Growth Stimulation of Durum Wheat and Common Buckwheat by Non-Thermal Atmospheric Pressure Plasma
Abstract
:1. Introduction
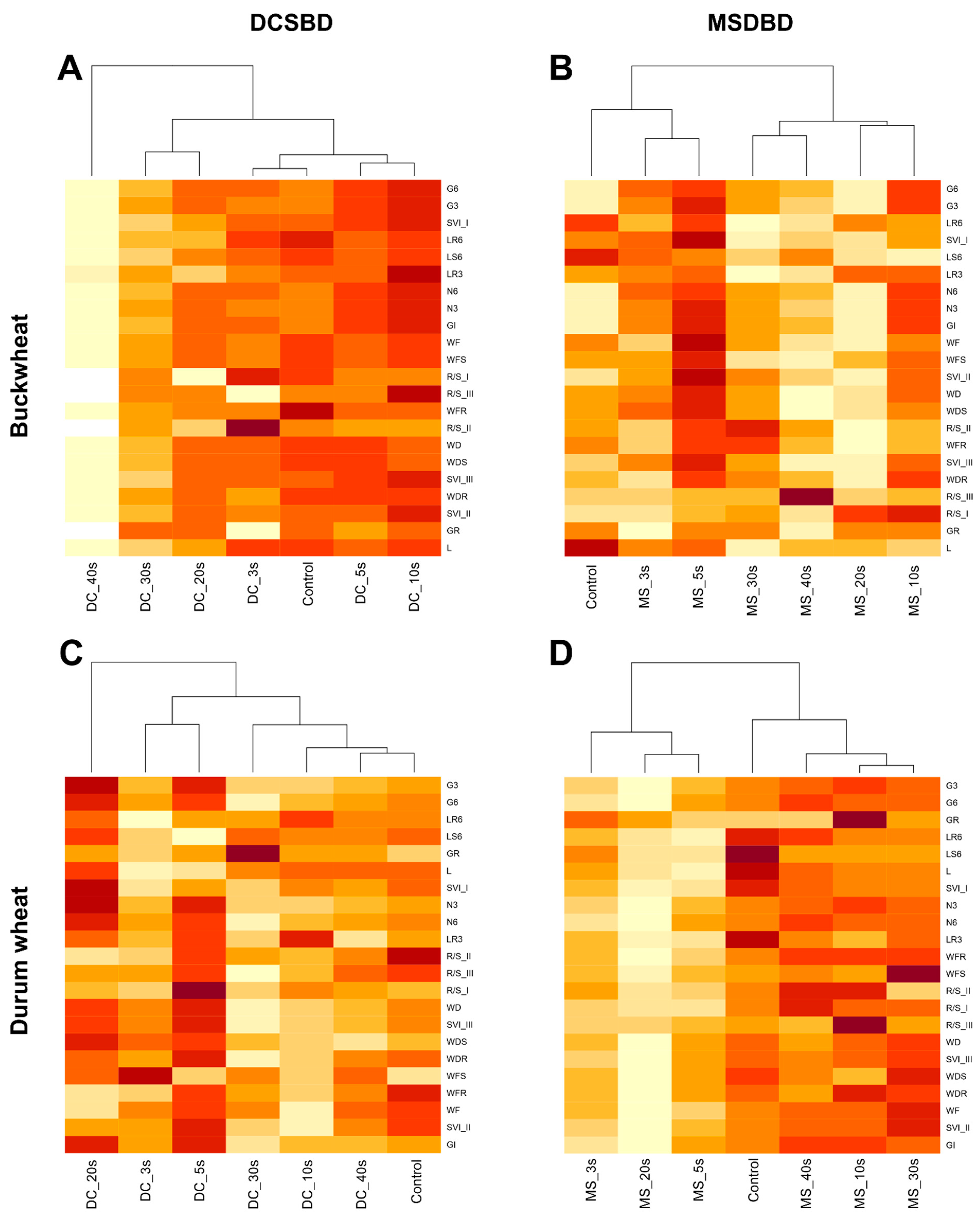
2. Results
2.1. Fruit Surface Diagnostic
2.2. Germination and Early Growth
3. Discussion
4. Materials and Methods
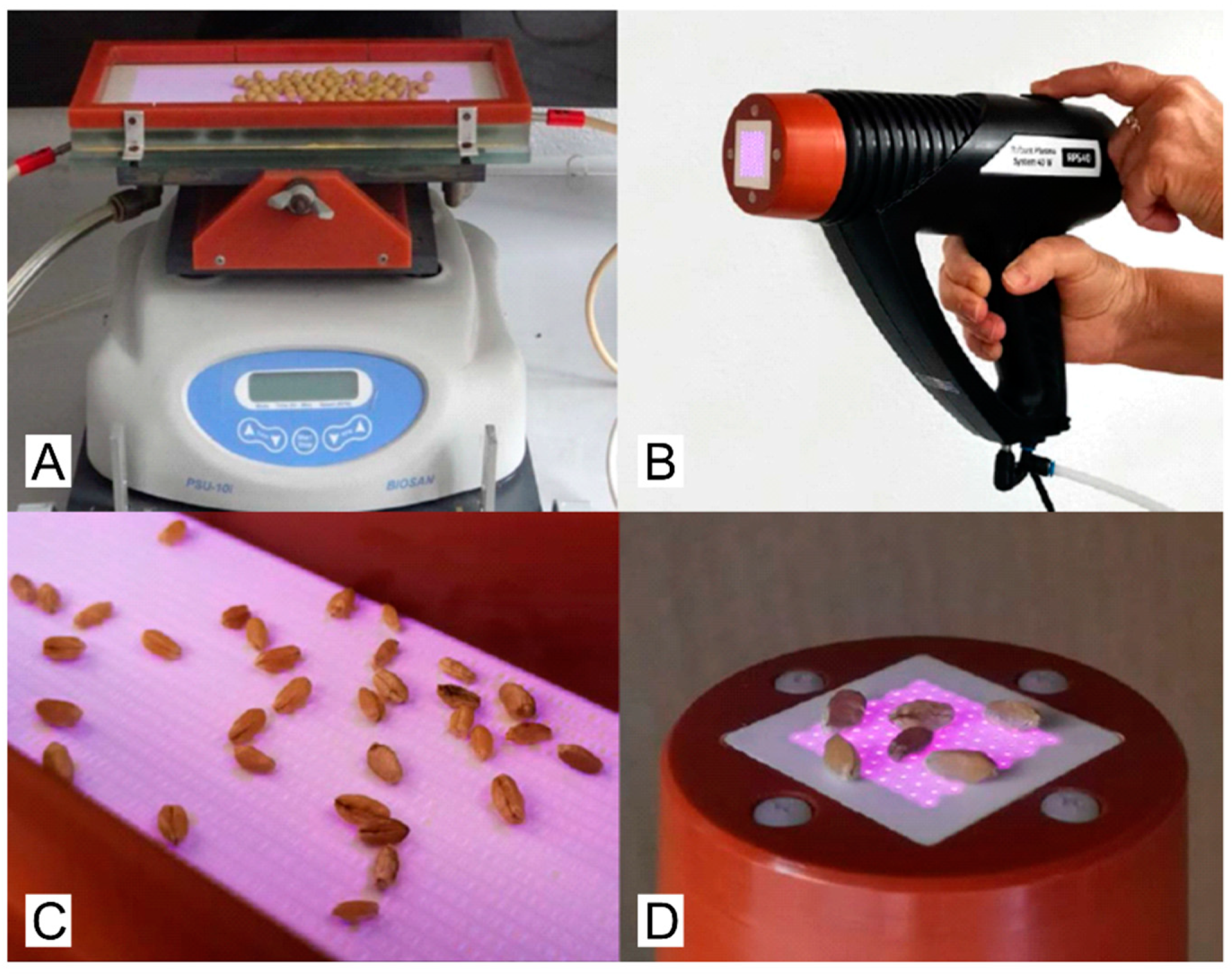
4.1. Description of Plasma Sources
4.2. Plant Material
4.3. Plasma Treatment
4.4. Seed Surface Diagnostics
4.5. Germination and Early Growth
4.6. Data Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Paterson, R.R.M.; Lima, N. How Will Climate Change Affect Mycotoxins in Food? Food Res. Int. 2010, 43, 1902–1914. [Google Scholar] [CrossRef]
- Macholdt, J.; Honermeier, B. Impact of Climate Change on Cultivar Choice: Adaptation Strategies of Farmers and Advisors in German Cereal Production. Agronomy 2016, 6, 40. [Google Scholar] [CrossRef]
- van Tilburg, A.J.; Hudson, P.F. Extreme Weather Events and Farmer Adaptation in Zeeland, the Netherlands: A European Climate Change Case Study from the Rhine Delta. Sci. Total Environ. 2022, 844, 157212. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, M.; Ceglar, A.; Dentener, F.; Toreti, A. Wheat Yield Loss Attributable to Heat Waves, Drought and Water Excess at the Global, National and Subnational Scales. Environ. Res. Lett. 2017, 12, 064008. [Google Scholar] [CrossRef]
- Dumalasová, V.; Bartoš, P. Reaction of Wheat, Alternative Wheat and Triticale Cultivars to Common Bunt. Czech J. Genet. Plant Breed. 2010, 46, 14–20. [Google Scholar] [CrossRef]
- Groth, S.; Wittmann, R.; Longin, C.F.H.; Böhm, V. Influence of Variety and Growing Location on Carotenoid and Vitamin E Contents of 184 Different Durum Wheat Varieties (Triticum turgidum ssp. Durum) in Germany. Eur. Food Res. Technol. 2020, 246, 2079–2092. [Google Scholar] [CrossRef]
- Rachoń, L.; Bobryk-Mamczarz, A.; Kiełtyka-Dadasiewicz, A. Hulled Wheat Productivity and Quality in Modern Agriculture against Conventional Wheat Species. Agriculture 2020, 10, 275. [Google Scholar] [CrossRef]
- Kolev, T.; Todorov, Z.; Mangova, M. The Variety-main Factor for Increasing Yield and Quality of Durum Wheat Grain. Sci. Papers Ser. A Agron. 2021, 64, 411–416. [Google Scholar]
- Erley, G.S.A.; Kaul, H.P.; Kruse, M.; Aufhammer, W. Yield and Nitrogen Utilization Efficiency of the Pseudocereals Amaranth, Quinoa, and Buckwheat under Differing Nitrogen Fertilization. Eur. J. Agron. 2005, 22, 95–100. [Google Scholar] [CrossRef]
- Nazco, R.; Peña, R.J.; Ammar, K.; Villegas, D.; Crossa, J.; Royo, C. Durum Wheat (Triticum durum Desf.) Mediterranean Landraces as Sources of Variability for Allelic Combinations at Glu-1/Glu-3 Loci Affecting Gluten Strength and Pasta Cooking Quality. Genet. Resour. Crop Evol. 2014, 61, 1219–1236. [Google Scholar] [CrossRef]
- Stasiak, M.; Gidzinski, D.; Jordan, M.; Dixon, M. Crop Selection for Advanced Life Support Systems in the ESA MELiSSA Program: Durum Wheat (Triticum turgidum Var Durum). Adv. Space Res. 2012, 49, 1684–1690. [Google Scholar] [CrossRef]
- Diffenbaugh, N.S.; Giorgi, F. Climate Change Hotspots in the CMIP5 Global Climate Model Ensemble. Clim. Change 2012, 114, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Royo, C.; Nazco, R.; Villegas, D. The Climate of the Zone of Origin of Mediterranean Durum Wheat (Triticum durum Desf.) Landraces Affects Their Agronomic Performance. Genet. Resour. Crop Evol. 2014, 61, 1345–1358. [Google Scholar] [CrossRef]
- Sabella, E.; Aprile, A.; Negro, C.; Nicolì, F.; Nutricati, E.; Vergine, M.; Luvisi, A.; De Bellis, L. Impact of Climate Change on Durum Wheat Yield. Agronomy 2020, 10, 793. [Google Scholar] [CrossRef]
- Ceglar, A.; Toreti, A.; Zampieri, M.; Royo, C. Global Loss of Climatically Suitable Areas for Durum Wheat Growth in the Future. Environ. Res. Lett. 2021, 16, 104049. [Google Scholar] [CrossRef]
- Bożek, K.S.; Żuk-Gołaszewska, K.; Bochenek, A.; Gołaszewski, J.; Kalaji, H.M. Modelling the Growth, Development and Yield of Triticum durum Desf under the Changes of Climatic Conditions in North-Eastern Europe. Sci. Rep. 2021, 11, 21753. [Google Scholar] [CrossRef] [PubMed]
- Angioloni, A.; Collar, C. Nutritional and Functional Added Value of Oat, Kamut ®, Spelt, Rye and Buckwheat versus Common Wheat in Breadmaking. J. Sci. Food Agric. 2011, 91, 1283–1292. [Google Scholar] [CrossRef]
- Unal, H.; Izli, G.; Izli, N.; Asik, B.B. Comparison of Some Physical and Chemical Characteristics of Buckwheat (Fagopyrum Esculentum Moench) Grains. CyTA-J. Food 2017, 15, 257–265. [Google Scholar] [CrossRef]
- Domingos, I.F.N.; Bilsborrow, P.E. The Effect of Variety and Sowing Date on the Growth, Development, Yield and Quality of Common Buckwheat (Fagopyrum Esculentum Moench). Eur. J. Agron. 2021, 126, 126264. [Google Scholar] [CrossRef]
- Sobhani, M.R.; Rahmikhdoev, G.; Mazaheri, D.; Majidian, M. Influence of Different Sowing Date and Planting Pattern and N Rate on Buckwheat Yield and Its Quality. Aust. J. Crop Sci. 2014, 8, 1402–1414. [Google Scholar]
- Mariotti, M.; Masoni, A.; Arduini, I. Forage and Grain Yield of Common Buckwheat in Mediterranean Conditions: Response to Sowing Time and Irrigation. Crop Pasture Sci. 2016, 67, 1000. [Google Scholar] [CrossRef]
- Siracusa, L.; Gresta, F.; Sperlinga, E.; Ruberto, G. Effect of Sowing Time and Soil Water Content on Grain Yield and Phenolic Profile of Four Buckwheat (Fagopyrum Esculentum Moench.) Varieties in a Mediterranean Environment. J. Food Compos. Anal. 2017, 62, 1–7. [Google Scholar] [CrossRef]
- Waman, A.A.; Bohra, P.; Norman, A. Chemical Pre-Treatments Improve Seed Germination and Seedling Growth in Semecarpus Kurzii: An Ethnomedicinally Important Plant. J. Res. 2018, 29, 1283–1289. [Google Scholar] [CrossRef]
- Araújo, S.D.S.; Paparella, S.; Dondi, D.; Bentivoglio, A.; Carbonera, D.; Balestrazzi, A. Physical Methods for Seed Invigoration: Advantages and Challenges in Seed Technology. Front. Plant Sci. 2016, 7, 646. [Google Scholar] [CrossRef] [PubMed]
- Campobenedetto, C.; Grange, E.; Mannino, G.; van Arkel, J.; Beekwilder, J.; Karlova, R.; Garabello, C.; Contartese, V.; Bertea, C.M. A Biostimulant Seed Treatment Improved Heat Stress Tolerance during Cucumber Seed Germination by Acting on the Antioxidant System and Glyoxylate Cycle. Front. Plant Sci. 2020, 11, 836. [Google Scholar] [CrossRef] [PubMed]
- Šimek, M.; Homola, T. Plasma-Assisted Agriculture: History, Presence, and Prospects—A Review. Eur. Phys. J. D 2021, 75, 210. [Google Scholar] [CrossRef]
- Attri, P.; Ishikawa, K.; Okumura, T.; Koga, K.; Shiratani, M. Plasma Agriculture from Laboratory to Farm: A Review. Processes 2020, 8, 1002. [Google Scholar] [CrossRef]
- Mitra, A.; Li, Y.F.; Klämpfl, T.G.; Shimizu, T.; Jeon, J.; Morfill, G.E.; Zimmermann, J.L. Inactivation of Surface-Borne Microorganisms and Increased Germination of Seed Specimen by Cold Atmospheric Plasma. Food Bioprocess Technol. 2014, 7, 645–653. [Google Scholar] [CrossRef]
- Zahoranová, A.; Henselová, M.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Medvecká, V.; Černák, M. Effect of Cold Atmospheric Pressure Plasma on the Wheat Seedlings Vigor and on the Inactivation of Microorganisms on the Seeds Surface. Plasma Chem. Plasma Process. 2016, 36, 397–414. [Google Scholar] [CrossRef]
- Li, Y.; Wang, T.; Meng, Y.; Qu, G.; Sun, Q.; Liang, D.; Hu, S. Air Atmospheric Dielectric Barrier Discharge Plasma Induced Germination and Growth Enhancement of Wheat Seed. Plasma Chem. Plasma Process. 2017, 37, 1621–1634. [Google Scholar] [CrossRef]
- Sandanuwan, T.; Attygalle, D.; Amarasinghe, S.; Weragoda, S.C.; Ranaweera, B.; Rathnayake, K.; Alankara, W. Shelf Life Extension of Cavendish Banana Fruit Using Cold Plasma Treatment. In Proceedings of the MERCon 2020—6th International Multidisciplinary Moratuwa Engineering Research Conference, Moratuwa, Sri Lanka, 29–30 July 2020. [Google Scholar]
- Takaki, K.; Takahashi, K.; Hamanaka, D.; Yoshida, R.; Uchino, T. Function of Plasma and Electrostatics for Keeping Quality of Agricultural Produce in Post-Harvest Stage. Jpn. J. Appl. Phys. 2020, 60, 010501. [Google Scholar] [CrossRef]
- Scholtz, V.; Pazlarova, J.; Souskova, H.; Khun, J.; Julak, J. Nonthermal Plasma—A Tool for Decontamination and Disinfection. Biotechnol. Adv. 2015, 33, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Guiyun, C.; Yushan, W.; Mingyue, Z.; Wanxing, M.; Xixian, X.; Ye, C. Cold Atmospheric Plasma Treatment Improves the γ-Aminobutyric Acid Content of Buckwheat Seeds Providing a New Anti-Hypertensive Functional Ingredient. Food Chem. 2022, 388, 133064. [Google Scholar] [CrossRef] [PubMed]
- Nedyalkova, S.; Bozhanova, V.; Benova, E.; Marinova, P.; Tsonev, I.; Bogdanov, T.; Koleva, M. Study on the Effect of Cold Plasma on the Germination and Growth of Durum Wheat Seeds Contaminated with Fusarium Graminearum. Int. J. Innov. Approaches Agric. Res. 2019, 3, 623–635. [Google Scholar] [CrossRef]
- Mravlje, J.; Kobal, T.; Regvar, M.; Starič, P.; Zaplotnik, R.; Mozetič, M.; Vogel-Mikuš, K. The Sensitivity of Fungi Colonising Buckwheat Grains to Cold Plasma Is Species Specific. J. Fungi 2023, 9, 609. [Google Scholar] [CrossRef] [PubMed]
- Scholtz, V.; Šerá, B.; Khun, J.; Šerý, M.; Julák, J. Effects of Nonthermal Plasma on Wheat Grains and Products. J. Food Qual. 2019, 2019, 7917825. [Google Scholar] [CrossRef]
- Homola, T.; Krumpolec, R.; Zemánek, M.; Kelar, J.; Synek, P.; Hoder, T.; Černák, M. An Array of Micro-Hollow Surface Dielectric Barrier Discharges for Large-Area Atmospheric-Pressure Surface Treatments. Plasma Chem. Plasma Process. 2017, 37, 1149–1163. [Google Scholar] [CrossRef]
- Dziadek, K.; Kopeć, A.; Pastucha, E.; Piątkowska, E.; Leszczyńska, T.; Pisulewska, E.; Witkowicz, R.; Francik, R. Basic Chemical Composition and Bioactive Compounds Content in Selected Cultivars of Buckwheat Whole Seeds, Dehulled Seeds and Hulls. J. Cereal Sci. 2016, 69, 1–8. [Google Scholar] [CrossRef]
- Devi, P.B.; Vijayabharathi, R.; Sathyabama, S.; Malleshi, N.G.; Priyadarisini, V.B. Health Benefits of Finger Millet (Eleusine coracana L.) Polyphenols and Dietary Fiber: A Review. J. Food Sci. Technol. 2014, 51, 1021–1040. [Google Scholar] [CrossRef]
- Kostryukov, S.G.; Matyakubov, H.B.; Masterova, Y.Y.; Kozlov, A.S.; Pryanichnikova, M.K.; Pynenkov, A.A.; Khluchina, N.A. Determination of Lignin, Cellulose, and Hemicellulose in Plant Materials by FTIR Spectroscopy. J. Anal. Chem. 2023, 78, 718–727. [Google Scholar] [CrossRef]
- Javier-Astete, R.; Jimenez-Davalos, J.; Zolla, G. Determination of Hemicellulose, Cellulose, Holocellulose and Lignin Content Using FTIR in Calycophyllum spruceanum (Benth.) K. Schum. And Guazuma crinita Lam. PLoS ONE 2021, 16, e0256559. [Google Scholar] [CrossRef] [PubMed]
- Tomeková, J.; Kyzek, S.; Medvecká, V.; Gálová, E.; Zahoranová, A. Influence of Cold Atmospheric Pressure Plasma on Pea Seeds: DNA Damage of Seedlings and Optical Diagnostics of Plasma. Plasma Chem. Plasma Process. 2020, 40, 1571–1584. [Google Scholar] [CrossRef]
- Švubová, R.; Kyzek, S.; Medvecká, V.; Slováková, Ľ.; Gálová, E.; Zahoranová, A. Novel Insight at the Effect of Cold Atmospheric Pressure Plasma on the Activity of Enzymes Essential for the Germination of Pea (Pisum sativum L. Cv. Prophet) Seeds. Plasma Chem. Plasma Process. 2020, 40, 1221–1240. [Google Scholar] [CrossRef]
- Ďurčányová, S.; Slováková, Ľ.; Klas, M.; Tomeková, J.; Ďurina, P.; Stupavská, M.; Kováčik, D.; Zahoranová, A. Efficacy Comparison of Three Atmospheric Pressure Plasma Sources for Soybean Seed Treatment: Plasma Characteristics, Seed Properties, Germination. Plasma Chem. Plasma Process. 2023, 43, 1863–1885. [Google Scholar] [CrossRef]
- Slavíček, P.; Štěpánová, V.; Fleischer, M.; Kelar, J.; Kelar Tučeková, Z.; Jurmanová, J.; Pazderka, M.; Prášil, V.; Prášil, J. The Multi-Hollow Surface Dielectric Barrier Discharge Usage for the Seeds’ Treatment Aimed to the Dustiness Decrease of Free-Floating Particles from Agrochemicals. Plasma Chem. Plasma Process. 2023, 43, 1887–1906. [Google Scholar] [CrossRef]
- Mildaziene, V.; Ivankov, A.; Sera, B.; Baniulis, D. Biochemical and Physiological Plant Processes Affected by Seed Treatment with Non-Thermal Plasma. Plants 2022, 11, 856. [Google Scholar] [CrossRef] [PubMed]
- Royo, C.; Maccaferri, M.; Álvaro, F.; Moragues, M.; Sanguineti, M.C.; Tuberosa, R.; Maalouf, F.; del Moral, L.F.G.; Demontis, A.; Rhouma, S.; et al. Understanding the Relationships between Genetic and Phenotypic Structures of a Collection of Elite Durum Wheat Accessions. Field Crops Res. 2010, 119, 91–105. [Google Scholar] [CrossRef]
- Monneveux, P.; Jing, R.; Misra, S.C. Phenotyping for Drought Adaptation in Wheat Using Physiological Traits. Front. Physiol. 2012, 3, 429. [Google Scholar] [CrossRef]
- Chaouachi, L.; Marín-Sanz, M.; Kthiri, Z.; Boukef, S.; Harbaoui, K.; Barro, F.; Karmous, C. The Opportunity of Using Durum Wheat Landraces to Tolerate Drought Stress: Screening Morpho-Physiological Components. AoB Plants 2023, 15, plad022. [Google Scholar] [CrossRef]
- Šerá, B.; Gajdovâ, I.; Černâk, M.; Gavril, B.; Hnatiuc, E.; Kováčik, D.; Kříha, V.; Sláma, J.; Šerý, M.; Špatenka, P. How Various Plasma Sources May Affect Seed Germination and Growth. In Proceedings of the 2012 13th International Conference on Optimization of Electrical and Electronic Equipment (OPTIM), Brasov, Romania, 24–26 May 2012; pp. 1365–1370. [Google Scholar]
- Ivankov, A.; Naučienė, Z.; Degutytė-Fomins, L.; Žūkienė, R.; Januškaitienė, I.; Malakauskienė, A.; Jakštas, V.; Ivanauskas, L.; Romanovskaja, D.; Šlepetienė, A.; et al. Changes in Agricultural Performance of Common Buckwheat Induced by Seed Treatment with Cold Plasma and Electromagnetic Field. Appl. Sci. 2021, 11, 4391. [Google Scholar] [CrossRef]
- Starič, P.; Remic, L.; Vogel-Mikuš, K.; Junkar, I.; Vavpetič, P.; Kelemen, M.; Pongrac, P. Exploring the Potential of Cold Plasma Treatment Followed by Zinc-Priming for Biofortification of Buckwheat Sprouts. Front. Nutr. 2023, 10, 1151101. [Google Scholar] [CrossRef] [PubMed]
- Černák, M.; Černáková, L.; Hudec, I.; Kováčik, D.; Zahoranová, A. Diffuse Coplanar Surface Barrier Discharge and Its Applications for In-Line Processing of Low-Added-Value Materials. Eur. Phys. J.-Appl. Phys. 2009, 47, 22806. [Google Scholar] [CrossRef]
- Šerá, B. Methodological Contribution on Seed Germination and Seedling Initial Growth Tests in Wild Plants. Not. Bot. Horti Agrobot. Cluj. Napoca 2023, 51, 13164. [Google Scholar] [CrossRef]

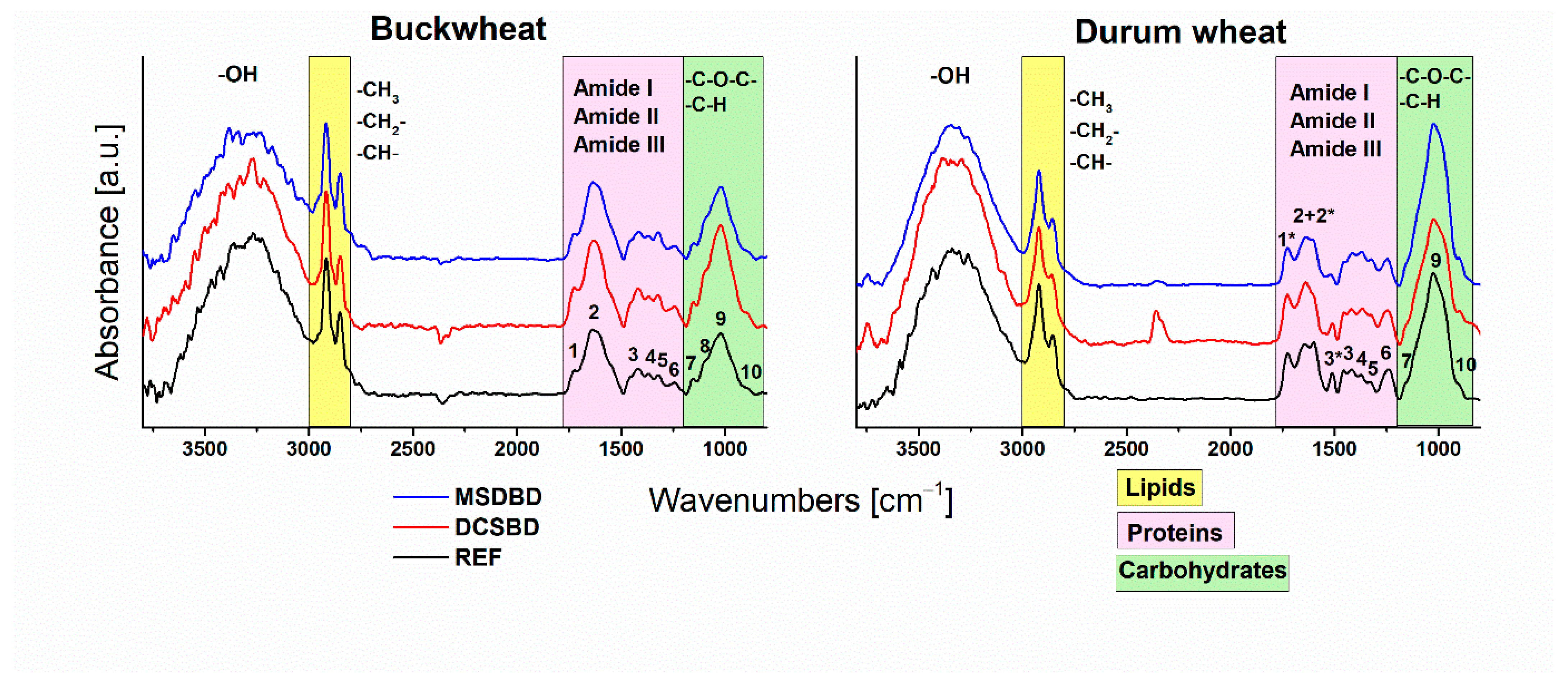

| Peak | Absorption Band [cm−1] | Assignments | Source of Peak |
|---|---|---|---|
| 1 + 1 * | 1760–1700 | C=O stretching vibrations of carbonyl, carboxyl groups | lignin, hemicellulose |
| 2 + 2 * | 1670–1530 | Amide I, Amide II (C=O, N–H, C–N vibrations), aromatic ring vibrations | proteins, lignin |
| 3 | 1460, 1415 | O-H in-plane bending, C-H in-plane bending in OCH3 (lignin), C–C stretching of aromatic ring (lignin), symmetrical bending vibrations in CH2 | lignin, cellulose, hemicellulose |
| 3 * | 1510 | aromatic skeletal vibrations | lignin |
| 4 | 1368 | C–H, O–H bending vibrations | cellulose, hemicellulose |
| 5 | 1320–1315 | CH2 wagging | cellulose, hemicellulose |
| 6 | 1244–1240 | C–O stretch, O–H in plane vibrations | cellulose, hemicellulose |
| 7 | 1153–1151 | C–O–C asymmetric stretching vibrations | cellulose, hemicellulose |
| 8 | 1096 | aromatic C–H in-plane deformation, C–O–C asymmetric stretching vibrations | lignin, cellulose, hemicellulose |
| 9 | 1030–1020 | C-O stretching | lignin, hemicellulose |
| 10 | 900–880 | C-H vibrations of aromatic ring, C-H vibrations of polysaccharide units | lignin, cellulose, hemicellulose |
| Plants | Apparatus | Treatment Time (s) | Germination on the 3rd Day (%) | Germination on the 6th Day (%) | Germination Rate (%) | Germination Index | Seedling Vigor Index I. (mm) | Seedling Vigor Index II. (mg) | Seedling Vigor Index III. (mg) | R/S_Length | R/S_Fresh_ Weight | R/S_Dry_ Weight |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | |||
| Buckwheat | control | 0 | 44.0 ± 5.5 ab | 44.0 ± 5.5 ab | 100 ± 0.0 a | 6.6 ± 0.8 ab | 45.1 ± 7.3 ab | 0.7 ± 0.2 ab | 0.07 ± 0.01 ab | 1.7 ± 0.1 a | 0.2 ± 0.1 a | 0.19 ± 0.03 a |
| DCSBD | 3 | 45.3 ± 5.9 ab | 47.3 ± 6.5 ab | 96.4 ± 2.6 a | 6.9 ± 0.9 ab | 46.0 ± 9.3 ab | 0.6 ± 0.2 ab | 0.07 ± 0.02 ab | 1.8 ± 0.3 a | 0.3 ± 0.2 a | 0.14 ± 0.01 a | |
| 5 | 52.7 ± 2.9 a | 53.3 ± 3.2 a | 98.9 ± 1.1 a | 7.9 ± 0.4 a | 49.3 ± 3.7 ab | 0.7 ± 0.1 ab | 0.09 ± 0.01 ab | 1.6 ± 0.2 a | 0.2 ± 0.0 a | 0.19 ± 0.01 a | ||
| 10 | 59.3 ± 4.3 a | 59.3 ± 4.4 a | 100 ± 0.0 a | 8.9 ± 0.6 a | 58.2 ± 3.8 b | 0.9 ± 0.1 b | 0.09 ± 0.02 b | 1.5 ± 0.2 a | 0.2 ± 0.0 a | 0.23 ± 0.02 a | ||
| 20 | 48.7 ± 1.7 ab | 48.7 ± 1.8 ab | 100 ± 0.0 a | 7.3 ± 0.3 ab | 34.9 ± 4.2 ad | 0.7 ± 0.1 ab | 0.07 ± 0.01 ab | 1.1 ± 0.1 a | 0.1 ± 0.0 a | 0.19 ± 0.03 a | ||
| 30 | 34.0 ± 2.9 b | 34.0 ± 2.10 b | 100 ± 0.0 a | 5.1 ± 0.4 b | 19.9 ± 3.3 cd | 0.4 ± 0.1 ac | 0.03 ± 0.00 c | 1.5 ± 0.2 a | 0.2 ± 0.0 a | 0.20 ± 0.03 a | ||
| 40 | 5.3 ±1.7 c | 5.3 ±1.8 c | - | 0.8 ± 0.3 c | 2.1 ± 0.9 c | 0.0 ± 0.0 c | 0.00 ± 0.00 c | - | - | - | ||
| control | 0 | 44.0 ± 5.5 a | 44.0 ± 5.5 a | 100 ± 0.0 a | 6.6 ± 0.8 a | 45.1 ± 7.3 ab | 0.7 ± 0.2 a | 0.07 ± 0.01 a | 1.7 ± 0.1 a | 0.2 ± 0.1 a | 0.19 ± 0.03 a | |
| MSDBD | 3 | 54.7 ± 3.3 a | 56.7 ± 3.8 a | 96.7 ± 1.3 a | 8.3 ± 0.5 a | 47.6 ± 4.7 ab | 0.9 ± 0.1 a | 0.09 ± 0.02 a | 1.8 ± 0.1 a | 0.2 ± 0.0 a | 0.16 ± 0.02 a | |
| 5 | 60.7 ± 6.0 a | 60.7 ± 6.1 a | 100 ± 0.0 a | 9.1 ± 0.9 a | 55.7 ± 7.4 b | 1.1 ± 0.2 a | 0.12 ± 0.02 a | 2.4 ± 0.3 ab | 0.2 ± 0.1 a | 0.21 ± 0.05 a | ||
| 10 | 58.7 ± 3.4 a | 58.7 ± 3.5 a | 100 ± 0.0 a | 8.8 ± 0.5 a | 41.4 ± 2.8 ab | 1.0 ± 0.1 a | 0.10 ± 0.01 a | 3.8 ± 0.1 c | 0.2 ± 0.1 a | 0.25 ± 0.04 a | ||
| 20 | 44.7 ± 5.6 a | 44.7 ± 5.7 a | 100 ± 0.0 a | 6.7 ± 0.8 a | 32.9 ± 3.6 a | 0.7 ± 0.1 a | 0.06 ± 0.01 a | 3.6 ± 0.2 bc | 0.1 ± 0.0 a | 0.19 ± 0.01 a | ||
| 30 | 52.7 ± 8.1 a | 52.7 ± 8.2 a | 100 ± 0.0 a | 7.9 ± 1.2 a | 31.6 ± 4.5 a | 0.9 ± 0.3 a | 0.09 ± 0.03 a | 2.6 ± 0.5 ab | 0.3 ± 0.1 a | 0.23 ± 0.02 a | ||
| 40 | 49.3 ± 3.4 a | 50.7 ± 3.7 a | 97.6 ± 1.5 a | 7.5 ± 0.5 a | 38.2 ± 2.3 ab | 0.8 ± 0.2 a | 0.06 ± 0.01 a | 1.7 ± 0.3 a | 0.2 ± 0.0 a | 0.76 ± 0.58 a | ||
| Hard wheat | control | 0 | 88.0 ± 3.4 a | 88.7 ± 3.4 a | 99.3 ± 0.7 a | 13.2 ± 0.5 a | 147.7 ± 5.8 a | 3.1 ± 0.2 a | 0.37 ± 0.02 ab | 1.0 ± 0.0 a | 1.1 ± 0.1 b | 1.00 ± 0.06 a |
| DCSBD | 3 | 87.3 ± 1.6 a | 88.0 ± 1.7 a | 99.3 ± 0.7 a | 13.1 ± 0.2 a | 135.8 ± 7.1 a | 2.9 ± 0.1 a | 0.36 ± 0.02 abc | 1.0 ± 0.1 a | 0.8 ± 0.0 a | 0.86 ± 0.05 ab | |
| 5 | 91.3 ± 2.3 a | 91.3 ± 2.3 a | 100.0 ± 0.0 a | 13.7 ± 0.3 a | 143.1 ± 5.6 a | 3.1 ± 0.1 a | 0.42 ± 0.03 b | 1.2 ± 0.0 a | 1.0 ± 0.1 ab | 1.01 ± 0.05 a | ||
| 10 | 86.7 ± 2.8 a | 86.7 ± 2.8 a | 100.0 ± 0.0 a | 13.0 ± 0.4 a | 144.8 ± 6.3 a | 2.7 ± 0.2 a | 0.32 ± 0.02 ac | 1.0 ± 0.1 a | 0.8 ± 0.1 ab | 0.79 ± 0.03 ab | ||
| 20 | 92.0 ± 1.7 a | 92.0 ± 1.7 a | 100.0 ± 0.0 a | 13.8 ± 0.3 a | 155.8 ± 3.8 a | 2.9 ± 0.1 a | 0.40 ± 0.02 ab | 1.0 ± 0.1 a | 0.7 ± 0.1 a | 0.86 ± 0.05 ab | ||
| 30 | 83.3 ± 4.2 a | 86.0 ± 4.0 a | 103.5 ± 3.5 a | 12.8 ± 0.6 a | 138.3 ± 9.4 a | 2.8 ± 0.3 a | 0.28 ± 0.02 b | 1.0 ± 0.1 a | 0.8 ± 0.1 ab | 0.63 ± 0.05 b | ||
| 40 | 87.3 ± 3.4 a | 87.3 ± 3.4 a | 100.0 ± 0.0 a | 13.1 ± 0.5 a | 144.3 ± 5.4 a | 3.0 ± 0.2 a | 0.34 ± 0.02 abc | 1.0 ± 0.0 a | 0.9 ± 0.1 ab | 0.96 ± 0.10 a | ||
| control | 0 | 88.0 ± 3.4 a | 88.7 ± 3.4 a | 99.3 ± 0.7 a | 13.2 ± 0.5 a | 147.7 ± 5.8 b | 3.1 ± 0.2 a | 0.37 ± 0.02 a | 1.0 ± 0.0 ab | 1.1 ± 0.1 a | 1.00 ± 0.06 a | |
| MSDBD | 3 | 82.7 ± 4.4 a | 83.3 ± 4.3 a | 101.0 ± 1.6 a | 12.5 ± 0.6 a | 117.3 ± 15.2 b | 2.6 ± 0.3 a | 0.31 ± 0.04 a | 0.9 ± 0.1 ab | 1.0 ± 0.1 a | 0.95 ± 0.09 a | |
| 5 | 86.0 ± 3.6 a | 86.7 ± 3.9 a | 99.3 ± 0.7 a | 12.9 ± 0.6 a | 104.8 ± 7.7 ab | 2.7 ± 0.3 a | 0.34 ± 0.03 a | 0.9 ± 0.1 a | 0.9 ± 0.1 a | 0.98 ± 0.04 a | ||
| 10 | 89.3 ± 2.7 a | 91.3 ± 0.8 a | 102.6 ± 3.6 a | 13.6 ± 0.1 a | 129.0 ± 8.0 ab | 3.2 ± 0.1 a | 0.37 ± 0.02 a | 1.0 ± 0.1 ab | 1.1 ± 0.0 a | 1.19 ± 0.03 a | ||
| 20 | 80.0 ± 11.3 a | 80.0 ± 11.3 a | 100.0 ± 0.0 a | 12.0 ± 1.7 a | 99.8 ± 15.3 a | 2.3 ± 0.4 a | 0.26 ± 0.05 a | 0.9 ± 0.0 ab | 0.9 ± 0.1 a | 0.93 ± 0.11 a | ||
| 30 | 90.0 ± 1.1 a | 90.0 ± 1.1 a | 100.0 ± 0.0 a | 13.5 ± 0.2 a | 130.0 ± 7.8 ab | 3.5 ± 0.3 a | 0.39 ± 0.01 a | 1.0 ± 0.0 ab | 0.9 ± 0.1 a | 1.00 ± 0.03 a | ||
| 40 | 90.0 ± 2.4 a | 90.1 ± 2.4 a | 99.3 ± 0.7 a | 13.5 ± 0.4 a | 136.2 ± 5.1 ab | 3.3 ± 0.3 a | 0.35 ± 0.02 a | 1.0 ± 0.0 b | 1.1 ± 0.1 a | 0.96 ± 0.03 a |
| Plants | Apparatus | Treatment Time (s) | Length of Root on the 3rd Day (mm) | Length of Root on the 6th Day (mm) | Length of Shoot (mm) | Length of Seedling (mm) | Weight of Fresh Root (mg) | Weight of Fresh Shoot (mg) | Weight of Fresh Seedling (mg) | Weight of Dried Root (mg) | Weight of Dried Shoot (mg) | Weight of Dried Seedling (mg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | |||
| Buckwheat | control | 0 | 24.1 ± 2.4 ab | 63.3 ± 4.8 a | 37.4 ± 3.5 a | 100.7 ± 7.7 a | 0.3 ± 0.1 b | 1.4 ± 0.2 a | 1.6 ± 0.3 a | 0.03 ± 0.01 a | 0.13 ± 0.01 ab | 0.16 ± 0.02 a |
| DCSBD | 3 | 22.0 ± 3.1 ab | 58.9 ± 4.0 a | 36.1 ± 5.5 ab | 95.0 ± 7.7 a | 0.2 ± 0.0 ab | 1.0 ± 0.2 a | 1.2 ± 0.2 a | 0.02 ± 0.00 a | 0.13 ± 0.02 ab | 0.14 ± 0.02 a | |
| 5 | 24.0 ± 2.4 ab | 55.7 ± 4.0 ac | 36.5 ± 1.7 a | 92.3 ± 3.0 a | 0.2 ± 0.0 ab | 1.2 ± 0.1 a | 1.4 ± 0.1 a | 0.03 ± 0.00 a | 0.14 ± 0.00 b | 0.16 ± 0.01 a | ||
| 10 | 30.2 ± 0.7 b | 58.8 ± 2.7 a | 39.7 ± 3.7 a | 98.5 ± 2.8 a | 0.2 ± 0.0 ab | 1.3 ± 0.1 a | 1.5 ± 0.1 a | 0.03 ± 0.00 a | 0.13 ± 0.02 ab | 0.15 ± 0.02 a | ||
| 20 | 15.2 ± 0.9 ac | 37.0 ± 4.9 bc | 34.0 ± 2.4 ab | 70.9 ± 6.4 a | 0.2 ± 0.1 ab | 1.2 ± 0.1 a | 1.4 ± 0.2 a | 0.00 ± 0.01 b | 0.12 ± 0.01 ab | 0.14 ± 0.01 a | ||
| 30 | 17.9 ± 2.3 ac | 34.3 ± 3.5 b | 24.0 ± 4.2 ab | 58.3 ± 7.4 bc | 0.2 ± 0.0 ab | 0.9 ± 0.1 a | 1.0 ± 0.1 a | 0.02 ± 0.00 ab | 0.08 ± 0.00 a | 0.10 ± 0.01 a | ||
| 40 | 10.4 ± 4.2 c | 13.7 ± 6.0 d | 17.0 ± 6.8 b | 30.8 ± 12.7 b | 0.0 ± 0.0 a | 0.1 ± 0.0 b | 0.1 ± 0.0 b | 0.00 ± 0.00 b | 0.01 ± 0.00 c | 0.01 ± 0.00 b | ||
| control | 0 | 24.1 ± 2.4 a | 63.3 ± 4.8 a | 37.4 ± 3.5 c | 100.7 ± 7.7 b | 0.3 ± 0.1 a | 1.4 ± 0.2 a | 1.6 ± 0.3 a | 0.03 ± 0.01 a | 0.13 ± 0.01 a | 0.16 ± 0.02 a | |
| MSDBD | 3 | 25.1 ± 2.0 a | 54.3 ± 5.1 a | 23.0 ± 1.9 ac | 84.3 ± 6.7 ab | 0.2 ± 0.1 a | 1.3 ± 0.1 a | 1.6 ± 0.1 a | 0.02 ± 0.00 a | 0.14 ± 0.02 a | 0.16 ± 0.02 a | |
| 5 | 26.0 ± 3.8 a | 64.1 ± 7.3 a | 27.4 ± 3.7 abc | 91.6 ± 9.8 ab | 0.4 ± 0.1 a | 1.5 ± 0.2 a | 1.8 ± 0.2 a | 0.03 ± 0.01 a | 0.12 ± 0.01 a | 0.18 ± 0.02 a | ||
| 10 | 25.8 ± 1.8 a | 56.5 ± 4.6 a | 14.7 ± 1.0 b | 71.2 ± 5.6 ab | 0.3 ± 0.1 a | 1.4 ± 0.1 a | 1.7 ± 0.1 a | 0.03 ± 0.01 a | 0.14 ± 0.01 a | 0.17 ± 0.01 a | ||
| 20 | 26.4 ± 1.9 a | 58.8 ± 3.9 a | 16.3 ± 1.0 ab | 75.1 ± 4.7 ab | 0.1 ± 0.0 a | 1.3 ± 0.2 a | 1.5 ± 0.2 a | 0.02 ± 0.00 a | 0.11 ± 0.01 a | 0.13 ± 0.02 a | ||
| 30 | 18.5 ± 1.3 a | 42.8 ± 4.4 a | 20.2 ± 5.8 ab | 63.0 ± 7.3 a | 0.4 ± 0.1 a | 1.3 ± 0.2 a | 1.6 ± 0.3 a | 0.03 ± 0.01 a | 0.13 ± 0.02 a | 0.16 ± 0.03 a | ||
| 40 | 20.6 ± 2.2 a | 46.6 ± 4.6 a | 29.3 ± 2.7 ac | 75.9 ± 3.3 ab | 0.3 ± 0.1 a | 1.3 ± 0.2 a | 1.5 ± 0.3 a | 0.03 ± 0.01 a | 0.09 ± 0.02 a | 0.12 ± 0.01 a | ||
| Hard wheat | control | 0 | 45.0 ± 2.9 a | 84.4 ± 1.4 a | 82.2 ± 1.7 a | 166.6 ± 1.5 a | 1.8 ± 0.1 a | 1.7 ± 0.1 a | 3.4 ± 0.2 a | 0.21 ± 0.01 ab | 0.21 ± 0.01 a | 0.41 ± 0.01 ab |
| DCSBD | 3 | 43.7 ± 2.8 a | 77.7 ± 5.9 a | 76.5 ± 3.1 a | 154.2 ± 7.3 a | 1.4 ± 0.1 a | 1.9 ± 0.1 a | 3.3 ± 0.1 a | 0.19 ± 0.01 ab | 0.22 ± 0.01 a | 0.41 ± 0.01 ab | |
| 5 | 47.1 ± 2.4 a | 83.7 ± 1.3 a | 72.9 ± 3.4 a | 156.6 ± 4.5 a | 1.7 ± 0.1 a | 1.7 ± 0.1 a | 3.4 ± 0.2 a | 0.23 ± 0.01 b | 0.23 ± 0.01 a | 0.46 ± 0.02 b | ||
| 10 | 47.8 ± 1.8 a | 86.6 ± 6.4 a | 80.9 ± 1.7 a | 167.5 ± 7.7 a | 1.4 ± 0.1 a | 1.7 ± 0.1 a | 3.1 ± 0.2 a | 0.16 ± 0.01 ac | 0.20 ± 0.01 a | 0.36 ± 0.02 ac | ||
| 20 | 46.5 ± 0.7 a | 85.9 ± 3.1 a | 83.5 ± 2.5 a | 169.4 ± 2.7 a | 1.4 ± 0.1 a | 1.8 ± 0.0 a | 3.2 ± 0.1 a | 0.20 ± 0.01 ab | 0.23 ± 0.00 a | 0.43 ± 0.01 ab | ||
| 30 | 42.9 ± 2.5 a | 83.5 ± 6.1 a | 82.2 ± 2.3 a | 165.8 ± 7.1 a | 1.5 ± 0.1 a | 1.8 ± 0.1 a | 3.4 ± 0.2 a | 0.13 ± 0.01 c | 0.21 ± 0.01 a | 0.34 ± 0.01 b | ||
| 40 | 42.1 ± 1.9 a | 84.7 ± 5.7 a | 81.5 ± 3.5 a | 166.2 ± 8.9 a | 1.6 ± 0.1 a | 1.8 ± 0.1 a | 3.4 ± 0.1 a | 0.19 ± 0.01 ab | 0.20 ± 0.01 a | 0.39 ± 0.02 ab | ||
| control | 0 | 45.0 ± 2.9 b | 84.4 ± 1.4 b | 82.2 ± 1.7 c | 166.6 ± 1.5 b | 1.8 ± 0.1 a | 1.7 ± 0.1 ab | 3.4 ± 0.2 ab | 0.21 ± 0.01 ab | 0.21 ± 0.01 a | 0.41 ± 0.01 ab | |
| MSDBD | 3 | 35.9 ± 3.3 ab | 67.9 ± 7.8 ab | 71.5 ± 5.3 ab | 139.4 ± 12.9 ab | 1.5 ± 0.2 a | 1.6 ± 0.1 ab | 3.4 ± 0.3 ab | 0.18 ± 0.02 ab | 0.19 ± 0.01 a | 0.37 ± 0.03 ab | |
| 5 | 33.1 ± 1.9 a | 57.0 ± 3.5 a | 63.9 ± 4.0 a | 120.8 ± 5.9 a | 1.5 ± 0.1 a | 1.6 ± 0.1 ab | 3.4 ± 0.2 ab | 0.19 ± 0.01 ab | 0.19 ± 0.01 a | 0.38 ± 0.02 ab | ||
| 10 | 36.2 ± 1.1 ab | 74.0 ± 2.6 ab | 69.9 ± 3.6 ab | 143.9 ± 5.5 ab | 1.9 ± 0.1 a | 1.7 ± 0.1 ab | 3.6 ± 0.1 ab | 0.23 ± 0.01 a | 0.19 ± 0.01 a | 0.42 ± 0.01 a | ||
| 20 | 31.4 ± 1.0 a | 60.2 ± 4.3 a | 64.5 ± 2.4 a | 124.8 ± 6.6 a | 1.3 ± 0.2 a | 1.4 ± 0.2 a | 2.7 ± 0.3 a | 0.14 ± 0.01 b | 0.16 ± 0.02 a | 0.31 ± 0.03 b | ||
| 30 | 39.7 ± 2.0 ab | 74.0 ± 4.3 ab | 70.2 ± 4.4 ab | 144.3 ± 8.4 ab | 1.9 ± 0.2 a | 2.0 ± 0.1 b | 3.9 ± 0.3 b | 0.22 ± 0.01 a | 0.21 ± 0.00 a | 0.43 ± 0.01 a | ||
| 40 | 39.4 ± 1.1 ab | 80.0 ± 2.7 b | 70.4 ± 2.2 ab | 150.4 ± 4.8 ab | 1.9 ± 0.1 a | 1.7 ± 0.1 ab | 3.6 ± 0.2 ab | 0.19 ± 0.00 ab | 0.20 ± 0.01 a | 0.39 ± 0.02 ab |
| Treatment Time (s) | DCSBD | MSDBD | Suma | |
|---|---|---|---|---|
| Buckwheat | 3 | 1× | 1× | 2× |
| 5 | 2× | 12× | 14× | |
| 10 | 5× | 4× | 9× | |
| 20 | 1× | 1× | 2× | |
| 30 | 1× | 4× | 5× | |
| 40 | 0× | 2× | 2× | |
| Sum | 10× | 24× | 34× | |
| Durum wheat | 3 | 0× | 0× | 0× |
| 5 | 5× | 0× | 5× | |
| 10 | 2× | 4× | 6× | |
| 20 | 7× | 0× | 7× | |
| 30 | 1× | 4× | 5× | |
| 40 | 0× | 2× | 2× | |
| Sum | 15× | 10× | 25× |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tunklová, B.; Šerá, B.; Šrámková, P.; Ďurčányová, S.; Šerý, M.; Kováčik, D.; Zahoranová, A.; Hnilička, F. Growth Stimulation of Durum Wheat and Common Buckwheat by Non-Thermal Atmospheric Pressure Plasma. Plants 2023, 12, 4172. https://doi.org/10.3390/plants12244172
Tunklová B, Šerá B, Šrámková P, Ďurčányová S, Šerý M, Kováčik D, Zahoranová A, Hnilička F. Growth Stimulation of Durum Wheat and Common Buckwheat by Non-Thermal Atmospheric Pressure Plasma. Plants. 2023; 12(24):4172. https://doi.org/10.3390/plants12244172
Chicago/Turabian StyleTunklová, Barbora, Božena Šerá, Petra Šrámková, Sandra Ďurčányová, Michal Šerý, Dušan Kováčik, Anna Zahoranová, and František Hnilička. 2023. "Growth Stimulation of Durum Wheat and Common Buckwheat by Non-Thermal Atmospheric Pressure Plasma" Plants 12, no. 24: 4172. https://doi.org/10.3390/plants12244172
APA StyleTunklová, B., Šerá, B., Šrámková, P., Ďurčányová, S., Šerý, M., Kováčik, D., Zahoranová, A., & Hnilička, F. (2023). Growth Stimulation of Durum Wheat and Common Buckwheat by Non-Thermal Atmospheric Pressure Plasma. Plants, 12(24), 4172. https://doi.org/10.3390/plants12244172








