Evaluation of Salt Stress-Induced Changes in Polyamine, Amino Acid, and Phytoalexin Profiles in Mature Fruits of Grapevine Cultivars Grown in Tunisian Oases
Abstract
:1. Introduction
2. Results
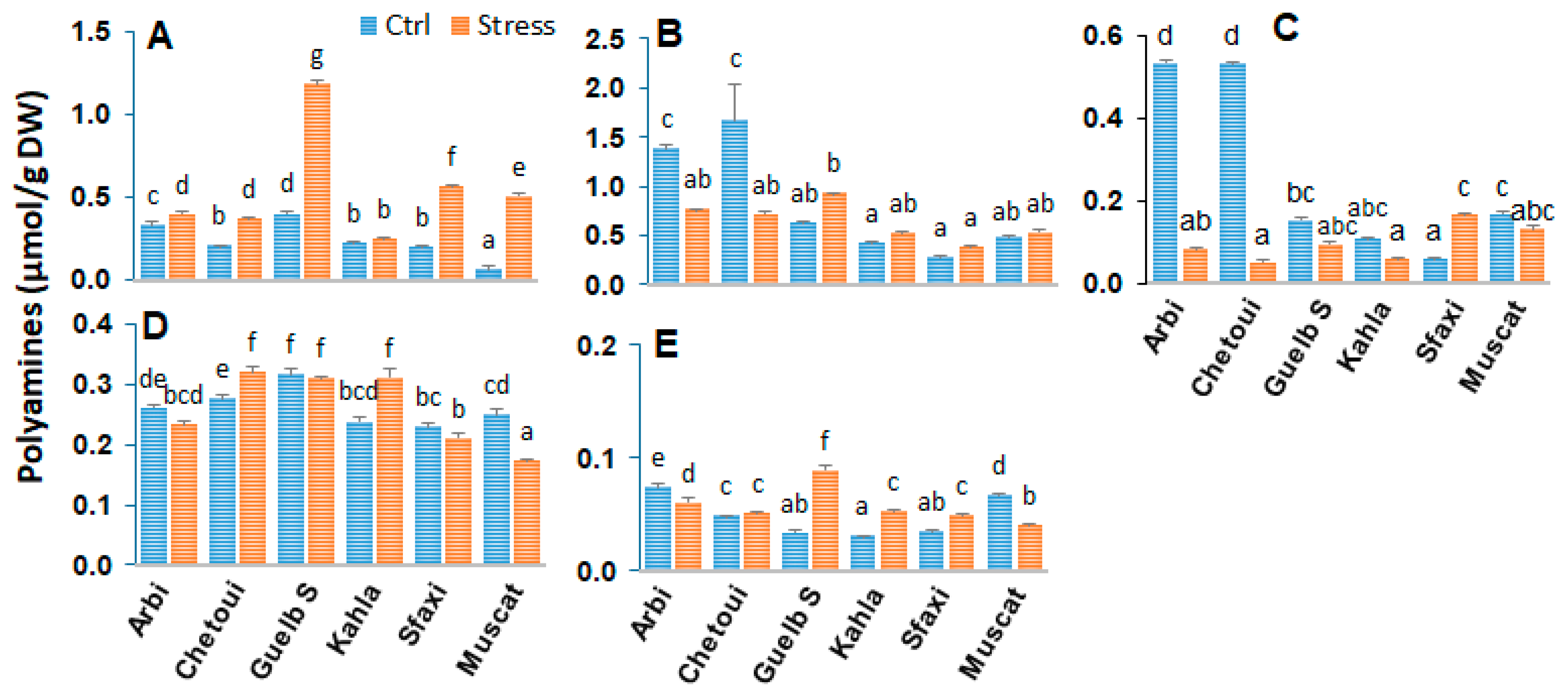
2.1. Polyamines in Berry Skin
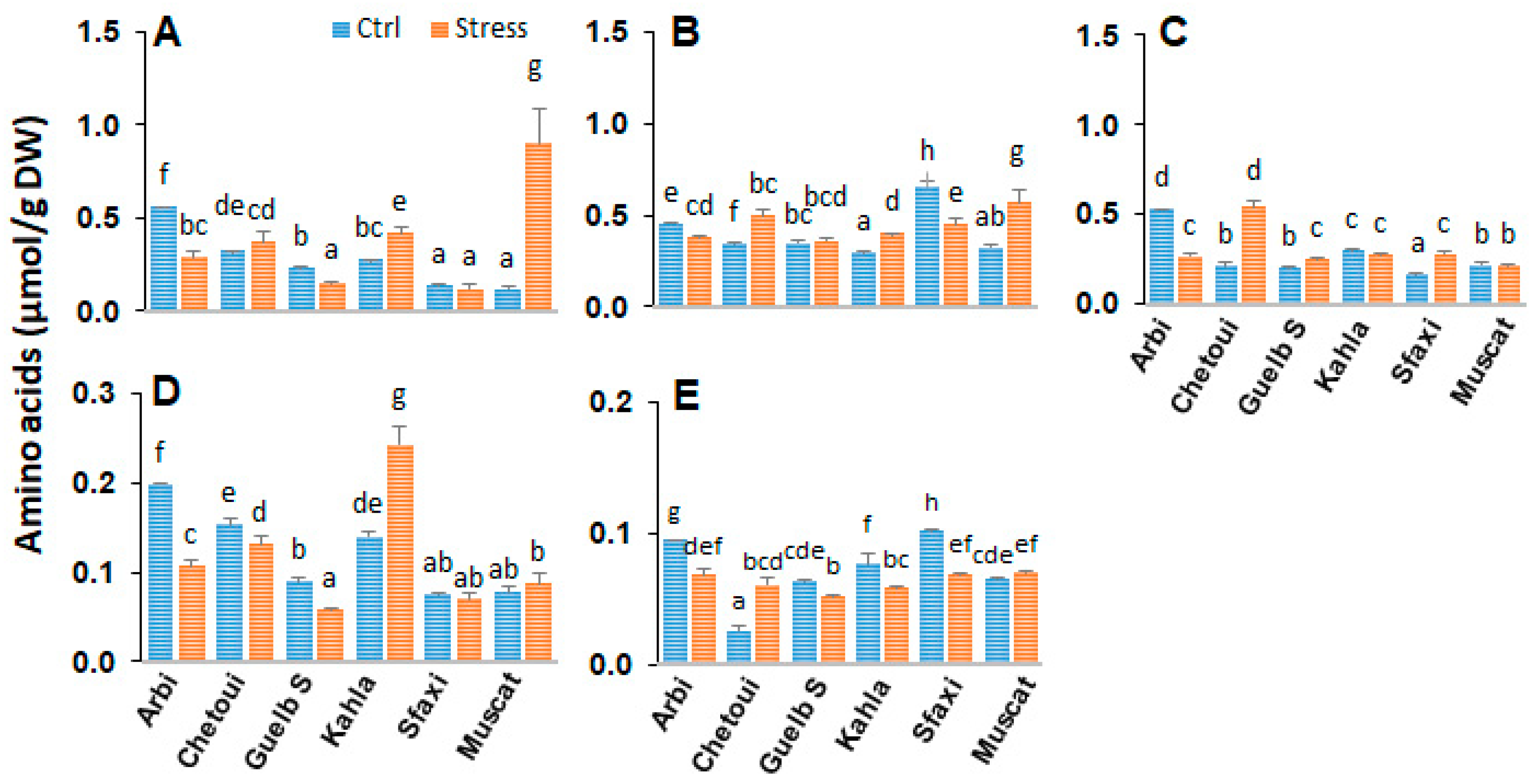
2.2. Amino Acids in Berry Skin
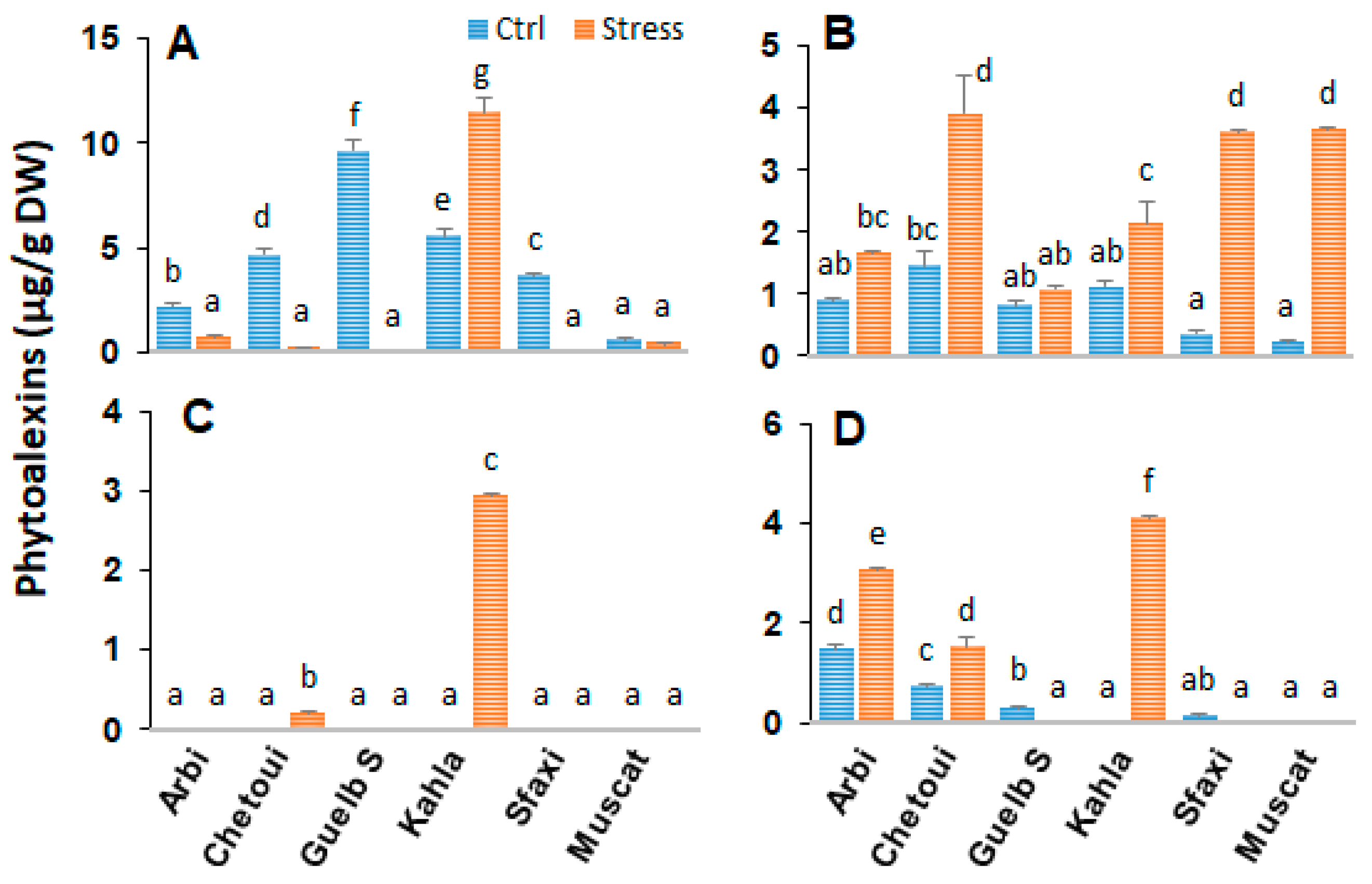
2.3. Stilbenes in Berry Skin
2.4. PCA Plot and Heatmap for Berry Skin Metabolites
2.5. Polyamines in Berry Pulp
2.6. Amino Acids in Berry Pulp
2.7. PCA Plot and Heatmap for Berry Pulp Metabolites
2.8. Polyamines in Berry Seeds
2.9. Amino Acids in Berry Seeds
2.10. Stilbenes in Berry Seeds
2.11. PCA Plot and Heatmap for Berry Seed Metabolites
3. Discussion
3.1. Polyamine Titers in Response to Salt Stress
3.2. Amino Acid Patterns in Response to Salt Stress
3.3. The Accumulation of Stilbenes as a Part of Adaptation to Salt Stress
4. Materials and Methods
4.1. Site Selection and Climate of the Oases
4.2. Sample Collection
4.3. Amino Acid Analysis
4.4. Polyamine Analysis
4.5. Stilbene Analysis
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Negrào, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhou-Tsang, A.; Wu, Y.; Henderson, S.; Walker, A.; Borneman, A.; Walker, R.; Gilliham, M. Grapevine salt tolerance. Aust. J. Grape Wine Res. 2021, 27, 149–168. [Google Scholar] [CrossRef]
- Daldoul, S.; Gargouri, M.; Weinert, C.; Jarrar, A.; Egert, B.; Mliki, A.; Nick, P. A Tunisian wild grape leads to metabolic fingerprints of salt tolerance. Plant Physiol. 2023, 193, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Zörb, C.; Geilfus, C.M.; Dietz, K.J. Salinity and crop yield. Plant Biol. Suppl. 2018, 1, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.; Abdelghany, A.E.; Zhang, H.; Feng, H.; Zhang, Y.; Yu, S.; Zhang, F.; Li, Z.; Fan, J. Exogenous silicon application improves fruit yield and quality of drip-irrigated greenhouse tomato by regulating physiological characteristics and growth under combined drought and salt stress. Sci. Hortic. 2023, 321, 112352. [Google Scholar] [CrossRef]
- Atak, A.; Ergönül, O.; Dilli, Y.; Kesgin, M.; Altındişli, A. Grapevine breeding studies in Turkey. Acta Hortic. 2023, 1370, 145–152. [Google Scholar] [CrossRef]
- Jeandet, P.; Vannozzi, A.; Sobarzo-Sánchez, E.; Uddin, S.; Bru, R.; Martínez-Márquez, A.; Clément, C.; Cordelier, S.; Manayi, A.; Rasekhian, M.; et al. Phytostilbenes as agrochemicals: Biosynthesis, bioactivity, metabolic engineering and biotechnology. Nat. Prod. Rep. 2021, 28, 1282–1329. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- OIV: International Organization of Vine and Wine (2019) Report on the World Viti-Vinicultural Situation. Available online: https://www.oecd-ilibrary.org/governance/international-regulatory-co-operation/international-organisation-of-vine-and-wine-oiv_9789264244047-44-en (accessed on 15 January 2020).
- Atak, A. New Perspectives in Grapevine Breeding, in Plant Breeding—New Perspectives; Wang, H., Ed.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar] [CrossRef]
- Louime, L.; Vasanthaiah, L.K.; Basha, S.M.; Lu, J. Perspective of biotic and abiotic stress research in grapevines (Vitis sp.). Int. J. Fruit Sci. 2010, 10, 79–86. [Google Scholar] [CrossRef]
- Gray, D.J.; Li, Z.T.; Dhekney, S.A. Precision breeding of grapevine (Vitis vinifera L.) for improved traits. Plant Sci. 2014, 228, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Paschalidis, K.A.; Moschou, P.N.; Aziz, A.; Toumi, I.; Roubelakis-Angelakis, K.A. Polyamines in grapevine: An update. In Grapevine Molecular Physiology & Biotechnology; Roubelakis-Angelakis, K.A., Ed.; Springer Publisher: New York, NY, USA, 2009; pp. 207–228. ISBN 978-90-481-2304-9. [Google Scholar]
- Degu, A.; Hochberg, U.; Sikron, N.; Venturini, L.; Buson, G.; Ghan, R.; Plaschkes, I.; Batushansky, A.; Chalifa-Caspi, V.; Mattivi, F.; et al. Metabolite and transcript profiling of berry skin during fruit development elucidates differential regulation between Cabernet Sauvignon and Shiraz cultivars at branching points in the polyphenol pathway. BMC Plant Biol. 2014, 14, 188. [Google Scholar] [CrossRef] [PubMed]
- Hatmi, S.; Villaume, S.; Trotel-Aziz, P.; Barka, E.A.; Clément, C.; Aziz, A. Osmotic stress and ABA affect immune response and susceptibility of grapevine berries to gray mold. Front. Plant Sci. 2018, 9, 1010. [Google Scholar] [CrossRef]
- Deluc, L.G.; Grimplet, J.; Wheatley, M.D.; Tillett, R.L.; Quilici, D.R.; Osborne, C.; Schooley, D.A.; Schlauch, K.A.; Cushman, J.C.; Cramer, G.R. Transcriptomic and metabolite analyses of Cabernet Sauvignon grape berry development. BMC Genom. 2007, 8, 429. [Google Scholar] [CrossRef]
- Fortes, A.M.; Agudelo-Romero, P.; Silva, M.S.; Ali, K.; Sousa, L.; Maltese, F.; Choi, Y.H.; Grimplet, J.; Zapater, J.M.M.; Verpoorte, R.; et al. Transcript and metabolite analysis in Trincadeira cultivar reveal novel information regarding the dynamics of grape ripening. BMC Plant Biol. 2011, 11, 149. [Google Scholar] [CrossRef]
- Gatto, P.; Vrhovsek, U.; Muth, J.; Segala, C.; Romualdi, C.; Fontana, P.; Pruefer, D.; Stefanini, M.; Moser, C.; Mattivi, F. Ripening and genotype control stilbene accumulation in healthy grapes. J. Agric. Food Chem. 2008, 56, 11773–11785. [Google Scholar] [CrossRef]
- Suzuki, M.; Nakabayashi, R.; Mori, T.; Saito, K.; Shiratake, K. The metabolic profile of grape berry skin and a comparison of metabolomes before veraison and at harvest. Plant Biotechnol. 2015, 32, 267–272. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, Y.Y. Grape phytochemicals and associated health benefits. Crit. Rev. Food Sci. Nutr. 2013, 53, 1202–1225. [Google Scholar] [CrossRef]
- Hatmi, S.; Gruau, C.; Trotel-Aziz, P.; Villaume, S.; Rabenoelina, F.; Baillieul, F.; Eullaffroy, P.; Clément, C.; Ferchichi, A.; Aziz, A. Drought stress tolerance in grapevine involves activation of polyamine oxidation contributing to improved immune response and low susceptibility to Botrytis cinerea. J. Exp. Bot. 2015, 66, 775–787. [Google Scholar] [CrossRef]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savoure, A. Diversity, distribution, and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J.; Colmer, T.D. Plant salt tolerance: Adaptations in halophytes. Ann. Bot. 2015, 115, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Szabados, L.; Savoure, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.S.; Ali, M.; Ahmad, M.; Siddique, K.H. Polyamines: Natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol. Adv. 2011, 29, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Pál, M.; Szalai, G.; Janda, T. Speculation: Polyamines are important in abiotic stress signaling. Plant Sci. 2015, 237, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Hatmi, S.; Trotel-Aziz, P.; Villaume, S.; Couderchet, M.; Clément, C.; Aziz, A. Osmotic stress-induced polyamine oxidation mediates defense responses and reduces stress-enhanced grapevine susceptibility to Botrytis cinerea. J. Exp. Bot. 2014, 65, 75–88. [Google Scholar] [CrossRef]
- Ben Maachia, S.; Habib, A.; Sahli, A.; Sebei, H.; Harbi, M. Situation de la vigne et étude de l’effet de la salinité des eaux d’irrigation sur la fertilité des cépage s représentatifs des oasis de Tozeur. Rev. Régions Arid. 2014, 35, 1165–1170. [Google Scholar]
- Hannah, L.; Roehrdanz, P.R.; Ikegamib, M.; Shepard, A.V.; Shawc, M.R.; Tabord, G.; Zhi, L.; Marquetf, P.A.; Hijmansj, R.J. Climate change, wine, and conservation. Proc. Natl. Acad. Sci. USA 2013, 110, 6907–6912. [Google Scholar] [CrossRef]
- Walker, R.R.; Blackmore, D.H.; Clingeleffer, P.R. Impact of rootstock on yield and ion concentrations in petioles, juice, and wine of Shiraz and Chardonnay in different viticultural environments with different irrigation water salinity. Aust. J. Grape Wine Res. 2010, 16, 243–257. [Google Scholar] [CrossRef]
- Sato, S.; Sakaguchi, S.; Furukawa, H.; Ikeda, H. Effects of NaCl application to hydroponic nutrient solution on fruit characteristic of tomato (Lycopersicon esculentum Mill.). Sci. Hortic. 2006, 109, 248–253. [Google Scholar] [CrossRef]
- Kusano, T.; Berberich, T.; Tateda, C.; Takahashi, Y. Polyamines: Essential factors for growth and survival. Planta 2008, 228, 367–381. [Google Scholar] [CrossRef]
- Angelini, R.; Cona, A.; Federico, R.; Fincato, P.; Tavladoraki, P.; Tisi, A. Plant amine oxidases “on the move”: An update. Plant Physiol. Biochem. 2010, 48, 560–564. [Google Scholar] [CrossRef]
- Moschou, P.N.; Paschalidis, K.A.; Roubelakis-Angelakis, K.A. Plant polyamine catabolism: The state of the art. Plant Signal. Behav. 2008, 3, 1061–1066. [Google Scholar] [CrossRef]
- Ikbal, F.E.; Hernández, J.A.; Barba-Espín, G.; Koussa, T.; Aziz, A.; Faize, M.; Diaz-Vivancos, P. Enhanced salt-induced antioxidative responses involve a contribution of polyamine biosynthesis in grapevine plants. J. Plant Physiol. 2014, 171, 79–788. [Google Scholar] [CrossRef]
- Shabala, S.; Cuin, T.A.; Pottosin, I. Polyamines prevent NaCl-induced K1 efflux from pea mesophyll by blocking non-selective cation channels. FEBS Lett. 2007, 581, 1993–1999. [Google Scholar] [CrossRef]
- Zhao, F.; Song, C.P.; He, J.; Zhu, H. Polyamines improve K+/Na+ homeostasis in barley seedlings by regulating root ion channel activities. Plant Physiol. 2007, 145, 1061–1072. [Google Scholar] [CrossRef]
- Freitas, V.S.; Miranda, R.D.S.; Costa, J.H.; Oliveira, D.F.D.; Paula, S.D.O.; Miguel, E.D.C.; Freire, R.S.; Prisco, J.T.; Gomes-Filho, E. Ethylene triggers salt tolerance in maize genotypes by modulating polyamine catabolism enzymes associated with H2O2 production. Environ. Exp. Bot. 2018, 145, 75–86. [Google Scholar] [CrossRef]
- Wang, W.; Liu, J.H. CsPAO4 of Citrus sinensis functions in polyamine terminal catabolism and inhibits plant growth under salt stress. Sci. Rep. 2016, 6, 31384. [Google Scholar] [CrossRef]
- Wang, W.; Paschalidis, K.; Feng, J.-C.; Song, J.; Liu, J.-H. Polyamine catabolism in plants: A universal process with diverse functions. Front. Plant Sci. 2019, 10, 561. [Google Scholar] [CrossRef]
- Yu, Z.; Jia, D.; Liu, T. Polyamine oxidases play various roles in plant development and abiotic stress tolerance. Plants 2019, 8, 184. [Google Scholar] [CrossRef]
- Golestan Hashemi, F.S.; Ismail, M.R.; Rafii, M.Y.; Aslani, F.; Miah, G.; Muharam, F.M. Critical multifunctional role of the betaine aldehyde dehydrogenase gene in plants. Biotechnol. Biotechnol. Equip. 2018, 32, 815–829. [Google Scholar] [CrossRef]
- Verslues, P.E.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.; Zhu, J.-K. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006, 45, 523–539. [Google Scholar] [CrossRef]
- Du Plessis, K.; Young, P.R.; Eyéghé-Bickong, H.A.; Vivier, M.A. The transcriptional responses and metabolic consequences of acclimation to elevated light exposure in grapevine berries. Front. Plant Sci. 2017, 8, 1261. [Google Scholar] [CrossRef]
- Majumdar, R.; Shao, L.; Minocha, R.; Long, S.; Minocha, S.C. Ornithine: The overlooked molecule in the regulation of polyamine metabolism. Plant Cell Physiol. 2013, 54, 990–1004. [Google Scholar] [CrossRef]
- Podlešáková, K.; Ugena, L.; Spíchal, L.; Doležal, K.; De Diego, N. Phytohormones and polyamines regulate plant stress responses by altering GABA pathway. New Biotechnol. 2018, 88, 53–65. [Google Scholar] [CrossRef]
- Jeandet, P.; Uddin, M.S.; Clément, C.; Aziz, A.; Jacquard, C.; Khan, H.; Shah, M.A.; Barka, E.A.; Koffas, M.; Nabavi, S.M.; et al. Production of high molecular-ordered stilbene oligomers for the study of their biological activity: Total synthesis, bio-catalyzed synthesis and production by plant systems. Nat. Prod. Rep. 2023, 40, 1045–1057. [Google Scholar] [CrossRef]
- Jeandet, P.; Trotel-Aziz, P.; Jacquard, C.; Clément, C.; Mohan, C.; Morkunas, I.; Khan, H.; Aziz, A. Use of elicitors and beneficial bacteria to induce and prime the stilbene phytoalexin response: Main experiments and applications to grapevine disease resistance. Agronomy 2023, 13, 2225. [Google Scholar] [CrossRef]
- Kostopoulou, Z.; Therios, I.; Molassiotis, A. Resveratrol and its combination with α-tocopherol mediate salt adaptation in citrus seedlings. Plant Physiol. Biochem. 2014, 78, 1–9. [Google Scholar] [CrossRef]
- Valletta, A.; Iozia, L.M.; Leonelli, F. Impact of environmental factors on stilbene biosynthesis. Plants 2021, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Souid, I.; Toumi, I.; Hermosín-Gutiérre, I.; Nasri, S.; Mliki, A.; Ghorbel, A. The effect of salt stress on resveratrol et piceid accumulation in two Vitis vinifera L. cultivars. Physiol. Mol. Biol. Plant 2019, 25, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Keylor, M.H.; Matsuura, B.S.; Stephenson, C.R.J. Chemistry and biology of resveratrol-derived natural products. Chem. Rev. 2015, 115, 8976–9027. [Google Scholar] [CrossRef]
- Creasy, L.L.; Coffee, M. Phytoalexin production potential of grape berries. J. Am. Soc. Hortic. Sci. 1988, 113, 230–234. [Google Scholar] [CrossRef]
- Jeandet, P.; Bessis, R.; Gautheron, B. The production of resveratrol (3,5,4′-trihydroxystilbene) by grape berries in different developmental stages. Am. J. Enol. Vitic. 1991, 42, 41–46. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Duan, W.; Xi, H.; Wang, L.; Li, S. Accumulation and transportation of resveratrol in grapevines treated by ultraviolet-C irradiation. Life Sci. J. 2013, 10, 2535–2542. [Google Scholar]
- Li, T.; Li, Y.; Sun, Z.; Xi, X.; Sha, G.; Ma, C.; Tian, Y.; Wang, C.; Zheng, X. Resveratrol alleviates the KCl salinity stress of Malus hupehensis Rhed. Front. Plant Sci. 2021, 12, 650485. [Google Scholar] [CrossRef]
- Dhaouadi, L. Etude Expérimentale et Comparative de Différents Systèmes d’irrigation sous Palmier Dattier dans les Oasis Tunisiennes. Ph.D. Thesis, Université de Carthage, Tunis, Tunisia, 2017; 179p. [Google Scholar]
- Aziz, A.; Brun, O.; Audran, J.C. Involvement of polyamines in the control of fruitlet physiological abscission in grapevine (Vitis vinifera). Physiol. Plant. 2001, 113, 50–58. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habib, A.; Ben Maachia, S.; Namsi, A.; Harbi Ben Slimane, M.; Jeandet, P.; Aziz, A. Evaluation of Salt Stress-Induced Changes in Polyamine, Amino Acid, and Phytoalexin Profiles in Mature Fruits of Grapevine Cultivars Grown in Tunisian Oases. Plants 2023, 12, 4031. https://doi.org/10.3390/plants12234031
Habib A, Ben Maachia S, Namsi A, Harbi Ben Slimane M, Jeandet P, Aziz A. Evaluation of Salt Stress-Induced Changes in Polyamine, Amino Acid, and Phytoalexin Profiles in Mature Fruits of Grapevine Cultivars Grown in Tunisian Oases. Plants. 2023; 12(23):4031. https://doi.org/10.3390/plants12234031
Chicago/Turabian StyleHabib, Abir, Sihem Ben Maachia, Ahmed Namsi, Mounira Harbi Ben Slimane, Philippe Jeandet, and Aziz Aziz. 2023. "Evaluation of Salt Stress-Induced Changes in Polyamine, Amino Acid, and Phytoalexin Profiles in Mature Fruits of Grapevine Cultivars Grown in Tunisian Oases" Plants 12, no. 23: 4031. https://doi.org/10.3390/plants12234031





