Chemical Compositions of Essential Oil Extracted from Eight Thyme Species and Potential Biological Functions
Abstract
:1. Introduction
2. Results
2.1. Phenotypic Evaluation of Different Thyme Species
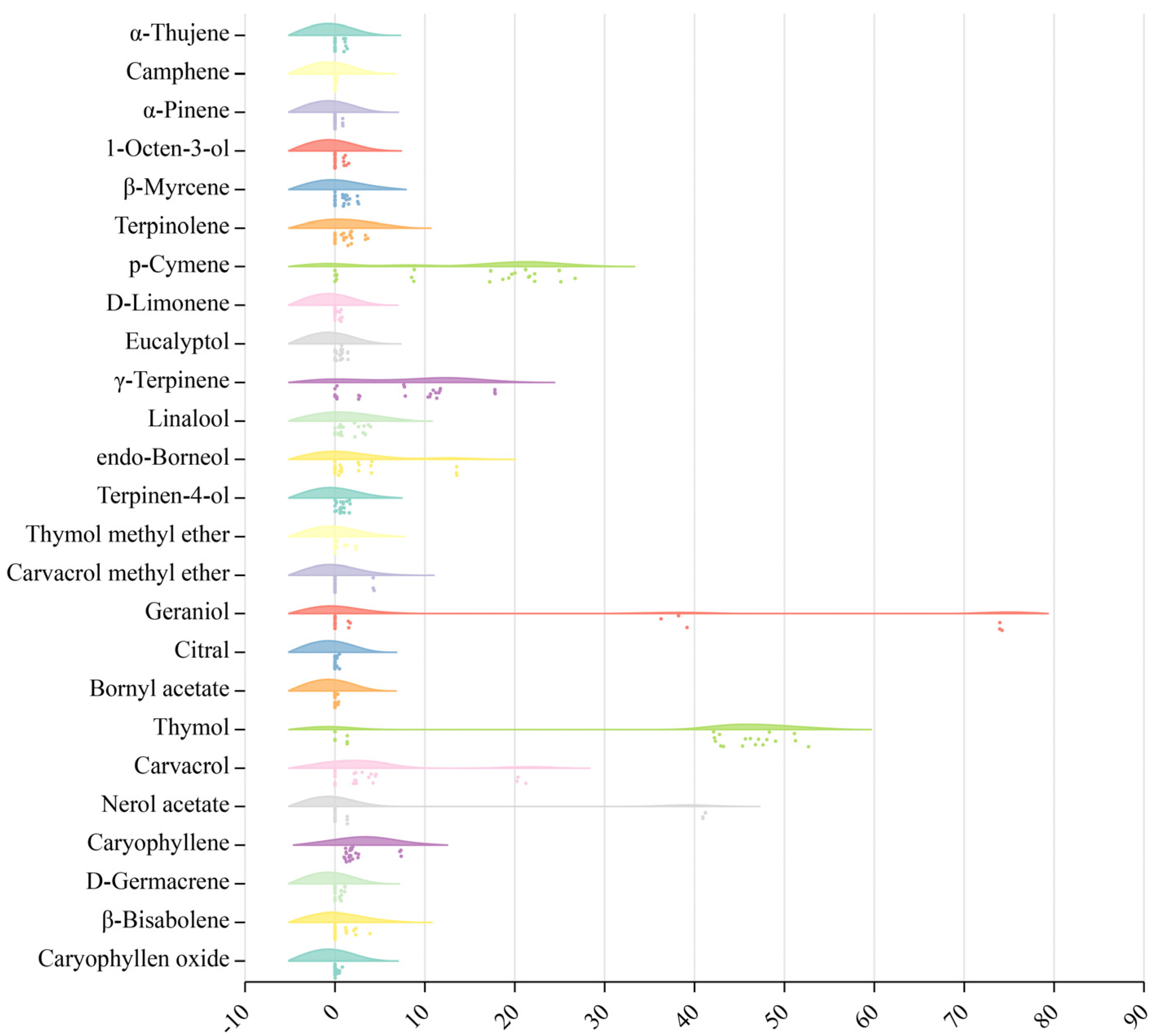
2.2. Essential Oil Components from Eight Thyme Species
2.3. Multivariate Statistical Analysis of Essential Oil Components from Eight Thyme Species
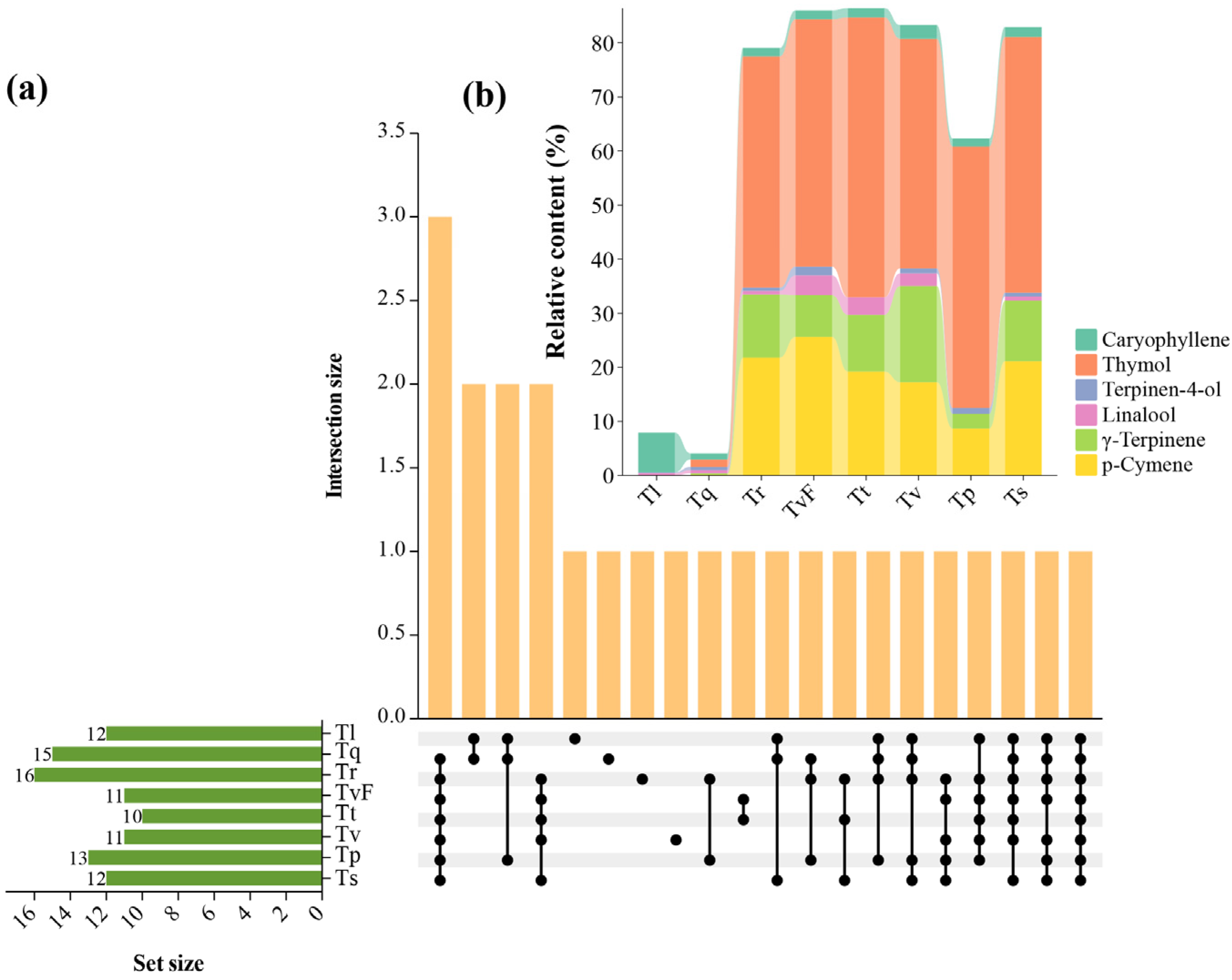
2.4. Characterization of Shared and Unique TEO Components from Eight Thyme Species
2.5. Variation Analysis of TEO Components from Eight Thyme Species
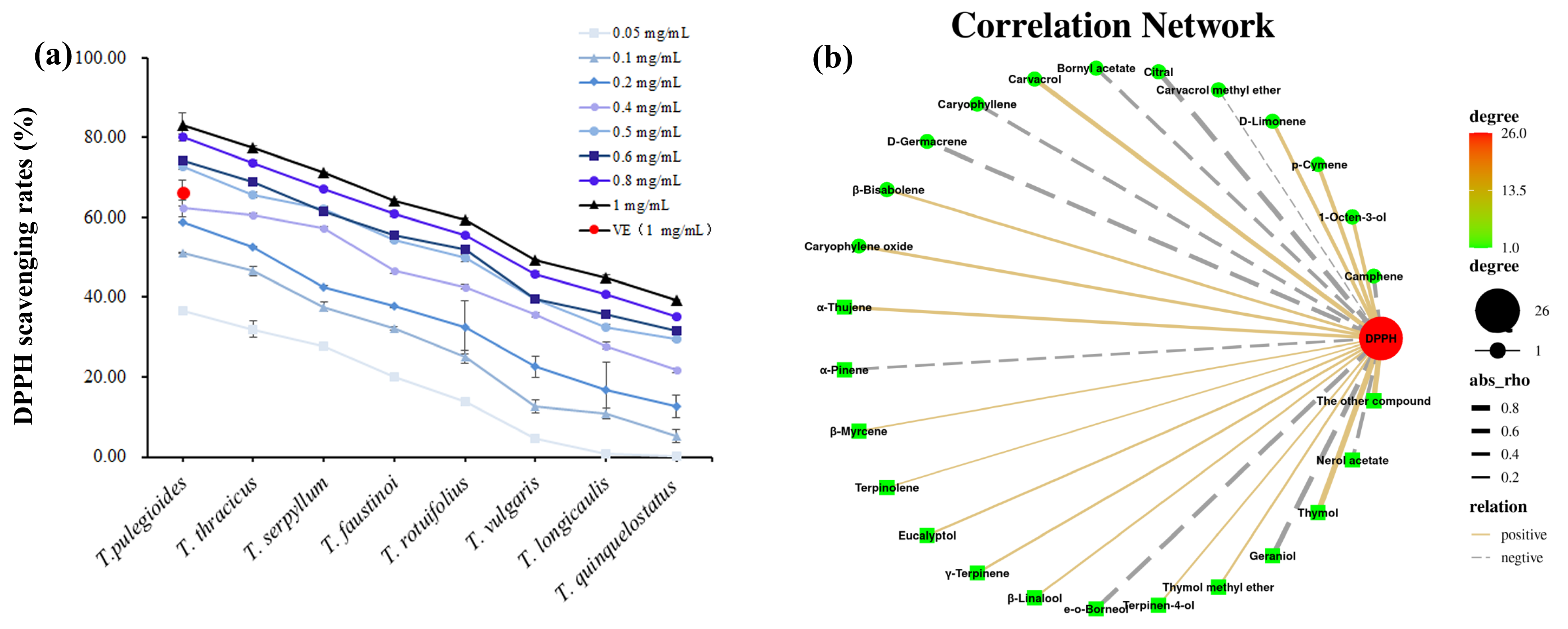
2.6. Evaluation of the Antioxidant Activity of Eight Thyme Species
2.7. Other Potential Biological Functions of TEO
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Extraction of Essential Oils
4.3. Gas Chromatography Mass Spectrometry Analysis of Essential Oils
4.4. In Vitro Antioxidant Activity
4.5. Antibacterial Activity of the Main Components against E. coli and S. aureus
4.6. Cell Migration Ability Test
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salehi, B.; Abu-Darwish, M.S.; Tarawneh, A.H.; Cabral, C.; Gadetskaya, A.V.; Salgueiro, L.; Hosseinabadi, T.; Rajabi, S.; Chanda, W.; Sharifi-Rad, M.; et al. Thymus spp. plants-food applications and phytopharmacy properties. Trends Food Sci. Technol. 2019, 85, 287–306. [Google Scholar] [CrossRef]
- Hong, J.Y.; Kim, H.; Jeon, W.-J.; Baek, S.; Ha, I.-H. Antioxidative effects of Thymus quinquecostatus Celak through mitochondrial biogenesis improvement in RAW 264.7 macrophages. Antioxidants 2020, 9, 548. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Lee, S.J.; Hwang, J.W.; Kim, E.K.; Kim, S.E.; Kim, E.H.; Moon, S.H.; Jeon, B.T.; Park, P.J. In vitro protective effects of Thymus quinquecostatus Celak extracts on T-BHP-induced cell damage through antioxidant activity. Food Chem. Toxicol. 2012, 50, 4191–4198. [Google Scholar] [CrossRef]
- Evans, J.D.; Martin, S.A. Effects of thymol on ruminal microorganisms. Curr. Microbiol. 2000, 41, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Hu, C.L.; Zhang, Z.P.; Chen, R.; Yang, S.B.; Miao, Z.Y.; Sun, L.N.; Wang, Y.Q. Development and validation an LC-MS/MS method to quantify (+)-borneol in rat plasma: Application to a pharmacokinetic study. J. Chromatogr. B 2019, 1109, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Cutillas, A.B.; Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Thyme essential oils from Spain: Aromatic profile ascertained by GC–MS, and their antioxidant, anti-lipoxygenase and antimicrobial activities. J. Food Drug Anal. 2018, 26, 529–544. [Google Scholar] [CrossRef]
- Pinto, L.; Tapia-Rodríguez, M.R.; Baruzzi, F.; Ayala-Zavala, J.F. Plant antimicrobials for food quality and safety: Recent views and future challenges. Foods 2023, 12, 2315. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia; Council of Europe: Strasburg, France, 2002. [Google Scholar]
- Falleh, H.; Ben Jemaa, M.; Saada, M.; Ksouri, R. Essential Oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Marchese, A.; Izadi, M.; Curti, V.; Daglia, M.; Nabavi, S.F. Plants belonging to the genus thymus as antibacterial agents: From farm to pharmacy. Food Chem. 2015, 173, 339–347. [Google Scholar] [CrossRef]
- Danute, M.; Genovaite, B. The Main citral-geraniol and carvacrol chemotypes of the essential oil of Thymus pulegioides L. growing wild in Vilnius district (Lithuania). J. Agric. Food Chem. 1999, 47, 3787–3790. [Google Scholar]
- Horváth, G.; Szabó, L.G.; Héthelyi, E.; Lemberkovics, E. Essential oil composition of three cultivated Thymus chemotypes from Hungary. J. Essent. Oil Res. 2006, 18, 315–317. [Google Scholar] [CrossRef]
- Kim, M.; Moon, J.C.; Kim, S.; Sowndhararajan, K. Morphological, chemical, and genetic characteristics of Korean native thyme Bak-Ri-Hyang (Thymus quinquecostatus Celak). Antibiotics 2020, 9, 289. [Google Scholar] [CrossRef] [PubMed]
- Trendafilova, A.; Todorova, M.; Ivanova, V.; Zhelev, P.; Aneva, I. Essential oil composition of five thymus species from Bulgaria. Chem. Biodivers. 2021, 18, e21004. [Google Scholar] [CrossRef]
- Thompson, J.D.; Chalchat, J.C.; Michet, A.; Linhart, Y.B.; Ehlers, B. Qualitative and quantitative variation in monoterpene co-occurrence and composition in the essential oil of Thymus vulgaris chemotypes. J. Chem. Ecol. 2003, 29, 859–880. [Google Scholar] [CrossRef] [PubMed]
- Petruzzi, L.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Microbial spoilage of foods: Fundamentals. In The Microbiological Quality of Food; Bevilacqua, A., Corbo, M.R., Sinigaglia, M., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 1–21. [Google Scholar]
- Pakbin, B.; Brück, W.M.; Brück, T.B.; Allahyari, S.; Tamai, I.A. A quantitative prevalence of Escherichia coli O157 in different food samples using real-time qPCR method. Food Sci. Nutr. 2023, 11, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, I.M.; Miranda, N.; Hook, W.; Mendoza, J.; Kumfert, Q.; Barnes, T.; Sung, K.; Khan, S.; Nawaz, M.; Banerjee, P.; et al. A Single-Laboratory Performance Evaluation of MALDI-TOF MS in Rapid Identification of Staphylococcus aureus, Cronobacter sakazakii, Vibrio parahaemolyticus, and Some Closely Related Bacterial Species of Public Health Importance. J. AOAC Int. 2023, 106, 1574–1588. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Khan, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.A.; Manzoor, N. Fungicidal activity of thymol and carvacrol by disrupting ergosterol biosynthesis and membrane integrity against Candida. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 41–50. [Google Scholar] [CrossRef]
- De Lira Mota, K.; De Oliveira Pereira, F.; De Oliveira, W.; Lima, I.; De Oliveira Lima, E. Antifungal activity of Thymus vulgaris L. Essential oil and its constituent phytochemicals against Rhizopus oryzae: Interaction with ergosterol. Molecules 2012, 17, 14418–14433. [Google Scholar] [CrossRef]
- Chavan, P.S.; Tupe, S.G. Antifungal activity and mechanism of action of carvacrol and thymol against vineyard and wine spoilage yeasts. Food Control 2014, 46, 115–120. [Google Scholar] [CrossRef]
- Ultee, A.; Kets, E.P.W.; Smid, E.J. Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 1999; 65, 4606–4610. [Google Scholar]
- Yanishlieva, N.V.; Marinova, E.M.; Gordon, M.H.; Raneva, V.G. Antioxidant activity and mechanism of action of thymol and carvacrol in two lipid systems. Food Chem. 1999, 64, 59–66. [Google Scholar] [CrossRef]
- Su, L.; Yin, J.J.; Charles, D.; Zhou, K.; Moore, J.; Yu, L. Total phenolic contents, chelating capacities, and radical-scavenging properties of black peppercorn, nutmeg, rosehip, cinnamon and oregano leaf. Food Chem. 2007, 100, 990–997. [Google Scholar] [CrossRef]
- Embuscado, M.E. Spices and herbs: Natural sources of antioxidants—A mini review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Hao, Y.; Kang, J.; Guo, X.; Yang, R.; Chen, Y.; Li, J.; Shi, L. Comparison of nutritional compositions and essential oil profiles of different parts of a dill and two fennel cultivars. Foods 2021, 10, 1784. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and thyme essential oil—New insights into selected therapeutic applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef]
- Ben El Hadj Ali, I.; Guetat, A.; Boussaid, M. Chemical and Genetic Variability of Thymus algeriensis Boiss. et Reut. (Lamiaceae), a north African endemic species. Ind. Crops Prod. 2012, 40, 277–284. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of thymus species collected from different regions of Iran. Food Chem. 2017, 220, 153–161. [Google Scholar] [CrossRef]
- Mockut, D.; Bernotien, G. The alpha-terpenyl acetate chemotype of essential oil of Thymus pulegioides L. Biochem. Syst. Ecol. 2001, 29, 69–76. [Google Scholar] [CrossRef]
- Groendahl, E.; Ehlers, B.K.; Keefover-Ring, K. A new cis-sabinene hydrate chemotype detected in large thyme (Thymus pulegioides L.) growing wild in Denmark. J. Essent. Oil Res. 2008, 20, 40–41. [Google Scholar] [CrossRef]
- Mártonfi, P.; Grejtovský, A.; Repčák, M. Chemotype pattern differentiation of Thymus pulegioides on different substrates. Biochem. Syst. Ecol. 1994, 22, 819–825. [Google Scholar] [CrossRef]
- Senatore, F. Influence of harvesting time on yield and composition of the essential oil of a thyme (Thymus pulegioides L.) growing wild in campania (southern Italy). J. Agric. Food Chem. 1996, 44, 1327–1332. [Google Scholar] [CrossRef]
- Ložienė, K.; Venskutonis, P.R. Influence of environmental and genetic factors on the stability of essential oil composition of Thymus pulegioides. Biochem. Syst. Ecol. 2005, 33, 517–525. [Google Scholar] [CrossRef]
- Novak, J.; Lukas, B.; Franz, C. Temperature Influences thymol and carvacrol differentially in Origanum spp. (Lamiaceae). J. Essent. Oil Res. 2010, 22, 412–415. [Google Scholar] [CrossRef]
- Karimi, A.; Krähmer, A.; Herwig, N.; Schulz, H.; Hadian, J.; Meiners, T. Variation of secondary metabolite profile of zataria multiflora boiss. Populations linked to geographic, climatic, and edaphic factors. Front. Plant Sci. 2020, 11, 969. [Google Scholar] [CrossRef] [PubMed]
- Mastro, G.D.; Tarraf, W.; Verdini, L.; Brunetti, G.; Ruta, C. Essential oil diversity of Origanum vulgare L. Populations from southern Italy. Food Chem. 2017, 235, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gioffrè, G.; Ursino, D.; Labate, M.L.C.; Giuffrè, A.M. The peel essential oil composition of bergamot fruit (Citrus bergamia, Risso) of Reggio Calabria (Italy): A review. Emir. J. Food Agric. 2020, 32, 835. [Google Scholar] [CrossRef]
- Hassiotis, C.N.; Ntana, F.; Lazari, D.M.; Poulios, S.; Vlachonasios, K.E. Environmental and developmental factors affect essential oil production and quality of Lavandula angustifolia during flowering period. Ind. Crops Prod. 2014, 62, 359–366. [Google Scholar] [CrossRef]
- Andrea, B. Factors affecting chemical variability of essential oils: A review of recent developments. Nat. Prod. Commun. 2009, 4, 1147–1154. [Google Scholar]
- Hao, Y.; Kang, J.; Yang, R.; Li, H.; Cui, H.; Bai, H.; Tsitsilin, A.; Li, J.; Shi, L. Multidimensional exploration of essential oils generated via eight oregano cultivars: Compositions, chemodiversities, and antibacterial capacities. Food Chem. 2022, 374, 131629. [Google Scholar] [CrossRef]
- Lukas, B.; Schmiderer, C.; Novak, J. Essential oil diversity of European Origanum vulgare L. (Lamiaceae). Phytochemistry 2015, 119, 32–40. [Google Scholar] [CrossRef]
- Hammerbacher, A.; Coutinho, T.A.; Gershenzon, J. Roles of plant volatiles in defence against microbial pathogens and microbial exploitation of volatiles. Plant Cell Environ. 2019, 42, 2827–2843. [Google Scholar] [CrossRef]
- Moore, B.D.; Andrew, R.L.; Külheim, C.; Foley, W.J. Explaining intraspecific diversity in plant secondary metabolites in an ecological context. New Phytol. 2014, 201, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2020, 60, 3042–3053. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.P.; Kong, N.Q.; Wang, L.; Luo, Z.; Yin, J.; Chen, Y.L. Nanocomplexation between thymol and soy protein isolate and its improvements on stability and antibacterial properties of thymol. Food Chem. 2021, 334, 127594. [Google Scholar] [CrossRef] [PubMed]
- Cometa, S.; Bonifacio, M.A.; Bellissimo, A.; Pinto, L.; Petrella, A.; Vietro, N.D.; Iannaccone, G.; Baruzzi, F.; Giglio, E.D. A green approach to develop zeolite-thymol antimicrobial composites: Analytical characterization and antimicrobial activity evaluation. Heliyon 2022, 8, e09551. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef] [PubMed]
- Aristatile, B.; Al-Numair, K.S.; Veeramani, C.; Pugalendi, K.V. Effect of carvacrol on hepatic marker enzymes and antioxidant status in d-galactosamine-induced hepatotoxicity in rats. Fundam. Clin. Pharmacol. 2009, 23, 757–765. [Google Scholar] [CrossRef]
- Botsoglou, N.A.; Grigoropoulou, S.H.; Botsoglou, E.; Govaris, A.; Papageorgiou, G. The effects of dietary oregano essential oil and α-tocopheryl acetate on lipid oxidation in raw and cooked turkey during refrigerated storage. Meat Sci. 2003, 65, 1193–1200. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Park, C.; Yeo, H.; Park, Y.; Kim, Y.; Park, C.; Kim, J.; Park, S. Integrated analysis of transcriptome and metabolome and evaluation of antioxidant activities in Lavandula pubescens. Antioxidants 2021, 10, 1027. [Google Scholar] [CrossRef]
- Marques, J.D.L.; Volcão, L.M.; Funck, G.D.; Kroning, I.S.; Da Silva, W.P.; Fiorentini, Â.M.; Ribeiro, G.A. Antimicrobial Activity of Essential Oils of Origanum vulgare L. and Origanum majorana L. against Staphylococcus aureus Isolated from Poultry Meat. Ind. Crops Prod. 2015, 77, 444–450. [Google Scholar] [CrossRef]



| Compound | RI a | RT b | Relative Concentration (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TvF | Tp | Tr | Tt | Tl | Tq | Ts | Tv | |||
| α-Thujene | 920 | 921 | 1.06 ± 0.13 | - | - | 1.21 ± 0.12 | - | - | - | - |
| α-Pinene | 929 | 921 | - | - | - | - | - | - | - | 0.87 ± 0.01 |
| Camphene | 952 | 936 | - | - | - | - | - | 0.21 ± 0.02 | - | - |
| 1-Octen-3-ol | 980 | 968 | - | 1.30 ± 0.20 | 0.98 ± 0.04 | - | - | - | - | - |
| β-Myrcene | 991 | 982 | 1.28 ± 0.04 | - | 1.08 ± 0.11 | 1.50 ± 0.14 | - | - | 0.89 ± 0.04 | 2.55 ± 0.07 |
| Terpinolene | 1017 | 1008 | 0.98 ± 0.02 | - | 1.80 ± 0.06 | 1.62 ± 0.31 | - | - | 1.27 ± 0.50 | 3.48 ± 0.15 |
| p-Cymene | 1025 | 1018 | 25.60 ± 0.98 | 8.70 ± 0.16 | 21.81 ± 0.36 | 19.22 ± 0.50 | - | 0.17 ± 0.01 | 21.15 ± 1.1 | 17.25 ± 0.06 |
| D-Limonene | 1030 | 1021 | - | - | 0.44 ± 0.10 | 0.66 ± 0.11 | - | - | 0.63 ± 0.08 | - |
| Eucalyptol | 1032 | 1030 | 0.76 ± 0.06 | 0.82 ± 0.01 | 0.63 ± 0.08 | 0.66 ± 0.06 | 0.15 ± 0.02 | - | - | 1.43 ± 0.02 |
| γ-Terpinene | 1060 | 1058 | 7.74 ± 0.08 | 2.68 ± 0.07 | 11.66 ± 0.08 | 10.51 ± 0.14 | - | 0.21 ± 0.02 | 11.16 ± 0.20 | 17.80 ± 0.02 |
| Linalool | 1099 | 1097 | 3.64 ± 0.38 | - | 0.64 ± 0.01 | 3.25 ± 0.12 | 0.43 ± 0.01 | 0.61 ± 0.01 | 0.83 ± 0.08 | 2.34 ± 0.24 |
| endo-Borneol | 1167 | 1164 | - | - | 0.51 ± 0.07 | - | 4.06 ± 0.06 | 13.54 ± 0.01 | 2.63 ± 0.05 | - |
| Terpinen-4-ol | 1177 | 1176 | 1.63 ± 0.05 | 1.12 ± 0.18 | 0.62 ± 0.04 | - | 0.15 ± 0.01 | 0.61 ± 0.03 | 0.64 ± 0.08 | 0.93 ± 0.02 |
| Thymol methyl ether | 1235 | 1235 | - | 1.19 ± 0.13 | 2.34 ± 0.07 | - | - | 0.20 ± 0.01 | - | - |
| Carvacrol methyl ether | 1244 | 1244 | - | - | 4.29 ± 0.06 | - | - | - | - | - |
| Citral | 1247 | 1247 | - | - | - | - | 0.23 ± 0.02 | 0.49 ± 0.01 | - | - |
| Geraniol | 1255 | 1254 | - | - | - | - | 37.89 ± 1.48 | 74.04 ± 0.15 | - | - |
| Bornyl acetate | 1285 | 1286 | - | - | - | - | 0.35 ± 0.03 | - | - | - |
| Thymol | 1291 | 1292 | 45.74 ± 0.44 | 48.32 ± 0.70 | 42.75 ± 0.56 | 51.68 ± 0.87 | - | 1.36 ± 0.00 | 47.31 ± 0.66 | 42.44 ± 0.30 |
| Carvacrol | 1299 | 1300 | 4.43 ± 0.18 | 20.61 ± 0.55 | 2.18 ± 0.16 | - | - | - | 3.60 ± 0.52 | 2.28 ± 0.06 |
| Nerol acetate | 1382 | 1385 | - | - | - | - | 41.02 ± 0.17 | 1.36 ± 0.01 | - | - |
| Caryophyllene | 1419 | 1422 | 1.64 ± 0.08 | 1.46 ± 0.44 | 1.56 ± 0.26 | 1.76 ± 0.09 | 7.30 ± 0.08 | 1.13 ± 0.12 | 1.80 ± 0.14 | 2.50 ± 0.16 |
| Germacrene D | 1481 | 1483 | - | - | - | - | 0.63 ± 0.10 | 1.09 ± 0.01 | 0.66 ± 0.08 | - |
| β-Bisabolene | 1509 | 1511 | - | 2.46 ± 1.36 | 1.26 ± 0.03 | - | 2.18 ± 0.12 | 0.01 ± 0.01 | - | - |
| Caryophyllene oxide | 1581 | 1578 | - | 0.61 ± 0.19 | - | - | 0.21 ± 0.02 | 0.01 ± 0.01 | - | - |
| Monoterpene hydrocarbons | 36.65 ± 1.06 | 11.38 ± 0.20 | 36.78 ± 0.24 | 34.72 ± 0.65 | - | 0.59 ± 0.03 | 41.94 ± 0.23 | 35.09 ± 1.13 | ||
| Oxygenated monoterpenes | 56.19 ± 0.40 | 74.34 ± 0.40 | 53.96 ± 0.71 | 55.58 + 0.83 | 84.26 ± 1.32 | 92.23 ± 0.14 | 49.42 ± 0.56 | 55.01 ± 0.73 | ||
| Sesquiterpene hydrocarbons | 1.64 ± 0.08 | 3.92 ± 1.36 | 2.81 ± 0.24 | 1.76 ± 0.09 | 10.11 ± 0.06 | 2.24 ± 0.13 | 2.50 ± 0.16 | 2.46 ± 0.16 | ||
| Oxygenated sesquiterpenes | - | 0.61 ± 0.19 | - | - | 0.21 ± 0.01 | 0.01 ± 0.01 | - | - | ||
| Total | 96.18 ± 1.49 | 91.54 ± 1.27 | 94.19 ± 0.64 | 92.19 ± 0.12 | 94.58 ± 1.37 | 95.06 ± 0.31 | 92.56 ± 0.68 | 93.85 ± 0.88 | ||
| EO yields (%) | 1.09 ± 0.12 | 0.53 ± 0.06 | 1.60 ± 0.05 | 1.63 ± 0.08 | 1.16 ± 0.05 | 0.78 ± 0.03 | 1.50 ± 0.06 | 1.00 ± 0.02 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Wei, Z.; Yang, R.; Zhang, Y.; Sun, M.; Bai, H.; Mo, M.; Yao, C.; Li, H.; Shi, L. Chemical Compositions of Essential Oil Extracted from Eight Thyme Species and Potential Biological Functions. Plants 2023, 12, 4164. https://doi.org/10.3390/plants12244164
Dong Y, Wei Z, Yang R, Zhang Y, Sun M, Bai H, Mo M, Yao C, Li H, Shi L. Chemical Compositions of Essential Oil Extracted from Eight Thyme Species and Potential Biological Functions. Plants. 2023; 12(24):4164. https://doi.org/10.3390/plants12244164
Chicago/Turabian StyleDong, Yanmei, Ziling Wei, Rui Yang, Yanan Zhang, Meiyu Sun, Hongtong Bai, Meiling Mo, Chunlei Yao, Hui Li, and Lei Shi. 2023. "Chemical Compositions of Essential Oil Extracted from Eight Thyme Species and Potential Biological Functions" Plants 12, no. 24: 4164. https://doi.org/10.3390/plants12244164





