhou, J.; Ahmed, N.; Wei, C.; Yuan, S.; Ashraf, M.; Zhang, L. Beneficial Role of Acetylcholine in Chlorophyll Metabolism and Photosynthetic Gas Exchange in Nicotiana Benthamiana Seedlings under Salinity Stress. Plant Biol. 2020, 22, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Zahra, N.; Al Hinai, M.S.; Hafeez, M.B.; Rehman, A.; Wahid, A.; Siddique, K.H.M.; Farooq, M. Regulation of Photosynthesis under Salt Stress and Associated Tolerance Mechanisms. Plant Physiol. Biochem. 2022, 178, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Zhao, X.; Liu, S.; Sun, F.; Zhang, C.; Xi, Y. Beneficial Effects of Melatonin in Overcoming Drought Stress in Wheat Seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhao, H.; Cao, K.; Hu, L.; Du, T.; Baluška, F.; Zou, Z. Beneficial Roles of Melatonin on Redox Regulation of Photosynthetic Electron Transport and Synthesis of D1 Protein in Tomato Seedlings under Salt Stress. Front. Plant Sci. 2016, 7, 1823. [Google Scholar] [CrossRef]
- Turk, H.; Erdal, S.; Genisel, M.; Atici, O.; Demir, Y.; Yanmis, D. The Regulatory Effect of Melatonin on Physiological, Biochemical and Molecular Parameters in Cold-Stressed Wheat Seedlings. Plant Growth Regul. 2014, 74, 139–152. [Google Scholar] [CrossRef]
- Brestic, M.; Zivcak, M.; Kunderlikova, K.; Sytar, O.; Shao, H.; Kalaji, H.M.; Allakhverdiev, S.I. Low PSI Content Limits the Photoprotection of PSI and PSII in Early Growth Stages of Chlorophyll B-Deficient Wheat Mutant Lines. Photosynth. Res. 2015, 125, 151–166. [Google Scholar] [CrossRef]
- Horton, P.; Ruban, A. Molecular Design of the Photosystem II Light-Harvesting Antenna: Photosynthesis and Photoprotection. J. Exp. Bot. 2005, 56, 365–373. [Google Scholar] [CrossRef]
- Jiang, Q.; Xu, Z.-S.; Wang, F.; Li, M.-Y.; Ma, J.; Xiong, A.-S. Effects of Abiotic Stresses on the Expression of Lhcb1 Gene and Photosynthesis of Oenanthe Javanica and Apium Graveolens. Biol. Plant 2014, 58, 256–264. [Google Scholar] [CrossRef]
- Lin, S.; Song, X.-F.; Mao, H.-T.; Li, S.-Q.; Gan, J.-Y.; Yuan, M.; Zhang, Z.-W.; Yuan, S.; Zhang, H.-Y.; Su, Y.-Q.; et al. Exogenous Melatonin Improved Photosynthetic Efficiency of Photosystem II by Reversible Phosphorylation of Thylakoid Proteins in Wheat under Osmotic Stress. Front. Plant Sci. 2022, 13, 966181. [Google Scholar] [CrossRef] [PubMed]
- Alboresi, A.; Ballottari, M.; Hienerwadel, R.; Giacometti, G.M.; Morosinotto, T. Antenna Complexes Protect Photosystem I from Photoinhibition. BMC Plant Biol. 2009, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- de Bianchi, S.; Betterle, N.; Kouril, R.; Cazzaniga, S.; Boekema, E.; Bassi, R.; Dall’Osto, L. Arabidopsis Mutants Deleted in the Light-Harvesting Protein Lhcb4 Have a Disrupted Photosystem II Macrostructure and Are Defective in Photoprotection. Plant Cell 2011, 23, 2659–2679. [Google Scholar] [CrossRef] [PubMed]
- Dall’Osto, L.; Caffarri, S.; Bassi, R. A Mechanism of Nonphotochemical Energy Dissipation, Independent from PsbS, Revealed by a Conformational Change in the Antenna Protein CP26. Plant Cell 2005, 17, 1217–1232. [Google Scholar] [CrossRef]
- Liang, X.; Qiao, D.; Huang, M.; Yi, X.; Bai, L.; Xu, H.; Wei, L.; Zeng, J.; Cao, Y. Identification of a Gene Encoding the Light-Harvesting Chlorophyll a/b Proteins of Photosystem I in Green Alga Dunaliella Salina. DNA Seq. 2008, 19, 137–145. [Google Scholar] [CrossRef]
- Jansson, S. A Guide to the Lhc Genes and Their Relatives in Arabidopsis. Trends Plant Sci. 1999, 4, 236–240. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W. Photoprotection in an Ecological Context: The Remarkable Complexity of Thermal Energy Dissipation. New Phytol. 2006, 172, 11–21. [Google Scholar] [CrossRef]
- Matsubara, S.; Krause, G.H.; Seltmann, M.; Virgo, A.; Kursar, T.A.; Jahns, P.; Winter, K. Lutein Epoxide Cycle, Light Harvesting and Photoprotection in Species of the Tropical Tree Genus Inga. Plant Cell Environ. 2008, 31, 548–561. [Google Scholar] [CrossRef]
- Olson, J.A.; Krinsky, N.I. Introduction: The Colorful, Fascinating World of the Carotenoids: Important Physiologic Modulators. FASEB J. 1995, 9, 1547–1550. [Google Scholar] [CrossRef]
- McElroy, J.S.; Kopsell, D.A.; Sorochan, J.C.; Sams, C.E. Response of Creeping Bentgrass Carotenoid Composition to High and Low Irradiance. Crop. Sci. 2006, 46, 2606–2612. [Google Scholar] [CrossRef]
- Frank, H.A.; Cogdell, R.J. Carotenoids in Photosynthesis. Photochem. Photobiol. 1996, 63, 257–264. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Gilmore, A.M.; Adams, W.W. Carotenoids 3: In Vivo Function of Carotenoids in Higher Plants. FASEB J. 1996, 10, 403–412. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the Thiobarbituric Acid-Reactive-Substances Assay for Estimating Lipid Peroxidation in Plant Tissues Containing Anthocyanin and Other Interfering Compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Diao, M.; Ma, L.; Wang, J.; Cui, J.; Fu, A.; Liu, H. Selenium Promotes the Growth and Photosynthesis of Tomato Seedlings Under Salt Stress by Enhancing Chloroplast Antioxidant Defense System. J. Plant Growth Regul. 2014, 33, 671–682. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant Responses of Rice Seedlings to Salinity Stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Ye, T.; Shi, H.; Wang, Y.; Chan, Z. Contrasting Changes Caused by Drought and Submergence Stresses in Bermudagrass (Cynodon Dactylon). Front. Plant Sci. 2015, 6, 951. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Qian, Y.; Tan, D.-X.; Reiter, R.J.; He, C. Melatonin Induces the Transcripts of CBF/DREB1s and Their Involvement in Both Abiotic and Biotic Stresses in Arabidopsis. J. Pineal Res. 2015, 59, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Jiang, D.; Liu, F.; Dai, T.; Jing, Q.; Cao, W. Effects of Salt and Waterlogging Stresses and Their Combination on Leaf Photosynthesis, Chloroplast ATP Synthesis, and Antioxidant Capacity in Wheat. Plant Sci. 2009, 176, 575–582. [Google Scholar] [CrossRef]
- Ke, D.; Sun, G.; Wang, Z. Effects of Superoxide Radicals on ACC Synthase Activity in Chilling-Stressed Etiolated Mungbean Seedlings. Plant Growth Regul. 2007, 51, 83–91. [Google Scholar] [CrossRef]
- Jabs, T.; Dietrich, R.A.; Dangl, J.L. Initiation of Runaway Cell Death in an Arabidopsis Mutant by Extracellular Superoxide. Science 1996, 273, 1853–1856. [Google Scholar] [CrossRef]
- Dunand, C.; Crèvecoeur, M.; Penel, C. Distribution of Superoxide and Hydrogen Peroxide in Arabidopsis Root and Their Influence on Root Development: Possible Interaction with Peroxidases. New Phytol. 2007, 174, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.R.; Bewley, J.D. Lipid Peroxidation Associated with Accelerated Aging of Soybean Axes. Plant Physiol. 1980, 65, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Polle, A.; Otter, T.; Seifert, F. Apoplastic Peroxidases and Lignification in Needles of Norway Spruce (Picea abies L.). Plant Physiol. 1994, 106, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Patra, H.K.; Kar, M.; Mishra, D. Catalase Activity in Leaves and Cotyledons During Plant Development and Senescence1)1) Part X of the Series “Studies on Leaf Senescence”. Supported in Part by a Grant from the Council of Scientific and Industrial Research, Government of India to D.M. Biochem. Und Physiol. Der Pflanz. 1978, 172, 385–390. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.; Li, X.; Xia, X.-J.; Zhou, Y.-H.; Shi, K.; Chen, Z.; Yu, J.-Q. H2O2 Mediates the Crosstalk of Brassinosteroid and Abscisic Acid in Tomato Responses to Heat and Oxidative Stresses. J. Exp. Bot. 2014, 65, 4371–4383. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-J.; Du, Y.-T.; Zhou, Y.-B.; Chen, J.; Xu, Z.-S.; Ma, Y.-Z.; Chen, M.; Min, D.-H. Overexpression of TaCOMT Improves Melatonin Production and Enhances Drought Tolerance in Transgenic Arabidopsis. Int. J. Mol. Sci. 2019, 20, 652. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol 2014, 15, 550. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, D.; Wang, J.; Lu, Z.; Liu, R.; Hong, Y.; Su, B.; Wang, Y.; Peng, Z.; Yu, C.; Gao, Y.; et al. Melatonin-Mediated Enhancement of Photosynthetic Capacity and Photoprotection Improves Salt Tolerance in Wheat. Plants 2023, 12, 3984. https://doi.org/10.3390/plants12233984
Yan D, Wang J, Lu Z, Liu R, Hong Y, Su B, Wang Y, Peng Z, Yu C, Gao Y, et al. Melatonin-Mediated Enhancement of Photosynthetic Capacity and Photoprotection Improves Salt Tolerance in Wheat. Plants. 2023; 12(23):3984. https://doi.org/10.3390/plants12233984
Chicago/Turabian StyleYan, Di, Jiajie Wang, Zhenzong Lu, Rui Liu, Yue Hong, Baocai Su, Ye Wang, Zhen Peng, Chunxin Yu, Yuerong Gao, and et al. 2023. "Melatonin-Mediated Enhancement of Photosynthetic Capacity and Photoprotection Improves Salt Tolerance in Wheat" Plants 12, no. 23: 3984. https://doi.org/10.3390/plants12233984




