3‴-O-Foliamenthoyl-Rutin, a New Flavonoid Glycoside from the Roots of Nymphoides peltata
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Structural Elucidation of Compounds 1–6
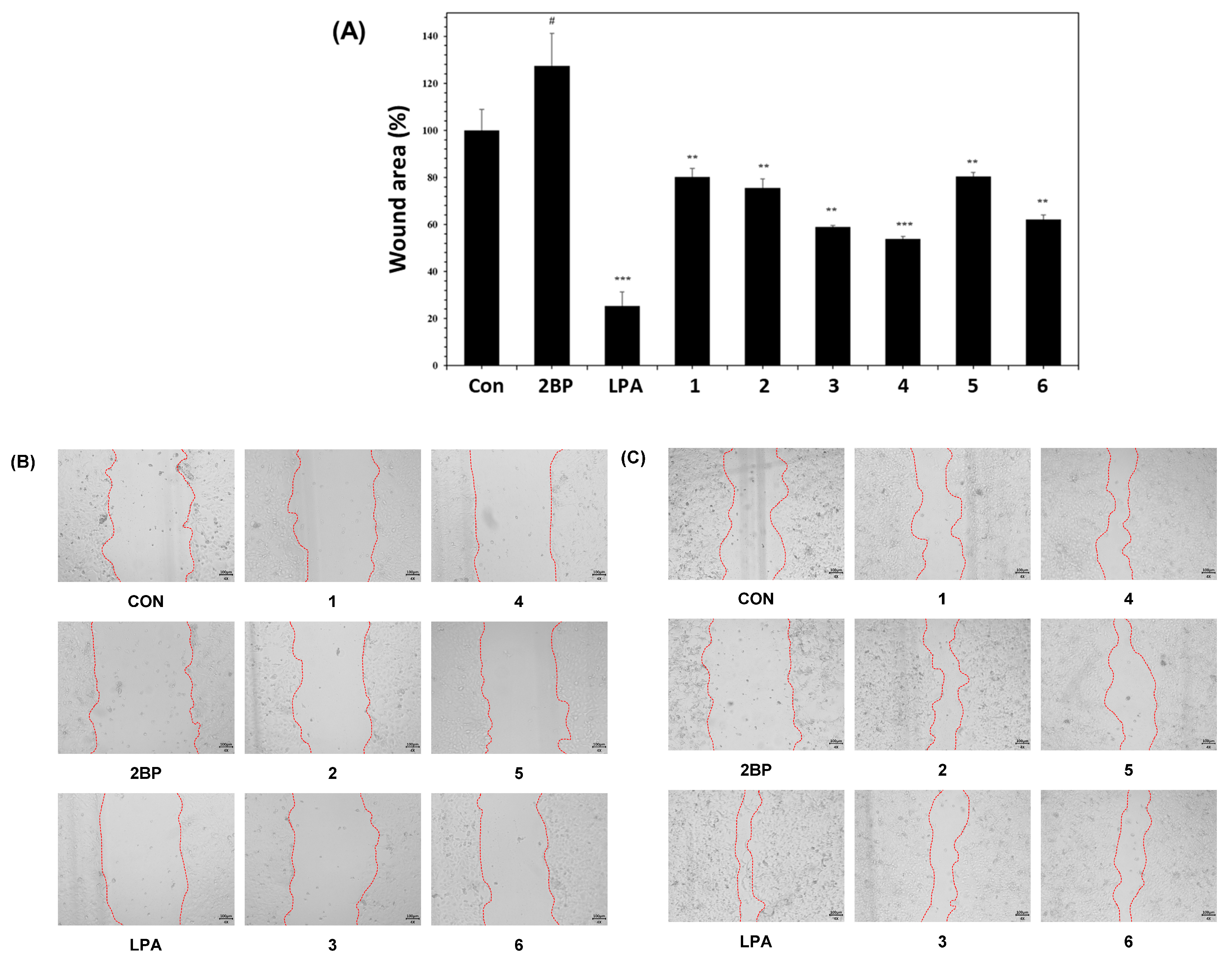
2.2. Wound-Healing Activities of Compounds 1–6
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3‴-O-Foliamenthoyl-Rutin (6)
3.4. Acid Hydrolysis and Absolute Configuration Determination of Sugar Moieties
3.5. Cell Culture
3.6. Wound-Healing Assay and MTT Assay
3.7. Statistics Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chuang, T.; Ornduff, R. Seed morphology and systematics of Menyanthaceae. AM. J. Bot. 1992, 79, 1396–1406. [Google Scholar] [CrossRef]
- Njuguna, A.W.; Li, Z.-Z.; Saina, J.K.; Munywoki, J.M.; Gichira, A.W.; Gituru, R.W.; Wang, Q.-F.; Chen, J.-M. Comparative analyses of the complete chloroplast genomes of nymphoides and menyanthes species (menyanthaceae). Aquat. Bot. 2019, 156, 73–81. [Google Scholar] [CrossRef]
- Uesugi, R.; Tani, N.; Goka, K.; Nishihiro, J.; Tsumura, Y.; Washitani, I. Isolation and characterization of highly polymorphic microsatellites in the aquatic plant, Nymphoides peltata (Menyanthaceae). Mole. Ecol. Notes. 2005, 5, 343–345. [Google Scholar] [CrossRef]
- National List of Species of Korea 2022. National Institute of Biological Resources. Available online: http://kbr.go.kr/ (accessed on 10 September 2023).
- Khan, Z.R.; Chowdhury, N.S.; Sharmin, S.; Sohrab, M.H. Medicinal values of aquatic plant genus Nymphoides grown in Asia: A review. Asian Pac. J. Trop. Biomed. 2018, 8, 113–119. [Google Scholar] [CrossRef]
- Nocchi, N.; Duarte, H.M.; Pereira, R.C.; Konno, T.U.P.; Soares, A.R. Effects of UV-B radiation on secondary metabolite production, antioxidant activity, photosynthesis and herbivory interactions in Nymphoides humboldtiana (Menyanthaceae). J. Photochem. Photobiol. B Biol. 2020, 212, 112021. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Tuenter, E.; Exarchou, V.; Upadhyay, A.; Cos, P.; Maes, L.; Apers, S.; Pieters, L. Phytochemical and pharmacological investigations on Nymphoides indica leaf extracts. Phytother. Res. 2016, 30, 1624–1633. [Google Scholar] [CrossRef] [PubMed]
- Murali, A.; Sudha, C.; Madhavan, V.; Yoganarasimhan, S. Anticonvulsant and Sedative Activity of Tagara (Nymphoides macrospermum). Pharm. Biol. 2007, 45, 407–410. [Google Scholar] [CrossRef]
- Du, Y.; Wang, R.; Zhang, H.; Liu, J. Antitumor constituents of the wetland plant Nymphoides peltata: A case study for the potential utilization of constructed wetland plant resources. Nat. Prod. Commun. 2015, 10, 1934578X1501000203. [Google Scholar] [CrossRef]
- Zhou, X.; Ning, K.; Ling, B.; Chen, X.; Cheng, H.; Lu, B.; Gao, Z.; Xu, J. Multiple injections of autologous adipose-derived stem cells accelerate the burn wound healing process and promote blood vessel regeneration in a rat model. Stem Cells Dev. 2019, 28, 1463–1472. [Google Scholar] [CrossRef]
- Mathias, E.; Srinivas, M.M. Pediatric thermal burns and treatment: A review of progress and future prospects. Medicines 2017, 4, 91. [Google Scholar] [CrossRef]
- Gautam, S.; Chou, C.-F.; Dinda, A.K.; Potdar, P.D.; Mishra, N.C. Surface modification of nanofibrous polycaprolactone/gelatin composite scaffold by collagen type I grafting for skin tissue engineering. Mater. Sci. Eng. C 2014, 34, 402–409. [Google Scholar] [CrossRef]
- Childs, D.R.; Murthy, A.S. Overview of wound healing and management. Surg. Clin. 2017, 97, 189–207. [Google Scholar] [CrossRef]
- Sun, M.-L.; Zhao, F.; Chen, X.-L.; Zhang, X.-Y.; Zhang, Y.-Z.; Song, X.-Y.; Sun, C.-Y.; Yang, J. Promotion of wound healing and prevention of frostbite injury in rat skin by exopolysaccharide from the arctic marine bacterium Polaribacter sp. SM1127. Mar. Drugs 2020, 18, 48. [Google Scholar] [CrossRef] [PubMed]
- Dorai, A.A. Wound care with traditional, complementary and alternative medicine. Indian J. Plast. Surg. 2012, 45, 418–424. [Google Scholar] [CrossRef]
- Yeng, N.K.; Shaari, R.; Nordin, M.L.; Sabri, J. Investigation of wound healing effect of Acalypha indica extract in sprague dawley rats. Biomed. Pharmacol. J. 2019, 12, 1857–1865. [Google Scholar] [CrossRef]
- Schmidt, C.A.; Murillo, R.; Bruhn, T.; Bringmann, G.; Goettert, M.; Heinzmann, B.; Brecht, V.; Laufer, S.A.; Merfort, I. Catechin derivatives from Parapiptadenia rigida with in vitro wound-healing properties. J. Nat. Prod. 2010, 73, 2035–2041. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.Q.; Joung, Y.K.; Lih, E.; Park, K.D. In situ forming and rutin-releasing chitosan hydrogels as injectable dressings for dermal wound healing. Biomacromolecules 2011, 12, 2872–2880. [Google Scholar] [CrossRef]
- Öz, B.E.; İşcan, G.S.; Akkol, E.K.; Süntar, İ.; Acıkara, Ö.B. Isoflavonoids as wound healing agents from Ononidis Radix. J. Ethnopharmacol. 2018, 211, 384–393. [Google Scholar] [CrossRef]
- Muralidhar, A.; Babu, K.S.; Sankar, T.R.; Reddanna, P.; Latha, J. Wound healing activity of flavonoid fraction isolated from the stem bark of Butea monosperma (Lam) in albino wistar rats. Eur. J. Exp. Biol. 2013, 3, 1–6. [Google Scholar]
- Mensah, A.; Sampson, J.; Houghton, P.; Hylands, P.; Westbrook, J.; Dunn, M.; Hughes, M.; Cherry, G. Effects of Buddleja globosa leaf and its constituents relevant to wound healing. J. Ethnopharmacol. 2001, 77, 219–226. [Google Scholar] [CrossRef]
- Muhammad, A.A.; Pauzi, N.A.S.; Arulselvan, P.; Abas, F.; Fakurazi, S. In vitro wound healing potential and identification of bioactive compounds from Moringa oleifera Lam. BioMed Res. Int. 2013, 2013, 974580. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-Y.; Park, N.-J.; Jegal, H.; Paik, J.-H.; Choi, S.; Kim, S.-N.; Yang, M.H. Nymphoides peltata root extracts improve atopic dermatitis by regulating skin inflammatory and anti-oxidative enzymes in 2, 4-dinitrochlorobenzene (DNCB)-induced SKH-1 hairless mice. Antioxidants 2023, 12, 873. [Google Scholar] [CrossRef] [PubMed]
- Blunder, M.; Orthaber, A.; Bauer, R.; Bucar, F.; Kunert, O. Efficient identification of flavones, flavanones and their glycosides in routine analysis via off-line combination of sensitive NMR and HPLC experiments. Food Chem. 2017, 218, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Hardiyanti, R.; Marpaung, L.; Adnyana, I.K.; Simanjuntak, P. Isolation of quercitrin from Dendrophthoe pentandra (L.) Miq leaves and it’s antioxidant and antibacterial activities. Rasayan. J. Chem. 2019, 12, 1822–1827. [Google Scholar] [CrossRef]
- Slimestad, R.; Torskangerpoll, K.; Nateland, H.S.; Johannessen, T.; Giske, N.H. Flavonoids from black chokeberries, Aronia melanocarpa. J. Food Compos. Anal. 2005, 18, 61–68. [Google Scholar] [CrossRef]
- Iwagawa, T.; Asai, H.; Hase, T.; Sako, S.; Su, R.; Hagiwara, N.; Kim, M. Monoterpenoids from Radermachia sinica. Phytochem 1990, 29, 1913–1916. [Google Scholar] [CrossRef]
- Itoh, A.; Tanahashi, T.; Nagakura, N.; Takenaka, Y.; Chen, C.-C.; Pelletier, J. Flavonoid glycosides from Adina racemosa and their inhibitory activities on eukaryotic protein synthesis. J. Nat. Prod. 2004, 67, 427–431. [Google Scholar] [CrossRef]
- Piipponen, M.; Li, D.; Landén, N.X. The immune functions of keratinocytes in skin wound healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Kirsner, R. Pathophysiology of acute wound healing. Clin. Dermatol. 2007, 25, 9–18. [Google Scholar] [CrossRef]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research techniques made simple: Analysis of collective cell migration using the wound healing assay. J. Investig. Dermatol. 2017, 137, e11–e16. [Google Scholar] [CrossRef]
- Mazereeuw-Hautier, J.; Gres, S.; Fanguin, M.; Cariven, C.; Fauvel, J.; Perret, B.; Chap, H.; Salles, J.-P.; Saulnier-Blache, J.-S. Production of lysophosphatidic acid in blister fluid: Involvement of a lysophospholipase D activity. J. Investig. Dermatol. 2005, 125, 421–427. [Google Scholar] [CrossRef]
- Mi, Y.; Zhong, L.; Lu, S.; Hu, P.; Pan, Y.; Ma, X.; Yan, B.; Wei, Z.; Yang, G. Quercetin promotes cutaneous wound healing in mice through Wnt/β-catenin signaling pathway. J. Ethnopharmacol. 2022, 290, 115066. [Google Scholar] [CrossRef]
- Chen, J.; Li, G.; Sun, C.; Peng, F.; Yu, L.; Chen, Y.; Tan, Y.; Cao, X.; Tang, Y.; Xie, X.; et al. Chemistry, pharmacokinetics, pharmacological activities, and toxicity of Quercitrin. Phytother. Res. 2022, 36, 1545–1575. [Google Scholar] [CrossRef] [PubMed]
- Süntar, I.P.; Akkol, E.K.; Yalçin, F.N.; Koca, U.; Keleş, H.; Yesilada, E. Wound healing potential of Sambucus ebulus L. leaves and isolation of an active component, quercetin 3-O-glucoside. J. Ethnopharmacol. 2010, 129, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-Y.; Huang, C.-N.; Liao, C.-K.; Chang, H.-M.; Kuan, Y.-H.; Tseng, T.-J.; Yen, K.-J.; Yang, K.-L.; Lin, H.-C. Effects of Rutin on Wound Healing in Hyperglycemic Rats. Antioxidants 2020, 9, 1122. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, M.; Chen, F.; Han, R.; Du, K.; Zhang, Y.; Li, M.; Si, Y.; Feng, W. Six New Coumarin Glycosides from the Aerial Parts of Gendarussa vulgaris. Molecules 2019, 24, 1456. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, J.K.; Yu, J.S.; Jeong, S.Y.; Choi, J.H.; Kim, J.-C.; Ko, Y.-J.; Kim, S.-H.; Kim, K.H. Ginkwanghols A and B, osteogenic coumaric acid-aliphatic alcohol hybrids from the leaves of Ginkgo biloba. Arch. Pharm. Res. 2021, 44, 514–524. [Google Scholar] [CrossRef]
- Yu, J.S.; Jeong, S.Y.; Li, C.; Oh, T.; Kwon, M.; Ahn, J.S.; Ko, S.K.; Ko, Y.J.; Cao, S.; Kim, K.H. New phenalenone derivatives from the Hawaiian volcanic soil-associated fungus Penicillium herquei FT729 and their inhibitory effects on indoleamine 2,3-dioxygenase 1 (IDO1). Arch. Pharm. Res. 2022, 45, 105–113. [Google Scholar] [CrossRef]

 ) and HMBC (
) and HMBC ( ) correlations for compound 6.
) correlations for compound 6.
| Position | 6 | |
|---|---|---|
| δCc | δH (J in Hz) b | |
| 2 | 157.0 C | |
| 3 | 133.8 C | |
| 4 | 177.8 C | |
| 5 | 161.7 C | |
| 6 | 99.1 CH | 6.16 (d, 2.0) |
| 7 | 164.5 C | |
| 8 | 94.0 CH | 6.36 (d, 2.0) |
| 9 | 156.9 C | |
| 10 | 104.5 C | |
| 1′ | 121.7 C | |
| 2′ | 116.7 CH | 7.52 (d, 2.0) |
| 3′ | 145.2 C | |
| 4′ | 148.9 C | |
| 5′ | 115.7 CH | 6.84 (d, 8.0) |
| 6′ | 122.0 CH | 7.53 (dd, 8.0, 2.0) |
| 1″ | 101.8 CH | 5.34 (d, 7.0) |
| 2″ | 74.5 CH | 3.23 (m) |
| 3″ | 76.9 CH | 3.23 (m) |
| 4″ | 71.8 CH | 3.24 (m) |
| 5″ | 76.3 CH | 3.29 (m) |
| 6″ | 68.0 CH2 | 3.76 (br d, 10.5); 3.33 (overlap) |
| 1‴ | 101.3 CH | 4.41 (br s) |
| 2‴ | 68.3 CH | 3.60 (br s) |
| 3‴ | 74.7 CH | 4.63 (dd, 9.5, 3.5) |
| 4‴ | 69.4 CH | 3.37 (m) |
| 5‴ | 68.9 CH | 3.43 (m) |
| 6‴ | 18.1 CH3 | 1.03 (d, 6.0) |
| 1⁗ | 167.3 C | |
| 2⁗ | 128.1 C | |
| 3⁗ | 142.0 CH | 6.70 (t, 6.5) |
| 4⁗ | 27.1 CH2 | 2.25 (dt, 6.5, 7.0) |
| 5⁗ | 38.0 CH2 | 2.07 (t, 7.0) |
| 6⁗ | 135.6 C | |
| 7⁗ | 126.1 CH | 5.31 (t, 6.5) |
| 8⁗ | 58.0 CH2 | 3.94 (d, 6.5) |
| 9⁗ | 16.5 CH3 | 1.61 (s) |
| 10⁗ | 12.8 CH3 | 1.77 (s) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.-Y.; Lee, B.S.; Jo, B.-G.; Heo, S.P.; Keem, M.-J.; Kwon, T.-H.; Kim, S.-N.; Kim, K.H.; Yang, M.H. 3‴-O-Foliamenthoyl-Rutin, a New Flavonoid Glycoside from the Roots of Nymphoides peltata. Plants 2023, 12, 4083. https://doi.org/10.3390/plants12244083
Kim T-Y, Lee BS, Jo B-G, Heo SP, Keem M-J, Kwon T-H, Kim S-N, Kim KH, Yang MH. 3‴-O-Foliamenthoyl-Rutin, a New Flavonoid Glycoside from the Roots of Nymphoides peltata. Plants. 2023; 12(24):4083. https://doi.org/10.3390/plants12244083
Chicago/Turabian StyleKim, Tae-Young, Bum Soo Lee, Beom-Geun Jo, Seong Pil Heo, Min-Ji Keem, Taek-Hwan Kwon, Su-Nam Kim, Ki Hyun Kim, and Min Hye Yang. 2023. "3‴-O-Foliamenthoyl-Rutin, a New Flavonoid Glycoside from the Roots of Nymphoides peltata" Plants 12, no. 24: 4083. https://doi.org/10.3390/plants12244083
APA StyleKim, T.-Y., Lee, B. S., Jo, B.-G., Heo, S. P., Keem, M.-J., Kwon, T.-H., Kim, S.-N., Kim, K. H., & Yang, M. H. (2023). 3‴-O-Foliamenthoyl-Rutin, a New Flavonoid Glycoside from the Roots of Nymphoides peltata. Plants, 12(24), 4083. https://doi.org/10.3390/plants12244083








